Quality Dynamics of Beef Bottom Round During 2-Month Frozen Storage (−18 °C) and Week-Long Refrigeration (4 °C)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Used
2.2. Beef Samples
2.3. Quality Assessment
2.3.1. Color
2.3.2. pH
2.3.3. Cooking Loss
2.3.4. Thawing Loss
2.3.5. Centrifugal Loss
2.3.6. Drip Loss
2.3.7. Moisture Content
2.3.8. Shear Force
2.3.9. Thiobarbituric Acid-Reactive Substances (TBARSs)
2.3.10. Texture Profile Analysis (TPA)
2.4. GC-MS Analysis
2.5. Statistical Analysis
3. Results and Discussion
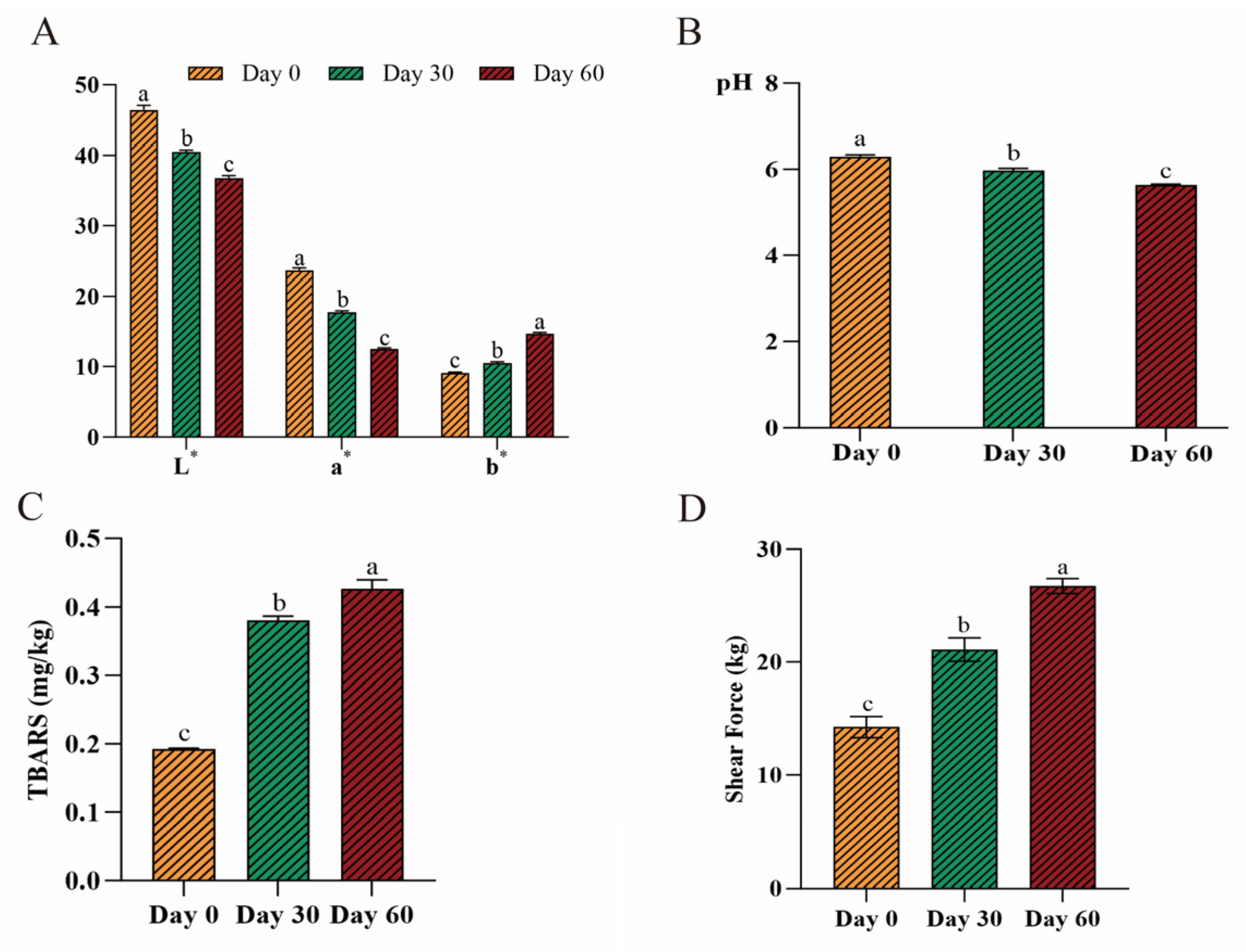
3.1. Quality Parameters of Beef During Long-Term Frozen Storage
3.1.1. Color and pH
3.1.2. TBARS
3.1.3. Shear Force
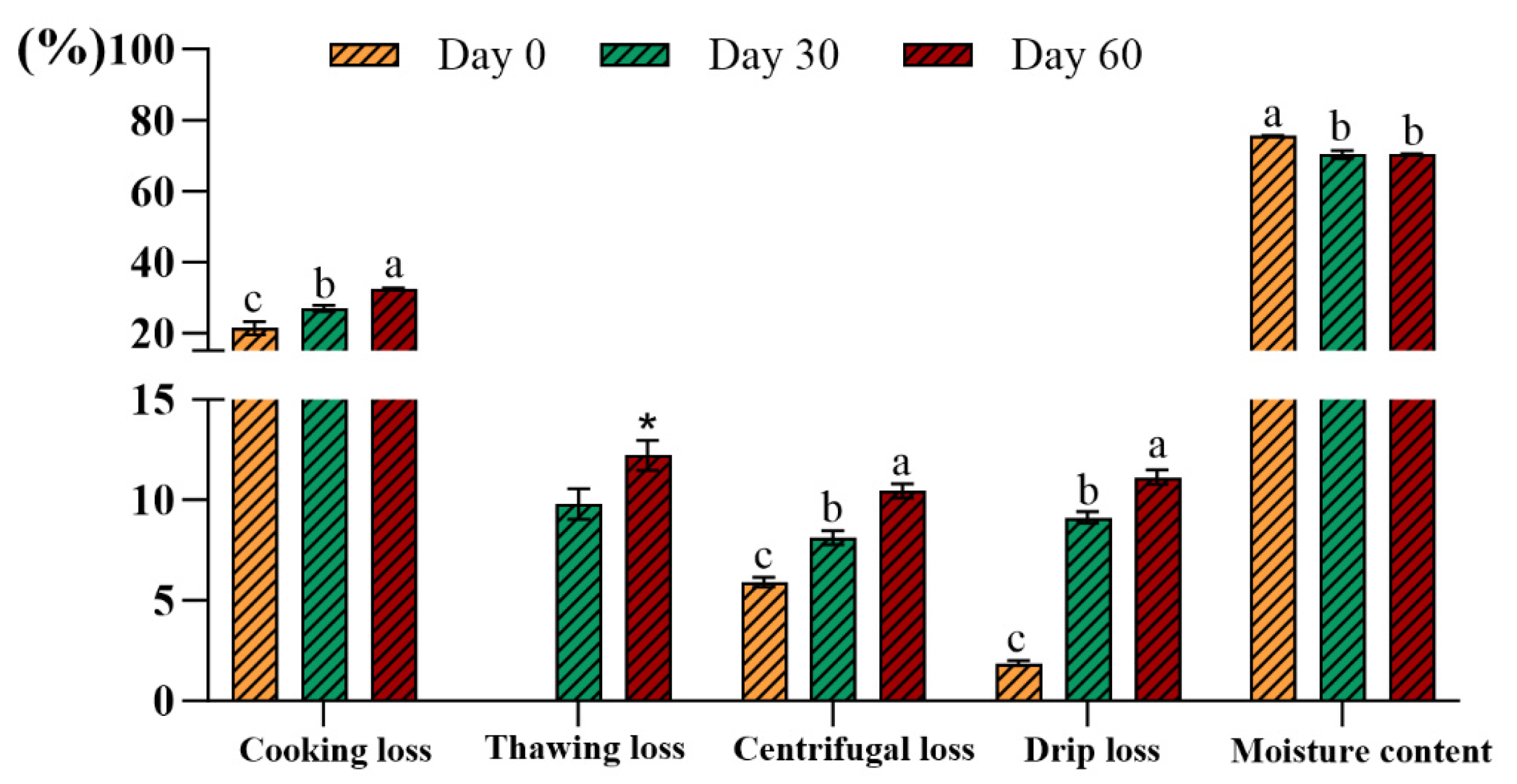
3.1.4. Cooking Loss, Thawing Loss, Centrifugal Loss, Drip Loss, and Moisture Content
3.1.5. TPA
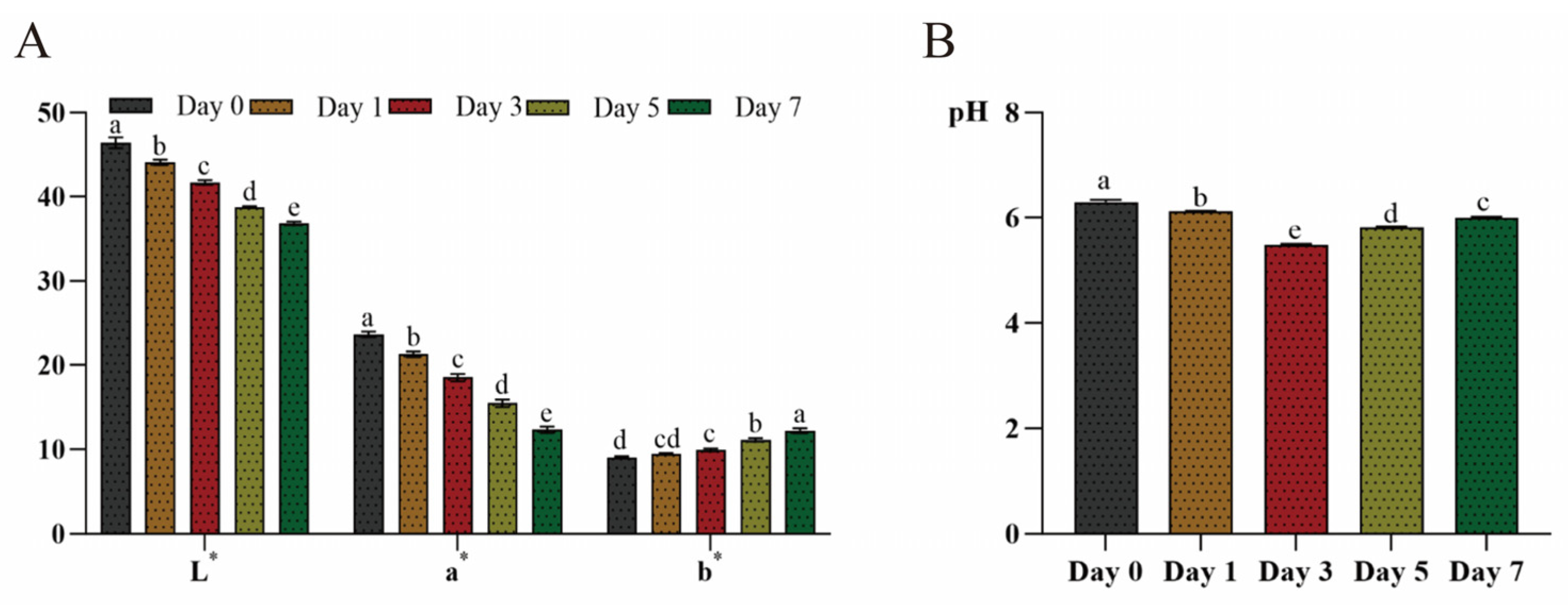
3.2. Quality Parameters of Beef Samples During Refrigerated Storage
3.2.1. pH and Color
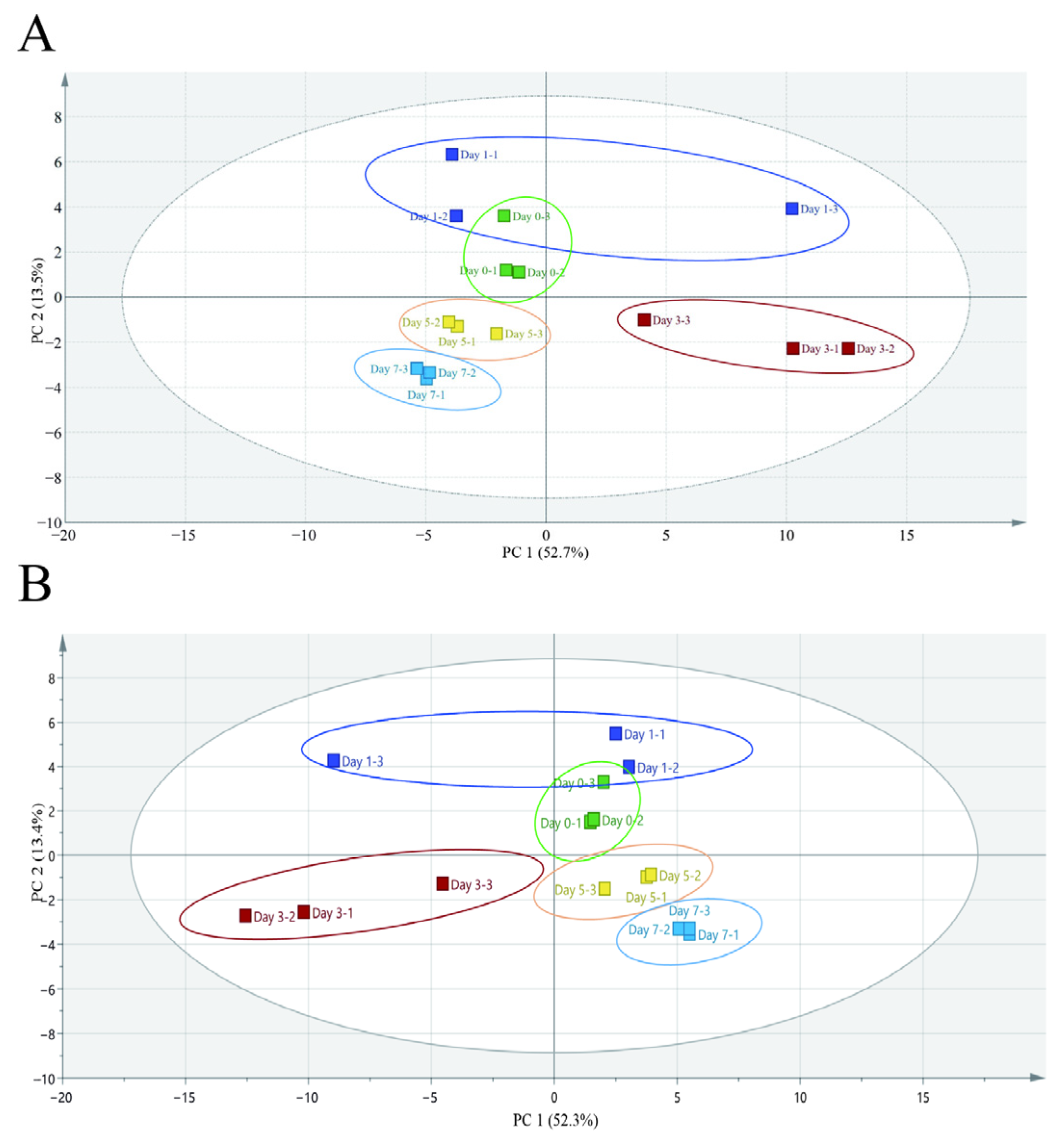
3.2.2. Unsupervised and Supervised Multigroup Analysis
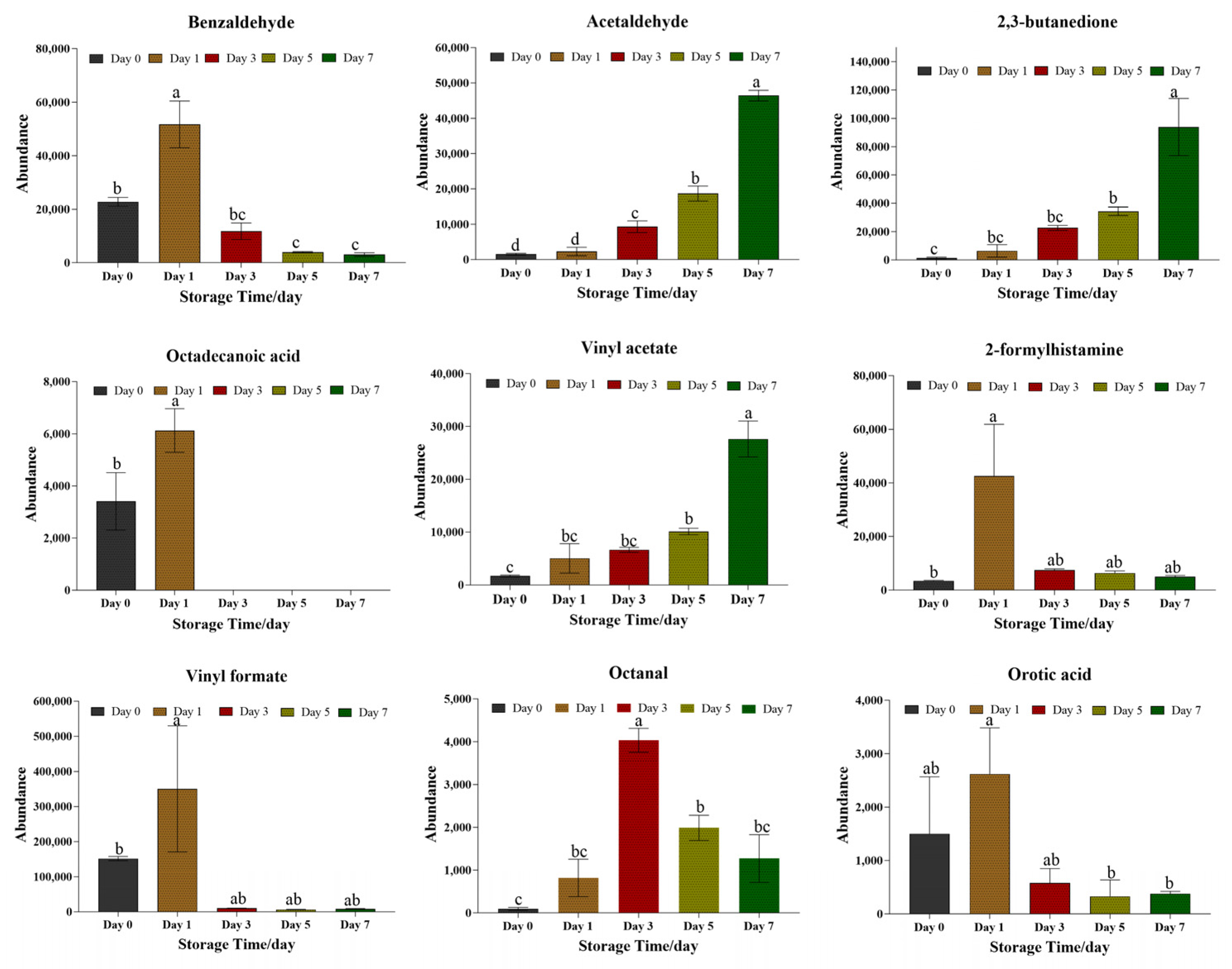
3.2.3. Analysis of Volatile Compounds for Quality Evaluation of Beef Bottom Round Samples Under Refrigeration Conditions (4 °C)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komarek, A.M.; Dunston, S.; Enahoro, D.; Godfray, H.C.J.; Herrero, M.; Mason-D’Croz, D.; Rich, K.M.; Scarborough, P.; Springmann, M.; Sulser, T.B.; et al. Income, consumer preferences, and the future of livestock-derived food demand. Glob. Environ. Chang. 2021, 70, 102343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Liu, Y.; Dong, P.; Liang, R.; Hopkins, D.L.; Holman, B.W.B.; Luo, X.; Zhu, L.; Yang, Z.; et al. Chinese consumer perception and purchasing behavior of beef—Mainly in North and East China. Meat Sci. 2025, 220, 109696. [Google Scholar] [CrossRef]
- Cho, S.; Kang, S.M.; Seong, P.; Kang, G.; Kim, Y.; Kim, J.; Lee, S.; Kim, S. Effect of aging time on physicochemical meat quality and sensory property of hanwoo bull beef. Korean J. Food Sci. Anim. Resour. 2016, 36, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-H.; Yoo, M.; Jung, T.-H.; Jeon, W.-M.; Han, K.-S. Evaluation of the Digestibility of Korean Hanwoo Beef Cuts Using the in vitro Physicochemical Upper Gastrointestinal System. Korean J. Food Sci. Anim. Resour. 2017, 37, 682–689. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Muscle Fiber Properties in Cattle and Their Relationships with Meat Qualities: An Overview. J. Agric. Food Chem. 2020, 68, 6021–6039. [Google Scholar] [CrossRef]
- Vahmani, P.; Mapiye, C.; Prieto, N.; Rolland, D.C.; McAllister, T.A.; Aalhus, J.L.; Dugan, M.E. The scope for manipulating the polyunsaturated fatty acid content of beef: A review. J. Anim. Sci. Biotechnol. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Realini, C.E.; Kim, Y.H.B.; Farouk, M.M. Challenges and processing strategies to produce high quality frozen meat. Meat Sci. 2023, 205, 109311. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Ruiz, J.; Martín, D.; Grau, R.; Antequera, T. Influence of pre-cure freezing on the profile of volatile compounds during the processing of Iberian hams. J. Sci. Food Agric. 2010, 90, 882–890. [Google Scholar] [CrossRef]
- Zhu, M.; Li, H.; Zong, J.; Zhang, S.; Ma, H. Influence of 7-day subfreezing storage on physicochemical, nutritional, and microstructural attributes of porcine longissimus thoracis et lumborum muscle. J. Food Sci. 2024, 89, 5633–5645. [Google Scholar] [CrossRef]
- Stanislawczyk, R.; Rudy, M.; Gil, M. The influence of frozen storage and selected substances on the quality of horse meat. Meat Sci. 2019, 155, 74–78. [Google Scholar] [CrossRef]
- Al-Dalali, S.; He, Z.; Du, M.; Sun, H.; Zhao, D.; Xu, B. Effect of frozen storage on the untargeted and targeted metabolites of flavored roasted beef using UHPLC–MS/MS and GC–MS. Food Chem. 2025, 469, 142511. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.237-2016; Determination of pH in Foods. National Health and Family Planning Commission (NHFPC) of China: Beijing, China, 2016.
- Yu, Q.; Gu, X.; Liu, Q.; Wen, R.; Sun, C. Effect of wet-aging on meat quality and exudate metabolome changes in different beef muscles. Food Res. Int. 2024, 184, 114260. [Google Scholar] [CrossRef]
- Deng, S.; Han, Y.; Gao, T.; Ye, K.; Liu, J. Effect of temperature fluctuation during frozen storage on beef quality. J. Food Process. Preserv. 2021, 45, e15043. [Google Scholar] [CrossRef]
- Yu, Q.; Li, S.; Cheng, B.; Brad Kim, Y.H.; Sun, C. Investigation of changes in proteomes of beef exudate and meat quality attributes during wet-aging. Food Chem. X 2023, 17, 100608. [Google Scholar] [CrossRef]
- Yuan, H. Brad Kim; Charlotte Liesse; Robert Kemp; Prabhu Balan. Evaluation of combined effects of ageing period and freezing rate on quality attributes of beef loins. Meat Sci. 2015, 110, 40–45. [Google Scholar]
- GB 5009.3-2016; Determination of Moisture in Foods. National Health and Family Planning Commission (NHFPC) of China: Beijing, China, 2016.
- Silva, D.R.; Torres Filho, R.A.; Cazedey, H.P.; Fontes, P.R.; Ramos, A.L.; Ramos, E.M. Comparison of Warner-Bratzler shear force values between round and square cross-section cores from cooked beef and pork Longissimus muscle. Meat Sci. 2015, 103, 1–6. [Google Scholar] [CrossRef]
- Piranavatharsan, U.; Jinadasa, B.K.K.K.; Jayasinghe, C.V.L. Validation of thiobarbituric acid reactive substances (TBARS) method for measuring secondary lipid oxidation products in fresh Indian mackerel (Rastrelliger kanagurta). Food Humanit. 2023, 1, 1194–1199. [Google Scholar] [CrossRef]
- Wang, W.; Lin, H.; Guan, W.; Song, Y.; He, X.; Zhang, D. Effect of static magnetic field-assisted thawing on the quality, water status, and myofibrillar protein characteristics of frozen beef steaks. Food Chem. 2024, 436, 137709. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Wang, Y.; Hao, S.; Zhang, K.; Tian, J.; Jin, Y. Effect of changes in the structure of myoglobin on the color of meat products. Food Mater. Res. 2024, 4, e011. [Google Scholar] [CrossRef]
- Ishiwatari, N.; Fukuoka, M.; Sakai, N. Effect of protein denaturation degree on texture and water state of cooked meat. J. Food Eng. 2013, 117, 361–369. [Google Scholar] [CrossRef]
- Pong, C.H.; Chiou, T.Z.; Ho, M.I. Effect of polyethylene package on the metmyoglobin reductase activity and color of tuna muscle during low temperature storage. Fish. Sci. 2010, 66, 384–389. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Bernardez-Morales, G.M.; Douglas, S.L.; Nichols, B.W.; Barrazueta-Cordero, R.J.; Belk, A.D.; Brandebourg, T.D.; Reyes, T.M.; Sawyer, J.T. Vacuum Packaging Can Protect Ground Beef Color and Oxidation during Cold Storage. Foods 2024, 13, 2841. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Powrie, W.D. Chemical effects during storage of frozen foods. J. Chem. Educ. 1984, 61, 340–347. [Google Scholar] [CrossRef][Green Version]
- Ali, S.; Zhang, W.; Rajput, N.; Khan, M.A.; Li, C.; Zhou, G. Effect of multiple freeze–thaw cycles on the quality of chicken breast meat. Food Chem. 2015, 173, 808–814. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Moxnes, J.F.; Sandbakk, O. The kinetics of lactate production and removal during whole-body exercise. Theor. Biol. Med. Model. 2012, 9, 7. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Wu, C.; Feng, Y.; Cui, J.; Yao, Z.; Xu, H.; Wang, S. Detection of Quality Deterioration of Packaged Raw Beef Based on Hyperspectral Technology. Food Sci. Nutr. 2025, 13, e70022. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Żurek, J.; Rudy, M.; Duma-Kocan, P.; Stanisławczyk, R.; Gil, M. Impact of Kosher Slaughter Methods of Heifers and Young Bulls on Physical and Chemical Properties of Their Meat. Foods 2022, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, A.; Lundstrom, K.; Lindahl, G. Influence of vacuum or high-oxygen modified atmosphere packaging on quality of beef M. longissimus dorsi steaks after different ageing times. Meat Sci. 2021, 87, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.B.; Coombs, C.E.O.; Hopkins, D.L. The quality of aged beef and aged-then-frozen lamb meat after up to 2 years of frozen storage at −12 or −18 °C. Meat Sci. 2025, 224, 109790. [Google Scholar] [CrossRef]
- Zequan, X.; Zirong, W.; Jiankun, L.; Xin, M.; Hopkins, D.L.; Holman, B.W.B.; Bekhit, A.E.-D.A. The effect of freezing time on the quality of normal and pale, soft and exudative (PSE)-like pork. Meat Sci. 2019, 152, 1–7. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Sun, D.-W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, Y.H.B.; Puolanne, E.; Ertbjerg, P. Role of freezing-induced myofibrillar protein denaturation in the generation of thaw loss: A review. Meat Sci. 2022, 190, 108841. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Xie, F.; Li, X.; Schroyen, M.; Chen, L.; Hou, C. Changes in water holding capacity of chilled fresh pork in controlled freezing-point storage assisted by different modes of electrostatic field action. Meat Sci. 2023, 204, 109269. [Google Scholar] [CrossRef]
- Jiang, S.; Carroll, L.; Rasmussen, L.M.; Davies, M.J. Oxidation of protein disulfide bonds by singlet oxygen gives rise to glu-tathionylated proteins. Redox Biol. 2021, 38, 101822. [Google Scholar] [CrossRef]
- Masson, P.; Lushchekina, S. Conformational Stability and Denaturation Processes of Proteins Investigated by Electrophoresis under Extreme Conditions. Molecules 2022, 27, 6861. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, M. Effects of Thawing Conditions on the Physicochemical and Microbiological Quality of Thawed Beef. Prev. Nutr. Food Sci. 2024, 29, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, K.; Wang, Y.; Chen, T. Effect of cytoskeleton on ice crystal growth in cells during freezing. Int. J. Food Prop. 2018, 21, 2400–2410. [Google Scholar] [CrossRef]
- Teo, K.Y.; DeHoyos, T.O.; Dutton, J.C.; Grinnell, F.; Han, B. Effects of freezing-induced cell-fluid-matrix interactions on the cells and extracellular matrix of engineered tissues. Biomaterials 2011, 32, 5380–5390. [Google Scholar] [CrossRef] [PubMed]
- Bak, K.H.; Bolumar, T.; Karlsson, A.H.; Lindahl, G.; Orlien, V. Effect of high pressure treatment on the color of fresh and processed meats: A review. Crit. Rev. Food Sci. 2017, 59, 228–252. [Google Scholar] [CrossRef]
- Wang, Y.; Domínguez, R.; Lorenzo, J.M.; Bohrer, B.M. The Relationship between Lipid Content in Ground Beef Patties with Rate of Discoloration and Lipid Oxidation during Simulated Retail Display. Foods 2021, 10, 1892. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 16, 96–123. [Google Scholar] [CrossRef]
- Chauhan, S.S.; England, E.M. Postmortem glycolysis and glycogenolysis: Insights from species comparisons. Meat Sci. 2018, 144, 118–126. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Q.; Fu, M.; Chen, Q.; Han, C. 2,3-Butanedione inhibited the swelling and microbial growth, maintained flavor and quality attributes of vacuum-packed lotus root. Food Control 2024, 166, 100757. [Google Scholar] [CrossRef]
- Kasai, H.; Kawai, K. Free radical-mediated acetaldehyde formation by model reactions of dietary components: Effects of meat, wine, cooking oil and coffee. Genes Environ. 2021, 43, 28. [Google Scholar] [CrossRef]
- Bai, J.; Baker, S.M.; Goodrich-Schneider, R.M.; Montazeri, N.; Sarnoski, P.J. Aroma Profile Characterization of Mahi-Mahi and Tuna for Determining Spoilage Using Purge and Trap Gas Chromatography-Mass Spectrometry. J. Food Sci. 2019, 84, 481–489. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Formation of phenylacetic acid and benzaldehyde by degradation of phenylalanine in the presence of lipid hydroperoxides: New routes in the amino acid degradation pathways initiated by lipid oxidation products. Food Chem. X 2019, 2, 100037. [Google Scholar] [CrossRef] [PubMed]
- Loffler, M.; Carrey, E.A.; Zameitat, E. Orotic Acid, More Than Just an Intermediate of Pyrimidine de novo Synthesis. J. Genet. Genom. 2015, 42, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Pang, Y.; Shen, G.; Bai, B.; Yang, Y.; Zeng, M. Identification and selection of volatile compounds derived from lipid oxidation as indicators for quality deterioration of frozen white meat and red meat using HS-SPME-GC-MS combined with OPLS-DA. Food Chem. 2025, 463, 141112. [Google Scholar] [CrossRef] [PubMed]
| Specification | Unit of Measure | Storage Time | ||
|---|---|---|---|---|
| Day 0 | Day 30 | Day 60 | ||
| Hardness | N | 328.21 a ± 33.61 | 163.11 b ± 21.05 | 74.70 c ± 7.53 |
| Adhesiveness | g/s | 212.27 a ± 24.96 | 144.89 b ± 4.76 | 59.36 c ± 4.60 |
| Springiness | - | 0.86 a ± 0.03 | 0.44 b ± 0.02 | 0.36 c ± 0.01 |
| Cohesiveness | - | 0.43 b ± 0.04 | 0.47 ab ± 0.02 | 0.55 a ± 0.01 |
| Gumminess | N | 156.21 a ± 12.19 | 82.50 b ± 7.8 | 51.99 c ± 6.54 |
| Chewiness | N | 58.52 a ± 5.45 | 30.60 b ± 4.81 | 22.41 b ± 2.16 |
| Resilience | - | 0.29 ± 0.02 | 0.29 ± 0.05 | 0.31 ± 0.03 |
| Count | Compound | Formula | Flavors | Matched Pathways |
|---|---|---|---|---|
| 1 | Benzaldehyde | C7H6O | Almond, burnt sugar, cherry, strong, sharp, sweet, bitter | Degradation of phenylalanine, Aminobenzoate degradation, Microbial metabolism in diverse environments, Degradation of aromatic compounds |
| 2 | 2,3-butanedione | C4H6O2 | Sweet, buttery | Butanoate metabolism |
| 3 | Acetaldehyde | C2H4O | Pungent, ether, whiskey, ethereal, aldehydic, fruity | Glycolysis/Gluconeogenesis, Phenylalanine metabolism, Benzoate degradation, Taurine and hypotaurine metabolism, Glycerophospholipid metabolism, Microbial metabolism in diverse environments, Degradation of aromatic compounds |
| 4 | Octadecanoic acid | C18H36O2 | Rich, smooth | Fatty acid biosynthesis Biosynthesis of unsaturated fatty acids Biosynthesis of plant secondary metabolites |
| 5 | Vinyl formate | C3H4O2 | NA | NA |
| 6 | 2-formylhistamine | C6H9N3O | Cabbage, garlic, amine, urine | NA |
| 7 | Vinyl acetate | C4H6O2 | NA | NA |
| 8 | Octanal | C8H16O | Aldehydic, citrus, fat | NA |
| 9 | Orotic acid | C5H4N2O4 | Sour, fruity | Pyrimidine metabolism, Biosynthesis of cofactors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Hou, W.; Zhu, M.; Akan, O.D.; Xing, Y.; Yu, Y.; Li, B.; Zhu, H. Quality Dynamics of Beef Bottom Round During 2-Month Frozen Storage (−18 °C) and Week-Long Refrigeration (4 °C). Metabolites 2025, 15, 294. https://doi.org/10.3390/metabo15050294
Song Y, Hou W, Zhu M, Akan OD, Xing Y, Yu Y, Li B, Zhu H. Quality Dynamics of Beef Bottom Round During 2-Month Frozen Storage (−18 °C) and Week-Long Refrigeration (4 °C). Metabolites. 2025; 15(5):294. https://doi.org/10.3390/metabo15050294
Chicago/Turabian StyleSong, Yue, Wenbo Hou, Mengliu Zhu, Otobong Donald Akan, Yanxia Xing, Yang Yu, Bo Li, and He Zhu. 2025. "Quality Dynamics of Beef Bottom Round During 2-Month Frozen Storage (−18 °C) and Week-Long Refrigeration (4 °C)" Metabolites 15, no. 5: 294. https://doi.org/10.3390/metabo15050294
APA StyleSong, Y., Hou, W., Zhu, M., Akan, O. D., Xing, Y., Yu, Y., Li, B., & Zhu, H. (2025). Quality Dynamics of Beef Bottom Round During 2-Month Frozen Storage (−18 °C) and Week-Long Refrigeration (4 °C). Metabolites, 15(5), 294. https://doi.org/10.3390/metabo15050294







