Metabolic Markers Demonstrate the Heterogeneity of Walking Ability in Non-Disabled Community-Dwelling Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Walking Ability Index (WAI)
2.3. Metabolomics
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
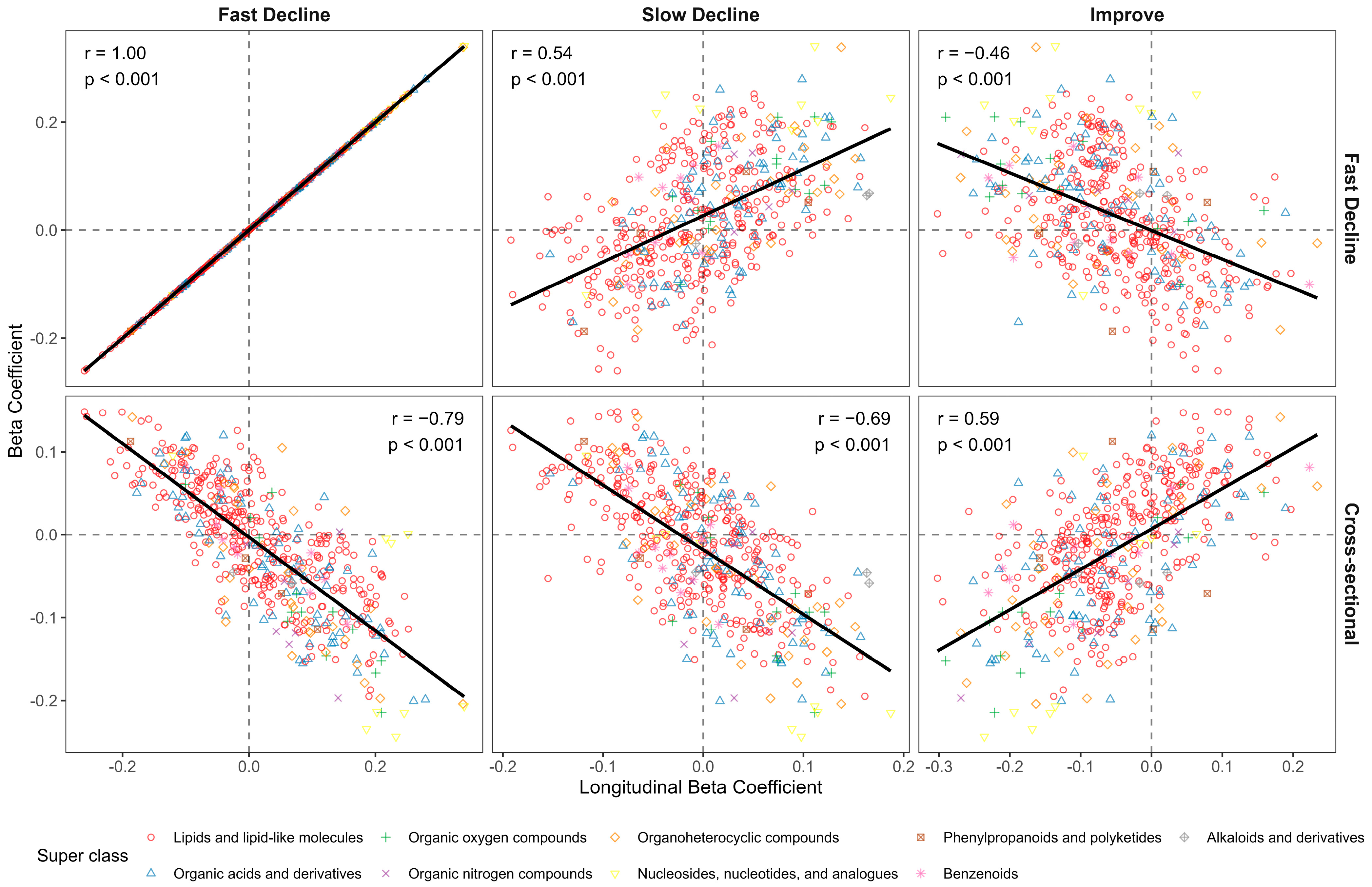
3.2. Cross-Sectional Metabolite Associations
3.3. Longitudinal Metabolite Associations
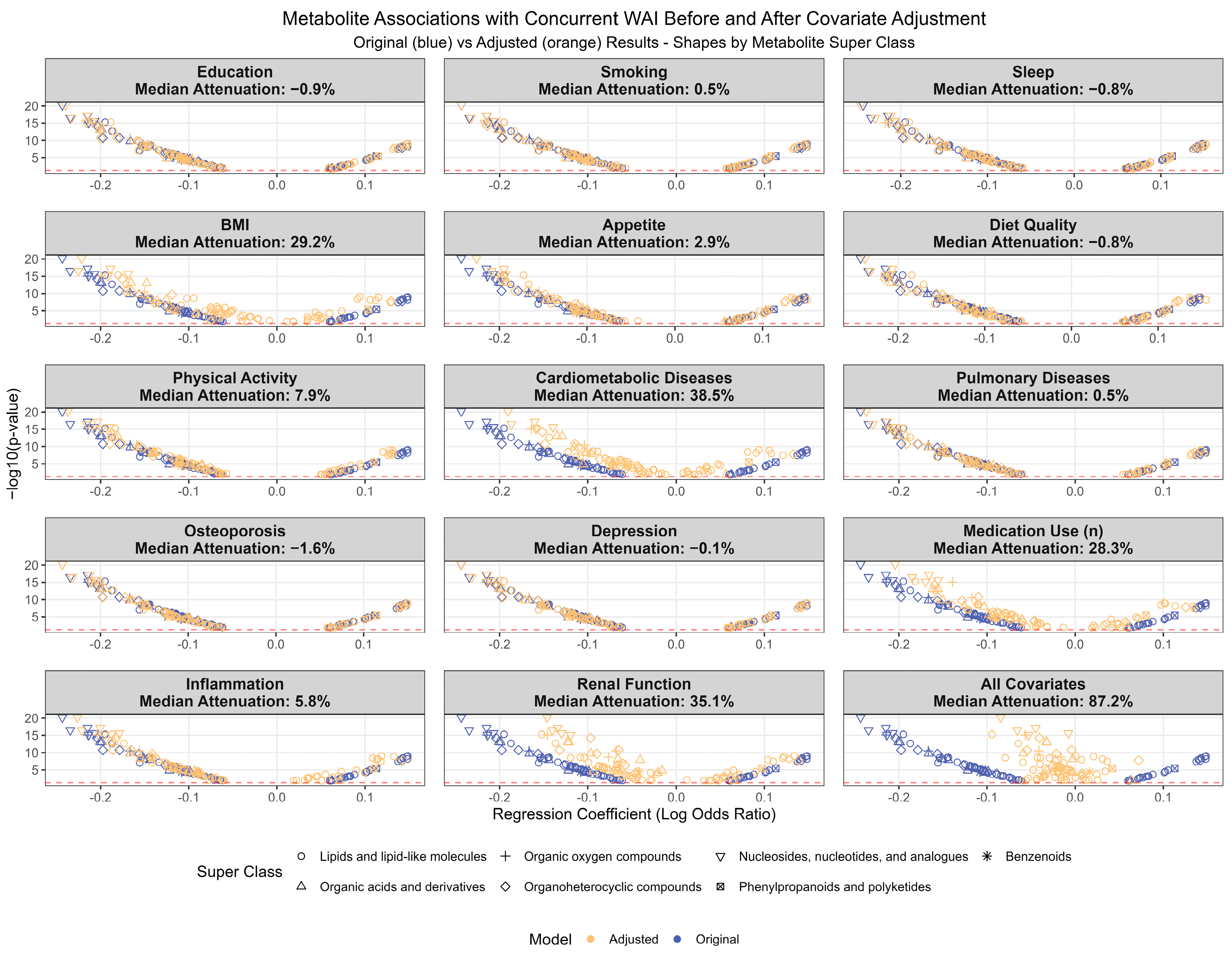
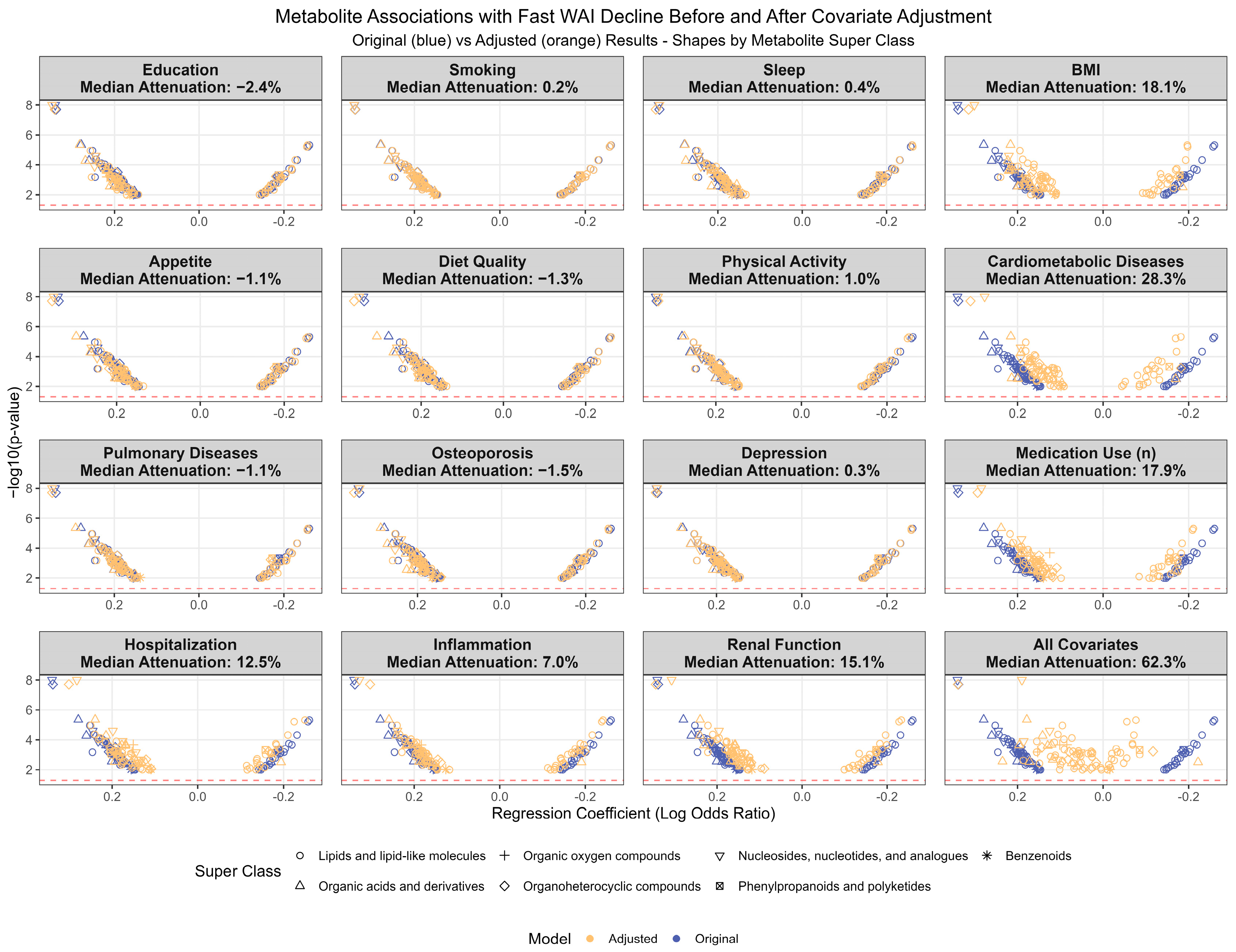
3.4. Investigation of Potential Confounders
3.5. Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WAI | Walking Ability Index |
| TG | Triacylglycerol |
| DG | Diacylglycerol |
| LPC | Lysophosphatidylcholine |
| CE | Cholesterol Ester |
References
- Jylhä, M.; Guralnik, J.M.; Balfour, J.; Fried, L.P. Walking difficulty, walking speed, and age as predictors of self-rated health: The women’s health and aging study. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M609–M617. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; Van Rompaey, V.; Saeys, W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; Moaddel, R.; Sun, K.; Fabbri, E.; Zhang, P.; Khadeer, M.; Salem, N., Jr.; Ferrucci, L.; Semba, R.D. Targeted Metabolomics Shows Low Plasma Lysophosphatidylcholine 18:2 Predicts Greater Decline of Gait Speed in Older Adults: The Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Marron, M.M.; Wendell, S.G.; Boudreau, R.M.; Clish, C.B.; Santanasto, A.J.; Tseng, G.C.; Zmuda, J.M.; Newman, A.B. Metabolites Associated with Walking Ability Among the Oldest Old from the CHS All Stars Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Moore, S.; Playdon, M.; Kritchevsky, S.; Newman, A.B.; Satterfield, S.; Ayonayon, H.; Clish, C.; Gerszten, R.; Harris, T.B. Metabolites Associated with Risk of Developing Mobility Disability in the Health, Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 73–80. [Google Scholar] [CrossRef]
- Duan-Porter, W.; Vo, T.N.; Ullman, K.; Langsetmo, L.; Strotmeyer, E.S.; Taylor, B.C.; Santanasto, A.J.; Cawthon, P.M.; Newman, A.B.; Simonsick, E.M.; et al. Hospitalization-Associated Change in Gait Speed and Risk of Functional Limitations for Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1657–1663. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Atkinson, H.H.; Rosano, C.; Simonsick, E.M.; Williamson, J.D.; Davis, C.; Ambrosius, W.T.; Rapp, S.R.; Cesari, M.; Newman, A.B.; Harris, T.B.; et al. Cognitive function, gait speed decline, and comorbidities: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 844–850. [Google Scholar] [CrossRef]
- Simonsick, E.M.; Newman, A.B.; Ferrucci, L.; Satterfield, S.; Harris, T.B.; Rodondi, N.; Bauer, D.C. Subclinical hypothyroidism and functional mobility in older adults. Arch. Intern. Med. 2009, 169, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Simonsick, E.M.; Newman, A.B.; Nevitt, M.C.; Kritchevsky, S.B.; Ferrucci, L.; Guralnik, J.M.; Harris, T. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M644–M649. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Colangelo, L.A.; Perry, A.S.; Marron, M.M.; Yaffe, K.; Sedaghat, S.; Lima, J.A.C.; Tian, Q.; Clish, C.B.; Newman, A.B.; et al. Implications of metabolism on multi-systems healthy aging across the lifespan. Aging Cell 2024, 23, e14090. [Google Scholar] [CrossRef]
- Simonsick, E.M.; Schrack, J.A.; Santanasto, A.J.; Studenski, S.A.; Ferrucci, L.; Glynn, N.W. Pittsburgh Fatigability Scale: One-Page Predictor of Mobility Decline in Mobility-Intact Older Adults. J. Am. Geriatr. Soc. 2018, 66, 2092–2096. [Google Scholar] [CrossRef]
- Murthy, V.L.; Nayor, M.; Carnethon, M.; Reis, J.P.; Lloyd-Jones, D.; Allen, N.B.; Kitchen, R.; Piaggi, P.; Steffen, L.M.; Vasan, R.S.; et al. Circulating metabolite profile in young adulthood identifies long-term diabetes susceptibility: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Diabetologia 2022, 65, 657–674. [Google Scholar] [CrossRef]
- Shah, R.V.; Steffen, L.M.; Nayor, M.; Reis, J.P.; Jacobs, D.R.; Allen, N.B.; Lloyd-Jones, D.; Meyer, K.; Cole, J.; Piaggi, P.; et al. Dietary metabolic signatures and cardiometabolic risk. Eur. Heart J. 2023, 44, 557–569. [Google Scholar] [CrossRef]
- Marron, M.M.; Harris, T.B.; Boudreau, R.M.; Clish, C.B.; Moore, S.C.; Murphy, R.A.; Murthy, V.L.; Sanders, J.L.; Shah, R.V.; Tseng, G.C.; et al. Metabolites Associated with Vigor to Frailty Among Community-Dwelling Older Black Men. Metabolites 2019, 9, 83. [Google Scholar] [CrossRef]
- Lee, J.S.; Kritchevsky, S.B.; Tylavsky, F.A.; Harris, T.; Everhart, J.; Simonsick, E.M.; Rubin, S.M.; Newman, A.B.; Health, A.; Body Composition, S. Weight-loss intention in the well-functioning, community-dwelling elderly: Associations with diet quality, physical activity, and weight change. Am. J. Clin. Nutr. 2004, 80, 466–474. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B.; et al. Inflammatory markers and onset of cardiovascular events: Results from the Health ABC study. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef]
- Thas, O.; Neve, J.D.; Clement, L.; Ottoy, J.P. Probabilistic Index Models. J. Roy. Stat. Soc. B 2012, 74, 623–671. [Google Scholar] [CrossRef]
- Sarraj, A.; Hassan, A.E.; Abraham, M.G.; Ortega-Gutierrez, S.; Kasner, S.E.; Hussain, M.S.; Chen, M.; Churilov, L.; Johns, H.; Sitton, C.W.; et al. Endovascular Thrombectomy for Large Ischemic Stroke Across Ischemic Injury and Penumbra Profiles. JAMA 2024, 331, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Chung, K.W. Advances in Understanding of the Role of Lipid Metabolism in Aging. Cells 2021, 10, 880. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Fielding, R.A. Metabolites Associated with Circulating Interleukin-6 in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1277–1283. [Google Scholar] [CrossRef]
- Wen, C.J.; Koh, Y.C.; Tung, Y.C.; Ho, P.Y.; Hsieh, S.C.; Lo, Y.C.; Tsai, J.S.; Pan, M.H. Cross-Sectional Study: New Approach for Diagnostic Identification of Non-Robust Older Adult. Mol. Nutr. Food Res. 2023, 67, e2300056. [Google Scholar] [CrossRef]
- Jorg, M.; Plehn, J.E.; Kristen, M.; Lander, M.; Walz, L.; Lietz, C.; Wijns, J.; Pichot, F.; Rojas-Charry, L.; Wirtz Martin, K.M.; et al. N1-methylation of adenosine (m(1)A) in ND5 mRNA leads to complex I dysfunction in Alzheimer’s disease. Mol. Psychiatry 2024, 29, 1427–1439. [Google Scholar] [CrossRef]
- Marron, M.M.; Yao, S.; Shah, R.V.; Murthy, V.L.; Newman, A.B. Metabolomic characterization of vigor to frailty among community-dwelling older Black and White men and women. GeroScience 2024, 46, 2371–2389. [Google Scholar] [CrossRef]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines--old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal muscle energy metabolism in obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Holtzer, R.; Oh-Park, M.; Derby, C.A.; Lipton, R.B.; Wang, C. Inflammatory markers and gait speed decline in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leung, K.S.; Chow, S.K.; Cheung, W.H. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J. Orthop. Translat 2017, 10, 94–101. [Google Scholar] [CrossRef]
- Holecek, M. Aspartic Acid in Health and Disease. Nutrients 2023, 15, 4023. [Google Scholar] [CrossRef]
- Li, Q.; You, Y.; Zeng, Y.; Wang, X.; Pan, Z.; Pang, J.; Chen, Q.; Zhou, Y.; Jin, Y.; Yang, Y.; et al. Associations between plasma tryptophan and indole-3-propionic acid levels and mortality in patients with coronary artery disease. Am. J. Clin. Nutr. 2022, 116, 1070–1077. [Google Scholar] [CrossRef]
- Shin, H.E.; Won, C.W.; Kim, M. Metabolomic profiles to explore biomarkers of severe sarcopenia in older men: A pilot study. Exp. Gerontol. 2022, 167, 111924. [Google Scholar] [CrossRef]
- Sigurdsson, M.I.; Kobayashi, H.; Amrein, K.; Nakahira, K.; Rogers, A.J.; Pinilla-Vera, M.; Baron, R.M.; Fredenburgh, L.E.; Lasky-Su, J.A.; Christopher, K.B. Circulating N-formylmethionine and metabolic shift in critical illness: A multicohort metabolomics study. Crit. Care 2022, 26, 321. [Google Scholar] [CrossRef]
- Nierenberg, J.L.; He, J.; Li, C.; Gu, X.; Shi, M.; Razavi, A.C.; Mi, X.; Li, S.; Bazzano, L.A.; Anderson, A.H.; et al. Serum metabolites associate with physical performance among middle-aged adults: Evidence from the Bogalusa Heart Study. Aging 2020, 12, 11914–11941. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular basis for redox control by the human cystine/glutamate antiporter system xc−. Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef]
- Marco-Bonilla, M.; Fresnadillo, M.; Largo, R.; Herrero-Beaumont, G.; Mediero, A. Energy Regulation in Inflammatory Sarcopenia by the Purinergic System. Int. J. Mol. Sci. 2023, 24, 16904. [Google Scholar] [CrossRef]
- Holloway, G.P.; Holwerda, A.M.; Miotto, P.M.; Dirks, M.L.; Verdijk, L.B.; van Loon, L.J.C. Age-Associated Impairments in Mitochondrial ADP Sensitivity Contribute to Redox Stress in Senescent Human Skeletal Muscle. Cell Rep. 2018, 22, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, A.; Rogge, E.; Vandendriessche, B.; Shiva, S.; Brouckaert, P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014, 5, e1102. [Google Scholar] [CrossRef]
- Stachon, P.; Geis, S.; Peikert, A.; Heidenreich, A.; Michel, N.A.; Unal, F.; Hoppe, N.; Dufner, B.; Schulte, L.; Marchini, T.; et al. Extracellular ATP Induces Vascular Inflammation and Atherosclerosis via Purinergic Receptor Y2 in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1577–1586. [Google Scholar] [CrossRef]
- Kunkel, G.T.; Maceyka, M.; Milstien, S.; Spiegel, S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 688–702. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Trieu, K.; Bhat, S.; Dai, Z.; Leander, K.; Gigante, B.; Qian, F.; Korat, A.V.A.; Sun, Q.; Pan, X.F.; Laguzzi, F.; et al. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: A cohort study, systematic review, and meta-analysis. PLoS Med. 2021, 18, e1003763. [Google Scholar] [CrossRef]
- Iggman, D.; Arnlov, J.; Cederholm, T.; Riserus, U. Association of Adipose Tissue Fatty Acids with Cardiovascular and All-Cause Mortality in Elderly Men. JAMA Cardiol. 2016, 1, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Caldow, M.K.; Ham, D.J.; Trieu, J.; Chung, J.D.; Lynch, G.S.; Koopman, R. Glycine Protects Muscle Cells From Wasting in vitro via mTORC1 Signaling. Front. Nutr. 2019, 6, 172. [Google Scholar] [CrossRef] [PubMed]
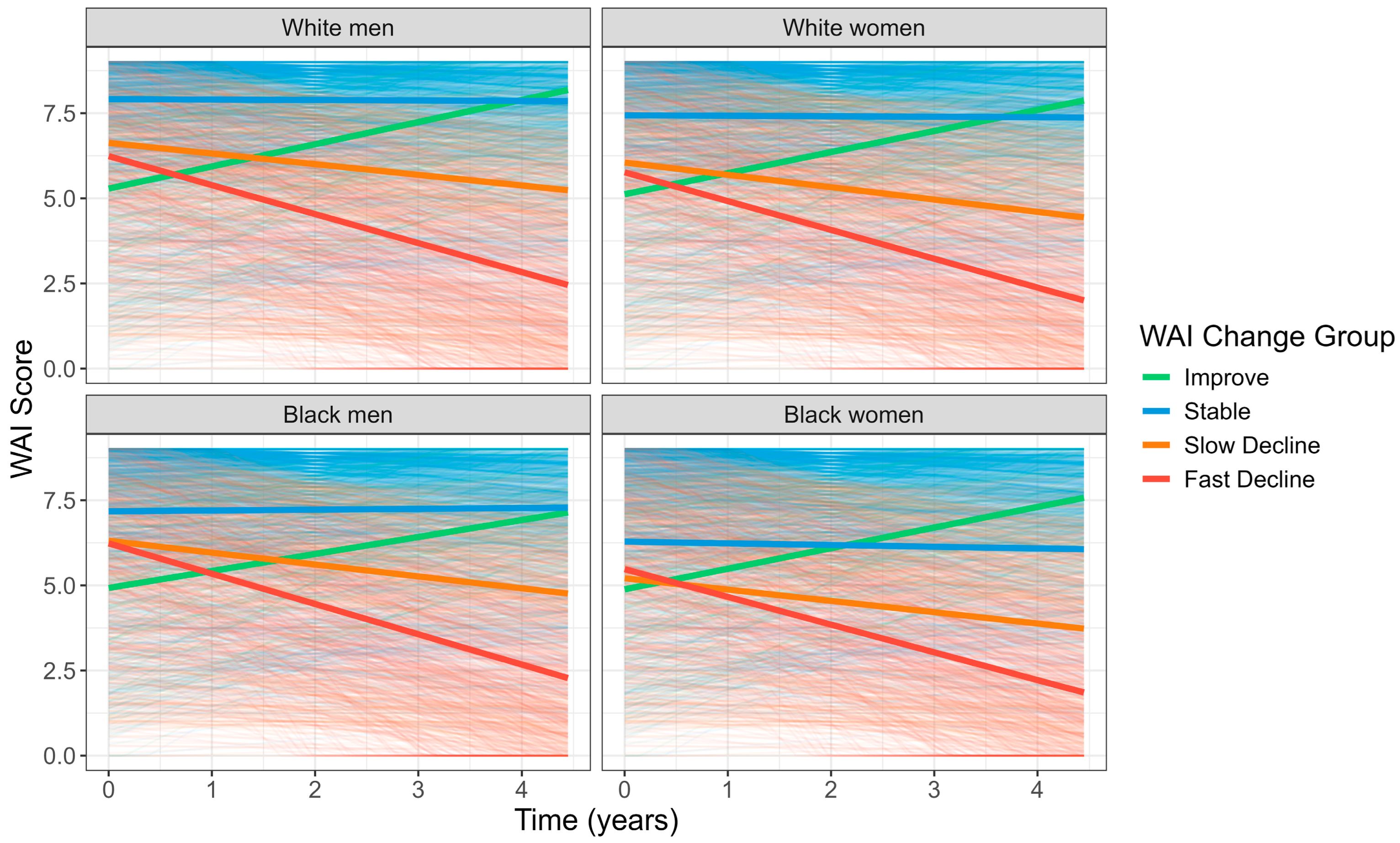


| Year 2 WAI | |||||
|---|---|---|---|---|---|
| Characteristic | Overall N = 2334 | 0–5 N = 623 | 6–8 N = 851 | 9 N = 860 | p-Value |
| WAI change over 4 years | <0.001 | ||||
| Improve | 148 (6%) | 113 (18%) | 35 (4%) | 0 (0%) | |
| Stable | 1203 (52%) | 232 (37%) | 403 (47%) | 568 (66%) | |
| Slow Decline | 520 (22%) | 166 (27%) | 187 (22%) | 167 (19%) | |
| Fast Decline | 463 (20%) | 112 (18%) | 226 (27%) | 125 (15%) | |
| Age, year | 74.6 (2.9) | 75.0 (2.9) | 74.5 (2.8) | 74.5 (2.8) | <0.001 |
| Race | <0.001 | ||||
| White | 1471 (63%) | 326 (52%) | 522 (61%) | 623 (72%) | |
| Black | 863 (37%) | 297 (48%) | 329 (39%) | 237 (28%) | |
| Sex | <0.001 | ||||
| Men | 1155 (49%) | 253 (41%) | 391 (46%) | 511 (59%) | |
| Women | 1179 (51%) | 370 (59%) | 460 (54%) | 349 (41%) | |
| Race and sex | <0.001 | ||||
| White men | 782 (34%) | 146 (23%) | 262 (31%) | 374 (43%) | |
| White women | 689 (30%) | 180 (29%) | 260 (31%) | 249 (29%) | |
| Black men | 373 (16%) | 107 (17%) | 129 (15%) | 137 (16%) | |
| Black women | 490 (21%) | 190 (30%) | 200 (24%) | 100 (12%) | |
| More than high school education | 1787 (77%) | 425 (68%) | 660 (78%) | 702 (82%) | <0.001 |
| Smoker | 210 (9%) | 72 (12%) | 86 (10%) | 52 (6%) | <0.001 |
| Sleep hours/night | 6.9 (1.3) | 6.7 (1.5) | 6.8 (1.3) | 7.0 (1.2) | 0.005 |
| BMI, kg/m2 | 27.3 (4.8) | 29.0 (5.4) | 27.2 (4.8) | 26.0 (3.9) | <0.001 |
| BMI category | <0.001 | ||||
| <25 kg/m2 | 786 (34%) | 147 (24%) | 289 (34%) | 350 (41%) | |
| 25–30 kg/m2 | 977 (42%) | 230 (37%) | 364 (43%) | 383 (45%) | |
| ≥30 kg/m2 | 571 (24%) | 246 (39%) | 198 (23%) | 127 (15%) | |
| Appetite | <0.001 | ||||
| Very good | 970 (42%) | 195 (32%) | 347 (41%) | 428 (50%) | |
| Good | 851 (37%) | 230 (38%) | 300 (36%) | 321 (38%) | |
| Moderate to poor | 485 (21%) | 182 (30%) | 196 (23%) | 107 (13%) | |
| Healthy Eating Index, 0–100 | 69.6 (12.2) | 67.7 (12.4) | 69.4 (12.0) | 71.2 (12.1) | <0.001 |
| Physical activity (Kcal/kg/Week) | 3.0 [0.4–9.5] | 0.8 [0.0–4.0] | 2.4 [0.3–7.5] | 7.5 [2.0–15.8] | <0.001 |
| Cardiovascular disease | 628 (27%) | 224 (36%) | 218 (26%) | 186 (22%) | <0.001 |
| Hypertension | 1237 (53%) | 421 (68%) | 459 (54%) | 357 (42%) | <0.001 |
| Diabetes | 911 (39%) | 289 (46%) | 330 (39%) | 292 (34%) | <0.001 |
| Cancer | 430 (18%) | 115 (18%) | 141 (17%) | 174 (20%) | 0.15 |
| Peripheral artery disease | 108 (5%) | 55 (9%) | 36 (4%) | 17 (2%) | <0.001 |
| Osteoporosis | 235 (10%) | 74 (12%) | 95 (11%) | 66 (8%) | 0.012 |
| Depression | 221 (10%) | 77 (12%) | 77 (9%) | 67 (8%) | 0.010 |
| Pulmonary disease | 261 (11%) | 112 (18%) | 88 (10%) | 61 (7%) | <0.001 |
| Total prescription medications | 3.0 [1.0–5.0] | 4.0 [2.0–6.0] | 3.0 [1.0–5.0] | 2.0 [1.0–4.0] | <0.001 |
| C-reactive protein (ug/mL) | 2.8 [1.2–6.2] | 3.9 [1.6–7.8] | 3.0 [1.3–6.3] | 2.1 [1.0–4.9] | <0.001 |
| Interleukin-6 (pg/mL) | 2.3 [1.5–3.9] | 2.9 [1.9–4.9] | 2.3 [1.5–3.8] | 2.0 [1.3–3.3] | <0.001 |
| Cystatin C (mg/dL) | 1.0 [0.9–1.1] | 1.0 [0.9–1.2] | 1.0 [0.9–1.1] | 1.0 [0.8–1.1] | <0.001 |
| Creatinine (mg/dL) | 1.0 [0.9–1.2] | 1.0 [0.9–1.2] | 1.0 [0.9–1.1] | 1.0 [0.9–1.1] | 0.2 |
| WAI Change Groups | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Overall N = 2334 | Fast Decline N = 463 | Slow Decline N = 520 | Stable N = 1203 | Improve N = 148 | p-Value |
| Initial WAI (Year 2) | 6.68 (2.56) | 6.82 (1.97) | 6.28 (2.79) | 7.20 (2.40) | 3.34 (2.04) | <0.001 |
| Age, year | 74.6 (2.9) | 74.7 (2.9) | 74.8 (2.8) | 74.6 (2.9) | 74.4 (2.8) | 0.4 |
| Race | 0.001 | |||||
| White | 1471 (63%) | 277 (60%) | 302 (58%) | 804 (67%) | 88 (59%) | |
| Black | 863 (37%) | 186 (40%) | 218 (42%) | 399 (33%) | 60 (41%) | |
| Sex | 0.051 | |||||
| Men | 1155 (49%) | 222 (48%) | 234 (45%) | 626 (52%) | 73 (49%) | |
| Women | 1179 (51%) | 241 (52%) | 286 (55%) | 577 (48%) | 75 (51%) | |
| Race and sex | 0.006 | |||||
| White men | 782 (34%) | 139 (30%) | 150 (29%) | 445 (37%) | 48 (32%) | |
| White women | 689 (30%) | 138 (30%) | 152 (29%) | 359 (30%) | 40 (27%) | |
| Black men | 373 (16%) | 83 (18%) | 84 (16%) | 181 (15%) | 25 (17%) | |
| Black women | 490 (21%) | 103 (22%) | 134 (26%) | 218 (18%) | 35 (24%) | |
| More than high school education | 1787 (77%) | 327 (71%) | 391 (75%) | 957 (80%) | 112 (76%) | <0.001 |
| Smoker | 210 (9.0%) | 38 (8.2%) | 55 (11%) | 98 (8.2%) | 19 (13%) | 0.13 |
| Sleep hours/night | 6.9 (1.3) | 6.8 (1.4) | 6.8 (1.4) | 6.9 (1.3) | 6.7 (1.1) | 0.10 |
| BMI, kg/m2 | 27.3 (4.8) | 28.4 (5.2) | 27.8 (5.1) | 26.6 (4.4) | 27.4 (4.8) | <0.001 |
| BMI category | <0.001 | |||||
| <25 kg/m2 | 786 (34%) | 130 (28%) | 159 (31%) | 453 (38%) | 44 (30%) | |
| 25–30 kg/m2 | 977 (42%) | 179 (39%) | 205 (39%) | 529 (44%) | 64 (43%) | |
| ≥30 kg/m2 | 571 (24%) | 154 (33%) | 156 (30%) | 221 (18%) | 40 (27%) | |
| Appetite | 0.013 | |||||
| Very good | 970 (42%) | 189 (42%) | 204 (40%) | 531 (45%) | 46 (31%) | |
| Good | 851 (37%) | 160 (35%) | 190 (37%) | 439 (37%) | 62 (42%) | |
| Moderate to poor | 485 (21%) | 104 (23%) | 120 (23%) | 221 (19%) | 40 (27%) | |
| Healthy Eating Index, 0–100 | 69.6 (12.2) | 68.6 (12.5) | 70.2 (11.8) | 69.8 (12.3) | 69.3 (11.6) | 0.2 |
| Energy expenditure (Kcal/kg/Week) | 3.0 [0.4–9.5] | 1.8 [0.1–6.8] | 1.8 [0.1–7.5] | 4.2 [0.7–12.0] | 2.6 [0.1–9.1] | <0.001 |
| Cardiovascular disease | 628 (27%) | 154 (33%) | 151 (29%) | 290 (24%) | 33 (22%) | <0.001 |
| Hypertension | 1237 (53%) | 290 (63%) | 305 (59%) | 568 (47%) | 74 (50%) | <0.001 |
| Diabetes | 911 (39%) | 212 (46%) | 219 (42%) | 423 (35%) | 57 (39%) | <0.001 |
| Cancer | 430 (18%) | 90 (19%) | 97 (19%) | 219 (18%) | 24 (16%) | 0.8 |
| Peripheral artery disease | 108 (4.7%) | 30 (6.6%) | 33 (6.5%) | 37 (3.2%) | 8 (5.5%) | 0.003 |
| Osteoporosis | 235 (10%) | 55 (12%) | 57 (11%) | 106 (9.0%) | 17 (12%) | 0.2 |
| Depression | 221 (9.5%) | 48 (10%) | 56 (11%) | 100 (8.4%) | 17 (11%) | 0.3 |
| Pulmonary disease | 261 (11%) | 73 (16%) | 72 (14%) | 105 (8.8%) | 11 (7.4%) | <0.001 |
| Total prescription medications | 3.0 [1.0–5.0] | 3.0 [2.0–5.0] | 3.0 [1.0–5.0] | 2.0 [1.0–4.0] | 3.0 [1.0–4.0] | <0.001 |
| Number of hospitalizations during follow-up | <0.001 | |||||
| None | 1212 (52%) | 168 (36%) | 241 (46%) | 731 (61%) | 72 (49%) | |
| Once | 584 (25%) | 132 (29%) | 139 (27%) | 271 (23%) | 42 (28%) | |
| More than once | 538 (23%) | 163 (35%) | 140 (27%) | 201 (17%) | 34 (23%) | |
| C-reactive protein (ug/mL) | 2.8 [1.2–6.2] | 3.4 [1.5–7.5] | 3.0 [1.2–7.1] | 2.6 [1.1–5.6] | 3.2 [1.2–6.3] | <0.001 |
| Interleukin-6 (pg/mL) | 2.3 [1.5–3.9] | 2.8 [1.8–4.4] | 2.5 [1.7–4.1] | 2.0 [1.4–3.6] | 2.4 [1.5–4.1] | <0.001 |
| Cystatin C (mg/dL) | 1.0 [0.9–1.1] | 1.0 [0.9–1.2] | 1.0 [0.9–1.1] | 1.0 [0.9–1.1] | 1.0 [0.9–1.1] | <0.001 |
| Creatinine (mg/dL) | 1.0 [0.9–1.2] | 1.0 [0.9–1.2] | 1.0 [0.9–1.2] | 1.0 [0.9–1.2] | 1.0 [0.9–1.1] | 0.8 |
| Pathway | Reference Metabolome Using All Metabolites in Pathway Library | Reference Metabolome Using Metabolites Measured in the Health ABC Study | Impact | ||
|---|---|---|---|---|---|
| Match Status | p-Value | Match Status | p-Value | ||
| Top pathways involving 81 consistent metabolites | |||||
| Arginine biosynthesis | 3/14 | 0.000 | 3/10 | 0.193 | 0.00 |
| Citrate cycle (TCA cycle) | 3/20 | 0.001 | 3/6 | 0.049 | 0.12 |
| Pyruvate metabolism | 3/23 | 0.002 | 3/3 | 0.003 | 0.03 |
| Nicotinate and nicotinamide metabolism | 2/15 | 0.012 | 2/5 | 0.176 | 0.00 |
| Histidine metabolism | 2/16 | 0.013 | 2/6 | 0.240 | 0.00 |
| Alanine, aspartate, and glutamate metabolism | 2/28 | 0.038 | 2/9 | 0.432 | 0.23 |
| Glyoxylate and dicarboxylate metabolism | 2/32 | 0.049 | 2/8 | 0.369 | 0.03 |
| Glycerophospholipid metabolism | 2/36 | 0.061 | 2/7 | 0.305 | 0.12 |
| Tyrosine metabolism | 2/42 | 0.080 | 2/4 | 0.117 | 0.03 |
| Ascorbate and aldarate metabolism | 1/9 | 0.098 | 1/2 | 0.292 | 0.52 |
| Top pathways involving 18 longitudinal-only metabolites | |||||
| Primary bile acid biosynthesis | 3/46 | 0.001 | 3/9 | 0.016 | 0.02 |
| Purine metabolism | 3/70 | 0.004 | 3/7 | 0.007 | 0.09 |
| One carbon pool by folate | 2/26 | 0.007 | 2/7 | 0.076 | 0.04 |
| Valine, leucine, and isoleucine biosynthesis | 1/8 | 0.040 | 1/6 | 0.310 | 0.00 |
| Taurine and hypotaurine metabolism | 1/8 | 0.040 | 1/2 | 0.136 | 0.43 |
| Pantothenate and CoA biosynthesis | 1/20 | 0.096 | 1/5 | 0.310 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Mao, Z.; Marron, M.M.; Simonsick, E.M.; Murthy, V.L.; Shah, R.V.; Newman, A.B. Metabolic Markers Demonstrate the Heterogeneity of Walking Ability in Non-Disabled Community-Dwelling Older Adults. Metabolites 2025, 15, 334. https://doi.org/10.3390/metabo15050334
Yao S, Mao Z, Marron MM, Simonsick EM, Murthy VL, Shah RV, Newman AB. Metabolic Markers Demonstrate the Heterogeneity of Walking Ability in Non-Disabled Community-Dwelling Older Adults. Metabolites. 2025; 15(5):334. https://doi.org/10.3390/metabo15050334
Chicago/Turabian StyleYao, Shanshan, Ziling Mao, Megan M. Marron, Eleanor M. Simonsick, Venkatesh L. Murthy, Ravi V. Shah, and Anne B. Newman. 2025. "Metabolic Markers Demonstrate the Heterogeneity of Walking Ability in Non-Disabled Community-Dwelling Older Adults" Metabolites 15, no. 5: 334. https://doi.org/10.3390/metabo15050334
APA StyleYao, S., Mao, Z., Marron, M. M., Simonsick, E. M., Murthy, V. L., Shah, R. V., & Newman, A. B. (2025). Metabolic Markers Demonstrate the Heterogeneity of Walking Ability in Non-Disabled Community-Dwelling Older Adults. Metabolites, 15(5), 334. https://doi.org/10.3390/metabo15050334






