Storage Profiling: Evaluating the Effect of Modified Atmosphere Packaging on Metabolomic Changes of Strawberries (Fragaria × ananassa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Acquisition and Storage Design
2.3. Determination of the Viable Counts
2.4. Determination of the Water Content
2.5. Near-Infrared Spectroscopy Measurements
2.6. LC-ESI-IM-qTOF Measurements of Nonpolar Metabolites
2.6.1. Sample Preparation
2.6.2. LC-MS Conditions
2.6.3. LC-MS Data Processing
2.7. Multivariate Data Analysis
3. Results and Discussion
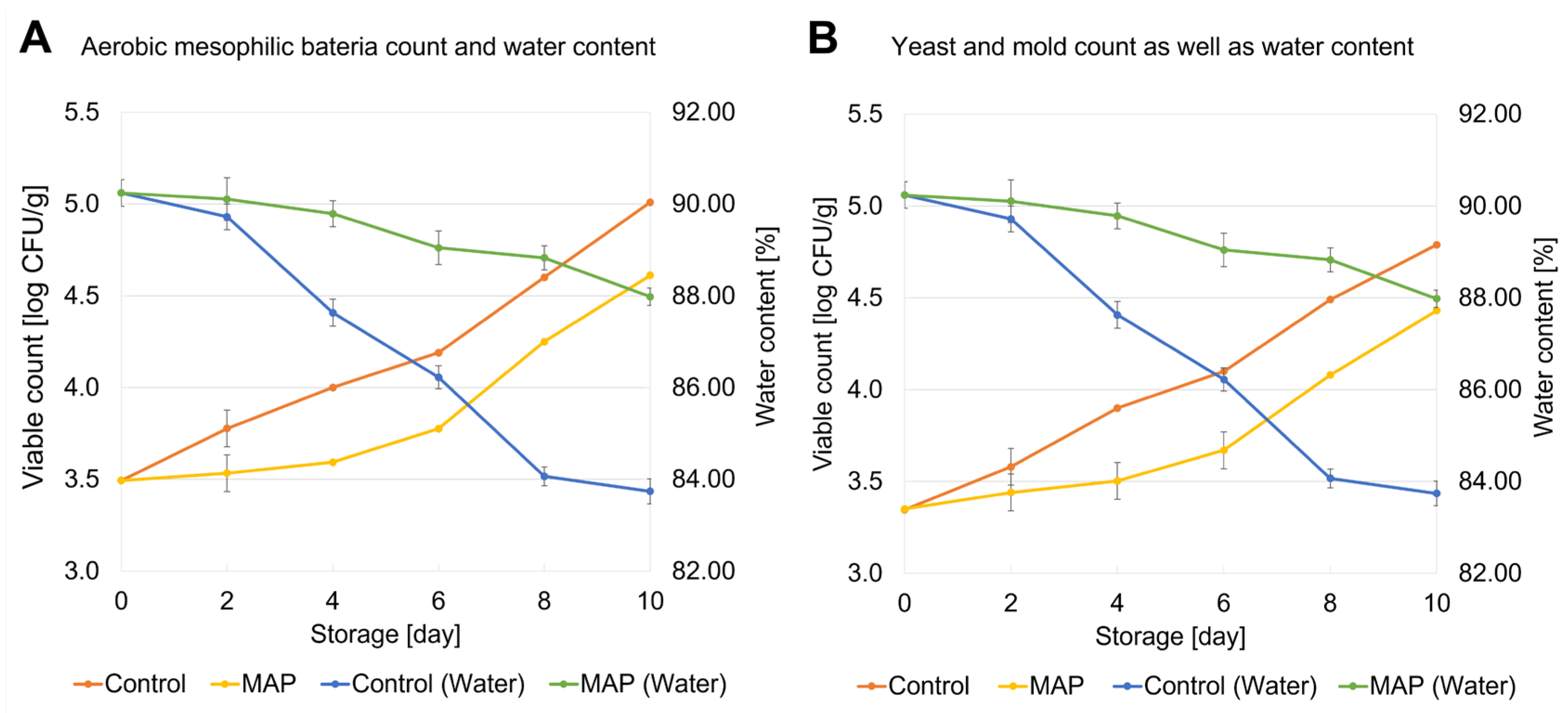
3.1. Changes in Viable Counts and Water Content During Storage
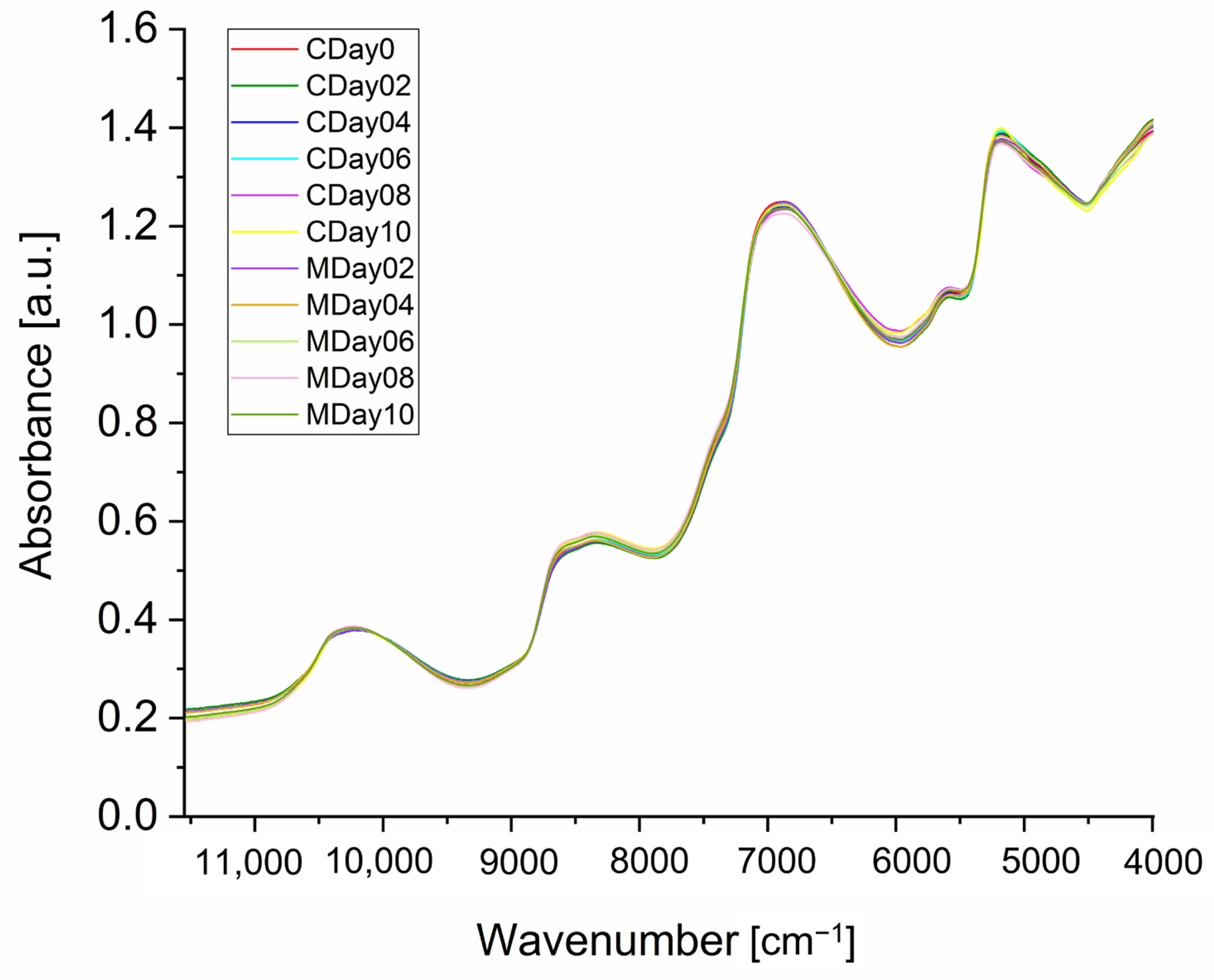
3.2. NIR Spectroscopy of Strawberries
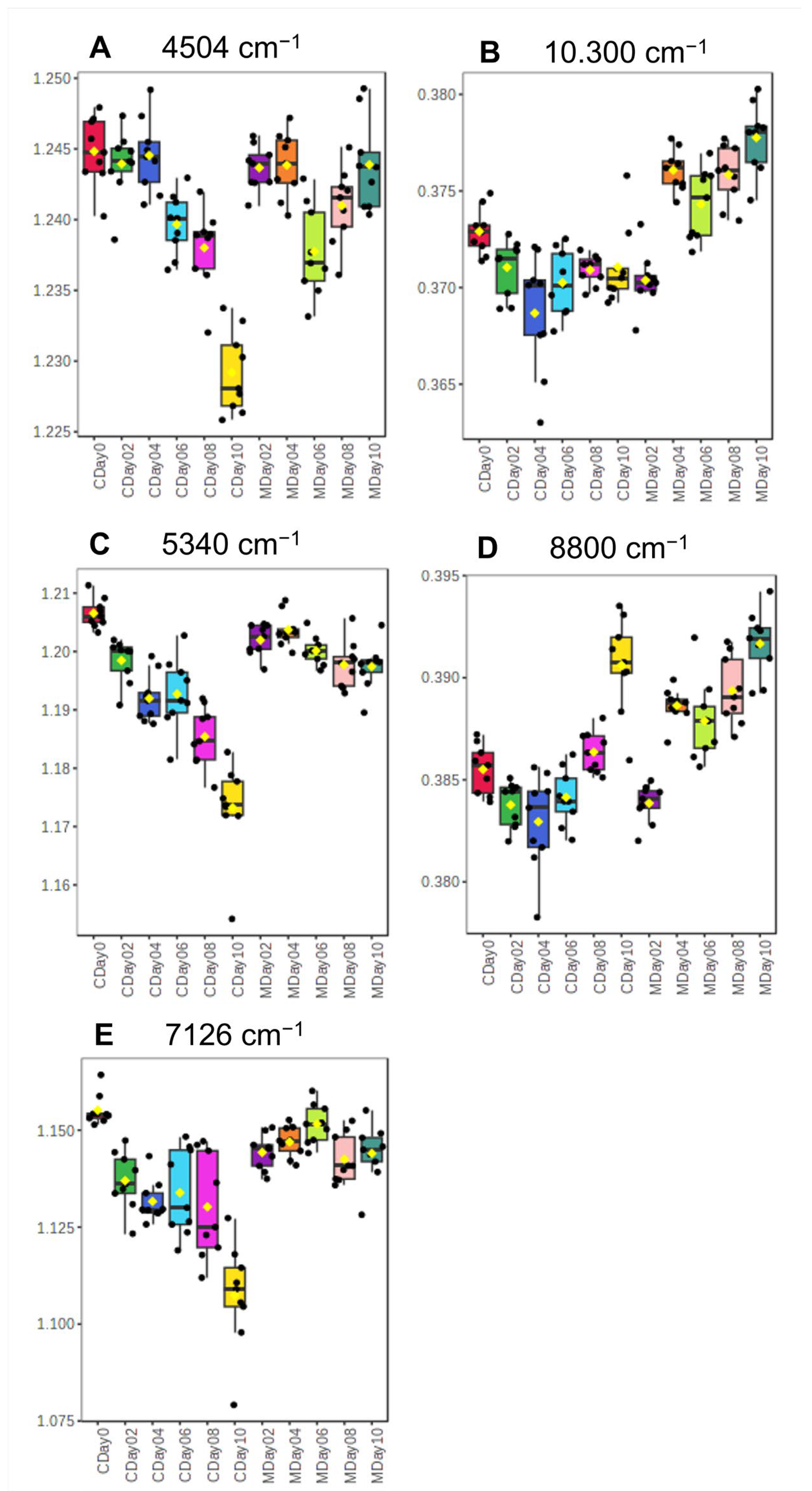
3.2.1. Spectra Interpretation
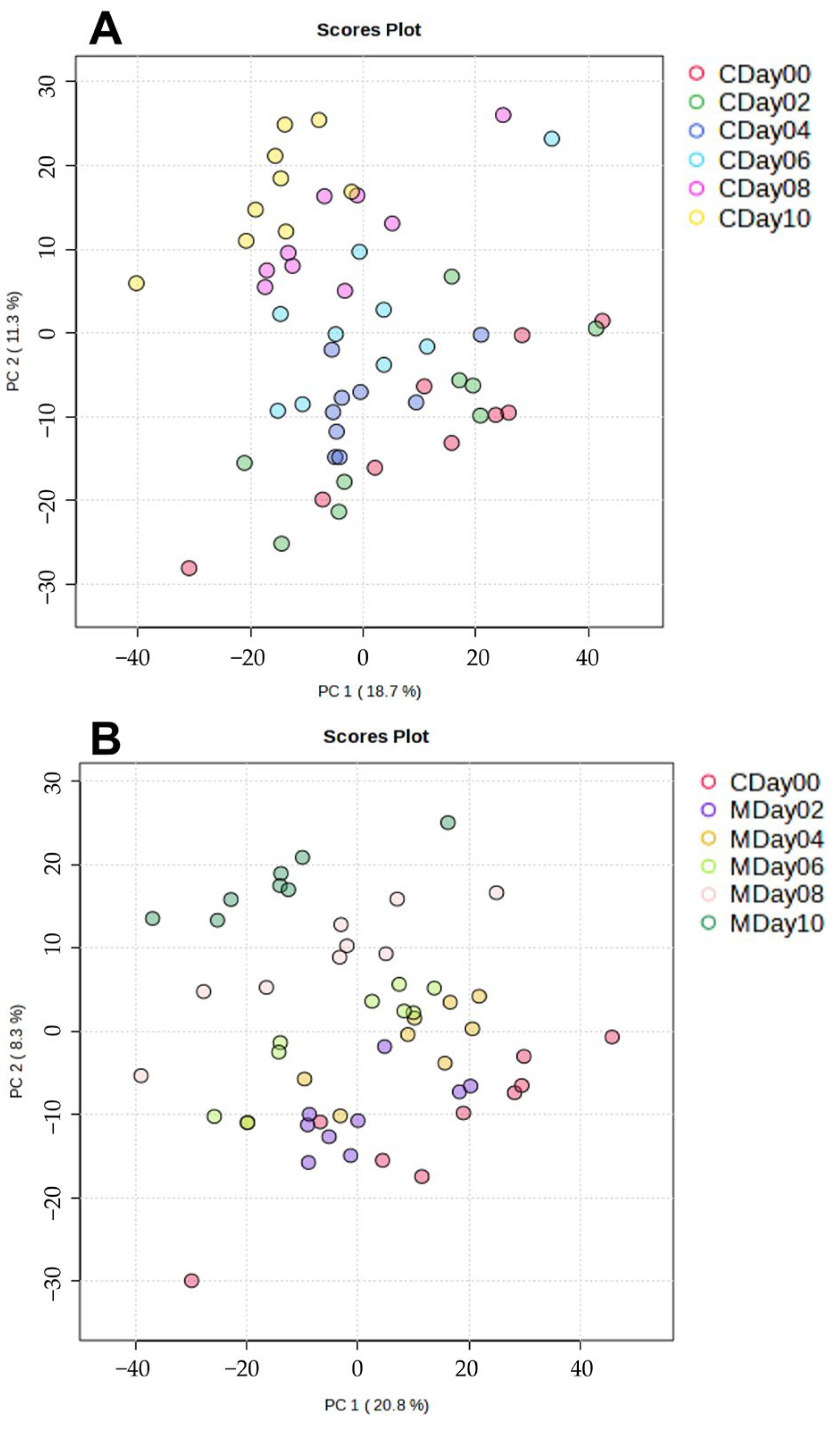
3.2.2. Application of Multivariate Data Analysis
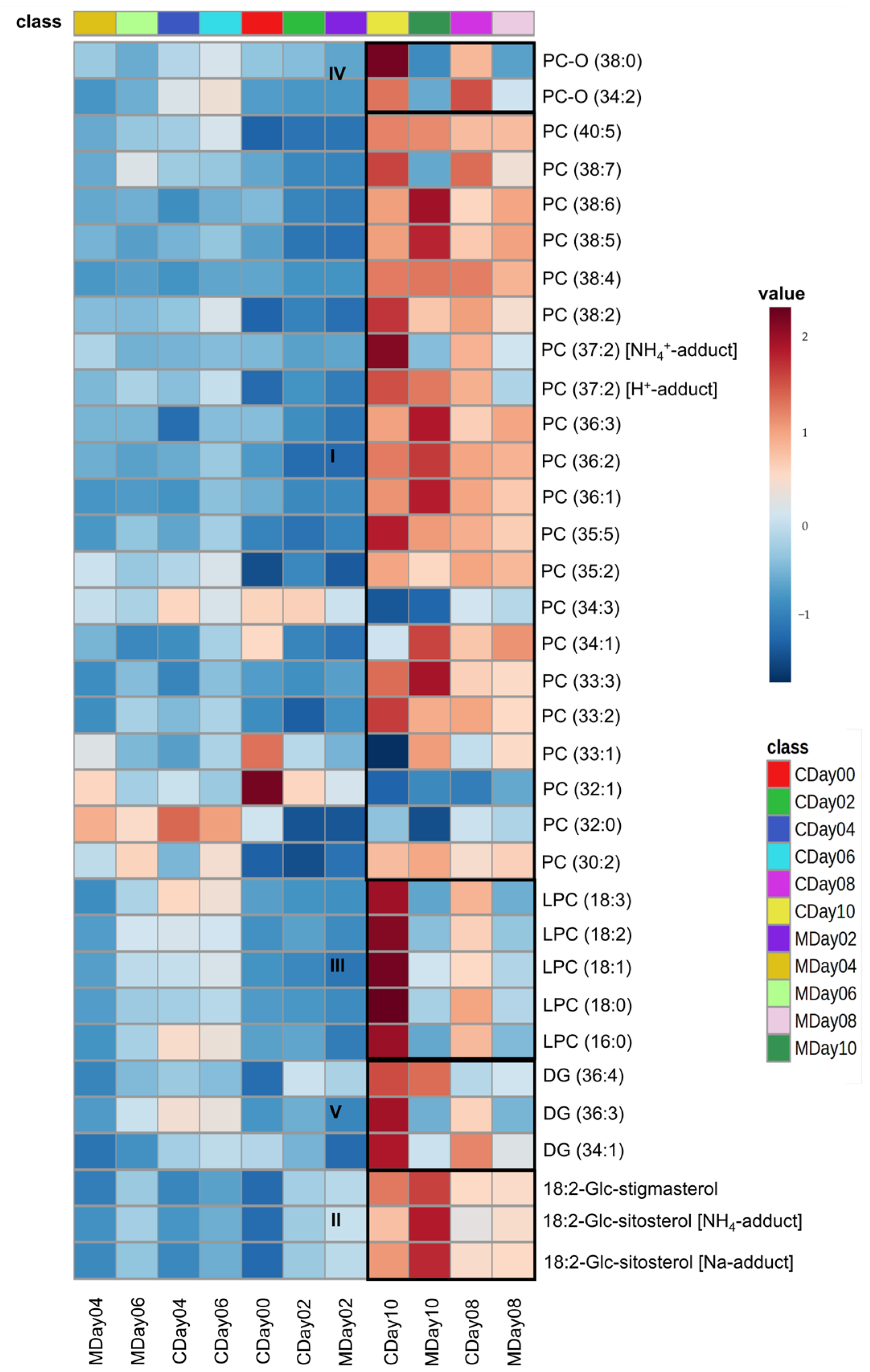
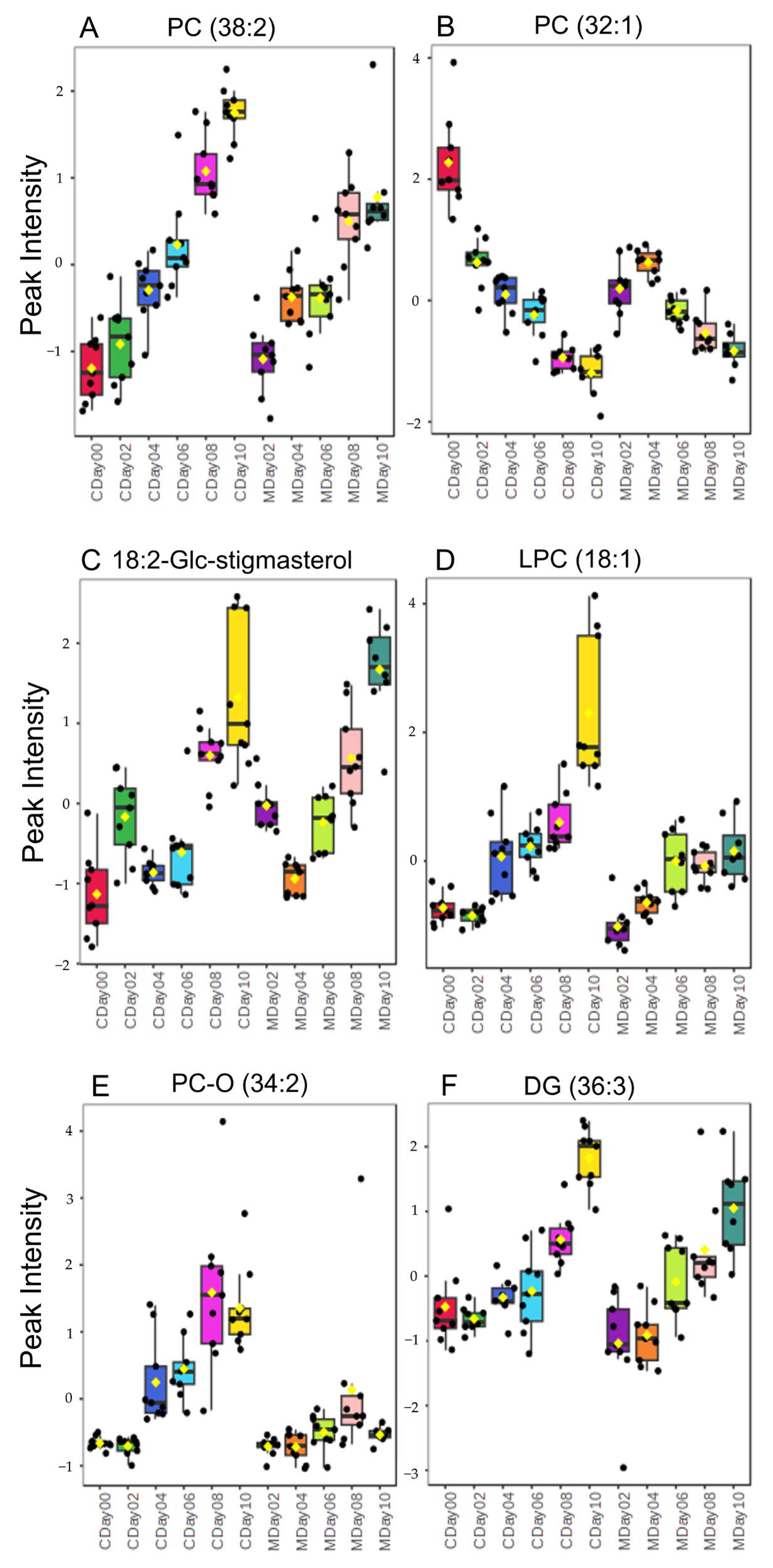
3.3. Lipidomic Profiling via LC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.-S.; Park, K.-J.; Choi, J.H.; Lim, J.-H.; Kim, H.-J. Metabolomic analysis of strawberries at different maturities according to postharvest storage period. Sci. Hortic. 2023, 321, 112283. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Drobek, M.; Cybulska, J.; Gałązka, A.; Feledyn-Szewczyk, B.; Marzec-Grządziel, A.; Sas-Paszt, L.; Gryta, A.; Trzciński, P.; Zdunek, A.; Frąc, M. The use of interactions between microorganisms in strawberry cultivation (Fragaria x ananassa Duch.). Front. Plant Sci. 2021, 12, 780099. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Dong, Y.; Jiang, S.; Cao, J.; Meng, R. Postharvest biological control of gray mold decay of strawberry with Rhodotorula glutinis. Biol. Control 2007, 40, 287–292. [Google Scholar] [CrossRef]
- Allende, A.; Marín, A.; Buendía, B.; Tomás-Barberán, F.; Gil, M.I. Impact of combined postharvest treatments (UV-C light, gaseous O3, superatmospheric O2 and high CO2) on health promoting compounds and shelf-life of strawberries. Postharvest Biol. Technol. 2007, 46, 201–211. [Google Scholar] [CrossRef]
- Mahecha-Rubiano, Y.R.; Garavito, J.; Castellanos, D.A. Configuration of a combined active packaging system with modified atmospheres and antimicrobial control to preserve fresh strawberry fruits. Food Packag. Shelf Life 2024, 46, 101390. [Google Scholar] [CrossRef]
- Peano, C.; Giuggioli, N.R.; Girgenti, V. Effect of different packaging materials on postharvest quality of cv. Envie2 strawberry. Int. Food Res. J. 2014, 21, 1165–1170. [Google Scholar]
- Sandhya. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Abu-Zahra, T.R. Effect of Cold Storage and Modified Atmosphere Packaging on Strawberry (Fragaria X Ananassa Duch.) cv.“Arben” Fruit Keeping Quality. Biosci. Biotechnol. Res. Asia 2017, 14, 1251–1258. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, K.; Xiao, H.; Tu, S.; Sun, K.; Sun, Y.; Pan, L.; Tu, K. Near-infrared hyperspectral imaging rapidly detects the decay of postharvest strawberry based on water-soluble sugar analysis. Food Anal. Methods 2019, 12, 936–946. [Google Scholar] [CrossRef]
- Shen, F.; Zhang, B.; Cao, C.; Jiang, X. On-line discrimination of storage shelf-life and prediction of post-harvest quality for strawberry fruit by visible and near infrared spectroscopy. J. Food Process Eng. 2018, 41, e12866. [Google Scholar] [CrossRef]
- Mancini, M.; Mazzoni, L.; Gagliardi, F.; Balducci, F.; Duca, D.; Toscano, G.; Mezzetti, B.; Capocasa, F. Application of the non-destructive NIR technique for the evaluation of strawberry fruits quality parameters. Foods 2020, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Šimkova, K.; Grohar, M.C.; Pelacci, M.; Veberic, R.; Jakopic, J.; Hudina, M. The effect of freezing, frozen storage and thawing on the strawberry fruit composition. Int. J. Fruit Sci. 2024, 24, 186–199. [Google Scholar] [CrossRef]
- Šimkova, K.; Veberič, R.; Hudina, M.; Jakopič, J. Berry size and weight as factors influencing the chemical composition of strawberry fruit. J. Food Compos. Anal. 2024, 123, 105509. [Google Scholar] [CrossRef]
- Joshi, P.; Pahariya, P.; Al-Ani, M.F.; Choudhary, R. Monitoring and prediction of sensory shelf-life in strawberry with ultraviolet-visible-near-infrared (UV-VIS-NIR) spectroscopy. Appl. Food Res. 2022, 2, 100123. [Google Scholar] [CrossRef]
- Saad, A.; Azam, M.M.; Amer, B.M.A. Quality analysis prediction and discriminating strawberry maturity with a hand-held Vis–NIR spectrometer. Food Anal. Methods 2022, 15, 689–699. [Google Scholar] [CrossRef]
- Sánchez, M.-T.; de la Haba, M.J.; Benítez-López, M.; Fernández-Novales, J.; Garrido-Varo, A.; Pérez-Marín, D. Non-destructive characterization and quality control of intact strawberries based on NIR spectral data. J. Food Eng. 2012, 110, 102–108. [Google Scholar] [CrossRef]
- Giuggioli, N.R.; Girgenti, V.; Baudino, C.; Peano, C. Influence of modified atmosphere packaging storage on postharvest quality and aroma compounds of strawberry fruits in a short distribution chain. J. Food Process. Preserv. 2015, 39, 3154–3164. [Google Scholar] [CrossRef]
- Mawele Shamaila, M.; Powrie, W.D.; Skura, B.J. Analysis of volatile compounds from strawberry fruit stored under modified atmosphere packaging (MAP). J. Food Sci. 1992, 57, 1173–1176. [Google Scholar] [CrossRef]
- Shamaila, M.; Powrie, W.D.; Skura, B.J. Sensory evaluation of strawberry fruit stored under modified atmosphere packaging (MAP) by quantitative descriptive analysis. J. Food Sci. 1992, 57, 1168–1184. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Zamindar, N.; Paidari, S.; Ibrahim, S.A.; Mohammadi Nafchi, A. The synergistic effects of aloe vera gel and modified atmosphere packaging on the quality of strawberry fruit. J. Food Process. Preserv. 2021, 45, e16003. [Google Scholar] [CrossRef]
- Błaszczyk, J.; Bieniasz, M.; Nawrocki, J.; Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Zaleski, T.; Knaga, J.; Bogdał, S. The effect of harvest date and storage conditions on the quality of remontant strawberry cultivars grown in a gutter system under covers. Agriculture 2022, 12, 1193. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Huang, P.-H.; Chan, Y.-J.; Chen, S.-J.; Lu, W.-C.; Li, P.-H. A new strategy to design novel modified atmosphere packaging formulation maintains the qualities of postharvest strawberries (Fragaria ananassa) during low-temperature storage. J. Food Saf. 2023, 43, e13082. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, M.; Wei, X.; Xu, C.; Luo, Z.; Mao, L. Consolidated cold and modified atmosphere package system for fresh strawberry supply chains. LWT Food Sci. Technol. 2019, 109, 207–215. [Google Scholar] [CrossRef]
- Olennikov, D.N. Coumarins of lovage roots (Levisticum officinale WDJ Koch): LC-MS profile, quantification, and stability during postharvest storage. Metabolites 2022, 13, 3. [Google Scholar] [CrossRef]
- Pott, D.M.; de Abreu e Lima, F.; Soria, C.; Willmitzer, L.; Fernie, A.R.; Nikoloski, Z.; Osorio, S.; Vallarino, J.G. Metabolic reconfiguration of strawberry physiology in response to postharvest practices. Food Chem. 2020, 321, 126747. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Arndt, M.; Rurik, M.; Drees, A.; Ahlers, C.; Feldmann, S.; Kohlbacher, O.; Fischer, M. Food authentication: Determination of the geographical origin of almonds (Prunus dulcis MILL.) via near-infrared spectroscopy. Microchem. J. 2021, 160, 105702. [Google Scholar] [CrossRef]
- Arndt, M.; Drees, A.; Ahlers, C.; Fischer, M. Determination of the geographical origin of walnuts (Juglans regia L.) using near-infrared spectroscopy and chemometrics. Foods 2020, 9, 1860. [Google Scholar] [CrossRef]
- Shakiba, N.; Gerdes, A.; Holz, N.; Wenck, S.; Bachmann, R.; Schneider, T.; Seifert, S.; Fischer, M.; Hackl, T. Determination of the geographical origin of hazelnuts (Corylus avellana L.) by Near-Infrared spectroscopy (NIR) and a Low-Level Fusion with nuclear magnetic resonance (NMR). Microchem. J. 2022, 174, 107066. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Geladi, P.; MacDougall, D.; Martens, H. Linearization and scatter-correction for near-infrared reflectance spectra of meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Engel, J.; Gerretzen, J.; Szymańska, E.; Jansen, J.J.; Downey, G.; Blanchet, L.; Buydens, L.M.C. Breaking with trends in pre-processing? TrAC Trends Anal. Chem. 2013, 50, 96–106. [Google Scholar] [CrossRef]
- Creydt, M.; Arndt, M.; Hudzik, D.; Fischer, M. Plant metabolomics: Evaluation of different extraction parameters for nontargeted UPLC-ESI-QTOF-mass spectrometry at the example of white Asparagus officinalis. J. Agric. Food Chem. 2018, 66, 12876–12887. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W. Lmsd: Lipid maps structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef]
- Zhou, Z.; Tu, J.; Xiong, X.; Shen, X.; Zhu, Z.-J. LipidCCS: Prediction of collision cross-section values for lipids with high precision to support ion mobility–mass spectrometry-based lipidomics. Anal. Chem. 2017, 89, 9559–9566. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, gkae253. [Google Scholar] [CrossRef]
- Jalali, A.; Linke, M.; Geyer, M.; Mahajan, P.V. Shelf life prediction model for strawberry based on respiration and transpiration processes. Food Packag. Shelf Life 2020, 25, 100525. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Combined effect of active coating and MAP to prolong the shelf life of minimally processed kiwifruit (Actinidia deliciosa cv. Hayward). Food Res. Int. 2011, 44, 1224–1230. [Google Scholar] [CrossRef]
- Afifi, E.H. Effect of active and passive modified atmosphere packaging on quality attributes of strawberry fruits during cold storage. Arab. Univ. J. Agric. Sci. 2016, 24, 157–168. [Google Scholar] [CrossRef]
- Ozkaya, O.; Dündar, O.; Scovazzo, G.C.; Volpe, G. Evaluation of quality parameters of strawberry fruits in modified atmosphere packaging during storage. Afr. J. Biotechnol. 2009, 8, 789–793. [Google Scholar]
- (EC) No. 2073/2005; No. 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Commission Regulation (EC): Brussels, Belgium, 2005.
- Wang, W.; Hu, W.; Ding, T.; Ye, X.; Liu, D. Shelf-life prediction of strawberry at different temperatures during storage using kinetic analysis and model development. J. Food Process. Preserv. 2018, 42, e13693. [Google Scholar] [CrossRef]
- Siro, I.; Devlieghere, F.; Jacxsens, L.; Uyttendaele, M.; Debevere, J. The microbial safety of strawberry and raspberry fruits packaged in high-oxygen and equilibrium-modified atmospheres compared to air storage. Int. J. Food. 2006, 41, 93–103. [Google Scholar] [CrossRef]
- de Filho, M.J.; Scolforo, C.Z.; Saraiva, S.H.; Pinheiro, C.J.G.; Silva, P.I.; Della Lucia, S.M. Physicochemical, microbiological and sensory acceptance alterations of strawberries caused by gamma radiation and storage time. Sci. Hortic. 2018, 238, 187–194. [Google Scholar] [CrossRef]
- Ladika, G.; Strati, I.F.; Tsiaka, T.; Cavouras, D.; Sinanoglou, V.J. On the assessment of strawberries’ shelf-life and quality, based on image analysis, physicochemical methods, and chemometrics. Foods 2024, 13, 234. [Google Scholar] [CrossRef]
- Pelletier, W.; Brecht, J.K.; do Nascimento Nunes, M.C.; Emond, J.-P. Quality of strawberries shipped by truck from California to Florida as influenced by postharvest temperature management practices. HortTechnology 2011, 21, 482–493. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Workman, J.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared; CRC: Boca Raton, FL, USA, 2012; ISBN 1439875251. [Google Scholar]
- Amodio, M.L.; Ceglie, F.; Chaudhry, M.M.A.; Piazzolla, F.; Colelli, G. Potential of NIR spectroscopy for predicting internal quality and discriminating among strawberry fruits from different production systems. Postharvest Biol. Technol. 2017, 125, 112–121. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K. Measurement and modeling of respiration rate of guava (CV. Baruipur) for modified atmosphere packaging. Int. J. Food Prop. 2011, 14, 609–628. [Google Scholar] [CrossRef]
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marengo, E. Impact of beneficial microorganisms on strawberry growth, fruit production, nutritional quality, and volatilome. Front. Plant Sci. 2018, 9, 1611. [Google Scholar] [CrossRef] [PubMed]
- Badillo, G.M.; Segura-Ponce, L.A. Classic and reaction-diffusion models used in modified atmosphere packaging (MAP) of fruit and vegetables. Food Eng. Rev. 2020, 12, 209–228. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K. Modeling of respiration rate of litchi fruit under aerobic conditions. Food Bioprocess Technol. 2011, 4, 272–281. [Google Scholar] [CrossRef]
- Cybulska, J.; Drobek, M.; Panek, J.; Cruz-Rubio, J.M.; Kurzyna-Szklarek, M.; Zdunek, A.; Frąc, M. Changes of pectin structure and microbial community composition in strawberry fruit (Fragaria × ananassa Duch.) during cold storage. Food Chem. 2022, 381, 132151. [Google Scholar] [CrossRef]
- Frick, A.A.; Weyermann, C. An untargeted lipidomic approach for qualitative determination of latent fingermark glycerides using UPLC-IMS-QToF-MS E. Analyst 2019, 144, 3590–3600. [Google Scholar] [CrossRef]
- Pi, J.; Wu, X.; Feng, Y. Fragmentation patterns of five types of phospholipids by ultra-high-performance liquid chromatography electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Anal. Methods 2016, 8, 1319–1332. [Google Scholar] [CrossRef]
- Song, C.; Wang, K.; Xiao, X.; Liu, Q.; Yang, M.; Li, X.; Feng, Y.; Li, S.; Shi, L.; Chen, W. Membrane lipid metabolism influences chilling injury during cold storage of peach fruit. Food Res. Int. 2022, 157, 111249. [Google Scholar] [CrossRef]
- Hong, K.; Yao, Q.; Golding, J.B.; Pristijono, P.; Zhang, X.; Hou, X.; Yuan, D.; Li, Y.; Chen, L.; Song, K. Low temperature storage alleviates internal browning of ‘Comte de Paris’ winter pineapple fruit by reducing phospholipid degradation, phosphatidic acid accumulation and membrane lipid peroxidation processes. Food Chem. 2023, 404, 134656. [Google Scholar] [CrossRef]
- Aktas, M.; Wessel, M.; Hacker, S.; Klüsener, S.; Gleichenhagen, J.; Narberhaus, F. Phosphatidylcholine biosynthesis and its significance in bacteria interacting with eukaryotic cells. Eur. J. Cell Biol. 2010, 89, 888–894. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; López-Lara, I.M.; Geiger, O. Biosynthesis of phosphatidylcholine in bacteria. Prog. Lipid Res. 2003, 42, 115–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Phospholipase D in hormonal and stress signaling. Curr. Opin. Plant Biol. 2002, 5, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Lu, S.; Fadlalla, T.; Iqbal, S.; Yue, H.; Yang, B.; Hong, Y.; Wang, X.; Guo, L. The functions of phospholipases and their hydrolysis products in plant growth, development and stress responses. Prog. Lipid Res. 2022, 86, 101158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Pan, X.; Qu, H.; Underhill, S., Jr. Low temperature alters plasma membrane lipid composition and ATPase activity of pineapple fruit during blackheart development. J. Bioenerg. Biomembr. 2014, 46, 59–69. [Google Scholar] [CrossRef]
- Whitaker, B.D. Changes in lipids of tomato fruit stored at chilling and non-chilling temperatures. Phytochemistry 1991, 30, 757–761. [Google Scholar] [CrossRef]
- Bartley, I.M. Changes in sterol and phospholipid composition of apples during storage at low temperature and low oxygen concentration. J. Sci. Food Agric. 1986, 37, 31–36. [Google Scholar] [CrossRef]
- Lee, S.; Suh, S.; Kim, S.; Crain, R.C.; Kwak, J.M.; Nam, H.-G.; Lee, Y. Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J. 1997, 12, 547–556. [Google Scholar] [CrossRef]
- Narváez-Vásquez, J.; Florin-Christensen, J.; Ryan, C.A. Positional specificity of a phospholipase a activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. Plant Cell 1999, 11, 2249–2260. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Zdunek, A.; Cybulska, J. The effect of cultivation method of strawberry (Fragaria x ananassa Duch.) cv. Honeoye on structure and degradation dynamics of pectin during cold storage. Molecules 2020, 25, 4325. [Google Scholar] [CrossRef]
- Saint Angelo, A.J.; Vercellotti, J.; Jacks, T.; Legendre, M. Lipid oxidation in foods. Crit. Rev. Food Sci. Nutr. 1996, 36, 175–224. [Google Scholar] [CrossRef]
- Loesel, H.; Shakiba, N.; Wenck, S.; Le Tan, P.; Karstens, T.-O.; Creydt, M.; Seifert, S.; Hackl, T.; Fischer, M. Food monitoring: Limitations of accelerated storage to predict molecular changes in hazelnuts (Corylus avellana L.) under Realistic conditions using UPLC-ESI-IM-QTOF-MS. Metabolites 2023, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- Gülen, H.; Çetinkaya, C.; Kadıoğlu, M.; Kesici, M.; Cansev, A.; Eriş, A. Peroxidase activity and lipid peroxidation in strawberry (Fragaria x ananassa) plants under low temperature. J. Biol. Environ. Sci. 2008, 2, 95–100. [Google Scholar]


| Day 2 | True | Prediction | Day 4 | True | Prediction | Day 6 | True | Prediction |
|---|---|---|---|---|---|---|---|---|
| MDay2-1 | 2 | 2 | MDay4-1 | 4 | 2 | MDay6–1 | 6 | 4 |
| MDay2-2 | 2 | 2 | MDay4-2 | 4 | 3 | MDay6–2 | 6 | 3 |
| MDay2-3 | 2 | 3 | MDay4-3 | 4 | 3 | MDay6–3 | 6 | 3 |
| MDay2-4 | 2 | 3 | MDay4-4 | 4 | 3 | MDay6–4 | 6 | 4 |
| MDay2-5 | 2 | 2 | MDay4-5 | 4 | 4 | MDay6–5 | 6 | 4 |
| MDay2-6 | 2 | 2 | MDay4-6 | 4 | 4 | MDay6–6 | 6 | 4 |
| MDay2-7 | 2 | 2 | MDay4-7 | 4 | 2 | MDay6–7 | 6 | 3 |
| MDay2-8 | 2 | 1 | MDay4-8 | 4 | 1 | MDay6–8 | 6 | 8 |
| MDay2-9 | 2 | 3 | MDay4-9 | 4 | 4 | MDay6–9 | 6 | 6 |
| Mean value | 2 | Mean value | 3 | Mean value | 4 | |||
| σ | 1 | σ | 1 | σ | 1 | |||
| Day 8 | True | Prediction | Day 10 | True | Prediction | |||
| MDay8-1 | 8 | 6 | MDay10-1 | 10 | 5 | |||
| MDay8-2 | 8 | 5 | MDay10-2 | 10 | 2 | |||
| MDay8-3 | 8 | 6 | MDay10-3 | 10 | 5 | |||
| MDay8-4 | 8 | 6 | MDay10-4 | 10 | 5 | |||
| MDay8-5 | 8 | 6 | MDay10-5 | 10 | 8 | |||
| MDay8-6 | 8 | 6 | MDay10-6 | 10 | 5 | |||
| MDay8-7 | 8 | 7 | MDay10-7 | 10 | 7 | |||
| MDay8-8 | 8 | 2 | MDay10-8 | 10 | 5 | |||
| MDay8-9 | 8 | 3 | MDay10-9 | 10 | 4 | |||
| Mean value | 5 | Mean value | 5 | |||||
| σ | 1 | σ | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockelt, J.; Dammann, R.; Griese, J.; Weiss, A.; Fischer, M.; Creydt, M. Storage Profiling: Evaluating the Effect of Modified Atmosphere Packaging on Metabolomic Changes of Strawberries (Fragaria × ananassa). Metabolites 2025, 15, 330. https://doi.org/10.3390/metabo15050330
Brockelt J, Dammann R, Griese J, Weiss A, Fischer M, Creydt M. Storage Profiling: Evaluating the Effect of Modified Atmosphere Packaging on Metabolomic Changes of Strawberries (Fragaria × ananassa). Metabolites. 2025; 15(5):330. https://doi.org/10.3390/metabo15050330
Chicago/Turabian StyleBrockelt, Johannes, Robin Dammann, Jennifer Griese, Agnes Weiss, Markus Fischer, and Marina Creydt. 2025. "Storage Profiling: Evaluating the Effect of Modified Atmosphere Packaging on Metabolomic Changes of Strawberries (Fragaria × ananassa)" Metabolites 15, no. 5: 330. https://doi.org/10.3390/metabo15050330
APA StyleBrockelt, J., Dammann, R., Griese, J., Weiss, A., Fischer, M., & Creydt, M. (2025). Storage Profiling: Evaluating the Effect of Modified Atmosphere Packaging on Metabolomic Changes of Strawberries (Fragaria × ananassa). Metabolites, 15(5), 330. https://doi.org/10.3390/metabo15050330





