Peripheral Antinociception Induced by Carvacrol in the Formalin Test Involves the Opioid Receptor-NO-cGMP-K+ Channel Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Assessment of Nociception in the Formalin Test
2.4. Study Design
2.5. Statistics and Data Analysis
3. Results
3.1. The Antinociceptive Properties of Carvacrol
3.2. Effect of Naltrexone, Metformin, and NO-cGMP Pathway Inhibitors on Carvacrol Antinociception
3.3. Effect of K+ Channel Blockers on Carvacrol Antinociception
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ANOVA | Analysis of variance |
| ATP | Adenosine triphosphate |
| AUC | Area under the curve |
| cGMP | Cyclic Guanosine monophosphate |
| CINVESTAV.IPN | Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional |
| CL | Contralateral |
| DMSO | Dimethyl sulfoxide |
| GTP | Guanosine triphosphate |
| IL | Ipsilateral |
| IPN | Instituto Politécnico Nacional |
| IUPAC | International Union of Pure and Applied Chemistry |
| L-NAME | NG-L-nitro-arginine methyl ester |
| MDPI | Multidisciplinary Digital Publishing Institute |
| NO | Nitric Oxide |
| NOS | Nitric Oxide synthase |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| ODQ | 1 H-(1,2,4)-oxadiazolo (4,2-a) quinoxalin-1-one |
| PKG | Protein kinase G |
| sc | Subcutaneously |
| SEM | Standard error of the mean |
| SPSS | Statistical Package for the Social Sciences |
| sGC | Soluble guanylate cyclase |
| TEA | Tetraethylammonium chloride |
| VEH | Vehicle |
| IUPAC | The International Union of Pure and Applied Chemistry |
| 4-AP | 4-aminopyridine |
References
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Z.; Majlessi, N.; Choopani, S.; Naghdi, N. Neuroprotective effects of carvacrol against Alzheimer’s disease and other neurodegenerative diseases: A review. Avicenna J. Phytomed. 2022, 12, 371–387. [Google Scholar]
- Khazdair, M.R.; Ghorani, V.; Boskabady, M.H. Experimental and clinical evidence on the effect of carvacrol on respiratory, allergic, and immunologic disorders: A comprehensive review. Biofactors 2022, 48, 779–794. [Google Scholar] [CrossRef]
- Oliveira, I.S.; da Silva, F.V.; Viana, A.F.; dos Santos, M.R.; Quintans-Júnior, L.J.; Martins Mdo, C.; Nunes, P.H.; Oliveira Fde, A.; Oliveira Rde, C. Gastroprotective activity of carvacrol on experimentally induced gastric lesions in rodents. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 899–908. [Google Scholar] [CrossRef]
- Balci, C.N.; Firat, T.; Acar, N.; Kukner, A. Carvacrol treatment opens Kir6.2 ATP-dependent potassium channels and prevents apoptosis on rat testis following ischemia-reperfusion injury model. Rom. J. Morphol. Embryol. 2021, 62, 179–190. [Google Scholar] [CrossRef]
- Testai, L.; Chericoni, S.; Martelli, A.; Flamini, G.; Breschi, M.C.; Calderone, V. Voltage-operated potassium (Kv) channels contribute to endothelium-dependent vasorelaxation of carvacrol on rat aorta. J. Pharm. Pharmacol. 2016, 68, 1177–1183. [Google Scholar] [CrossRef]
- Menezes, P.M.N.; Brito, M.C.; de Paiva, G.O.; Dos Santos, C.O.; de Oliveira, L.M.; de Araújo Ribeiro, L.A.; de Lima, J.T.; Lucchese, A.M.; Silva, F.S. Relaxant effect of Lippia origanoides essential oil in guinea-pig trachea smooth muscle involves potassium channels and soluble guanylyl cyclase. J. Ethnopharmacol. 2018, 220, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Gonçalves, J.C.; Alves Ade, M.; de Araújo, A.E.; Cruz, J.S.; Araújo, D.A. Distinct effects of carvone analogues on the isolated nerve of rats. Eur. J. Pharmacol. 2010, 645, 108–112. [Google Scholar] [CrossRef]
- Parnas, M.; Peters, M.; Dadon, D.; Lev, S.; Vertkin, I.; Slutsky, I.; Minke, B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium 2009, 45, 300–339. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.G.; Namer, B.; Reeh, P.W.; Fischer, M.J.M. TRPA1 and TRPV1 antagonists do not inhibit human acidosis-induced pain. J. Pain 2017, 18, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.T.; Fujita, T.; Jiang, C.Y.; Kumamoto, E. Carvacrol presynaptically enhances spontaneous excitatory transmission and produces outward current in adult rat spinal substantia gelatinosa neurons. Brain Res. 2014, 1592, 44–54. [Google Scholar] [CrossRef]
- Joca, H.C.; Vieira, D.C.; Vasconcelos, A.P.; Araújo, D.A.; Cruz, J.S. Carvacrol modulates voltage-gated sodium channels kinetics in dorsal root ganglia. Eur. J. Pharmacol. 2015, 756, 22–29. [Google Scholar] [CrossRef]
- Lozon, Y.; Sultan, A.; Lansdell, S.J.; Prytkova, T.; Sadek, B.; Yang, K.H.; Howarth, F.C.; Millar, N.S.; Oz, M. Inhibition of human α7 nicotinic acetylcholine receptors by cyclic monoterpene carveol. Eur. J. Pharmacol. 2016, 776, 44–51. [Google Scholar] [CrossRef]
- Muñoz-Perez, V.M.; Ortiz, M.I.; Gerardo-Muñoz, L.S.; Cariño-Cortes, R.; Salas-Casas, A. Tocolytic effect of the monoterpenic phenol isomer, carvacrol, on the pregnant rat uterus. Chin. J. Physiol. 2020, 63, 204–210. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.; Rocha, R.F.; Moreira, J.C.; et al. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante Melo, F.H.; Rios, E.R.; Rocha, N.F.; Citó Mdo, C.; Fernandes, M.L.; de Sousa, D.P.; de Vasconcelos, S.M.; de Sousa, F.C. Antinociceptive activity of carvacrol (5-isopropyl-2-methylphenol) in mice. J. Pharm. Pharmacol. 2012, 64, 1722–1729. [Google Scholar] [CrossRef]
- de Oliveira, A.S.; Llanes, L.C.; Nunes, R.J.; Nucci-Martins, C.; de Souza, A.S.; Palomino-Salcedo, D.L.; Dávila-Rodríguez, M.J.; Ferreira, L.L.G.; Santos, A.R.S.; Andricopulo, A.D. Antioxidant activity, molecular docking, quantum studies and in vivo antinociceptive activity of sulfonamides derived from carvacrol. Front. Pharmacol. 2021, 12, 788850. [Google Scholar] [CrossRef]
- Abed, D.Z.; Sadeghian, R.; Mohammadi, S.; Akram, M. Thymus persicus (Ronniger ex Rech. f.) Jalas alleviates nociceptive and neuropathic pain behavior in mice: Multiple mechanisms of action. J. Ethnopharmacol. 2022, 283, 114695. [Google Scholar] [CrossRef]
- Mohammadifard, F.; Alimohammadi, S. Chemical composition and role of opioidergic system in antinociceptive effect of Ziziphora Clinopodioides essential oil. Basic Clin. Neurosci. 2018, 9, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Alizamani, E.; Ghorbanzadeh, B.; Naserzadeh, R.; Mansouri, M.T. Montelukast, a cysteinyl leukotriene receptor antagonist, exerts local antinociception in animal model of pain through the L-arginine/nitric oxide/cyclic GMP/K(ATP) channel pathway and PPARgamma receptors. Int. J. Neurosci. 2020, 131, 1004–1011. [Google Scholar] [CrossRef]
- Amarante, L.H.; Duarte, I.D. The kappa-opioid agonist (+/−)-bremazocine elicits peripheral antinociception by activation of the L-arginine/nitric oxide/cyclic GMP pathway. Eur. J. Pharmacol. 2002, 454, 19–23. [Google Scholar] [CrossRef]
- Ghorbanzadeh, B.; Kheirandish, V.; Mansouri, M.T. Involvement of the L-arginine/nitric oxide/cyclic GMP/KATP channel pathway and PPARgamma receptors in the peripheral antinociceptive effect of carbamazepine. Drug Res. 2019, 69, 650–657. [Google Scholar]
- Ghorbanzadeh, B.; Mansouri, M.T.; Naghizadeh, B.; Alboghobeish, S. Local antinociceptive action of fluoxetine in the rat formalin assay: Role of l-arginine/nitric oxide/cGMP/K(ATP) channel pathway. Can. J. Physiol. Pharmacol. 2018, 96, 165–172. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Granados-Soto, V.; Castañeda-Hernández, G. The NO-cGMP-K+ channel pathway participates in the antinociceptive effect of diclofenac, but not of indomethacin. Pharmacol. Biochem. Behav. 2003, 76, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; Medina-Tato, D.A.; Sarmiento-Heredia, D.; Palma-Martínez, J.; Granados-Soto, V. Possible activation of the NO-cyclic GMP-protein kinase G-K+ channels pathway by gabapentin on the formalin test. Pharmacol. Biochem. Behav. 2006, 83, 420–427. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Cariño-Cortés, R.; Castañeda-Hernández, G. Participation of the opioid receptor-Nitric oxide-cGMP-K(+) channel pathway in the peripheral antinociceptive effect of nalbuphine and buprenorphine in rats. Can. J. Physiol. Pharmacol. 2020, 98, 753–762. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Cariño-Cortés, R.; Muñoz-Pérez, V.M.; Salas-Casas, A.; Castañeda-Hernández, G. Role of the NO-cGMP-K+ channels pathway in the peripheral antinociception induced by α-bisabolol. Can. J. Physiol. Pharmacol. 2021, 99, 1048–1056. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Cariño-Cortés, R.; Muñoz-Pérez, V.M.; Medina-Solís, C.E.; Castañeda-Hernández, G. Citral inhibits the nociception in the rat formalin test: Effect of metformin and blockers of opioid receptor and the NO-cGMP-K+ channel pathway. Can. J. Physiol. Pharmacol. 2022, 100, 306–313. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Cariño-Cortés, R.; Castañeda-Hernández, G.; Medina-Solís, C.E. Effect of nitric oxide-cyclic GMP-K+ channel pathway blockers; naloxone and metformin; on the antinociception induced by the diuretic pamabrom. Can. J. Physiol. Pharmacol. 2023, 101, 41–51. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Mathie, A.; Peters, J.A.; Veale, E.L.; Striessnig, J.; Kelly, E.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; et al. The concise guide to pharmacology 2019/20: Ion channels. Br. J. Pharmacol. 2019, 176, S142–S228. [Google Scholar] [CrossRef] [PubMed]
- Dascal, N.; Kahanovitch, U. The roles of Gβγ and Gα in gating and regulation of GIRK Channels. Int. Rev. Neurobiol. 2015, 123, 27–85. [Google Scholar] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical assessment of inflammatory pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef]
- Ortiz, M.I. Metformin and phenformin block the peripheral antinociception induced by diclofenac and indomethacin on the formalin test. Life Sci. 2012, 90, 8–12. [Google Scholar] [CrossRef]
- Mónica, F.Z.; Bian, K.; Murad, F. The endothelium-dependent nitric oxide-cGMP pathway. Adv. Pharmacol. 2016, 77, 1–27. [Google Scholar]
- Xiao, S.; Li, Q.; Hu, L.; Yu, Z.; Yang, J.; Chang, Q.; Chen, Z.; Hu, G. Soluble guanylate cyclase stimulators and activators: Where are we and where to go? Mini Rev. Med. Chem. 2019, 19, 1544–1557. [Google Scholar] [CrossRef]
- Koesling, D.; Mergia, E.; Russwurm, M. Physiological functions of NO-sensitive guanylyl cyclase isoforms. Curr. Med. Chem. 2016, 23, 2653–2665. [Google Scholar] [CrossRef]
- Ocaña, M.; Cendán, C.M.; Cobos, E.J.; Entrena, J.M.; Baeyens, J.M. Potassium channels and pain: Present realities and future opportunities. Eur. J. Pharmacol. 2004, 500, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Busserolles, J.; Tsantoulas, C.; Eschalier, A.; López García, J.A. Potassium channels in neuropathic pain: Advances, challenges, and emerging ideas. Pain 2016, 157, S7–S14. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Jiang, B.Y.; Chen, C.C. Ion channels involved in substance P-mediated nociception and antinociception. Int. J. Mol. Sci. 2019, 20, 1596. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, X.; Jiang, L.; Hu, L.; Kong, H.; Han, Y.; Qian, C.; Song, C.; Qian, Y.; Liu, W. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J. Neuroinflamm. 2016, 13, 294. [Google Scholar] [CrossRef]
- Baeza-Flores, G.D.C.; Guzmán-Priego, C.G.; Parra-Flores, L.I.; Murbartián, J.; Torres-López, J.E.; Granados-Soto, V. Metformin: A prospective alternative for the treatment of chronic pain. Front. Pharmacol. 2020, 11, 558474. [Google Scholar] [CrossRef]
- Guzmán-Priego, C.G.; Méndez-Mena, R.; Baños-González, M.A.; Araiza-Saldaña, C.I.; Castañeda-Corral, G.; Torres-López, J.E. Antihyperalgesic effects of indomethacin; ketorolac; and metamizole in rats: Effects of metformin. Drug Dev. Res. 2017, 78, 98–104. [Google Scholar] [CrossRef]
- Alorfi, N.M. Pharmacological methods of pain management: Narrative review of medication used. Int. J. Gen. Med. 2023, 16, 3247–3256. [Google Scholar] [CrossRef]
- Vordenberg, S.E. Topical nonprescription pain medications for adults. JAMA 2023, 330, 2128. [Google Scholar] [CrossRef]
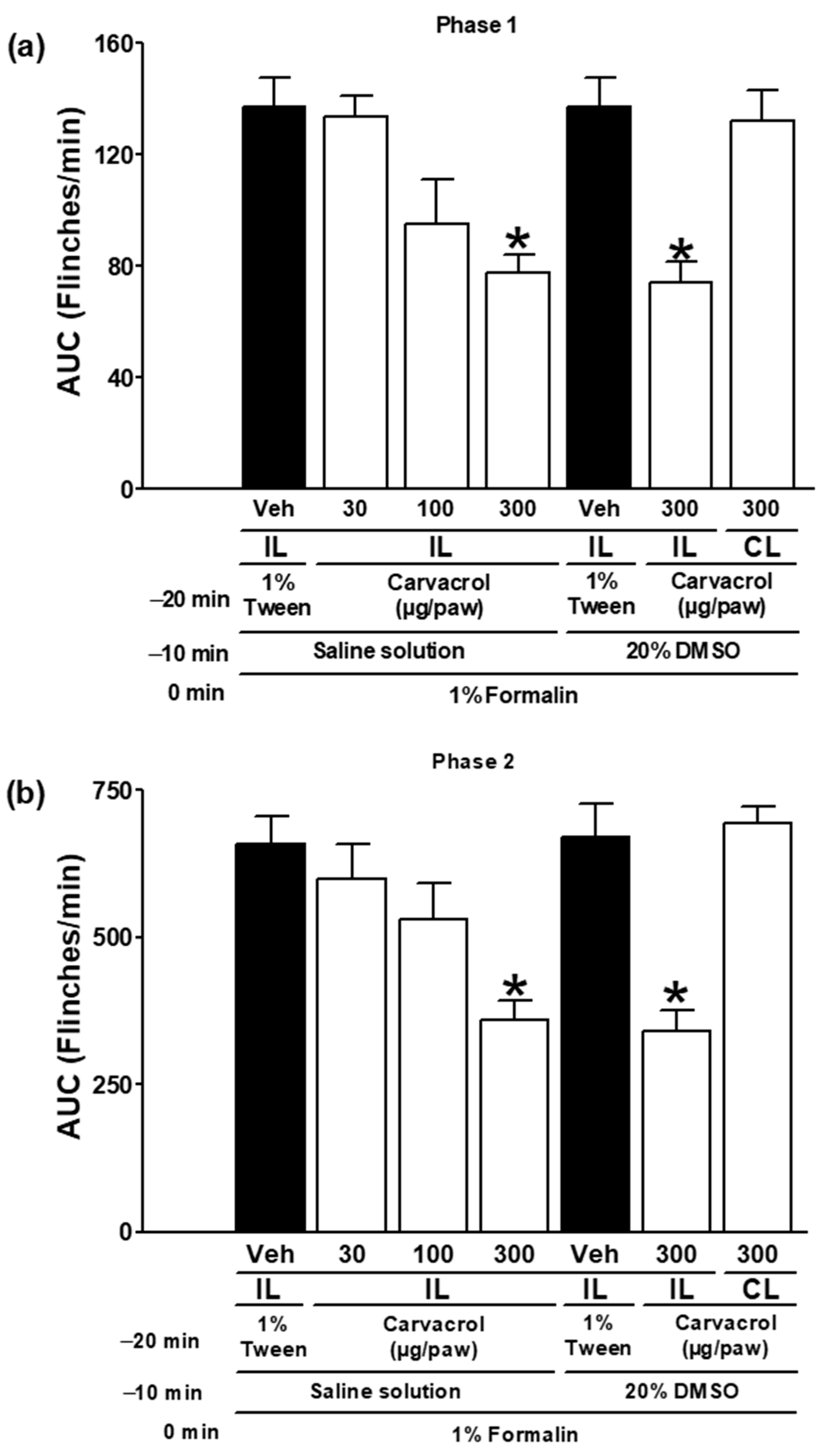

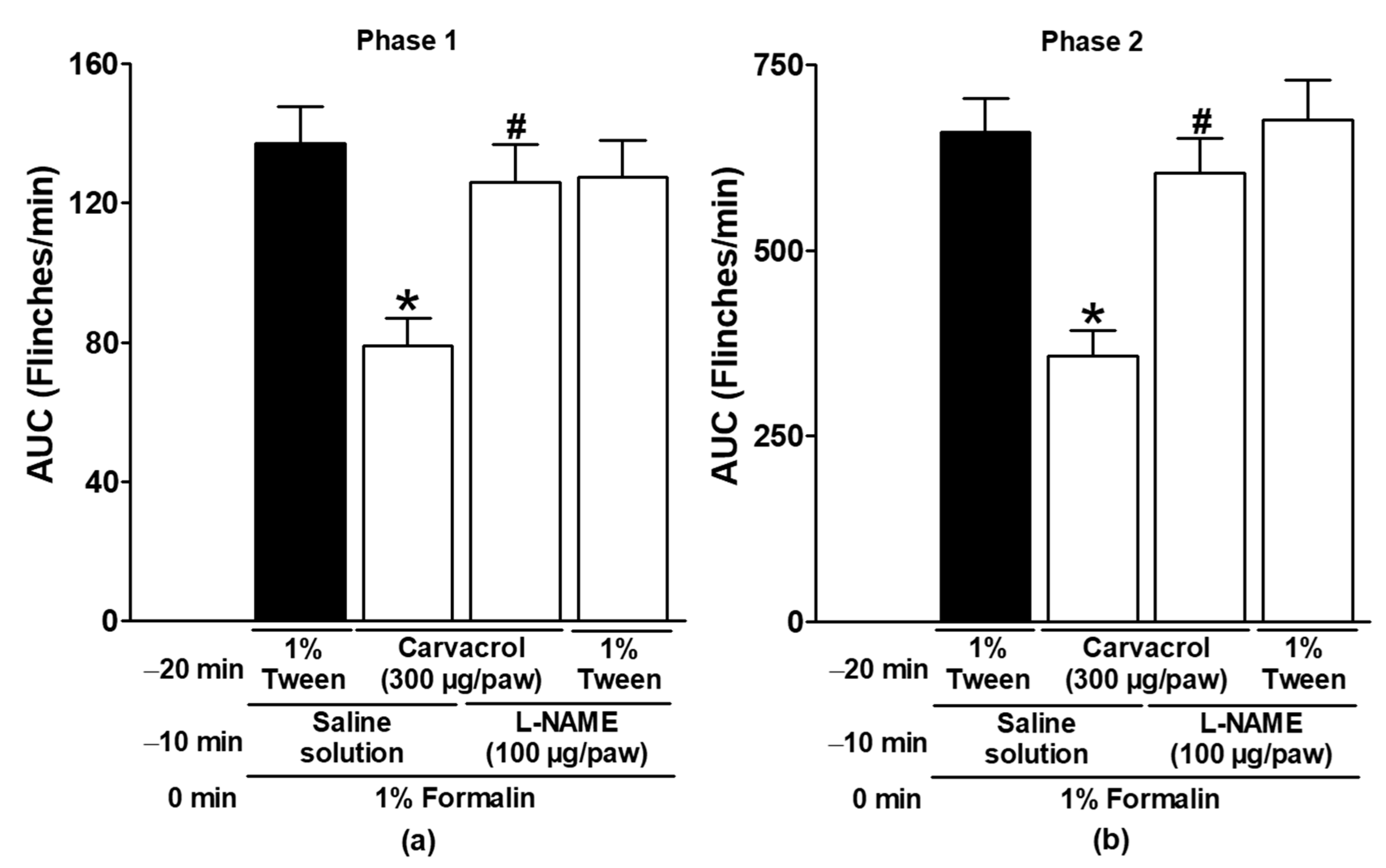
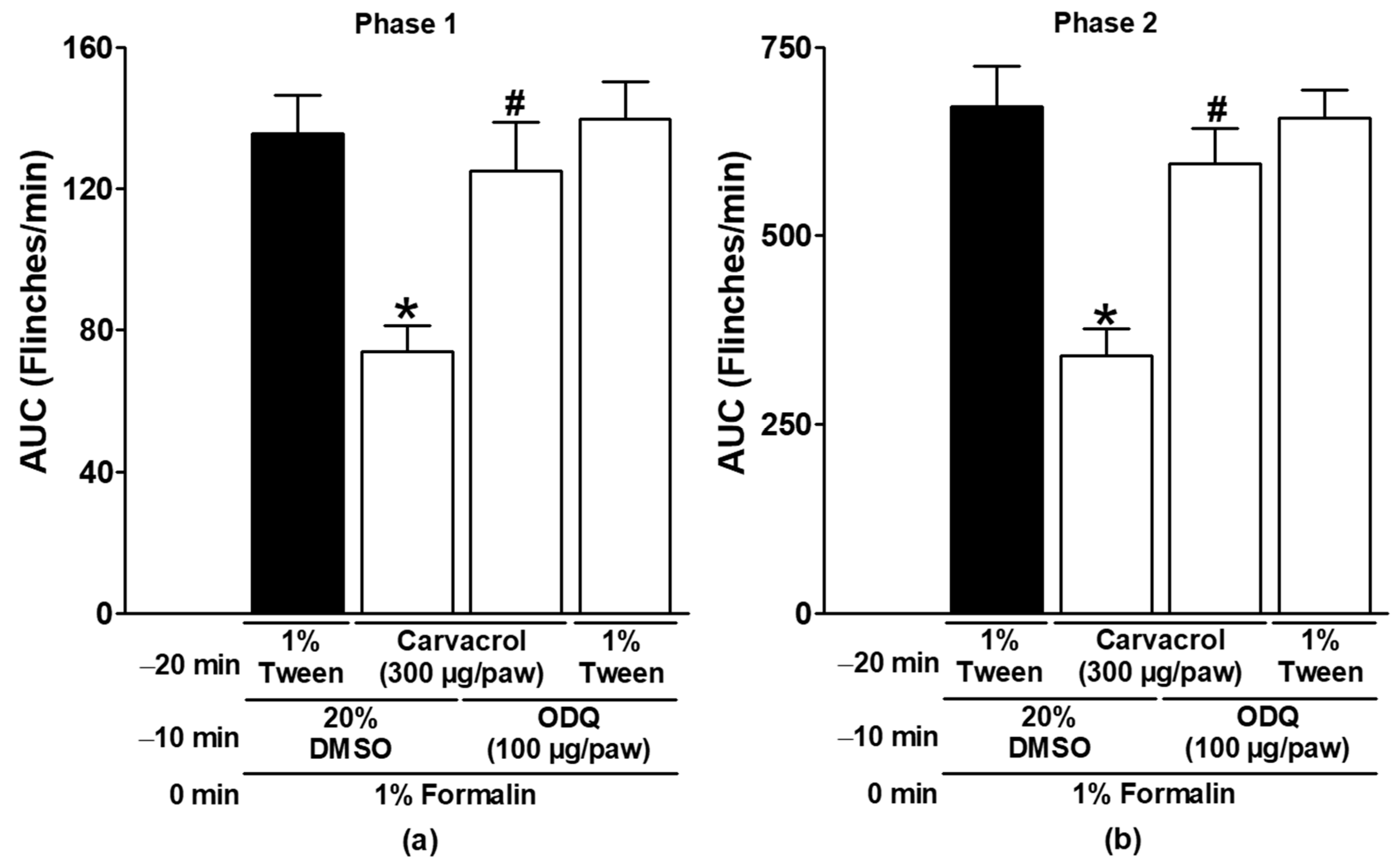
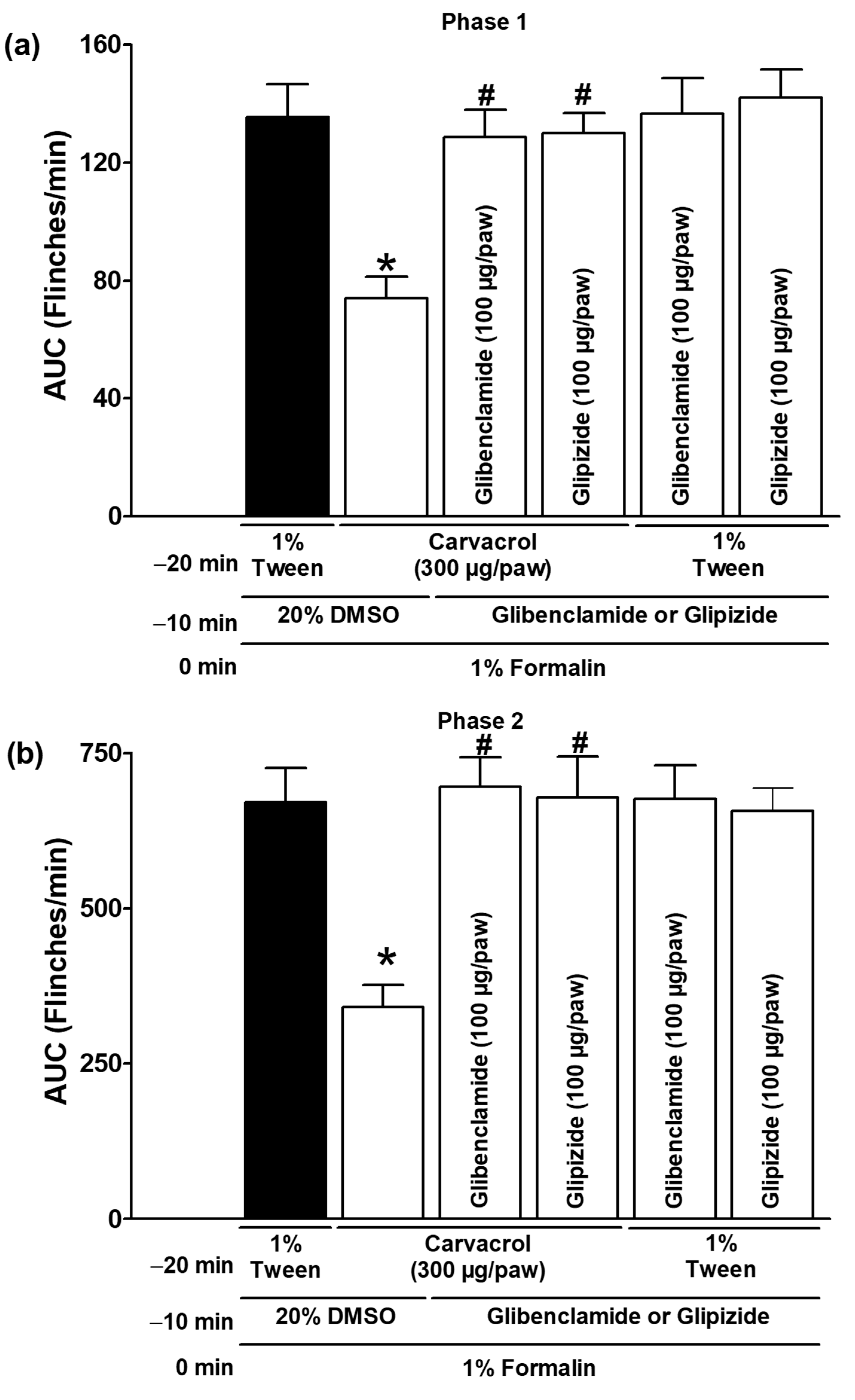

| −20 min Administrations in: IL = Right Paw CL = Left Paw | −10 min Administrations in the Right Paw | Zero Minute Injection of 1% Formalin in the Right Paw | Figures |
|---|---|---|---|
| 1% Tween solution (IL) | Saline solution | ✓ | Figure 1 |
| 1% Tween solution (IL) | 20% DMSO solution | ✓ | |
| Carvacrol (30–300 µg/paw, IL) | Saline solution | ✓ | |
| Carvacrol (300 µg/paw, IL) | 20% DMSO solution | ✓ | |
| Carvacrol (300 µg/paw, CL) | 20% DMSO solution | ✓ | |
| Carvacrol (300 µg/paw, IL) | Naltrexone (400 µg/paw) | ✓ | Figure 2 |
| Carvacrol (300 µg/paw, IL) | Metformin (400 µg/paw) | ✓ | |
| 1% Tween solution (IL) | Naltrexone (400 µg/paw) | ✓ | |
| 1% Tween solution (IL) | Metformin (400 µg/paw) | ✓ | |
| Carvacrol (300 µg/paw, IL) | L-NAME (100 µg/paw) | ✓ | Figure 3 |
| 1% Tween solution (IL) | L-NAME (100 µg/paw) | ✓ | |
| Carvacrol (300 µg/paw, IL) | ODQ (100 µg/paw) | ✓ | Figure 4 |
| 1% Tween solution (IL) | ODQ (100 µg/paw) | ✓ | |
| Carvacrol (300 µg/paw, IL) | Glibenclamide (100 µg/paw) | ✓ | Figure 5 |
| Carvacrol (300 µg/paw, IL) | Glipizide (100 µg/paw) | ✓ | |
| 1% Tween solution (IL) | Glibenclamide (100 µg/paw) | ✓ | |
| 1% Tween solution (IL) | Glipizide (100 µg/paw) | ✓ | |
| Carvacrol (300 µg/paw, IL) | 4-AP (100 µg/paw) | ✓ | Figure 6 |
| Carvacrol (300 µg/paw, IL) | TEA (100 µg/paw) | ✓ | |
| 1% Tween solution (IL) | 4-AP (100 µg/paw) | ✓ | |
| 1% Tween solution (IL) | TEA (100 µg/paw) | ✓ | |
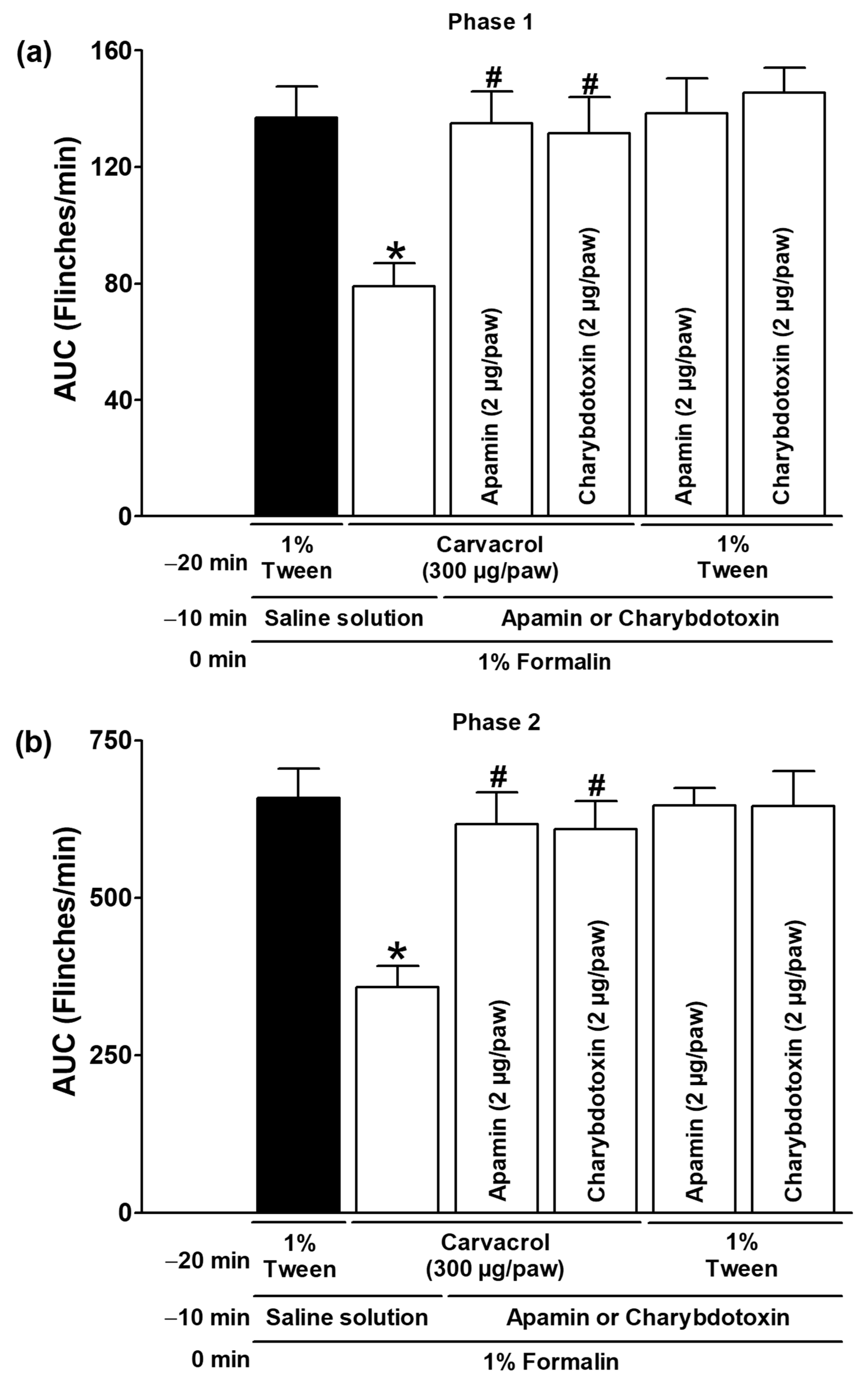
| Carvacrol (300 µg/paw, IL) | Apamin (2 µg/paw) | ✓ | Figure 7 |
| Carvacrol (300 µg/paw, IL) | Charybdotoxin (2 µg/paw) | ✓ | |
| 1% Tween solution (IL) | Apamin (2 µg/paw) | ✓ | |
| 1% Tween solution (IL) | Charybdotoxin (2 µg/paw) | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, M.I.; Cariño-Cortés, R.; Fernández-Martínez, E.; Muñoz-Pérez, V.M.; Castañeda-Hernández, G.; González-García, M.P. Peripheral Antinociception Induced by Carvacrol in the Formalin Test Involves the Opioid Receptor-NO-cGMP-K+ Channel Pathway. Metabolites 2025, 15, 314. https://doi.org/10.3390/metabo15050314
Ortiz MI, Cariño-Cortés R, Fernández-Martínez E, Muñoz-Pérez VM, Castañeda-Hernández G, González-García MP. Peripheral Antinociception Induced by Carvacrol in the Formalin Test Involves the Opioid Receptor-NO-cGMP-K+ Channel Pathway. Metabolites. 2025; 15(5):314. https://doi.org/10.3390/metabo15050314
Chicago/Turabian StyleOrtiz, Mario I., Raquel Cariño-Cortés, Eduardo Fernández-Martínez, Victor Manuel Muñoz-Pérez, Gilberto Castañeda-Hernández, and Martha Patricia González-García. 2025. "Peripheral Antinociception Induced by Carvacrol in the Formalin Test Involves the Opioid Receptor-NO-cGMP-K+ Channel Pathway" Metabolites 15, no. 5: 314. https://doi.org/10.3390/metabo15050314
APA StyleOrtiz, M. I., Cariño-Cortés, R., Fernández-Martínez, E., Muñoz-Pérez, V. M., Castañeda-Hernández, G., & González-García, M. P. (2025). Peripheral Antinociception Induced by Carvacrol in the Formalin Test Involves the Opioid Receptor-NO-cGMP-K+ Channel Pathway. Metabolites, 15(5), 314. https://doi.org/10.3390/metabo15050314







