Metabolic Characteristics of Obese Adolescents with Different Degrees of Weight Loss After Identical Exercise Training Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Subjects
2.2. Grouping of the Study Subjects
2.3. Exercise Intervention Protocol
2.3.1. Exercise Intervention
- −
- Work Interval: 2 min at 70–80% HRmax (moderate-to-high intensity).
- −
- Recovery Interval: 4 min at 50–70% HRmax (low-to-moderate intensity aerobic).
2.3.2. Dietary Control
2.4. Clinical Indicator Testing
2.4.1. Acquisition of Body Morphological Indicators
2.4.2. Acquisition of Fitness Level
2.4.3. Statistical Analysis of Clinical Indicators
2.5. Metabolomic Analysis
2.5.1. Sample Preparation
2.5.2. Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Analysis of Plasma Components
2.5.3. Differential Metabolite Screening
2.5.4. Discriminatory Efficacy Test for Differential Metabolites
2.5.5. Metabolic Pathway Analysis
3. Results
3.1. Effects of Exercise Intervention on Body Morphology and Fitness Level
3.2. Changes in Body Morphology and Fitness Level Between the HWL and LWL Groups
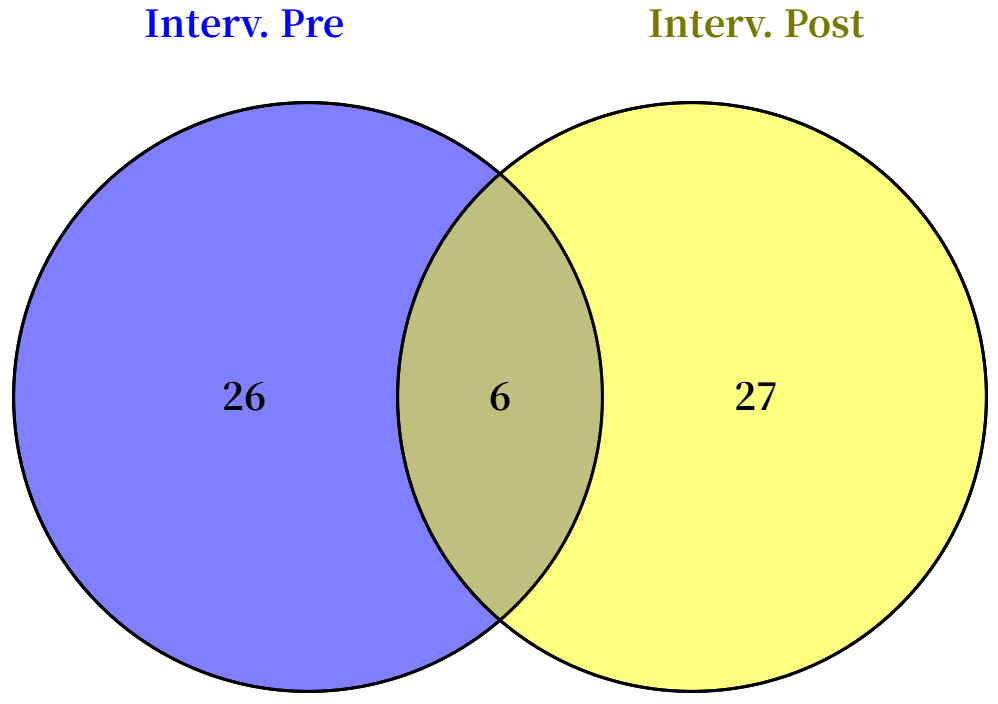
3.3. Identification of Differential Metabolites Between HWL and LWL Groups Before and After Intervention
3.4. Differential Metabolites Between the LWL and HWL Groups After Accounting for Baseline Differences
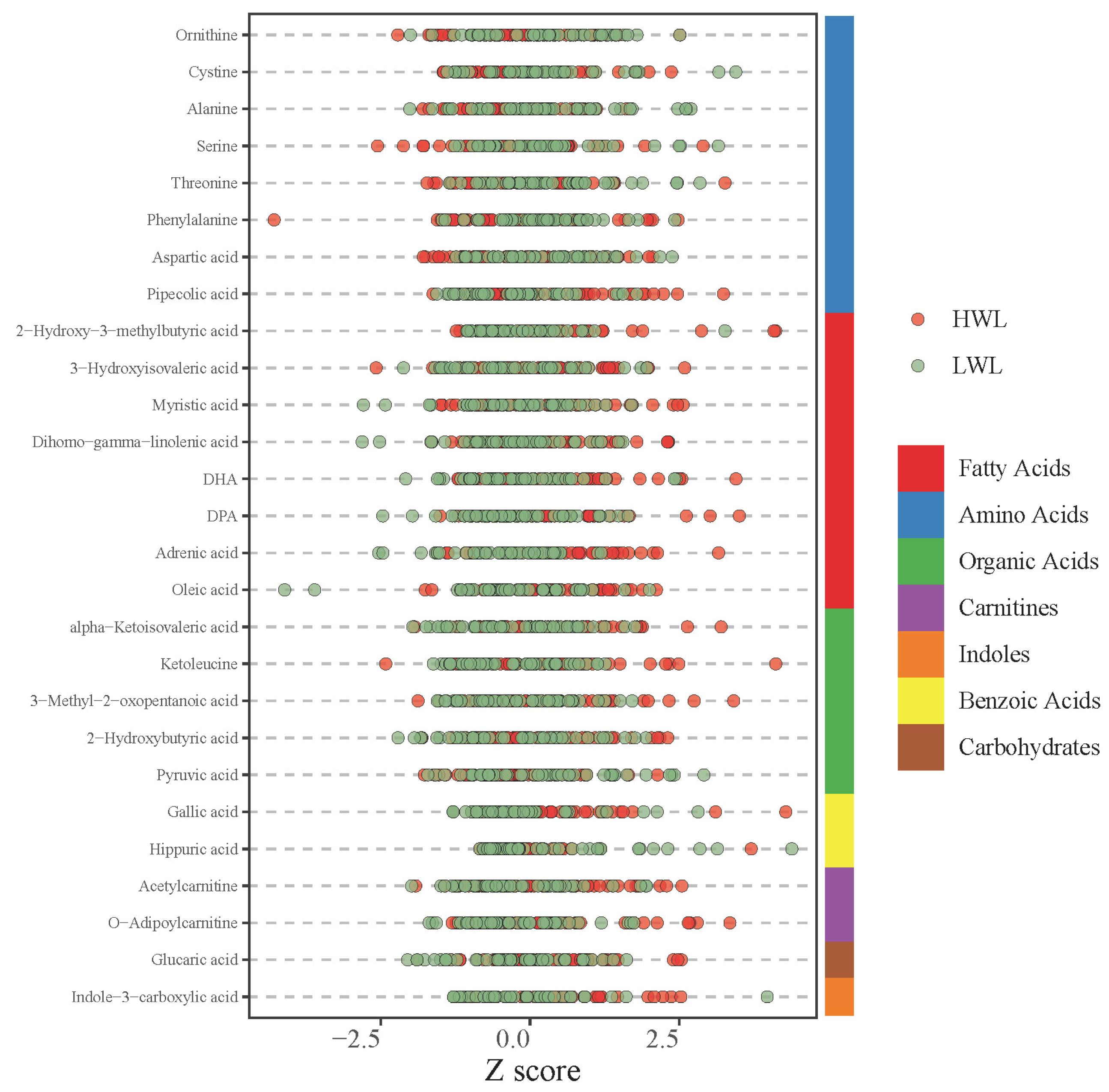
3.5. Class Distribution of the Differential Metabolites
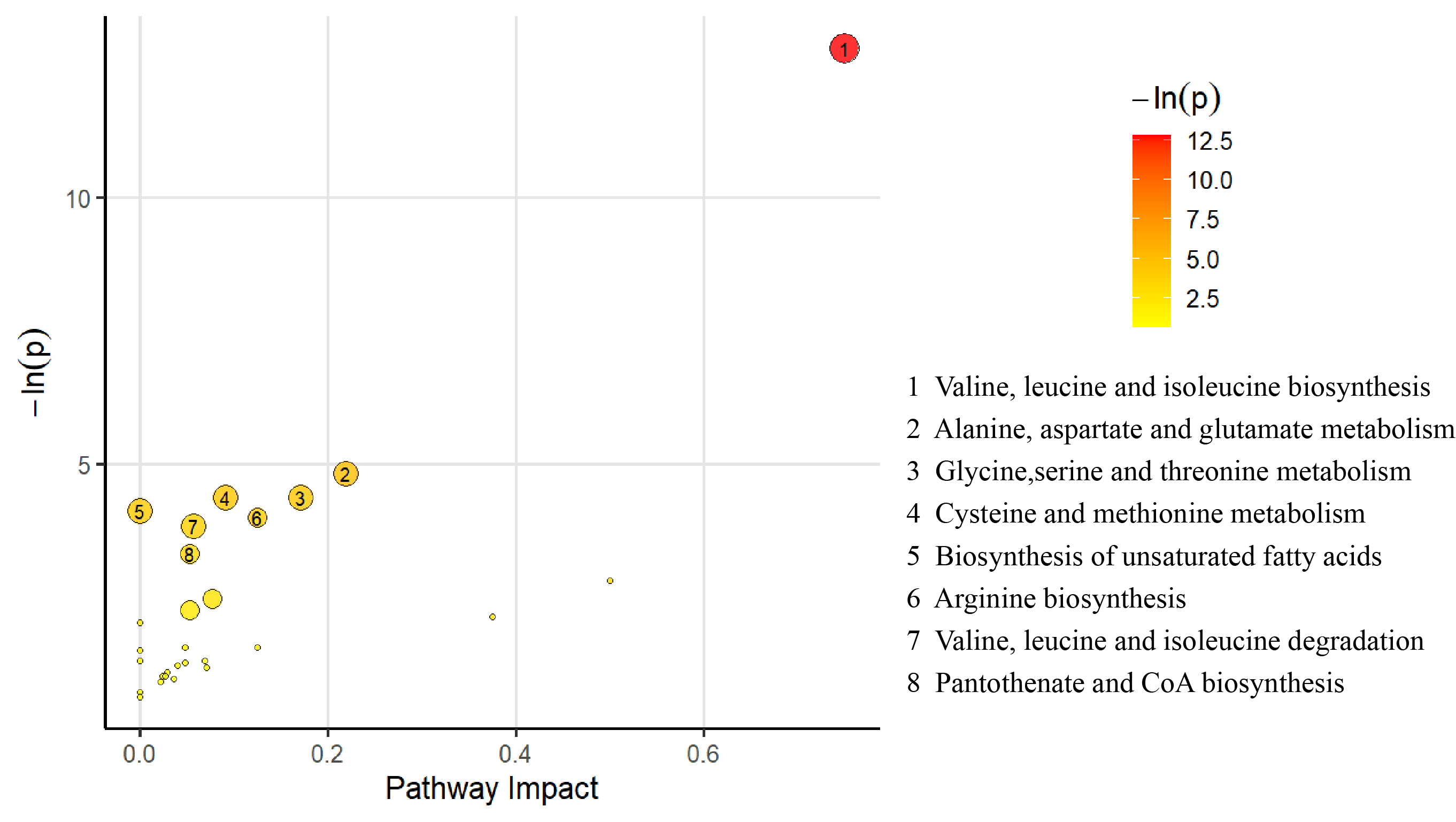
3.6. Pathway Analysis for the Differential Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LWL | Low weight loss |
| HWL | High weight loss |
| ROC | Receiver operating characteristic |
| AUC | Area Under the Curve |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DHA | docosahexaenoic acid |
| BCAA | Branched-chain Amino Acids |
| UFA | Unsaturated Fatty Acids |
| Arg | Arginine |
References
- Tully, L.; Arthurs, N.; Wyse, C.; Browne, S.; Case, L.; McCrea, L.; O’Connell, J.M.; O’Gorman, C.S.; Smith, S.M.; Walsh, A.; et al. Guidelines for treating child and adolescent obesity: A systematic review. Front. Nutr. 2022, 9, 902865. [Google Scholar] [CrossRef] [PubMed]
- WHO Organization. Obesity and Overweight. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 March 2024).
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and adolescent obesity. Nat. Rev. Dis. Primers 2023, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armstrong, S.C.; Eneli, I.; Osganian, S.K.; Wagner, B.E.; Waldrop, S.W.; Kelly, A.S. Pediatric Obesity Pharmacotherapy: State of the Science, Research Gaps, and Opportunities. Pediatrics 2024, 154, e2024067858. [Google Scholar] [CrossRef]
- Steinbeck, K.S.; Lister, N.B.; Gow, M.L.; Baur, L.A. Treatment of adolescent obesity. Nat. Rev. Endocrinol. 2018, 14, 331–334. [Google Scholar] [CrossRef]
- Moleres, A.; Campión, J.; Milagro, F.I.; Marcos, A.; Campoy, C.; Garagorri, J.M.; Gómez-Martínez, S.; Martínez, J.A.; Azcona-Sanjulián, M.C.; Martí, A. Differential DNA methylation patterns between high and low responders to a weight loss intervention in overweight or obese adolescents: The EVASYON study. Faseb J. 2013, 27, 2504–2512. [Google Scholar] [CrossRef]
- Pi, A.; Villivalam, S.D.; Kang, S. The Molecular Mechanisms of Fuel Utilization during Exercise. Biology 2023, 12, 1450. [Google Scholar] [CrossRef]
- Strasser, B.; Pesta, D. Resistance training for diabetes prevention and therapy: Experimental findings and molecular mechanisms. Biomed. Res. Int. 2013, 2013, 805217. [Google Scholar] [CrossRef]
- Laurens, C.; de Glisezinski, I.; Larrouy, D.; Harant, I.; Moro, C. Influence of Acute and Chronic Exercise on Abdominal Fat Lipolysis: An Update. Front. Physiol. 2020, 11, 575363. [Google Scholar] [CrossRef]
- Rodrigues, N.A.; Gobatto, C.A.; Forte, L.D.M.; Sousa, F.A.B.; Torsoni, A.S.; Fante, T.; Manchado-Gobatto, F.B. Load-matched acute and chronic exercise induce changes in mitochondrial biogenesis and metabolic markers. Appl. Physiol. Nutr. Metab. 2021, 46, 1196–1206. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Lundsgaard, A.M.; Kiens, B. Tuning fatty acid oxidation in skeletal muscle with dietary fat and exercise. Nat. Rev. Endocrinol. 2020, 16, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Howard, E.E. High-Quality Supplemental Protein Enhances Acute Muscle Protein Synthesis and Long-Term Strength Adaptations to Resistance Training in Young and Old Adults. J. Nutr. 2021, 151, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.D.; Rahnavard, A.; Kachroo, P.; Mendez, K.M.; Lawrence, W.; Julián-Serrano, S.; Hua, X.; Fuller, H.; Sinnott-Armstrong, N.; Tabung, F.K.; et al. An epidemiological introduction to human metabolomic investigations. Trends Endocrinol. Metab. 2023, 34, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Felder, T.K.; Ring-Dimitriou, S.; Auer, S.; Soyal, S.M.; Kedenko, L.; Rinnerthaler, M.; Cadamuro, J.; Haschke-Becher, E.; Aigner, E.; Paulweber, B.; et al. Specific circulating phospholipids, acylcarnitines, amino acids and biogenic amines are aerobic exercise markers. J. Sci. Med. Sport. 2017, 20, 700–705. [Google Scholar] [CrossRef]
- Parstorfer, M.; Poschet, G.; Kronsteiner, D.; Brüning, K.; Friedmann-Bette, B. Targeted Metabolomics in High Performance Sports: Differences between the Resting Metabolic Profile of Endurance- and Strength-Trained Athletes in Comparison with Sedentary Subjects over the Course of a Training Year. Metabolites 2023, 13, 833. [Google Scholar] [CrossRef]
- Yang, W.H.; Park, J.H.; Park, S.Y.; Park, Y. Energetic Contributions Including Gender Differences and Metabolic Flexibility in the General Population and Athletes. Metabolites 2022, 12, 965. [Google Scholar] [CrossRef]
- Kolodziej, F.; O’Halloran, K.D. Re-Evaluating the Oxidative Phenotype: Can Endurance Exercise Save the Western World? Antioxidants 2021, 10, 609. [Google Scholar] [CrossRef]
- Busebee, B.; Ghusn, W.; Cifuentes, L.; Acosta, A. Obesity: A Review of Pathophysiology and Classification. Mayo Clin. Proc. 2023, 98, 1842–1857. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129 (Suppl. S2), S102–S138. [Google Scholar] [CrossRef]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375–390.e311. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Cardel, M.I.; Kyle, T.K.; Jastreboff, A.M. Addressing psychosocial health in the treatment and care of adolescents with obesity. Obesity 2021, 29, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, K.M.; Korpelainen, R.; Vanhala, M.L.; Keinänen-Kiukaanniemi, S.M.; Korpelainen, J.T. Long-term effects on weight loss and maintenance by intensive start with diet and exercise. Scand. J. Med. Sci. Sports 2023, 33, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Videira-Silva, A.; Manco, L.; Sardinha, L.B.; Fonseca, H. Vigorous physical activity: A potential ally in adolescent obesity management. Eur. J. Sport. Sci. 2023, 23, 607–616. [Google Scholar] [CrossRef]
- Murphy, C.; Koehler, K. Energy deficiency impairs resistance training gains in lean mass but not strength: A meta-analysis and meta-regression. Scand. J. Med. Sci. Sports 2022, 32, 125–137. [Google Scholar] [CrossRef]
- Kokura, Y.; Ueshima, J.; Saino, Y.; Maeda, K. Enhanced protein intake on maintaining muscle mass, strength, and physical function in adults with overweight/obesity: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2024, 63, 417–426. [Google Scholar] [CrossRef]
- Leite, C.; Zovico, P.V.C.; Rica, R.L.; Barros, B.M.; Machado, A.F.; Evangelista, A.L.; Leite, R.D.; Barauna, V.G.; Maia, A.F.; Bocalini, D.S. Exercise-Induced Muscle Damage after a High-Intensity Interval Exercise Session: Systematic Review. Int. J. Environ. Res. Public. Health 2023, 20, 7082. [Google Scholar] [CrossRef]
- Nichols, S.; O’Doherty, A.; Carroll, S.; Ingle, L. Influence of appendicular skeletal muscle mass on resting metabolic equivalents in patients with cardiovascular disease: Implications for exercise training and prescription. Eur. J. Prev. Cardiol. 2020, 27, 1001–1003. [Google Scholar] [CrossRef]
- Martins, C.; Roekenes, J.A.; Rehfeld, J.F.; Hunter, G.R.; Gower, B.A. Metabolic adaptation is associated with a greater increase in appetite following weight loss: A longitudinal study. Am. J. Clin. Nutr. 2023, 118, 1192–1201. [Google Scholar] [CrossRef]
- McCarthy, D.; Berg, A. Weight Loss Strategies and the Risk of Skeletal Muscle Mass Loss. Nutrients 2021, 13, 2473. [Google Scholar] [CrossRef]
- Borfe, L.; Brand, C.; Schneiders, L.B.; Mota, J.; Cavaglieri, C.R.; Leite, N.; Renner, J.D.P.; Reuter, C.P.; Gaya, A.R. Effects and Responsiveness of a Multicomponent Intervention on Body Composition, Physical Fitness, and Leptin in Overweight/Obese Adolescents. Int. J. Environ. Res. Public. Health 2021, 18, 7267. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Kreouzi, M.; Pappas, A.; Nikolaou, M. Beyond Calories: Individual Metabolic and Hormonal Adaptations Driving Variability in Weight Management-A State-of-the-Art Narrative Review. Int. J. Mol. Sci. 2024, 25, 3438. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Sørensen, T.I.A.; Raubenheimer, D.; Dhurandhar, N.V.; Loos, R.J.F.; Bosy-Westphal, A.; Clemmensen, C.; Hjorth, M.F.; Allison, D.B.; Taubes, G.; et al. On the pathogenesis of obesity: Causal models and missing pieces of the puzzle. Nat. Metab. 2024, 6, 1856–1865. [Google Scholar] [CrossRef]
- Speakman, J.R.; Hall, K.D. Models of body weight and fatness regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220231. [Google Scholar] [CrossRef]
- Martin, C.K.; Johnson, W.D.; Myers, C.A.; Apolzan, J.W.; Earnest, C.P.; Thomas, D.M.; Rood, J.C.; Johannsen, N.M.; Tudor-Locke, C.; Harris, M.; et al. Effect of different doses of supervised exercise on food intake, metabolism, and non-exercise physical activity: The E-MECHANIC randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 583–592. [Google Scholar] [CrossRef]
- Hengist, A.; Sciarrillo, C.M.; Guo, J.; Walter, M.; Hall, K.D. Gut-derived appetite hormones do not explain energy intake differences in humans following low-carbohydrate versus low-fat diets. Obesity 2024, 32, 1689–1698. [Google Scholar] [CrossRef]
- Reistenbach-Goltz, F. Interindividual variability in perceived appetite and appetite-related hormone responses to eating and exercise in humans. Int. J. Obes. 2019, 48, 477–485. [Google Scholar]
- Abo, S.M.C.; Layton, A.T. Modeling sex-specific whole-body metabolic responses to feeding and fasting. Comput. Biol. Med. 2024, 181, 109024. [Google Scholar] [CrossRef]
- Christians, J.K.; Reue, K. The role of gonadal hormones and sex chromosomes in sex-dependent effects of early nutrition on metabolic health. Front Endocrinol (Lausanne) 2023, 14, 1304050. [Google Scholar] [CrossRef]
- Jiang, F.; Tian, L.; Fu, H.; Hasegawa, T.; Wei, L.J. Robust Alternatives to ANCOVA for Estimating the Treatment Effect via a Randomized Comparative Study. J. Am. Stat. Assoc. 2019, 114, 1854–1864. [Google Scholar] [CrossRef]
- Sato, H.; Taketomi, Y.; Miki, Y.; Murase, R.; Yamamoto, K.; Murakami, M. Secreted Phospholipase PLA2G2D Contributes to Metabolic Health by Mobilizing ω3 Polyunsaturated Fatty Acids in WAT. Cell Rep. 2020, 31, 107579. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.; Gallardo, A.; Romero, C.; Valenzuela, R.; Garcia-Diaz, D.F.; Duarte, L.; Bustamante, A.; Gasaly, N.; Gotteland, M.; Echeverria, F. n-3 polyunsaturated fatty acids in the regulation of adipose tissue browning and thermogenesis in obesity: Potential relationship with gut microbiota. Prostaglandins Leukot. Essent. Fat. Acids 2022, 177, 102388. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, X.; Wang, C.; Han, J.; Qi, W.; Zhang, H.; Liu, Z.; You, C. Lactobacillus paracasei BD5115-Derived 2-Hydroxy-3-Methylbutyric Acid Promotes Intestinal Epithelial Cells Proliferation by Upregulating the MYC Signaling Pathway. Front. Nutr. 2022, 9, 799053. [Google Scholar] [CrossRef]
- Martino, M.R.; Habibi, M.; Ferguson, D.; Brookheart, R.T.; Thyfault, J.P.; Meyer, G.A.; Lantier, L.; Hughey, C.C.; Finck, B.N. Disruption of hepatic mitochondrial pyruvate and amino acid metabolism impairs gluconeogenesis and endurance exercise capacity in mice. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E515–E527. [Google Scholar] [CrossRef]
- Kusy, K.; Matysiak, J.; Kokot, Z.J.; Ciekot-Sołtysiak, M.; Klupczyńska-Gabryszak, A.; Zarębska, E.A.; Plewa, S.; Dereziński, P.; Zieliński, J. Exercise-induced response of proteinogenic and non-proteinogenic plasma free amino acids is sport-specific: A comparison of sprint and endurance athletes. PLoS ONE 2024, 19, e0309529. [Google Scholar] [CrossRef]
- Leandro, J.; Houten, S.M. The lysine degradation pathway: Subcellular compartmentalization and enzyme deficiencies. Mol. Genet. Metab. 2020, 131, 14–22. [Google Scholar] [CrossRef]
- Park, J.M.; Harrison, C.E.; Ma, J.; Chen, J.; Ratnakar, J.; Zun, Z.; Liticker, J.; Reed, G.D.; Chhabra, A.; Haller, R.G.; et al. Hyperpolarized (13)C MR Spectroscopy Depicts in Vivo Effect of Exercise on Pyruvate Metabolism in Human Skeletal Muscle. Radiology 2021, 300, 626–632. [Google Scholar] [CrossRef]
- Ten Have, G.A.M.; Jansen, L.; Schooneman, M.G.; Engelen, M.; Deutz, N.E.P. Metabolic flux analysis of branched-chain amino and keto acids (BCAA, BCKA) and β-hydroxy β-methylbutyric acid across multiple organs in the pig. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E629–E640. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef]
- Wadsworth, B.J.; Leiwe, M.; Minogue, E.A.; Cunha, P.P.; Engman, V.; Brombach, C.; Asvestis, C.; Sah-Teli, S.K.; Marklund, E.; Koivunen, P.; et al. A 2-hydroxybutyrate-mediated feedback loop regulates muscular fatigue. Elife 2024, 12, 92707. [Google Scholar] [CrossRef] [PubMed]
- Ayyadurai, V.A.S.; Deonikar, P.; Fields, C. Mechanistic Understanding of D-Glucaric Acid to Support Liver Detoxification Essential to Muscle Health Using a Computational Systems Biology Approach. Nutrients 2023, 15, 733. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Masaki, H.; Fujita, K.; Murakami, H.; Shizuma, M.; Kiso, T.; Kiryu, T. Identification of Enzymes from Pseudogluconobacter saccharoketogenes Producing d-Glucaric Acid from d-Glucose. Biosci. Biotechnol. Biochem. 2021, 86, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Guerra, A.; Nouvenne, A.; Meschi, T.; Maggi, S. Disentangling the Complexity of Nutrition, Frailty and Gut Microbial Pathways during Aging: A Focus on Hippuric Acid. Nutrients 2023, 15, 1138. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91.e75. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Hu, Y.; Yin, F.; Liu, X.; Zhou, D. Gastrointestinal Digestion and Microbial Hydrolysis of Alkyl Gallates: Potential Sustained Release of Gallic Acid. Foods 2022, 11, 3936. [Google Scholar] [CrossRef]
- Gupta, R.D.; Mahant, S.A.; Wankhade, P.R.; Hemke, A.T.; Wadher, K.J.; Umekar, M.J. Gallic Acid: A Versatile Molecule with Promising Pharmacological Effect. Int. J. Pharmacogn. Life Sci. 2021, 2, 49–56. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakamura-Shinya, Y.; Ebina, K.; Komine, S.; Ra, S.G.; Ishikura, K.; Ohmori, H.; Honda, A. N-acetyltaurine and Acetylcarnitine Production for the Mitochondrial Acetyl-CoA Regulation in Skeletal Muscles during Endurance Exercises. Metabolites 2021, 11, 522. [Google Scholar] [CrossRef]
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, Fatty Acid Oxidation. In StatPearls; StatPearls Publishing. Copyright © 2025; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Demarquoy, J. Revisiting the Role of Carnitine in Heart Disease Through the Lens of the Gut Microbiota. Nutrients 2024, 16, 4244. [Google Scholar] [CrossRef]
- Liu, Y.; Pei, Z.; Pan, T.; Wang, H.; Chen, W.; Lu, W. Indole metabolites and colorectal cancer: Gut microbial tryptophan metabolism, host gut microbiome biomarkers, and potential intervention mechanisms. Microbiol. Res. 2023, 272, 127392. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Hu, Y.; Zhao, Y. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front. Pharmacol. 2021, 12, 769501. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Duffley, L.; Pulinilkunnil, T. Role of branched-chain amino acid-catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. Faseb j 2019, 33, 8711–8731. [Google Scholar] [CrossRef] [PubMed]
- Gumus Balikcioglu, P.; Ramaker, M.E.; Mason, K.A.; Huffman, K.M.; Johnson, J.L.; Ilkayeva, O.; Muehlbauer, M.J.; Freemark, M.; Kraus, W.E. Branched-Chain Amino Acid Catabolism and Cardiopulmonary Function Following Acute Maximal Exercise Testing in Adolescents. Front. Cardiovasc. Med. 2021, 8, 721354. [Google Scholar] [CrossRef] [PubMed]
- Duft, R.G.; Bonfante, I.L.P.; Palma-Duran, S.A.; Chacon-Mikahil, M.P.T.; Griffin, J.L.; Cavaglieri, C.R. Moderate-intensity Combined Training Induces Lipidomic Changes in Individuals With Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2024, 109, 2182–2198. [Google Scholar] [CrossRef]
- Dobrzyn, P. CoA in Health and Disease. Int. J. Mol. Sci. 2022, 23, 4371. [Google Scholar] [CrossRef]
| Body Morphology and Fitness Level | Pre-Intervention (n = 98) | Post-Intervention (n = 98) | p | Cohen’s d | 95%CI |
|---|---|---|---|---|---|
| Sex (male/female) | 54/44 | 54/44 | 1.000 | - | - |
| Age | 13.05 ± 1.58 | 13.05 ± 1.69 | 1.000 | - | - |
| Height (cm) | 164.6 ± 9.8 | 164.6 ± 9.6 | 1.000 | - | - |
| Weight (kg) | 80.65 (71.35, 90.15) | 72.35 (64.18, 81.23) | <0.001 | 0.55 | 8.31~9.7 |
| Body mass index (kg/m2) | 30.57 ± 4.41 | 27.26 ± 3.89 | <0.001 | 0.79 | 3.07~3.55 |
| Chest circumference (cm) | 102.7 ± 9.5 | 95.0 ± 8.8 | <0.001 | 0.83 | 6.74~8.57 |
| Waist circumference (cm) | 103.6 ± 11.8 | 94.7 ± 11.2 | <0.001 | 0.77 | 8.06~9.77 |
| Hip circumference (cm) | 107.9 ± 10.1 | 100.7 ± 9.6 | <0.001 | 0.73 | 6.60~7.87 |
| Waist–hip ratio | 0.96 ± 0.08 | 0.94 ± 0.09 | <0.001 | 0.23 | 0.01~0.02 |
| Waist–height ratio | 0.63 ± 0.07 | 0.58 ± 0.06 | <0.001 | 0.76 | 0.04~0.06 |
| Body fat percentage (%) | 32.68 ± 5.36 | 28.54 ± 6.04 | <0.001 | 0.72 | 3.77~4.51 |
| Body fat mass (kg) | 27.73 ± 9.11 | 21.71 ± 8.02 | <0.001 | 0.70 | 5.53~6.51 |
| Free fat weight (kg) | 55.64 ± 9.80 | 52.66 ± 9.08 | <0.001 | 0.31 | 2.67~3.30 |
| Body water (L) | 40.06 ± 7.06 | 37.91 ± 6.53 | <0.001 | 0.31 | 1.92~2.37 |
| Skeletal muscle mass (kg) | 50.92 ± 8.91 | 48.41 ± 8.30 | <0.001 | 0.29 | 2.22~2.79 |
| Body Morphology and Fitness Level | LWL (n = 46) | HWL (n = 52) | p | Cohen’s d | 95%CI |
|---|---|---|---|---|---|
| Sex (male/female) | 19/27 | 35/17 | 0.009 | - | - |
| Age | 13.15 ± 1.59 | 12.96 ± 1.79 | 0.581 | - | - |
| Height (cm) | 163.0 (160.0, 171.7) | 164.5 (159.0, 173.5) | 0.933 | - | - |
| Weight (kg) | 78.45 (71.13, 86.25) | 84.05 (72.58, 96.40) | 0.180 | 0.27 | −11.63~2.21 |
| Body mass index (kg/m2) | 28.95 (26.80, 31.70) | 30.40 (28.28, 33.80) | 0.108 | 0.32 | −3.19~0.32 |
| Chest circumferences (cm) | 101.0 (94.1, 105.0) | 102.0 (95.4, 109.3) | 0.388 | 0.19 | −5.85~2.29 |
| Waist circumference (cm) | 103.5 (94.6, 112.8) | 105.8 (97.8, 112.0) | 0.714 | 0.07 | −5.63~3.87 |
| Hip circumference (cm) | 105.0 (100.0, 110.8) | 110.3 (102.0, 115.5) | 0.182 | 0.27 | −6.77~1.30 |
| Waist–hip ratio | 0.98 (0.92, 1.01) | 0.95 (0.91, 0.99) | 0.292 | −0.2 | −0.01~0.04 |
| Waist–height ratio | 0.62 ± 0.07 | 0.63 ± 0.05 | 0.778 | 0.06 | −0.03~0.02 |
| Body fat percentage (%) | 33.15 (29.23, 36.58) | 32.70 (28.53, 35.98) | 0.903 | −0.02 | −2.03~2.29 |
| Body fat mass (kg) | 37.80 (34.85, 42.00) | 39.55 (36.10, 46.48) | 0.501 | 0.13 | −4.91~2.42 |
| Free fat weight (kg) | 25.75 (21.00, 30.68) | 27.60 (21.53, 34.15) | 0.081 | 0.36 | −7.36~0.43 |
| Body water (L) | 52.50 (48.45, 58.35) | 54.90 (50.15, 64.58) | 0.081 | 0.36 | −5.30~0.31 |
| Skeletal muscle mass (kg) | 48.15 (44.38, 53.75) | 50.10 (45.86, 59.43) | 0.077 | 0.36 | −6.72~0.35 |
| ∆body Morphology and Fitness Level | LWL (n = 46) | HWL (n = 52) | aov_p | Cohen’s d | 95%CI |
|---|---|---|---|---|---|
| ∆weight (kg) | 6.40 (5.30, 7.70) | 10.45 (8.95, 12.85) | <0.001 | 1.80 | −5.64~−3.55 |
| ∆body mass index (kg/m2) | 2.45 (2.08, 2.80) | 3.80 (3.30, 4.88) | <0.001 | 1.91 | −1.98~−1.29 |
| ∆chest circumference (cm) | 7.0 (5.0, 9.0) | 8.0 (5.5, 11.0) | 0.310 | −0.20 | −4.08~10.63 |
| ∆waist circumference (cm) | 6.7 ± 1.9 | 10.2 ± 4.6 | <0.001 | 0.80 | −4.76~−1.56 |
| ∆hip circumference (cm) | 4.5 (2.5, 7.7) | 5.0 (2.5, 8.3) | 0.029 | 0.52 | −2.60~−0.27 |
| ∆waist–hip ratio | 0.01 ± 0.02 | 0.03 ± 0.04 | 0.083 | 0.34 | −0.02~0.001 |
| ∆waist–height ratio | 0.04 (0.03, 0.05) | 0.06 (0.04, 0.09) | <0.001 | 0.76 | −0.02~−0.008 |
| ∆body fat percentage (%) | 3.35 ± 1.30 | 4.63 ± 2.00 | <0.001 | 0.73 | −2.50~−1.21 |
| ∆body fat mass (kg) | 4.63 ± 1.11 | 7.15 ± 2.03 | <0.001 | 1.49 | −3.68~−2.10 |
| ∆Free fat weight (kg) | 1.95 (1.50, 2.50) | 3.65 (2.80, 4.38) | <0.001 | 1.31 | −2.23~−1.17 |
| ∆body water (L) | 1.40 (1.10, 1.80) | 2.65 (2.00, 3.18) | <0.001 | 1.31 | −1.61~−0.84 |
| ∆skeletal muscle mass (kg) | 1.60 (1.18, 2.10) | 3.10 (2.30, 3.80) | <0.001 | 1.21 | −1.94~−0.96 |
| Metabolite | Class | LWL | HWL | aov_p |
|---|---|---|---|---|
| Glutamic acid | Amino Acids | 41.44 (34.14, 48.11) | 47.48 (35.95, 63.06) | 0.008 |
| beta-Alanine | Amino Acids | 4.06 ± 1.06 | 4.57 ± 1.14 | 0.024 |
| Dimethylglycine | Amino Acids | 5.03 (4, 5.83) | 5.74 (4.51, 6.86) | 0.005 |
| Hydroxyphenyllactic acid | Phenylpropanoic Acids | 1.56 (1.34, 1.78) | 1.70 (1.58, 2.14) | 0.006 |
| Indole-3-methyl acetate | Indoles | 0.12 (0.08, 0.21) | 0.09 (0.06, 0.14) | 0.019 |
| Indoleacetic acid | Indoles | 2.12 (1.63, 2.71) | 1.94 (1.40, 2.25) | 0.023 |
| Cinnamic acid | Phenylpropanoids | 0.07 (0.06, 0.08) | 0.06 (0.06, 0.07) | 0.001 |
| 2-Phenylpropionate | Phenylpropanoic Acids | 0.27 (0.22, 0.45) | 0.22 (0.12, 0.31) | 0.018 |
| Hydrocinnamic acid | Phenylpropanoic Acids | 0.28 (0.21, 0.43) | 0.22 (0.17, 0.31) | 0.031 |
| Benzenebutanoic acid | Benzenoids | 0.1 (0.09, 0.11) | 0.10 (0.09, 0.10) | 0.037 |
| Glyceric acid | Carbohydrates | 3.91 (3.11, 4.25) | 4.39 (3.76, 4.86) | 0.003 |
| N-Acetylserine | Amino Acids | 1.66 ± 0.29 | 1.77 ± 0.27 | 0.049 |
| N-Acetylneuraminic acid | Carbohydrates | 0.71 (0.67, 0.80) | 0.78 (0.69, 0.88) | 0.017 |
| Pyroglutamic acid | Amino Acids | 44.9 (36.23, 51.71) | 51.03 (38.19, 71.02) | 0.007 |
| Maltotriose | Carbohydrates | 3.48 (1.54, 4.46) | 1.87 (1.14, 3.44) | 0.045 |
| Rhamnose | Carbohydrates | 0.93 (0.75, 1.13) | 1.25 (0.88, 1.42) | 0.010 |
| Propionic acid | SCFAs | 2.41 (1.90, 3.46) | 3.15 (1.99, 5.63) | 0.028 |
| Homovanillic acid | Phenols | 1.14 (1.03, 1.31) | 1.25 (1.09, 1.48) | 0.006 |
| Butyric acid | SCFAs | 2.21 ± 0.50 | 2.47 ± 0.50 | 0.011 |
| Phenylacetylglutamine | Amino Acids | 1.21 (0.78, 2.02) | 0.92 (0.49, 1.37) | 0.020 |
| Valeric acid | SCFAs | 0.76 (0.58, 1.17) | 0.79 (0.52, 2.66) | 0.021 |
| 2-Methylpentanoic acid | SCFAs | 0.26 (0.15, 0.41) | 0.21 (0.13, 1.22) | 0.009 |
| Heptanoic acid | Fatty Acids | 0.44 ± 0.17 | 0.54 ± 0.22 | 0.016 |
| TCDCA | Bile Acids | 0.06 (0.03, 0.11) | 0.1 (0.04, 0.17) | 0.034 |
| Oxoglutaric acid | Organic Acids | 65.84 (58.41, 76.69) | 76.58 (65.22, 101.62) | 0.000 |
| Oxoadipic acid | Organic Acids | 0.13 (0.11, 0.15) | 0.14 (0.12, 0.17) | 0.026 |
| Undecylenic acid | Fatty Acids | 0.87 (0.58, 1.29) | 0.74 (0.45, 1.10) | 0.025 |
| Arachidonic acid | Fatty Acids | 90.04 ± 28.22 | 108.87 ± 28.60 | 0.001 |
| DPAn-6 | Fatty Acids | 2.00 (1.63, 2.50) | 2.42 (2.09, 2.82) | 0.008 |
| Heptadecanoic acid | Fatty Acids | 11.41 (7.62, 14.24) | 9.2 (6.79, 11.72) | 0.027 |
| Succinic acid | Organic Acids | 2.78 ± 0.46 | 3.00 ± 0.45 | 0.017 |
| Methylmalonylcarnitine | Carnitines | 0.04 (0.04, 0.04) | 0.04 (0.04, 0.05) | 0.007 |
| Metabolite | Class | LWL | HWL | aov_p |
|---|---|---|---|---|
| Alanine | Amino Acids | 305.71 ± 64.39 | 277.43 ± 45.39 | 0.011 |
| Aspartic acid | Amino Acids | 1.91 (1.47, 2.38) | 1.54 (1.10, 2.23) | 0.028 |
| Cystine | Amino Acids | 82.03 (69.04, 96.33) | 64.88 (47.02, 87.40) | 0.042 |
| Serine | Amino Acids | 176.4 (154.18, 187.24) | 161.88 (152.26, 181.80) | 0.047 |
| Ornithine | Amino Acids | 26.61 ± 5.08 | 24.56 ± 4.91 | 0.041 |
| Phenylalanine | Amino Acids | 51.24 (45.22, 55.64) | 45.55 (38.98, 51.16) | 0.031 |
| Pipecolic acid | Amino Acids | 7.41 (6.09, 8.43) | 7.68 (6.76, 9.89) | 0.015 |
| Threonine | Amino Acids | 61.3 (55.48, 69.54) | 52.29 (47.91, 64.84) | 0.001 |
| Gallic acid | Benzoic Acids | 0.53 (0.34, 0.78) | 0.81 (0.54, 1.22) | 0.043 |
| Glucaric acid | Carbohydrates | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.002 |
| 2-Hydroxy-3-methylbutyric acid | Fatty Acids | 22.59 (15.72, 31.04) | 27.22 (19.78, 38.25) | 0.035 |
| Hippuric acid | Benzoic Acids | 0.71 (0.28, 2.06) | 0.47 (0.29, 0.95) | 0.014 |
| Maltotriose | Carbohydrates | 1.67 (0.73, 3.13) | 1.05 (0.61, 1.76) | 0.008 |
| Acetylcarnitine | Carnitines | 73.54 (65.22, 96.24) | 86.9 (70.82, 110.28) | 0.034 |
| O-Adipoylcarnitine | Carnitines | 0.11 (0.1, 0.14) | 0.14 (0.11, 0.15) | 0.021 |
| 3-Hydroxyisovaleric acid | Fatty Acids | 1.13 ± 0.33 | 1.30 ± 0.36 | 0.011 |
| 3-Methyl-2-oxopentanoic acid | Organic Acids | 108.55 ± 22.36 | 118.9 ± 28.61 | 0.043 |
| Adrenic acid | Fatty Acids | 7.60 ± 2.13 | 9.47 ± 2.73 | 0.000 |
| Arachidonic acid | Fatty Acids | 106.42 ± 28.97 | 131.34 ± 30.65 | 0.000 |
| DHA | Fatty Acids | 44.89 (37.01, 59.12) | 53.52 (45.96, 74.57) | 0.009 |
| Dihomo-gamma-linolenic acid | Fatty Acids | 5.89 ± 1.90 | 7.00 ± 2.13 | 0.005 |
| Pyruvic acid | Organic Acids | 129.31 (86.48, 178.64) | 108.63 (84.75, 129.40) | 0.029 |
| DPA | Fatty Acids | 4.36 (3.61, 5.60) | 5.13 (4.12, 6.73) | 0.010 |
| DPAn-6 | Fatty Acids | 2.41 (1.87, 2.86) | 2.97 (2.34, 3.87) | 0.000 |
| Myristic acid | Fatty Acids | 33.40 ± 9.44 | 37.70 ± 10.96 | 0.040 |
| Undecylenic acid | Fatty Acids | 0.75 (0.49, 1.11) | 0.56 (0.39, 0.87) | 0.018 |
| Indole-3-carboxylic acid | Indoles | 0.10 (0.08, 0.13) | 0.13 (0.09, 0.16) | 0.045 |
| 2-Hydroxybutyric acid | Organic Acids | 187.38 ± 70.44 | 214.57 ± 64.22 | 0.025 |
| alpha-Ketoisovaleric acid | Organic Acids | 28.39 ± 5.00 | 30.73 ± 5.53 | 0.023 |
| Ketoleucine | Organic Acids | 292 (240, 327.52) | 306.47 (275.62, 342.72) | 0.015 |
| Oleic acid | Fatty Acids | 923.92 (841.54, 1060.98) | 1007.44 (891.57, 1107.10) | 0.029 |
| Homovanillic acid | Phenols | 1.19 (1.01, 1.31) | 1.32 (1.13, 1.45) | 0.030 |
| Hydroxyphenyllactic acid | Phenylpropanoic Acids | 1.63 (1.41, 1.96) | 1.89 (1.54, 2.06) | 0.015 |
| Metabolite | AUC | CI1 | CI2 | Thres | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| Threonine | 0.704 | 0.599921 | 0.80727 | 54.840 | 0.577 | 0.826 |
| Adrenic acid | 0.691 | 0.585515 | 0.797428 | 9.185 | 0.596 | 0.783 |
| Glucaric acid | 0.659 | 0.550605 | 0.766703 | 0.087 | 0.462 | 0.783 |
| Phenylalanine | 0.659 | 0.549571 | 0.768991 | 48.860 | 0.654 | 0.652 |
| 3-Hydroxyisovaleric acid | 0.658 | 0.548517 | 0.767118 | 1.077 | 0.808 | 0.522 |
| DHA | 0.648 | 0.538772 | 0.757214 | 45.804 | 0.750 | 0.522 |
| Gallic acid | 0.642 | 0.530811 | 0.75347 | 0.543 | 0.750 | 0.543 |
| Cystine | 0.637 | 0.524336 | 0.749076 | 67.288 | 0.538 | 0.804 |
| Aspartic acid | 0.632 | 0.520945 | 0.742851 | 1.389 | 0.462 | 0.826 |
| Indole-3-carboxylic acid | 0.631 | 0.520346 | 0.742614 | 0.131 | 0.538 | 0.739 |
| Pipecolic acid | 0.629 | 0.518154 | 0.739789 | 6.441 | 0.846 | 0.391 |
| Dihomo-gamma-linolenic acid | 0.628 | 0.517094 | 0.738759 | 7.064 | 0.500 | 0.804 |
| DPA | 0.628 | 0.517179 | 0.73951 | 4.894 | 0.577 | 0.674 |
| Alanine | 0.627 | 0.514406 | 0.738938 | 334.750 | 0.904 | 0.326 |
| Acetylcarnitine | 0.626 | 0.513978 | 0.737694 | 77.560 | 0.673 | 0.587 |
| Ketoleucine | 0.621 | 0.509903 | 0.732572 | 260.508 | 0.827 | 0.413 |
| O-Adipoylcarnitine | 0.62 | 0.507909 | 0.731639 | 0.109 | 0.750 | 0.500 |
| Ornithine | 0.62 | 0.506404 | 0.732727 | 25.511 | 0.635 | 0.674 |
| Oleic acid | 0.612 | 0.499796 | 0.724284 | 948.005 | 0.673 | 0.543 |
| 2-Hydroxy-3-methylbutyric acid | 0.610 | 0.497617 | 0.722283 | 26.585 | 0.538 | 0.674 |
| alpha-Ketoisovaleric acid | 0.607 | 0.4938 | 0.719411 | 24.856 | 0.923 | 0.283 |
| 2-Hydroxybutyric acid | 0.606 | 0.491841 | 0.719697 | 137.898 | 0.942 | 0.304 |
| 3-Methyl-2-oxopentanoic acid | 0.599 | 0.48667 | 0.712326 | 105.097 | 0.673 | 0.500 |
| Pyruvic acid | 0.598 | 0.480857 | 0.714795 | 127.578 | 0.750 | 0.522 |
| Serine | 0.594 | 0.481091 | 0.707873 | 165.999 | 0.538 | 0.674 |
| Hippuric acid | 0.593 | 0.477211 | 0.708826 | 1.895 | 0.962 | 0.283 |
| Myristic acid | 0.591 | 0.477502 | 0.703937 | 40.597 | 0.404 | 0.804 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Yang, G.; Qin, Y.; Wang, Y.; Liu, Z.; Zhang, Z.; Li, Z.; Wang, H.; Zhu, L. Metabolic Characteristics of Obese Adolescents with Different Degrees of Weight Loss After Identical Exercise Training Intervention. Metabolites 2025, 15, 313. https://doi.org/10.3390/metabo15050313
Xie X, Yang G, Qin Y, Wang Y, Liu Z, Zhang Z, Li Z, Wang H, Zhu L. Metabolic Characteristics of Obese Adolescents with Different Degrees of Weight Loss After Identical Exercise Training Intervention. Metabolites. 2025; 15(5):313. https://doi.org/10.3390/metabo15050313
Chicago/Turabian StyleXie, Xianyan, Gaoyuan Yang, Yulin Qin, Yu Wang, Zhijun Liu, Zhuofan Zhang, Ziyan Li, Huiguo Wang, and Lin Zhu. 2025. "Metabolic Characteristics of Obese Adolescents with Different Degrees of Weight Loss After Identical Exercise Training Intervention" Metabolites 15, no. 5: 313. https://doi.org/10.3390/metabo15050313
APA StyleXie, X., Yang, G., Qin, Y., Wang, Y., Liu, Z., Zhang, Z., Li, Z., Wang, H., & Zhu, L. (2025). Metabolic Characteristics of Obese Adolescents with Different Degrees of Weight Loss After Identical Exercise Training Intervention. Metabolites, 15(5), 313. https://doi.org/10.3390/metabo15050313





