Exploring the Medicinal Potential of Taraxacum Kok-Saghyz (TKS) Using Widely Targeted Metabolomics
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Metabolite Identification and Classification
3.2. Principal Component Analysis (PCA) and OPLS-DA
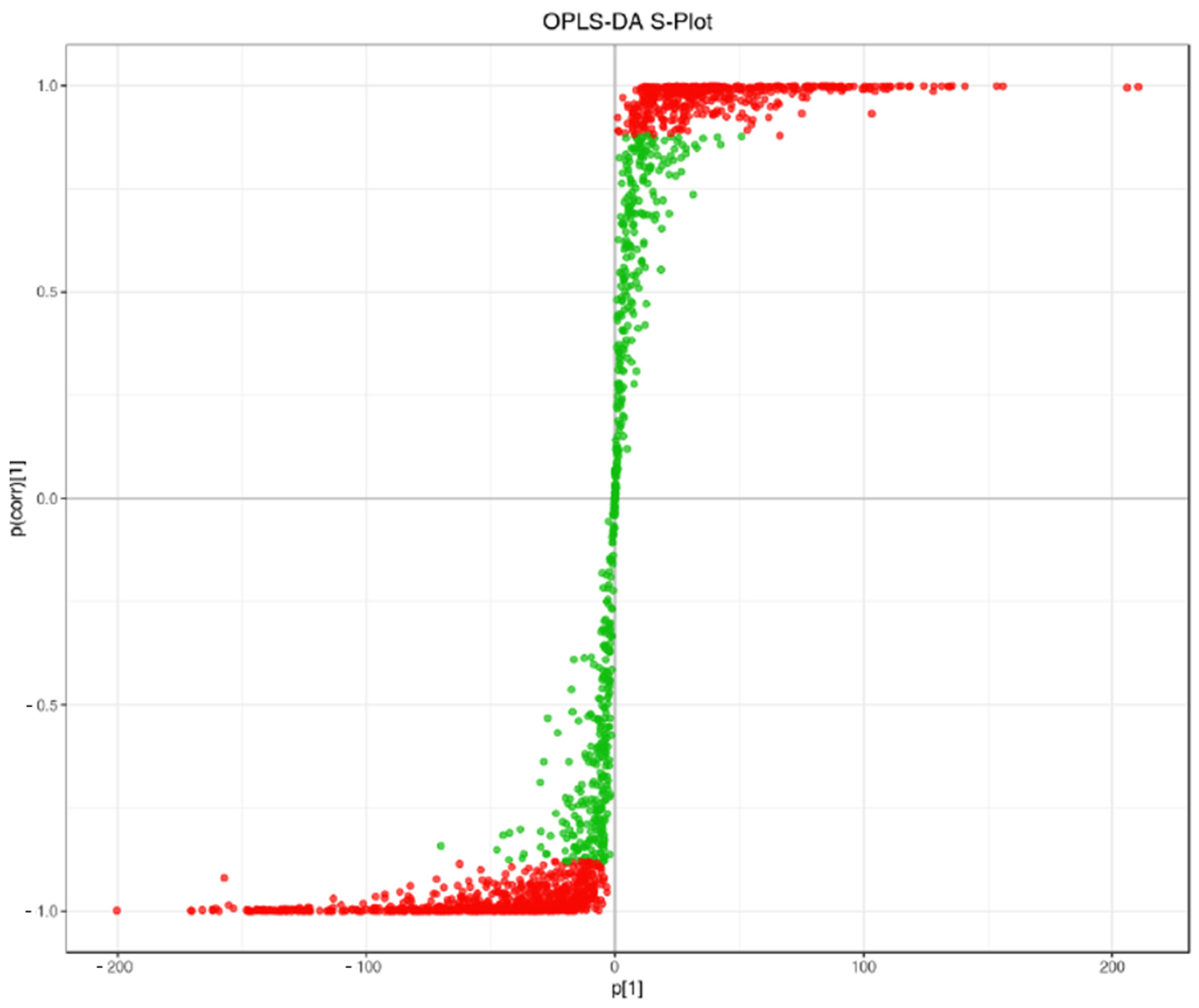
3.3. OPLS-DA and Differential Metabolite Analysis
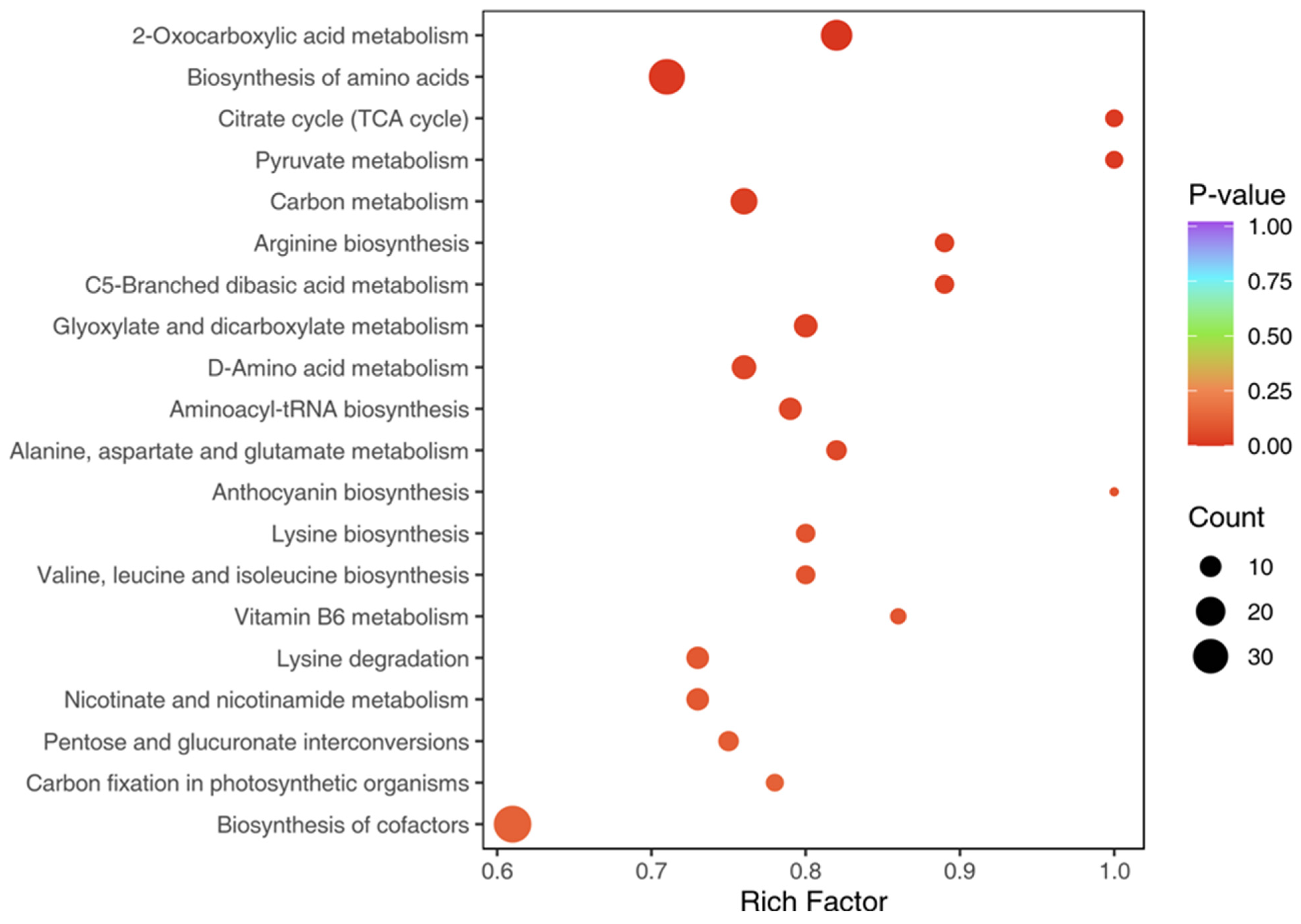
3.4. KEGG Pathway Analysis
3.5. Medicinal Potential of TKS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bZIP | Basic Leucine Zipper Protein |
| CUR | Curtain Gas |
| DOAJ | Directory of Open Access Journals |
| ESI | Electrospray Ionization |
| GSI | Ion Source Gas I |
| GSII | Ion Source Gas II |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LD | Linear Dichroism |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MRM | Multiple Reaction Monitoring |
| MWDB | Metware Biotechnology Inc. in-house Metabolomics Database |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PCA | Principal Component Analysis |
| SnRK2-ABA | SNF1-Related Protein Kinase 2-Abscisic Acid |
| TKS | Taraxacum kok-saghyz |
| UPLC-MS/MS | Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry |
| VIP | Variable Importance in Projection |
References
- Chen, Y.; Li, E.-M.; Xu, L.-Y. Guide to metabolomics analysis: A bioinformatics workflow. Metabolites 2022, 12, 357. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikström, C.; Wold, S. Multi- and Megavariate Data Analysis: Part I: Basic Principles and Applications, 2nd ed.; Umetrics Academy: Umeå, Sweden, 2006. [Google Scholar]
- Guo, S.; Qiu, S.; Cai, Y.; Wang, Z.; Yang, Q.; Tang, S.; Xie, Y.; Zhang, A. Mass spectrometry-based metabolomics for discovering active ingredients and exploring action mechanism of herbal medicine. Front. Chem. 2023, 11, 1142287. [Google Scholar] [CrossRef] [PubMed]
- Khoomrung, S.; Wanichthanarak, K.; Nookaew, I.; Thamsermsang, O.; Seubnooch, P.; Laohapand, T.; Akarasereenont, P. Metabolomics and integrative omics for the development of Thai traditional medicine. Front. Pharmacol. 2017, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Whaley, W.G.; Bowen, J.S. Russian dandelion (Taraxacum kok-saghyz): An emergency source of natural rubber. Econ. Bot. 1947, 1, 233–265. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Tan, Z.; Yu, Z.; Zhou, B.; Meng, L.; Shi, X. The phytochemical and pharmacological profile of taraxasterol. Front. Pharmacol. 2022, 13, 927365. [Google Scholar] [CrossRef]
- Chen, Y.; Fei, S.; Yu, X.; Tan, M. Taraxacum mongolicum extract alleviated H2O2-induced oxidative damage: The underlying mechanism revealed by metabolomics and lipidomics. Foods 2023, 12, 3314. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-obesity attributes; UHPLC-QTOF-MS/MS-based metabolite profiling and molecular docking insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A review of chemical constituents and pharmacological effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef]
- Kania-Dobrowolska, M.; Baraniak, J. Dandelion (Taraxacum officinale L.) as a source of biologically active compounds supporting the therapy of co-existing diseases in metabolic syndrome. Foods 2022, 11, 2858. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodríguez-Casado, A. Diverse biological activities of Taraxacum officinale Weber ex F.H. Wigg. Front. Pharmacol. 2012, 3, 120. [Google Scholar] [CrossRef]
- Ganzera, M.; Guggenberger, M.; Stuppner, H.; Zidorn, C. Altitudinal variation of secondary metabolite profiles in flowering heads of Matricaria chamomilla cv. BONA. Planta Medica 2008, 74, 453–457. [Google Scholar] [CrossRef]
- García-Carrasco, B.; Fernandez-Dacosta, R.; Dávalos, A.; Ordovás, J.M.; Rodriguez-Casado, A. In vitro hypolipidemic and antioxidant effects of leaf and root extracts of Taraxacum officinale. Med. Sci. 2015, 3, 38–54. [Google Scholar] [CrossRef]
- Deng, X.; Jiao, Y.; Hao, H.; Guo, Z.; An, G.; Zhang, W.; Xue, D.; Han, S. Dandelion extract suppresses the stem-like properties of triple-negative breast cancer cells by regulating CUEDC2/β-catenin/OCT4 signaling axis. J. Ethnopharmacol. 2025, 342, 119408. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, Z.; Yu, C.; Qi, X.; Fang, H.; Yu, X.; Li, L.; Bai, Y.; Liu, D.; Chen, Z.; et al. Identification and characterization of the TmSnRK2 family proteins related to chicoric acid biosynthesis in Taraxacum mongolicum. BMC Genom. 2025, 26, 276. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Shu, W.; Feng, W.; Meng, R.; Kong, L.; Cao, H.; Jiang, C.; Wang, S.; Wu, F.; et al. Taraxacum sinicum Kitag. (Binpu-3) root extract inhibits tumor invasion via Notch signaling in Drosophila and human breast cancer MDA-MB-231 cells. Front. Pharmacol. 2025, 16, 1494545. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Hall, B.; Rapinski, M.; Spoor, D.; Eid, H.; Saleem, A.; Arnason, J.T.; Foster, B.; Cuerrier, A.; Haddad, P.S.; Harris, C.S. A multivariate approach to ethnopharmacology: Antidiabetic plants of Eeyou Istchee. Front. Pharmacol. 2022, 12, 511078. [Google Scholar] [CrossRef]
- Hao, F.; Deng, X.; Yu, X.; Wang, W.; Yan, W.; Zhao, X.; Wang, X.; Bai, C.; Wang, Z.; Han, L. Taraxacum: A review of ethnopharmacology, phytochemistry and pharmacological activity. Am. J. Chin. Med. 2024, 52, 183–215. [Google Scholar] [CrossRef]
- Tanasa, M.-V.; Negreanu-Pirjol, T.; Olariu, L.; Negreanu-Pirjol, B.-S.; Lepadatu, A.-C.; Anghel, L.; Rosoiu, N. Bioactive compounds from vegetal organs of Taraxacum species (dandelion) with biomedical applications: A review. Int. J. Mol. Sci. 2025, 26, 450. [Google Scholar] [CrossRef]
- Mawalagedera, S.M.U.P.; Callahan, D.L.; Gaskett, A.C.; Rønsted, N.; Symonds, M.R.E. Combining evolutionary inference and metabolomics to identify plants with medicinal potential. Front. Ecol. Evol. 2019, 7, 267. [Google Scholar] [CrossRef]
- Chen, P.; Ding, S.; Yan, Z.; Liu, H.; Tu, J.; Chen, Y.; Zhang, X. Structural characteristic and in-vitro anticancer activities of dandelion leaf polysaccharides from pressurized hot water extraction. Nutrients 2023, 15, 80. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Hu, X.; Liu, Y.; Liu, Y.; Song, M.; Wu, R.; Wu, J. Isolation of a new polysaccharide from dandelion leaves and evaluation of its antioxidant, antibacterial, and anticancer activities. Molecules 2022, 27, 7641. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Cheng, M.; Jiang, R.; Zhao, X.; Zhu, J.; Liu, M.; Chao, X.; Zhang, C.; Zhou, B. Effects of dietary supplement with a Chinese herbal mixture on growth performance, antioxidant capacity, and gut microbiota in weaned pigs. Front. Vet. Sci. 2022, 9, 971647. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses (Supporting Information). J. Proteome Res. 2015, 14, 3322–3335. Available online: https://pubs.acs.org/doi/10.1021/acs.jproteome.5b00354 (accessed on 23 October 2023). [CrossRef] [PubMed]
- Ma, Q.; Chen, M.; Liu, Y.; Tong, Y.; Liu, T.; Wu, L.; Wang, J.; Han, B.; Zhou, L.; Hu, X. Lactobacillus acidophilus-fermented dandelion improves hyperuricemia and regulates gut microbiota. Fermentation 2023, 9, 352. [Google Scholar] [CrossRef]
- Nyamundanda, G.; Gormley, I.C.; Fan, Y.; Gallagher, W.M.; Brennan, L. MetSizeR: Selecting the optimal sample size for metabolomic studies using an analysis-based approach. BMC Bioinform. 2013, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, O.J.; Chindo, I.Y.; Mahmoud, A.A. Metabolite profiling of different solvent extracts of Moringa oleifera seeds and correlation with DPPH radical scavenging activity via 1H NMR-based metabolomics. J. Metabolomics Syst. Biol. 2023, 6, 1–21. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef]
- Cai, L.; Wan, D.; Yi, F.; Luan, L. Purification, preliminary characterization and hepatoprotective effects of polysaccharides from dandelion root. Molecules 2017, 22, 1409. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.E.; Shaaban, A.M.; Ibrahim, H.M.; Abouelmagd, F.; Mohamed, A.H. In vivo antischistosomicidal and immunomodulatory effects of dietary supplementation with Taraxacum officinale. J. Xenobiotics 2024, 14, 1003–1022. [Google Scholar] [CrossRef]
- Respondek, Z.; Isinkaralar, O.; Świsłowski, P.; Isinkaralar, K.; Rajfur, M. Biomonitoring with the use of the herbal plant Taraxacum officinale as a source of information on environmental contamination. Plants 2024, 13, 1805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Huang, Y.; Shen, J.; Xu, H.; Li, K. Integrative analysis of the metabolome and transcriptome reveals the mechanism of polyphenol biosynthesis in Taraxacum mongolicum. Front. Plant Sci. 2024, 15, 1418585. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, R.; Walasek-Janusz, M.; Caruso, G.; Pokluda, R.; Tallarita, A.V.; Golubkina, N.; Sękara, A. Multilateral use of dandelion in folk medicine of Central-Eastern Europe. Plants 2025, 14, 84. [Google Scholar] [CrossRef]
- Rehman, G.; Hamayun, M.; Iqbal, A.; Khan, S.A.; Khan, H.; Shehzad, A.; Khan, A.L.; Hussain, A.; Kim, H.-Y.; Ahmad, J.; et al. Effect of methanolic extract of dandelion roots on cancer cell lines and AMP-activated protein kinase pathway. Front. Pharmacol. 2017, 8, 875. [Google Scholar] [CrossRef]
- Qiao, Q.; Song, X.; Zhang, C.; Jiang, C.; Jiang, R. Structure and immunostimulating activity of polysaccharides derived from the roots and leaves of dandelion. Chem. Biol. Technol. Agric. 2024, 11, 51. [Google Scholar] [CrossRef]
- Cai, X.; Shao, Y.; Wang, Z.; Xu, Y.; Ren, Z.; Fu, L.; Zhu, Y. Antiviral activity of dandelion aqueous extract against pseudorabies virus both in vitro and in vivo. Front. Vet. Sci. 2023, 9, 1090398. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Z.; Wang, Y.; Liu, P.; Hu, K. Taraxacum officinale-derived exosome-like nanovesicles modulate gut metabolites to prevent intermittent hypoxia-induced hypertension. Biomed. Pharmacother. 2023, 161, 114572. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łę̨towska, A. Study of antioxidant activity of some medicinal plants having high content of caffeic acid derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef]
- Lee, B.-R.; Lee, J.-H.; An, H.-J. Effects of Taraxacum officinale on fatigue and immunological parameters in mice. Molecules 2012, 17, 13253–13265. [Google Scholar] [CrossRef] [PubMed]
- Ovadje, P.; Ammar, S.; Guerrero, J.-A.; Arnason, J.T.; Pandey, S. Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget 2016, 7, 70338–70358. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Wei, X.; Huang, H.; Wang, F.; Wang, Z.; Xie, J.; Wang, L.; Liu, D.; Hu, Z. Application of omics technologies in studies on antitumor effects of Traditional Chinese Medicine. Chin. Med. 2024, 19, 123. [Google Scholar] [CrossRef]
- Li, W.; Luo, F.; Wu, X.; Fan, B.; Yang, M.; Zhong, W.; Guan, D.; Wang, F.; Wang, Q. Anti-inflammatory effects and mechanisms of dandelion in RAW264.7 macrophages and zebrafish larvae. Front. Pharmacol. 2022, 13, 906927. [Google Scholar] [CrossRef]
- Zolotova, D.; Teterovska, R.; Bandere, D.; Lauberte, L.; Niedra, S. Antidiabetic properties of the root extracts of dandelion (Taraxacum officinale) and burdock (Arctium lappa). Plants 2024, 13, 1021. [Google Scholar] [CrossRef]
- Wang, S.; Hao, H.-F.; Jiao, Y.-N.; Fu, J.-L.; Guo, Z.-W.; Guo, Y.; Yuan, Y.; Li, P.-P.; Han, S.-Y. Dandelion extract inhibits triple-negative breast cancer cell proliferation by interfering with glycerophospholipids and unsaturated fatty acids metabolism. Front. Pharmacol. 2022, 13, 942996. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.; Zhang, L.; Xu, J.; Zhu, C.; Zhao, H.; Lv, G. Dandelion root extract suppressed gastric cancer cells proliferation and migration through targeting lncRNA-CCAT1. Biomed. Pharmacother. 2017, 93, 1010–1017. [Google Scholar] [CrossRef]
- Kang, M.-J.; Jang, S.-N.; Kang, I.-J.; Yang, G.-S.; Son, Y.G.; Kim, J.Y.; Goto, E.; Son, K.-H. Root-Zone Cooling Effects on Growth, Physiological, and Biochemical Responses of Taraxacum coreanum Under Hydroponics. J. Plant Growth Regul. 2025, 1–18. [Google Scholar] [CrossRef]
- Lis, B.; Jedrejek, D.; Rywaniak, J.; Soluch, A.; Stochmal, A.; Olas, B. Flavonoid preparations from Taraxacum officinale L. fruits—A phytochemical, antioxidant and hemostasis studies. Molecules 2020, 25, 5402. [Google Scholar] [CrossRef]
- Zhuang, X.; Shi, W.; Shen, T.; Cheng, X.; Wan, Q.; Fan, M.; Hu, D. Research updates and advances on flavonoids derived from dandelion and their antioxidant activities. Antioxidants 2024, 13, 1449. [Google Scholar] [CrossRef]
- Di Napoli, M.; Zucchetti, M. Taraxacum-derived metabolites: A comparative review of phytochemistry and biological roles across species. J. Nat. Prod. Res. 2021, 35, 875–889. [Google Scholar] [CrossRef]
- Shittu, R.O.; Ceesay, I.; Pwavodi, P.C. Antioxidant and Antimicrobial Activities of Dandelion Root Extract (Taraxacum officinale) and Its Cytotoxic Effect on MDA-MB-231 Breast Cancer Cells. Discov. Appl. Sci. 2025, 7, 136. [Google Scholar] [CrossRef]
- Jang, H.; Choi, M.; Lee, E.; Jang, K.-S. Comparative phytochemical profiling of methanolic extracts of different parts of white dandelion (Taraxacum coreanum) using hybrid ion-mobility Q-TOF MS. Mass Spectrom. Lett. 2024, 15, 95. [Google Scholar] [CrossRef]
- Ji, P.; Yang, X.; Zhao, X. Application of metabolomics in quality control of traditional Chinese medicines: A review. Front. Plant Sci. 2024, 15, 1463666. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Dong, R. Integrated microbiome-metabolomics analysis reveals the potential mechanism of dandelion root polysaccharides to ameliorate ulcerative colitis. Metabolites 2024, 14, 351. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Gigl, M.; Le, N.P.K.; Dawid, C.; Lamy, E. In vitro effect of Taraxacum officinale leaf aqueous extract on the interaction between ACE2 cell surface receptor and SARS-CoV-2 spike protein D614 and four mutants. Pharmaceuticals 2021, 14, 1055. [Google Scholar] [CrossRef]
- Choi, U.-K.; Lee, O.-H.; Yim, J.H.; Cho, C.-W.; Rhee, Y.K.; Lim, S.-I.; Kim, Y.-C. Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int. J. Mol. Sci. 2010, 11, 67–78. [Google Scholar] [CrossRef]
- Clare, B.A.; Conroy, R.S.; Spelman, K. The diuretic effect in human subjects of an extract of Taraxacum officinale folium over a single day. J. Altern. Complement. Med. 2009, 15, 929–934. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Yang, F.; Ye, X.-J.; Chen, M.-Y.; Li, H.-C.; Wang, Y.-F.; Zhong, M.-Y.; Zhong, C.-S.; Zeng, B.; Xu, L.-H.; He, X.-H.; et al. Inhibition of NLRP3 inflammasome activation and pyroptosis in macrophages by taraxasterol is associated with its regulation on mTOR signaling. Front. Immunol. 2021, 12, 632606. [Google Scholar] [CrossRef]
- Flores-Ocelotl, M.R.; Rosas-Murrieta, N.H.; Moreno, D.A.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Domínguez, F.; Santos-López, G. Taraxacum officinale and Urtica dioica extracts inhibit dengue virus serotype 2 replication in vitro. BMC Complement. Altern. Med. 2018, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Frolova, A.S.; Fokina, A.D.; Milentyeva, I.S.; Asyakina, L.K.; Proskuryakova, L.A.; Prosekov, A.Y. The biological active substances of Taraxacum officinale and Arctium lappa from the Siberian Federal District. Int. J. Mol. Sci. 2024, 25, 3263. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Antioxidant, prooxidant, and cytotoxic activities of solvent-fractionated dandelion (Taraxacum officinale) flower extracts in vitro. J. Agric. Food Chem. 2003, 51, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. New perspectives on the effect of dandelion, its food products and other preparations on the cardiovascular system and its diseases. Nutrients 2022, 14, 1350. [Google Scholar] [CrossRef]
- Ren, F.; Wu, K.; Yang, Y.; Yang, Y.; Wang, Y.; Li, J. Dandelion polysaccharide exerts anti-angiogenesis effect on hepatocellular carcinoma by regulating VEGF/HIF-1α expression. Front. Pharmacol. 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Todorova, M.; Petkova, N.; Dincheva, I. Non-polar phytochemical compounds from dandelion (Taraxacum officinale Weber ex FH Wigg.) flowers. Bulg. Chem. Commun. 2024, 56, 96–99. [Google Scholar] [CrossRef]
- Wu, X.; Li, N.; Dong, Z.; Yin, Q.; Zhou, T.; Zhu, L.; Yan, H.; Chen, Z.; Zhai, K. Extraction, purification, sulfated modification, and biological activities of dandelion root polysaccharides. Foods 2024, 13, 2393. [Google Scholar] [CrossRef]
- Aras, A.İ.; Arslan, E.; Özçay, B. In vitro cytotoxicity evaluation of dandelion root ethanol extract on PANC-1 cell line. Mutiara Med. J. Kedokt. Kesehat. 2024, 24, 66–70. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, Q.; Gong, Y.; Ou, Y.; Qi, Q.; Xie, Y.; Wang, X.; Hu, C.; Jiang, S.; et al. Effects of dietary supplementation with dandelion tannins or soybean isoflavones on growth performance, antioxidant function, intestinal morphology, and microbiota composition in Wenchang chickens. Front. Vet. Sci. 2023, 9, 1073659. [Google Scholar] [CrossRef]
- Piatkowska, E.; Biel, W.; Witkowicz, R.; Kepinska-Pacelik, J. Chemical composition and antioxidant activity of Asteraceae family plants. Appl. Sci. 2022, 12, 12293. [Google Scholar] [CrossRef]
- Wang, T.; Sun, J.; Wang, L.; Lin, Y.; Wu, Z.; Jia, Q.; Zhang, S.; An, J.; Ma, X.; Wu, Q.; et al. Therapeutic potential of isochlorogenic acid A from Taraxacum officinale in improving immune response and enhancing the efficacy of PD-1/PD-L1 blockade in triple-negative breast cancer. Front. Immunol. 2025, 16, 1529710. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Systematic investigation of the effects of seven plant extracts on the physiological parameters, yield, and nutritional quality of radish (Raphanus sativus var. sativus). Front. Plant Sci. 2021, 12, 651152. [Google Scholar] [CrossRef] [PubMed]
- Gurib-Fakim, A.; Mahomoodally, F.M. African flora as potential sources of medicinal plants: Towards the chemotherapy of major parasitic and other infectious diseases: A review. Jordan J. Biol. Sci. 2013, 6, 77–84. [Google Scholar] [CrossRef]
- Jin, X.; Xiao, J.; Lu, C.; Ma, W.; Fan, Y.; Xue, X.; Xia, Y.; Chen, N.; Liu, J.; Pei, X. Breastmilk microbiome changes associated with lactational mastitis and treatment with dandelion extract. Front. Microbiol. 2023, 14, 1247868. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Zhang, X.; Fu, H.; Han, X.; Guo, W.; Zhao, W.; Zhao, X.; Yu, C.; Li, H.; et al. Dandelion seed extract affects tumor progression and enhances the sensitivity of cisplatin in esophageal squamous cell carcinoma. Front. Pharmacol. 2022, 13, 897465. [Google Scholar] [CrossRef]

| Metabolite Class | Number Identified |
|---|---|
| Flavonoids | 450 |
| Alkaloids | 320 |
| Lipids | 275 |
| Amino Acids | 190 |
| Phenolic Compounds | 578 |
| Sample Comparison | Total Differential Metabolites | Upregulated | Downregulated |
|---|---|---|---|
| Root vs. Leaf | 964 | 355 | 609 |
| KEGG Pathway | Number of Metabolites |
|---|---|
| Amino Acid Biosynthesis | 112 |
| Flavonoid Biosynthesis | 89 |
| Lipid Metabolism | 135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.; Chu, J.S.-C.; Swiger, D.R. Exploring the Medicinal Potential of Taraxacum Kok-Saghyz (TKS) Using Widely Targeted Metabolomics. Metabolites 2025, 15, 306. https://doi.org/10.3390/metabo15050306
Tan M, Chu JS-C, Swiger DR. Exploring the Medicinal Potential of Taraxacum Kok-Saghyz (TKS) Using Widely Targeted Metabolomics. Metabolites. 2025; 15(5):306. https://doi.org/10.3390/metabo15050306
Chicago/Turabian StyleTan, Michele, Jeffrey Shih-Chieh Chu, and Daniel Robin Swiger. 2025. "Exploring the Medicinal Potential of Taraxacum Kok-Saghyz (TKS) Using Widely Targeted Metabolomics" Metabolites 15, no. 5: 306. https://doi.org/10.3390/metabo15050306
APA StyleTan, M., Chu, J. S.-C., & Swiger, D. R. (2025). Exploring the Medicinal Potential of Taraxacum Kok-Saghyz (TKS) Using Widely Targeted Metabolomics. Metabolites, 15(5), 306. https://doi.org/10.3390/metabo15050306







