Targeted and Non-Targeted Metabolomic Evaluation of Cerebrospinal Fluid in Early Phase Schizophrenia: A Pilot Study from the Hopkins First Episode Psychosis Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Metabolomic Analyses
2.2.1. Chemicals and Reagents
2.2.2. Sample Preparation
2.2.3. Non-Targeted Analysis
2.2.4. Targeted Analysis
2.2.5. Preparation of Standards
2.2.6. LC/MS/MS Analysis
2.2.7. Data Processing
3. Results
3.1. Participant Sample
3.2. Non-Targeted Data
3.3. Targeted Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef]
- Fuentes-Claramonte, P.; Estradé, A.; Solanes, A.; Ramella-Cravaro, V.; Garcia-Leon, M.A.; de Diego-Adeliño, J.; Molins, C.; Fung, E.; Valentí, M.; Anmella, G.; et al. Biomarkers for Psychosis: Are We There Yet? Umbrella Review of 1478 Biomarkers. Schizophr. Bull. Open 2024, 5, sgae018. [Google Scholar] [CrossRef]
- Cuthbert, B.N.; Insel, T.R. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med. 2013, 11, 126. [Google Scholar] [CrossRef]
- Hao, M.; Qin, Y.; Li, Y.; Tang, Y.; Ma, Z.; Tan, J.; Jin, L.; Wang, F.; Gong, X. Metabolome subtyping reveals multi-omics characteristics and biological heterogeneity in major psychiatric disorders. Psychiatry Res. 2023, 330, 115605. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, R.J. Transdiagnostic biomarker approaches to mental health disorders: Consideration of symptom complexity, comorbidity and context. Brain Behav. Immun. Health 2021, 16, 100303. [Google Scholar] [CrossRef] [PubMed]
- Weickert, C.S.; Weickert, T.W.; Pillai, A.; Buckley, P.F. Biomarkers in schizophrenia: A brief conceptual consideration. Dis. Markers 2013, 35, 3–9. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jaskiw, G.E.; Obrenovich, M.E.; Donskey, C.J. The phenolic interactome and gut microbiota: Opportunities and challenges in developing applications for schizophrenia and autism. Psychopharmacology 2019, 236, 1471–1489. [Google Scholar] [CrossRef]
- Jaskiw, G.E.; Xu, D.; Obrenovich, M.E.; Donskey, C.J. Small phenolic and indolic gut-dependent molecules in the primate central nervous system: Levels vs. bioactivity. Metabolomics 2022, 18, 8. [Google Scholar] [CrossRef]
- Obrenovich, M.E.; Donskey, C.J.; Schiefer, I.T.; Bongiovanni, R.; Li, L.; Jaskiw, G.E. Quantification of phenolic acid metabolites in humans by LC-MS: A structural and targeted metabolomics approach. Bioanalysis 2018, 10, 1591–1608. [Google Scholar] [CrossRef]
- Obrenovich, M.E.; Jaskiw, G.E.; Zhang, R.; Willard, B.; Donskey, C.J. Identification and Quantification by Targeted Metabolomics of Antibiotic-Responsive Urinary Small Phenolic Molecules Derived from the Intestinal Microbiota in Mice. Pathog. Immun. 2019, 4, 85–103. [Google Scholar] [CrossRef]
- Obrenovich, M.E.; Tackie-Yarboi, E.; Schiefer, I.T.; Donskey, C.J.; Jaskiw, G.E. Separating and identifying multiple structural isomers of 3-hydroxy-3-(3′-hydroxyphenyl)propanoic acid (3,3′-HPHPA). J. Liq. Chromatogr. Rel. Technol. 2024, 47, 284–291. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.V.; Choi, K.; Klemashevich, C.; Wu, C.; Prabakaran, D.; Pan, L.B.; Steinmeyer, S.; Mueller, C.; Yousofshahi, M.; Alaniz, R.C.; et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat. Commun. 2014, 5, 5492. [Google Scholar] [CrossRef]
- Murray, N.; Al Khalaf, S.; Bastiaanssen, T.F.S.; Kaulmann, D.; Lonergan, E.; Cryan, J.F.; Clarke, G.; Khashan, A.S.; O’Connor, K. Compositional and Functional Alterations in Intestinal Microbiota in Patients with Psychosis or Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2023, 49, 1239–1255. [Google Scholar] [CrossRef]
- Quintero, M.; Stanisic, D.; Cruz, G.; Pontes, J.G.M.; Costa, T.B.B.C.; Tasic, L. Metabolomic Biomarkers in Mental Disorders: Bipolar Disorder and Schizophrenia. In Reviews on Biomarker Studies in Psychiatric and Neurodegenerative Disorders; Guest, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 271–293. [Google Scholar]
- Lu, T.; Chen, Y.; Yoshiji, S.; Ilboudo, Y.; Forgetta, V.; Zhou, S.; Greenwood, C.M. Circulating metabolite abundances associated with risks of bipolar disorder, schizophrenia, and depression: A Mendelian randomization study. Biol. Psychiatry 2024, 96, 782–791. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, R.; Kirkbride, B.; Newbould, E.; Durkalski, V.; Jaskiw, G.E. Relationships between large neutral amino acid levels in plasma, cerebrospinal fluid, brain microdialysate and brain tissue in the rat. Brain Res. 2010, 1334, 45–57. [Google Scholar] [CrossRef]
- Davson, H.; Segal, M.B. The Physiology of the CSF and Blood-Brain Barriers; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Holmes, E.; Tsang, T.M.; Huang, J.T.; Leweke, F.M.; Koethe, D.; Gerth, C.W.; Nolden, B.M.; Gross, S.; Schreiber, D.; Nicholson, J.K.; et al. Metabolic profiling of CSF: Evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med. 2006, 3, e327. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; McEvoy, J.; Baillie, R.A.; Lee, D.; Yao, J.K.; Doraiswamy, P.M.; Krishnan, K.R. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol. Psychiatry 2007, 12, 934–945. [Google Scholar] [CrossRef]
- Panyard, D.J.; Kim, K.M.; Darst, B.F.; Deming, Y.K.; Zhong, X.; Wu, Y.; Kang, H.; Carlsson, C.M.; Johnson, S.C.; Asthana, S.; et al. Cerebrospinal fluid metabolomics identifies 19 brain-related phenotype associations. Commun. Biol. 2021, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, O.; Kokla, M.; Lehtonen, M.; Auriola, S.; Martiskainen, M.; Tiihonen, J.; Karhunen, P.J.; Hanhineva, K.; Kok, E. Changes in the metabolic profile of human male postmortem frontal cortex and cerebrospinal fluid samples associated with heavy alcohol use. Addict. Biol. 2021, 26, e13035. [Google Scholar] [CrossRef]
- Lin, H.T.; Cheng, M.L.; Lo, C.J.; Lin, G.; Lin, S.F.; Yeh, J.T.; Ho, H.Y.; Lin, J.R.; Liu, F.C. (1)H Nuclear Magnetic Resonance (NMR)-Based Cerebrospinal Fluid and Plasma Metabolomic Analysis in Type 2 Diabetic Patients and Risk Prediction for Diabetic Microangiopathy. J. Clin. Med. 2019, 8, 874. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, M.; Chang, Y.H.; Witmer, A.M.; Coughlin, J.M.; Schretlen, D.J.; Barker, P.B.; Yang, K.; Sawa, A. Reduction of N-acetyl aspartate (NAA) in association with relapse in early-stage psychosis: A 7-Tesla MRS study. Schizophrenia 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Kamath, V.; Lasutschinkow, P.; Ishizuka, K.; Sawa, A. Olfactory Functioning in First-Episode Psychosis. Schizophr. Bull. 2018, 44, 672–680. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- MassBank of North America (MoNA). Available online: https://mona.fiehnlab.ucdavis.edu/ (accessed on 12 January 2024).
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Liu, Y.; Xu, D. Sialic acid metabolism as a potential therapeutic target of atherosclerosis. Lipids Health Dis. 2019, 18, 173. [Google Scholar] [CrossRef]
- McIntosh, J.C.; Cooper, J.R. Function of N-Acetyl aspartic acid in the brain: Effects of certain drugs. Nature 1964, 203, 658. [Google Scholar] [CrossRef]
- Murray, H.C.; Low, V.F.; Swanson, M.E.; Dieriks, B.V.; Turner, C.; Faull, R.L.; Curtis, M.A. Distribution of PSA-NCAM in normal, Alzheimer’s and Parkinson’s disease human brain. Neuroscience 2016, 330, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Mealer, R.G.; Scolnick, E.M.; Smoller, J.W.; Cummings, R.D. Aberrant glycosylation in schizophrenia: A review of 25 years of post-mortem brain studies. Mol. Psychiatry 2020, 25, 3198–3207. [Google Scholar] [CrossRef]
- Bogoch, S. Cerebrospinal fluid neuraminic acid deficiency in schizophrenia; a preliminary report. Am. J. Psychiatry 1957, 114, 172. [Google Scholar] [CrossRef] [PubMed]
- Bogoch, S.; Dussik, K.T.; Lever, P.G. Clinical status and cerebrospinal fluid “total neuraminic acid”. AMA Arch. Gen. Psychiatry 1959, 1, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Robins, E.; Croninger, A.B.; Smith, K.; Moody, A.C. Studies on N-acetyl neuraminic acid in the cerebrospinal fluid in schizophrenia. Ann. N. Y. Acad. Sci. 1962, 96, 390–391. [Google Scholar] [CrossRef]
- Wood, P.L. Targeted lipidomics and metabolomics evaluations of cortical neuronal stress in schizophrenia. Schizophr. Res. 2019, 212, 107–112. [Google Scholar] [CrossRef]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, A.M. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef]
- Patel, T.; Blyth, J.C.; Griffiths, G.; Kelly, D.; Talcott, J.B. Moderate relationships between NAA and cognitive ability in healthy adults: Implications for cognitive spectroscopy. Front. Hum. Neurosci. 2014, 8, 39. [Google Scholar] [CrossRef]
- Rebelos, E.; Daniele, G.; Campi, B.; Saba, A.; Koskensalo, K.; Ihalainen, J.; Saukko, E.; Nuutila, P.; Backes, W.H.; Jansen, J.F.A.; et al. Circulating N-Acetylaspartate does not track brain NAA concentrations, cognitive function or features of small vessel disease in humans. Sci. Rep. 2022, 12, 11530. [Google Scholar] [CrossRef] [PubMed]
- Baycin-Hizal, D.; Gottschalk, A.; Jacobson, E.; Mai, S.; Wolozny, D.; Zhang, H.; Krag, S.S.; Betenbaugh, M.J. Physiologic and pathophysiologic consequences of altered sialylation and glycosylation on ion channel function. Biochem. Biophys. Res. Commun. 2014, 453, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, C.C.; Mäki-Marttunen, T.; Thompson, W.K.; Schork, A.J.; Bettella, F.; Djurovic, S.; Dale, A.M.; Andreassen, O.A.; Wang, Y. A molecule-based genetic association approach implicates a range of voltage-gated calcium channels associated with schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 454–467. [Google Scholar] [CrossRef]
- Bonfanti, L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog. Neurobiol. 2006, 80, 129–164. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Voineskos, D.; Daskalakis, Z.J.; Rajji, T.K.; Blumberger, D.M. A Review of Impaired Neuroplasticity in Schizophrenia Investigated with Non-invasive Brain Stimulation. Front. Psychiatry 2016, 7, 45. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Fraguas, D.; Diaz-Caneja, C.M.; Ayora, M.; Hernandez-Alvarez, F.; Rodriguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr. Bull. 2019, 45, 742–751. [Google Scholar] [CrossRef]
- He, Q.; You, Y.; Yu, L.; Yao, L.; Lu, H.; Zhou, X.; Wu, S.; Chen, L.; Chen, Y.; Zhao, X. Uric acid levels in subjects with schizophrenia: A systematic review and meta-analysis. Psychiatry Res. 2020, 292, 113305. [Google Scholar] [CrossRef]
- Bang, M.; Heo, Y.; Choi, T.K.; Lee, S.H. Positive Effects of Uric Acid on White Matter Microstructures and Treatment Response in Patients With Schizophrenia. Schizophr. Bull. 2024, 50, 815–826. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, T.; Liu, Z.; Liang, Y.; Li, F.; Li, Y.; Liu, W.; Li, F.; Shi, S.; Zhou, C.; et al. Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective cohort study. BMJ 2023, 380, e072691. [Google Scholar] [CrossRef]
- Abhijit, S.; Tripathi, S.J.; Bhagya, V.; Shankaranarayana Rao, B.S.; Subramanyam, M.V.; Asha Devi, S. Antioxidant action of grape seed polyphenols and aerobic exercise in improving neuronal number in the hippocampus is associated with decrease in lipid peroxidation and hydrogen peroxide in adult and middle-aged rats. Exp. Gerontol. 2018, 101, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, J.; Liu, X.; Ma, J. Relationship between efficacy and common metabolic parameters in first-treatment drug-naïve patients with early non-response schizophrenia: A retrospective study. Ann. Gen. Psychiatry 2023, 22, 6. [Google Scholar] [CrossRef]
- Chu, H.; Zhu, H.; Ma, J.; Jiang, Y.; Cui, C.; Yan, X.; Li, Q.; Zhang, X.; Chen, D.; Li, X.; et al. Mitochondrial Dysfunction and Metabolic Indicators in Patients with Drug-Naive First-Episode Schizophrenia: A Case-Control Study. Neuropsychiatr. Dis. Treat. 2024, 20, 2433–2442. [Google Scholar] [CrossRef]
- Farstad, M.; Skaug, O.E.; Solheim, D.M. Uric acid in the cerebrospinal fluid in psychiatric disorders. Acta Neurol. Scand. 1965, 41, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, C.; Dencker, S.J. Cerebrospinal uric acid in alcoholics. Acta Neurol. Scand. 1973, 49, 39–46. [Google Scholar] [CrossRef]
- Issa, F.; Gerhardt, G.A.; Bartko, J.J.; Suddath, R.L.; Lynch, M.; Gamache, P.H.; Freedman, R.; Wyatt, R.J.; Kirch, D.G. A multidimensional approach to analysis of cerebrospinal fluid biogenic amines in schizophrenia: I. Comparisons with healthy control subjects and neuroleptic-treated/unmedicated pairs analyses. Psychiatry Res. 1994, 52, 237–249. [Google Scholar] [CrossRef] [PubMed]
- León-Ortiz, P.; Rivera-Chávez, L.F.; Torres-Ruíz, J.; Reyes-Madrigal, F.; Carrillo-Vázquez, D.; Moncada-Habib, T.; Cassiano-Quezada, F.; Cadenhead, K.S.; Gómez-Martín, D.; de la Fuente-Sandoval, C. Systemic inflammation and cortical neurochemistry in never-medicated first episode-psychosis individuals. Brain. Behav. Immun. 2023, 111, 270–276. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Polikowska, A.; Serwin, N.; Michalczyk, A.; Stodolak, P.; Goszka, M.; Zoń, M.; Budkowska, M.; Tyburski, E.; Podwalski, P.; et al. The importance of oxidative biomarkers in diagnosis, treatment, and monitoring schizophrenia patients. Schizophr. Res. 2024, 270, 44–56. [Google Scholar] [CrossRef]
- Dunleavy, C.; Elsworthy, R.J.; Upthegrove, R.; Wood, S.J.; Aldred, S. Inflammation in first-episode psychosis: The contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr. Scand. 2022, 146, 6–20. [Google Scholar] [CrossRef]
- Degrell, I.; Nagy, E. Concentration gradients for HVA, 5-HIAA, ascorbic acid, and uric acid in cerebrospinal fluid. Biol. Psychiatry 1990, 27, 891–896. [Google Scholar] [CrossRef]
- Tohgi, H.; Abe, T.; Takahashi, S.; Kikuchi, T. The urate and xanthine concentrations in the cerebrospinal fluid in patients with vascular dementia of the Binswanger type, Alzheimer type dementia, and Parkinson’s disease. J. Neural Transm. Park. Dis. Dement. Sect. 1993, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.G.; DellaValle, B.; Bøgehave, H.; Mogensen, P.B.; Hahn, M.K.; Goth, C.K.; Sørensen, M.E.; Sigvard, A.K.; Tangmose, K.; Bojesen, K.B.; et al. Glycocalyx shedding patterns identifies antipsychotic-naïve patients with first-episode psychosis. Psychiatry Res. 2024, 339, 116037. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.L.; Yang, G.; Hernandez-Avila, M.; Przewozniak, K.; Zatonski, W.; Figueiredo, V.; Avila-Tang, E.; Ma, J.; Benowitz, N.L.; Samet, J.M. Cotinine concentration in smokers from different countries: Relationship with amount smoked and cigarette type. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1799–1804. [Google Scholar] [CrossRef]
- Malkawi, A.H.; Al-Ghananeem, A.M.; de Leon, J.; Crooks, P.A. Nicotine exposure can be detected in cerebrospinal fluid of active and passive smokers. J. Pharm. Biomed. Anal. 2009, 49, 129–132. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, T.; Rodríguez-Toscano, E.; Roldán, L.; Ferraro, L.; Parellada, M.; Calvo, A.; López, G.; Rapado-Castro, M.; La Barbera, D.; La Cascia, C.; et al. Tobacco use in first-episode psychosis, a multinational EU-GEI study. Psychol. Med. 2023, 53, 7265–7276. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.; Gartner, C.; Kisely, S.; Plever, S. Systematic review of tobacco smoking prevalence among young people in treatment for first-episode psychosis. Int. J. Ment. Health Nurs. 2024, 33, 1381–1387. [Google Scholar] [CrossRef]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 33–91. [Google Scholar] [CrossRef]
- Liu, X.; Smith, B.J.; Chen, C.; Callegari, E.; Becker, S.L.; Chen, X.; Cianfrogna, J.; Doran, A.C.; Doran, S.D.; Gibbs, J.P.; et al. Evaluation of cerebrospinal fluid concentration and plasma free concentration as a surrogate measurement for brain free concentration. Drug Metab. Dispos. 2006, 34, 1443–1447. [Google Scholar] [CrossRef]
- Gurpegui, M.; Aguilar, M.C.; Martinez-Ortega, J.M.; Diaz, F.J.; de Leon, J. Caffeine intake in outpatients with schizophrenia. Schizophr. Bull. 2004, 30, 935–945. [Google Scholar] [CrossRef]
- Williams, J.M.; Gandhi, K.K. Use of caffeine and nicotine in people with schizophrenia. Curr. Drug Abus. Rev. 2008, 1, 155–161. [Google Scholar] [CrossRef]
- Ringeisen, H.; Edlund, M.; Guyer, H.; Geiger, P.; Stambaugh, L.; Dever, J.; Liao, D.; Carr, C.; Peytchev, A.; Reed, W.; et al. Mental and Substance Use Disorders Prevalence Study (MDPS): Findings Report; RTI International: Durham, NC, USA, 2023. [Google Scholar]
- Kamiya, S.; Shirahase, H.; Nakamura, S.; Kanda, M.; Matsui, H.; Yoshimi, A.; Kasai, M.; Takahashi, K.; Kurahashi, K. A novel series of thromboxane A2 synthetase inhibitors with free radical scavenging and anti-peroxidative activities. Chem. Pharm. Bull. 2001, 49, 563–571. [Google Scholar] [CrossRef]
- Zeeli, S.; Weill, T.; Finkin-Groner, E.; Bejar, C.; Melamed, M.; Furman, S.; Zhenin, M.; Nudelman, A.; Weinstock, M. Synthesis and Biological Evaluation of Derivatives of Indoline as Highly Potent Antioxidant and Anti-inflammatory Agents. J. Med. Chem. 2018, 61, 4004–4019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Zeeshan, M.; Zhang, G.; Wang, C.; Han, X.; Yang, D. Integrative Genomics and Bioactivity-Guided Isolation of Novel Antimicrobial Compounds from Streptomyces sp. KN37 in Agricultural Applications. Molecules 2024, 29, 2040. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, M.; Taroncher, M.; Ruiz, M.J.; Rodríguez-Carrasco, Y.; Tolosa, J. In Vitro and Predictive Computational Toxicology Methods for the Neurotoxic Pesticide Amitraz and Its Metabolites. Brain Sci. 2023, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xia, Y. Pretreating and normalizing metabolomics data for statistical analysis. Genes Dis. 2024, 11, 100979. [Google Scholar] [CrossRef]
- Reveley, M.A.; De Belleroche, J.; Recordati, A.; Hirsch, S.R. Increased CSF amino acids and ventricular enlargement in schizophrenia: A preliminary study. Biol. Psychiatry 1987, 22, 413–420. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem. Res. 1998, 23, 635–644. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Mchaourab, A.S.; McClellan, F.; Elsworth, J.; Double, M.; Jaskiw, G.E. Large neutral amino acids levels in primate cerebrospinal fluid do not confirm competitive transport under baseline conditions. Brain Res. 2016, 1648, 372–379. [Google Scholar] [CrossRef]
- Potkin, S.G.; Cannon-Spoor, H.E.; DeLisi, L.E.; Neckers, L.M.; Wyatt, R.J. Plasma phenylalanine, tyrosine, and tryptophan in schizophrenia. Arch. Gen. Psychiatry 1983, 40, 749–752. [Google Scholar] [CrossRef]
- Szymanski, H.V.; Naylor, E.W.; Karoum, F. Plasma phenylethylamine and phenylalanine in chronic schizophrenic patients. Biol. Psychiatry 1987, 22, 194–198. [Google Scholar] [CrossRef]
- Okusaga, O.; Muravitskaja, O.; Fuchs, D.; Ashraf, A.; Hinman, S.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; Schiffman, J.; et al. Elevated levels of plasma phenylalanine in schizophrenia: A guanosine triphosphate cyclohydrolase-1 metabolic pathway abnormality? PLoS ONE 2014, 9, e85945. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, Z.; Sun, J.; Wang, H.; Cong, H.; Wei, Y.; Ma, Y.; Feng, K.; Yin, L.; Zhang, X. Increased serum phenylalanine/tyrosine ratio associated with the psychiatric symptom of anti-NMDAR encephalitis. Front. Neurol. 2024, 15, 1434139. [Google Scholar] [CrossRef] [PubMed]
- Teraishi, T.; Ozeki, Y.; Hori, H.; Sasayama, D.; Chiba, S.; Yamamoto, N.; Tanaka, H.; Iijima, Y.; Matsuo, J.; Kawamoto, Y.; et al. 13C-phenylalanine breath test detects altered phenylalanine kinetics in schizophrenia patients. Transl. Psychiatry 2012, 2, e119. [Google Scholar] [CrossRef] [PubMed]
- Teraishi, T.; Kajiwara, M.; Hori, H.; Sasayama, D.; Hidese, S.; Matsuo, J.; Ishida, I.; Kajiwara, Y.; Ozeki, Y.; Ota, M.; et al. 13C-phenylalanine breath test and serum biopterin in schizophrenia, bipolar disorder and major depressive disorder. J. Psychiatr. Res. 2018, 99, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.W.C.; Chang, W.C.; Lo, G.G.; Chan, K.W.S.; Lee, H.M.E.; Hui, L.M.C.; Suen, Y.N.; Leung, Y.L.E.; Au Yeung, K.M.P.; Chen, S.; et al. The role of dopamine dysregulation and evidence for the transdiagnostic nature of elevated dopamine synthesis in psychosis: A positron emission tomography (PET) study comparing schizophrenia, delusional disorder, and other psychotic disorders. Neuropsychopharmacology 2020, 45, 1870–1876. [Google Scholar] [CrossRef]
- Howes, O.D.; Shatalina, E. Integrating the Neurodevelopmental and Dopamine Hypotheses of Schizophrenia and the Role of Cortical Excitation-Inhibition Balance. Biol. Psychiatry 2022, 92, 501–513. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Shende, V.V.; Bauman, K.D.; Moore, B.S. The shikimate pathway: Gateway to metabolic diversity. Nat. Prod. Rep. 2024, 41, 604–648. [Google Scholar] [CrossRef]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef]
- Jia, M.; Li, D.; Wang, R.; Wang, A.; Strappe, P.; Wu, Q.; Shang, W.; Wang, X.; Zhuang, M.; Blanchard, C.; et al. Gut microbiota derived structural changes of phenolic compounds from colored rice and its corresponding fermentation property. Food Funct. 2022, 13, 10759–10768. [Google Scholar] [CrossRef]
- Li, D.; Wan, M.; Xue, L.; Zhang, Z.; Qiu, Y.; Mei, F.; Tang, N.; Yu, C.; Yu, Y.; Chen, T.; et al. Zinc promotes microbial p-coumaric acid production that protects against cholestatic liver injury. Cell Host Microbe 2024, 32, 2195–2211.e2199. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, R. p-Coumaric acid: A naturally occurring chemical with potential therapeutic applications. Curr. Org. Chem. 2022, 26, 1333–1349. [Google Scholar] [CrossRef]
- Cao, B.; Zeng, M.N.; Hao, F.X.; Hao, Z.Y.; Zhang, Z.K.; Liang, X.W.; Wu, Y.Y.; Zhang, Y.H.; Feng, W.S.; Zheng, X.K. P-coumaric acid ameliorates Aβ(25-35)-induced brain damage in mice by modulating gut microbiota and serum metabolites. Biomed. Pharmacother. 2023, 168, 115825. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, S.; Rashno, M.; Brooshghalan, S.E.; Salehi, I.; Sarihi, A.; Shahidi, S.; Rashidi, K.; Haddadi, R.; Komaki, A. p-Coumaric acid reverses spatial cognitive decline in a rat model of traumatic brain injury: Possible underlying mechanisms. J. Funct. Foods 2024, 120, 106381. [Google Scholar] [CrossRef]
- Stewart Campbell, A.; Needham, B.D.; Meyer, C.R.; Tan, J.; Conrad, M.; Preston, G.M.; Bolognani, F.; Rao, S.G.; Heussler, H.; Griffith, R.; et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: An open-label phase 1b/2a trial. Nat. Med. 2022, 28, 528–534. [Google Scholar] [CrossRef]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Funabashi, M.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.J.; et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Price, L.L.; Logvinenko, T.; Hatzis, C.; Padukone, N.; Reo, N.V.; Phillips, E.M.; Kirn, D.; Mills, J.; Fielding, R.A. Identification of serum analytes and metabolites associated with aerobic capacity. Eur. J. Appl. Physiol. 2013, 113, 1311–1320. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Hall, K.D.; Herrick, K.A.; Reedy, J.; Chung, S.T.; Stagliano, M.; Courville, A.B.; Sinha, R.; Freedman, N.D.; Hong, H.G.; et al. Metabolomic Profiling of an Ultraprocessed Dietary Pattern in a Domiciled Randomized Controlled Crossover Feeding Trial. J. Nutr. 2023, 153, 2181–2192. [Google Scholar] [CrossRef]
- Reavis, Z.W.; Mirjankar, N.; Sarangi, S.; Boyle, S.H.; Kuhn, C.M.; Matson, W.R.; Babyak, M.A.; Matson, S.A.; Siegler, I.C.; Kaddurah-Daouk, R.; et al. Sex and race differences of cerebrospinal fluid metabolites in healthy individuals. Metabolomics 2021, 17, 13. [Google Scholar] [CrossRef]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Krüger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef]
- Borovcanin, M.M.; Janicijevic, S.M.; Mijailovic, N.R.; Jovanovic, I.P.; Arsenijevic, N.N.; Vesic, K. Uric Acid Potential Role in Systemic Inflammation and Negative Symptoms After Acute Antipsychotic Treatment in Schizophrenia. Front. Psychiatry 2021, 12, 822579. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.K.; Reddy, R.; van Kammen, D.P. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1998, 80, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Karahalil, B.; Elkama, A.; Ak, M.; Nemutlu, E. Metabolomics mapping changed after olanzapine therapy in drug-naive schizophrenia patients-the significant impact of gene polymorphisms. Toxicol. Res. 2022, 11, 547–556. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Hunt, G.E.; Large, M.M.; Cleary, M.; Lai, H.M.X.; Saunders, J.B. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol Depend. 2018, 191, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Derkach, A.; Freedman, N.D.; Landi, M.T.; Albanes, D.; Weinstein, S.J.; Mondul, A.M.; Matthews, C.E.; Guertin, K.A.; Xiao, Q.; et al. Cigarette smoking behaviour and blood metabolomics. Int. J. Epidemiol. 2016, 45, 1421–1432. [Google Scholar] [CrossRef]
- Shang, P.; Ho, A.M.; Tufvesson-Alm, M.; Lindberg, D.R.; Grant, C.W.; Orhan, F.; Eren, F.; Bhat, M.; Engberg, G.; Schwieler, L.; et al. Identification of cerebrospinal fluid and serum metabolomic biomarkers in first episode psychosis patients. Transl. Psychiatry 2022, 12, 229. [Google Scholar] [CrossRef]
- Orhan, F.; Goiny, M.; Becklén, M.; Mathé, L.; Piehl, F.; Schwieler, L.; Fatouros-Bergman, H.; Farde, L.; Cervenka, S.; Sellgren, C.M.; et al. CSF dopamine is elevated in first-episode psychosis and associates to symptom severity and cognitive performance. Schizophr. Res. 2023, 257, 34–40. [Google Scholar] [CrossRef]
- Romer, T.B.; Jeppesen, R.; Christensen, R.H.B.; Benros, M.E. Biomarkers in the cerebrospinal fluid of patients with psychotic disorders compared to healthy controls: A systematic review and meta-analysis. Mol. Psychiatry 2023, 28, 2277–2290. [Google Scholar] [CrossRef]
- Whitehurst, T.S.; Osugo, M.; Townsend, L.; Shatalina, E.; Vava, R.; Onwordi, E.C.; Howes, O. Proton Magnetic Resonance Spectroscopy of N-acetyl Aspartate in Chronic Schizophrenia, First Episode of Psychosis and High-Risk of Psychosis: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 119, 255–267. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Jaskiw, G.E.; Obrenovich, M.E.; Kundrapu, S.; Donskey, C.J. Changes in the serum metabolome of patients treated with broad-spectrum antibiotics. Pathog. Immun. 2020, 5, 382–418. [Google Scholar] [CrossRef] [PubMed]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration: Silver Spring, MD, USA, 2016. [Google Scholar]

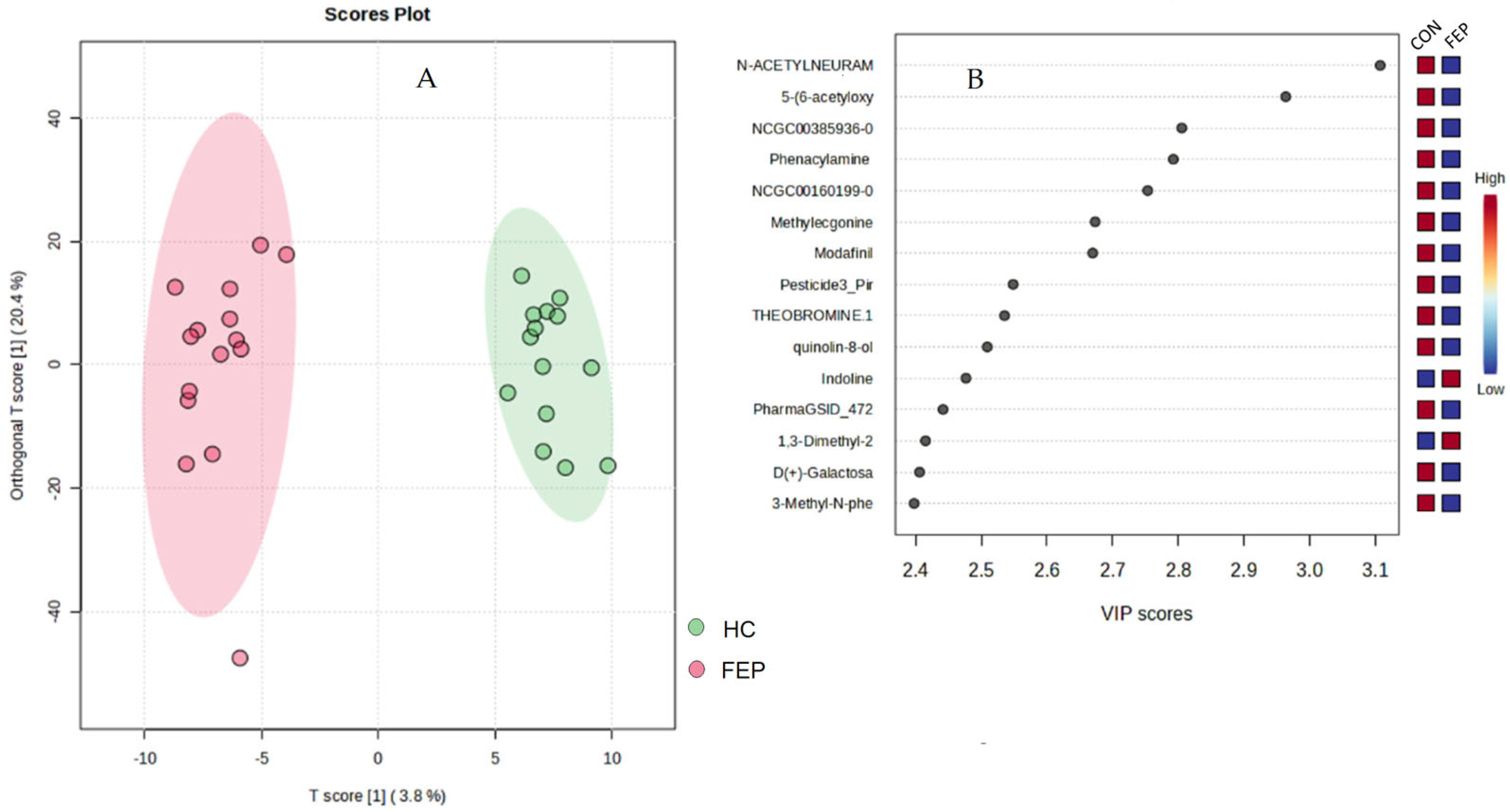


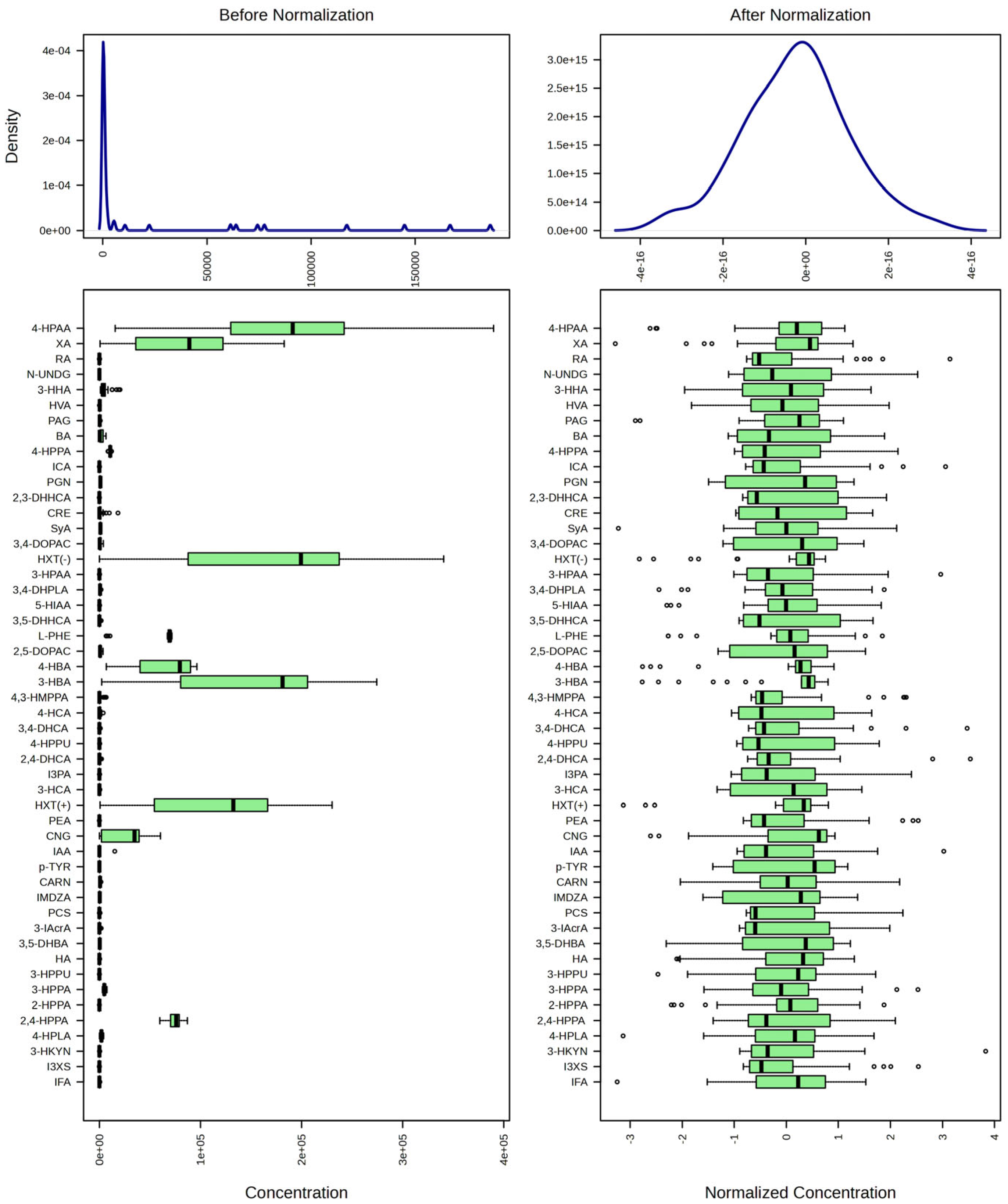
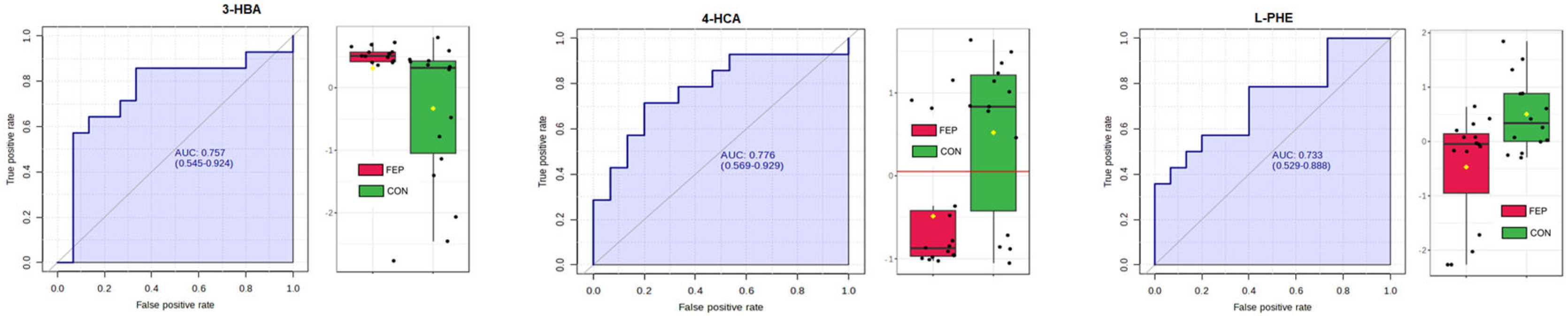
| Variable | HC | FEP | Significance |
|---|---|---|---|
| Age (y) | 23.21 + 1.0 | 23.47 + 0.96 | 0.86 |
| Gender M/F | 8/6 | 11/4 | 0.36 |
| Race A/B/W | 1/11/2 | 1/12/2 | 0.74 |
| Freezer time (y) | 5.95 + 0.35 | 5.27 + 0.19 | 0.1 |
| C18 Chromatography | POS ESI | NEG ESI | Total |
|---|---|---|---|
| Number of Features | 6745 | 451 | 7196 |
| Number of MS1 Level features matched to MoNA and MSDIAL MSP databases | 1339 | 110 | 1449 |
| FEP vs. HC | Raw p < 0.05 | Raw p > 0.05 | |
|---|---|---|---|
| Direction of change | ↑ | ↓ | |
| Number of features | 7 | 16 | 1425 |
| FC | log2 (FC) | Raw p Value | negLog (Raw p Value) | ||
|---|---|---|---|---|---|
| 1 | N-Acetylneuraminate | 0.45425 | −1.1384 | 0.000457 | 3.3399 |
| 2 | Phenacylamine Hydrochloride | 0.42157 | −1.2462 | 0.00119 | 2.9246 |
| 3 | 5-(6-acetyloxy-3,5,7-trimethoxy-4-oxochromen-2-yl)-2-methoxyphenyl acetate | 0.26672 | −1.9066 | 0.001964 | 2.7069 |
| 4 | Modafinil | 0.41774 | −1.2593 | 0.003386 | 2.4704 |
| 5 | Theobromine.1 | 0.14956 | −2.7412 | 0.005127 | 2.2902 |
| 6 | Indoline | 16.123 | 4.011 | 0.008778 | 2.0566 |
| 7 | Uric acid.1 | 11.927 | 3.5762 | 0.011072 | 1.9558 |
| 8 | N-(2,4-Dimethylphenyl)-N-methylimidoformamide | 6.2543 | 2.6448 | 0.01325 | 1.8778 |
| 9 | 1,3-Dimethyl-2-imidazolidinon | 8.713 | 3.1232 | 0.015998 | 1.7959 |
| 10 | NCGC00380117-01_C27H41NO4_(7E)-3-Isobutyl-4,5,8,12,12-pentamethyl-3,3a,4,6a,9,10,10a,13a,14,15-decahydro-1H-[1,3]dioxolo[7,8]cycloundeca[1,2-d]isoindole-1,16(2H)-dione | 0.26435 | −1.9195 | 0.01624 | 1.7894 |
| 11 | PharmaGSID_47259 | 0.47576 | −1.0717 | 0.019798 | 1.7034 |
| 12 | Procaine (Novocaine) HCl | 0.46382 | −1.1084 | 0.020015 | 1.6987 |
| 13 | Kainic Acid | 0.44111 | −1.1808 | 0.022828 | 1.6415 |
| 14 | N-2-Hydroxycyclopentyladenosine | 0.47246 | −1.0817 | 0.026159 | 1.5824 |
| 15 | N-Acetyl-L-aspartic acid.1 | 0.16743 | −2.5783 | 0.02893 | 1.5386 |
| 16 | Theophylline | 0.33052 | −1.5972 | 0.032693 | 1.4855 |
| 17 | Isoxanthopterin | 0.33413 | −1.5815 | 0.037612 | 1.4247 |
| 18 | tetradec-5-ynoic acid.1 | 0.29953 | −1.7392 | 0.038349 | 1.4162 |
| 19 | Cotinine | 5.1242 | 2.3573 | 0.040772 | 1.3896 |
| 20 | Theophylline | 0.17017 | −2.555 | 0.043098 | 1.3655 |
| 21 | Andrachcinidine | 2.0015 | 1.001 | 0.04501 | 1.3467 |
| 22 | 2-Hydroxypyridine | 2.7989 | 1.4848 | 0.045195 | 1.3449 |
| 23 | Procaine | 0.40754 | −1.295 | 0.046636 | 1.3313 |
| Abbreviation | IUPAC Name | CID | CAS | MW | Mean | MED | STDev | % “Non-0” Entries | RT (min) |
|---|---|---|---|---|---|---|---|---|---|
| TAU | 2-aminoethanesulfonic acid | 1123 | 107-35-7 | 125.15 | 384.99 | 323.94 | 189.55 | 69.0 | 1.64 |
| CARN | (3R)-3-hydroxy-4-(trimethylazaniumyl)butanoate | 10917 | 541-15-1 | 161.2 | 641.07 | 657.56 | 356.96 | 100.0 | 1.59 |
| IMDZA | 3-(1H-imidazol-5-yl)propanoic acid | 70630 | 1074-59-5 | 140.14 | IDNQ | 72.4 | 7.1 | ||
| 3-HPPU | 3-(3-hydroxyphenyl)-2-oxopropanoic acid | 5318321 | 4607-41-4 | 180.16 | IDNQ | 82.8 | 1.94 | ||
| CRE | 2-amino-3-methyl-4H-imidazol-5-one | 588 | 60-27-5 | 113.12 | 3005.73 | 1014.08 | 4852.72 | 58.6 | 1.86 |
| XANS | 9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purine-2,6-dione | 64959 | 146-80-5 | 284.23 | 57.40 | 57.40 | 3.4 | 6.97 | |
| 3-HKYN | 2-amino-4-(2-amino-3-hydroxyphenyl)-4-oxobutanoic acid | 89 | 484-78-6 | 224.21 | 298.07 | 298.07 | 272.57 | 6.9 | 6.61 |
| HXT | 1,7-dihydropurin-6-one | 135398638 | 68-94-0 | 136.11 | 166,783.98 | 199,278.78 | 106,273.24 | 100.0 | 14.3 |
| XA | 3,7-dihydropurine-2,6-dione | 1188 | 69-89-6 | 152.11 | 80,324.64 | 89,918.97 | 49,963.03 | 96.6 | 7.4 |
| 3,5-DHHCA | 3-(3,5-dihydroxyphenyl)propanoic acid | 161525 | 26539-01-5 | 182.17 | 726.12 | 352.90 | 669.53 | 37.9 | 7.16 |
| 3,4-DOPAC | 2-(3,4-dihydroxyphenyl)acetic acid | 547 | 102-32-9 | 168.15 | 1928.23 | 1589.08 | 1083.38 | 51.7 | 6.99 |
| 2,5-DOPAC | 2-(2,5-dihydroxyphenyl)acetic acid | 780 | 451-13-8 | 168.15 | IDNQ | 55.2 | 2.18 | ||
| 4-HPPU | 3-(4-hydroxyphenyl)-2-oxopropanoic acid | 979 | 156-39-8 | 180.16 | 160.14 | 141.83 | 99.18 | 41.4 | 6.98 |
| 3,4-DHCA | (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | 689043 | 331-39-5 | 180.16 | 544.50 | 482.37 | 352.43 | 13.8 | 6.98 |
| 3,4-DHPLA * | 3-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid | 439435 | 23028-17-3 | 198.174 | 768.68 | 664.07 | 338.54 | 89.7 | 6.49 |
| SyA | 4-hydroxy-3,5-dimethoxybenzoic acid | 10742 | 530-57-4 | 198.17 | IDNQ | 96.6 | 6.5 | ||
| p-TYR | (2S)-2-amino-3-(4-hydroxyphenyl)propanoic acid | 6057 | 60-18-4 | 181.19 | 288.89 | 296.45 | 180.88 | 62.1 | 2.06 |
| 2,4-DHCA | (E)-3-(2,4-dihydroxyphenyl)prop-2-enoic acid | 446611 | 614-86-8 | 180.16 | IDNQ | 6.9 | 6.98 | ||
| PGN * | benzene-1,3,5-triol | 359 | 108-73-6 | 126.11 | 1275.10 | 1307.20 | 347.87 | 62.1 | 7.01 |
| 5-HIAA | 2-(5-hydroxy-1H-indol-3-yl)acetic acid | 1826 | 54-16-0 | 191.18 | 119.18 | 102.34 | 68.34 | 89.7 | 7.09 |
| ICA | 1H-indol-3-ylmethanol | 3712 | 700-06-1 | 147.17 | IDNQ | 17.2 | 3.15 | ||
| BA | benzoic acid | 243 | 65-85-0 | 122.12 | 4055.59 | 3856.55 | 1198.60 | 41.4 | 2.5 |
| L-PHE | (2S)-2-Amino-3-phenylpropanoic acid | 6140 | 63-91-2 | 165.19 | 61,352.08 | 69,824.54 | 21,694.42 | 100.0 | 6.46 |
| 3-HHA | 2-[(3-hydroxybenzoyl)amino]acetic acid | 450268 | 1637-75-8 | 195.17 | 5637.01 | 3256.17 | 5456.52 | 100.0 | 1.7 |
| 4,3-HMPPA * | 3-(4-hydroxy-3-methoxyphenyl)propanoic acid | 14340 | 1135-23-5 | 196.2 | 4647.88 | 5043.02 | 2192.95 | 20.7 | 7.3 |
| HA | 2-benzamidoacetic acid | 464 | 495-69-2 | 179.17 | 152.50 | 137.09 | 71.06 | 79.3 | 7.05 |
| 4-HPLA | 2-hydroxy-3-(4-hydroxyphenyl)propanoic acid | 9378 | 306-23-0 | 182.17 | 2047.23 | 2023.46 | 1077.68 | 100.0 | 7.15 |
| 2,3-DHHCA | 3-(2,3-dihydroxyphenyl)propanoic acid | 20 | 3714-73-6 | 182.17 | 7.68 | 6.33 | 5.99 | 31.0 | 7.14 |
| HVA | 2-(4-hydroxy-3-methoxyphenyl)acetic acid | 1738 | 306-08-1 | 182.17 | 272.91 | 273.87 | 76.17 | 100.0 | 2.07 |
| 2-HBA | 2-hydroxybenzoic acid | 338 | 69-72-7 | 138.12 | 180.54 | 47.92 | 558.66 | 72.4 | 1.7 |
| CNG * | 2-[[(E)-3-phenylprop-2-enoyl]amino]acetic acid | 709625 | 16534-24-0 | 205.21 | 23,947.50 | 35,344.27 | 20,400.68 | 93.1 | 6.5 |
| ISA (I3XS) | 1H-indol-3-yl hydrogen sulfate | 10258 | 487-94-5 | 213.21 | 13.49 | 6.15 | 17.71 | 27.6 | 4.31 |
| 3-HPAA | 2-(3-hydroxyphenyl)acetic acid | 12122 | 621-37-4 | 152.15 | 157.58 | 115.06 | 107.09 | 13.8 | 7.34 |
| 2-HPAA | 2-(2-hydroxyphenyl)acetic acid | 11970 | 614-75-5 | 152.15 | 694.04 | 525.22 | 632.64 | 24.1 | 7.3 |
| 4-HPAA | 2-(4-Hydroxyphenyl)acetic acid | 127 | 156-38-7 | 152.15 | 205,728.25 | 200,232.56 | 86,633.30 | 89.7 | 7.33 |
| IAA | 2-(1H-indol-3-yl)acetic acid | 802 | 87-51-4 | 175.18 | 1582.66 | 307.76 | 4307.78 | 41.4 | 10.82 |
| 3-HBA | 3-hydroxybenzoic acid | 7420 | 99-06-9 | 138.12 | 161,373.60 | 194,364.15 | 77,760.59 | 89.7 | 7.65 |
| 3-HCA | 3-(3-Hydroxyphenyl)propanoic acid | 637541 | 14755-02-3 | 164.16 | 154.87 | 90.62 | 156.19 | 62.1 | 6.9 |
| 4-HCA | (2E)-3-(4-Hydroxyphenyl)prop-2-enoic acid | 637542 | 7400-08-0 | 164.16 | 1234.43 | 1094.32 | 986.64 | 44.8 | 7.59 |
| 4-HBA | 4-hydroxybenzoic acid | 135 | 99-96-7 | 138.12 | 73,092.90 | 81,531.21 | 20,714.32 | 86.2 | 7.19 |
| IFA | (E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoic acid | 736186 | 25522-33-2 | 194.18 | IDNQ | 96.6 | 7.7 | ||
| PAG | 2-[(2-phenylacetyl)amino]acetic acid | 68144 | 500-98-1 | 193.2 | IDNQ | 93.1 | 43.7 | ||
| RA | (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxypropanoic acid | 5281792 | 20283-92-5 | 360.3 | IDNQ | 24.1 | 6.9 | ||
| 2-HPPA | 3-(2-hydroxyphenyl)propanoic acid | 873 | 495-78-3 | 166.17 | 24.59 | 20.70 | 9.82 | 82.8 | 7.59 |
| 2,4-HPPA * | 2-(4-hydroxyphenyl)propanoic acid | 102526 | 938-96-5 | 166.17 | 74,309.51 | 75,951.21 | 7329.52 | 100.0 | 7.6 |
| 4-HPPA * | 3-(4-hydroxyphenyl)propanoic acid | 10394 | 501-97-3 | 166.17 | 10,635.33 | 10,773.43 | 888.97 | 100.0 | 7.84 |
| 3-HPPA | 3-(3-hydroxyphenyl)propanoic acid | 91 | 621-54-5 | 166.17 | 5044.83 | 4841.94 | 1227.72 | 100.0 | 7.18 |
| PEA-HCl | 2-phenylethanamine;hydrochloride | 9075 | 156-28-5 | 157.64 | 35.39 | 29.68 | 20.03 | 13.8 | 7.84 |
| 3-IACrA | (E)-3-(1H-indol-3-yl)prop-2-enoic acid | 15030923 | 29953-71-7 | 187.19 | 721.67 | 570.28 | 617.71 | 34.5 | 7.3 |
| PCS | (4-methylphenyl) hydrogen sulfate | 4615423 | 3233-58-7 | 188.2 | 302.30 | 266.79 | 255.42 | 27.6 | 7/7 |
| I3PA | 3-(1H-indol-3-yl)propanoic acid | 3744 | 830-96-6 | 189.21 | 217.42 | 212.92 | 35.39 | 17.2 | 3.79 |
| 3,5-DHBA | 3,5-dihydroxybenzoic acid | 7424 | 99-10-5 | 154.12 | IDNQ | 100.0 | 8.27 | ||
| 4-EPS * | (4-ethylphenyl) hydrogen sulfate | 20822573 | 85734-98-1 | 202.23 | 76.45 | 81.51 | 46.05 | 44.8 | 7.28 |
| N-UNDG * | 2-(undecanoylamino)acetic acid | 454092 | 83871-09-4 | 243.34 | 185.89 | 181.04 | 53.80 | 34.5 | 10.49 |
| Compound | V | Wilcoxon p | FDR | % Non-Zero Entries |
|---|---|---|---|---|
| 4-HCA | 47 | 0.010482 | 0.48178 | 44.8% |
| 3-OHBA | 159 | 0.017844 | 0.48178 | 89.7% |
| PHE(+) | 57 | 0.036742 | 0.66136 | 100% |
| 4-HBA | 147 | 0.069659 | 0.75231 | 86.2% |
| 3-HPPA | 147 | 0.069659 | 0.75231 | 100% |
| 3-HHA | 67 | 0.10231 | 0.78924 | 100% |
| PAG | 67 | 0.10231 | 0.78924 | 93.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaskiw, G.E.; Obrenovich, M.E.; Donskey, C.J.; Briggs, F.B.S.; Chung, S.S.; Kalinina, A.I.; Bolomey, A.; Hayes, L.N.; Yang, K.; Yolken, R.H.; et al. Targeted and Non-Targeted Metabolomic Evaluation of Cerebrospinal Fluid in Early Phase Schizophrenia: A Pilot Study from the Hopkins First Episode Psychosis Project. Metabolites 2025, 15, 275. https://doi.org/10.3390/metabo15040275
Jaskiw GE, Obrenovich ME, Donskey CJ, Briggs FBS, Chung SS, Kalinina AI, Bolomey A, Hayes LN, Yang K, Yolken RH, et al. Targeted and Non-Targeted Metabolomic Evaluation of Cerebrospinal Fluid in Early Phase Schizophrenia: A Pilot Study from the Hopkins First Episode Psychosis Project. Metabolites. 2025; 15(4):275. https://doi.org/10.3390/metabo15040275
Chicago/Turabian StyleJaskiw, George E., Mark E. Obrenovich, Curtis J. Donskey, Farren B. S. Briggs, Sun Sunnie Chung, Anastasiya I. Kalinina, Austin Bolomey, Lindsay N. Hayes, Kun Yang, Robert H. Yolken, and et al. 2025. "Targeted and Non-Targeted Metabolomic Evaluation of Cerebrospinal Fluid in Early Phase Schizophrenia: A Pilot Study from the Hopkins First Episode Psychosis Project" Metabolites 15, no. 4: 275. https://doi.org/10.3390/metabo15040275
APA StyleJaskiw, G. E., Obrenovich, M. E., Donskey, C. J., Briggs, F. B. S., Chung, S. S., Kalinina, A. I., Bolomey, A., Hayes, L. N., Yang, K., Yolken, R. H., & Sawa, A. (2025). Targeted and Non-Targeted Metabolomic Evaluation of Cerebrospinal Fluid in Early Phase Schizophrenia: A Pilot Study from the Hopkins First Episode Psychosis Project. Metabolites, 15(4), 275. https://doi.org/10.3390/metabo15040275






