Recent Advances in Pretreatment Methods and Detection Techniques for Veterinary Drug Residues in Animal-Derived Foods
Abstract
1. Introduction
2. Pretreatment Methods
2.1. Liquid–Liquid Extraction (LLE) Technology
2.2. SPE
2.3. Immunoaffinity Chromatography (IAC)
2.4. QuEChERS
2.5. Molecular Imprinting Technology (MIT)
3. Chromatographic Detection Techniques
3.1. GC-MS
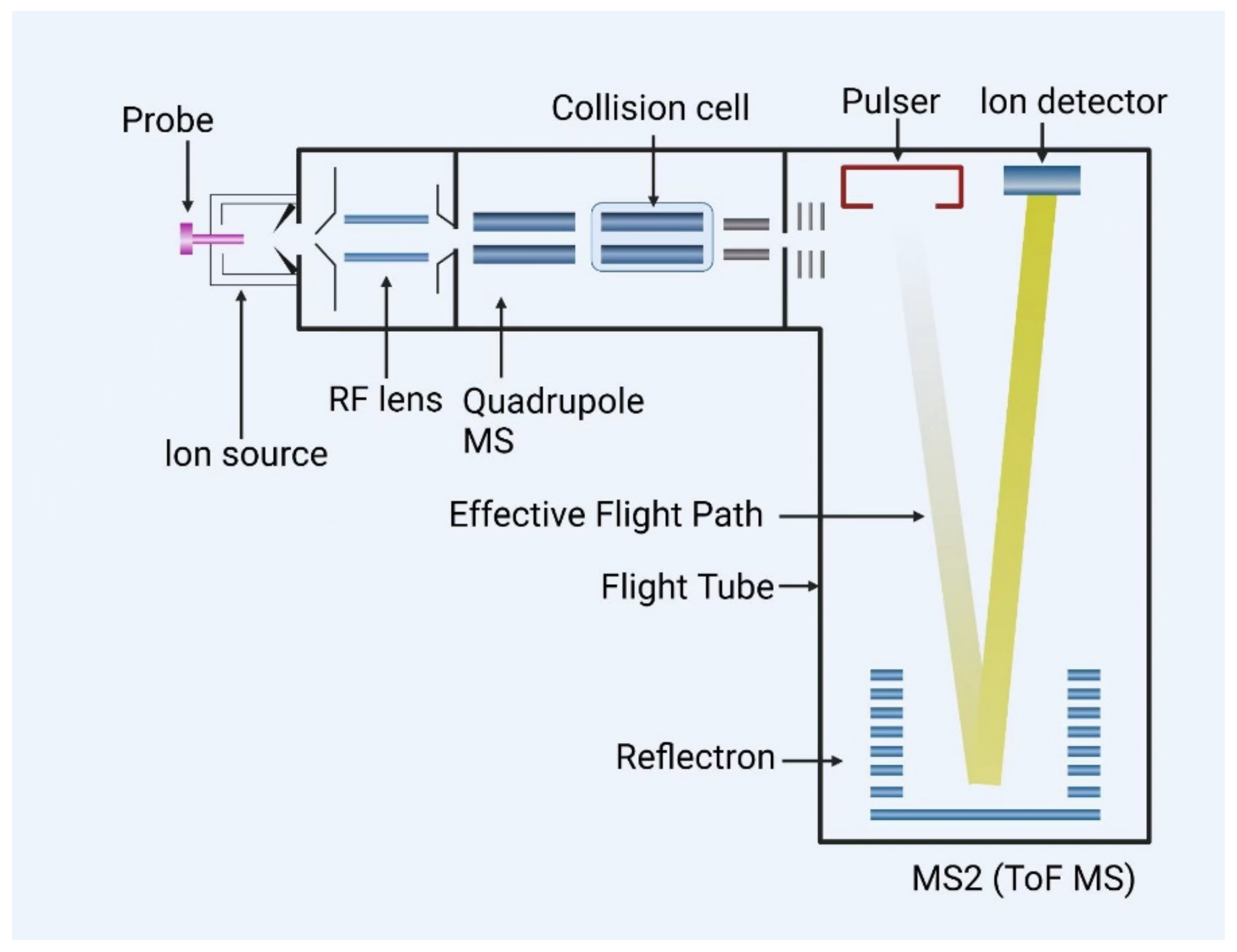
3.2. Liquid Chromatography Quadrupole-Time-of-Flight Mass Spectrometry (LC-QTOF-MS)
3.3. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
3.4. Liquid Chromatography Coupled to Ion Trap Mass Spectrometry (LC-IT-MS)
3.5. CE-MS
4. Rapid Detection Techniques
4.1. Immunoassay Analysis Techniques
4.1.1. GICA
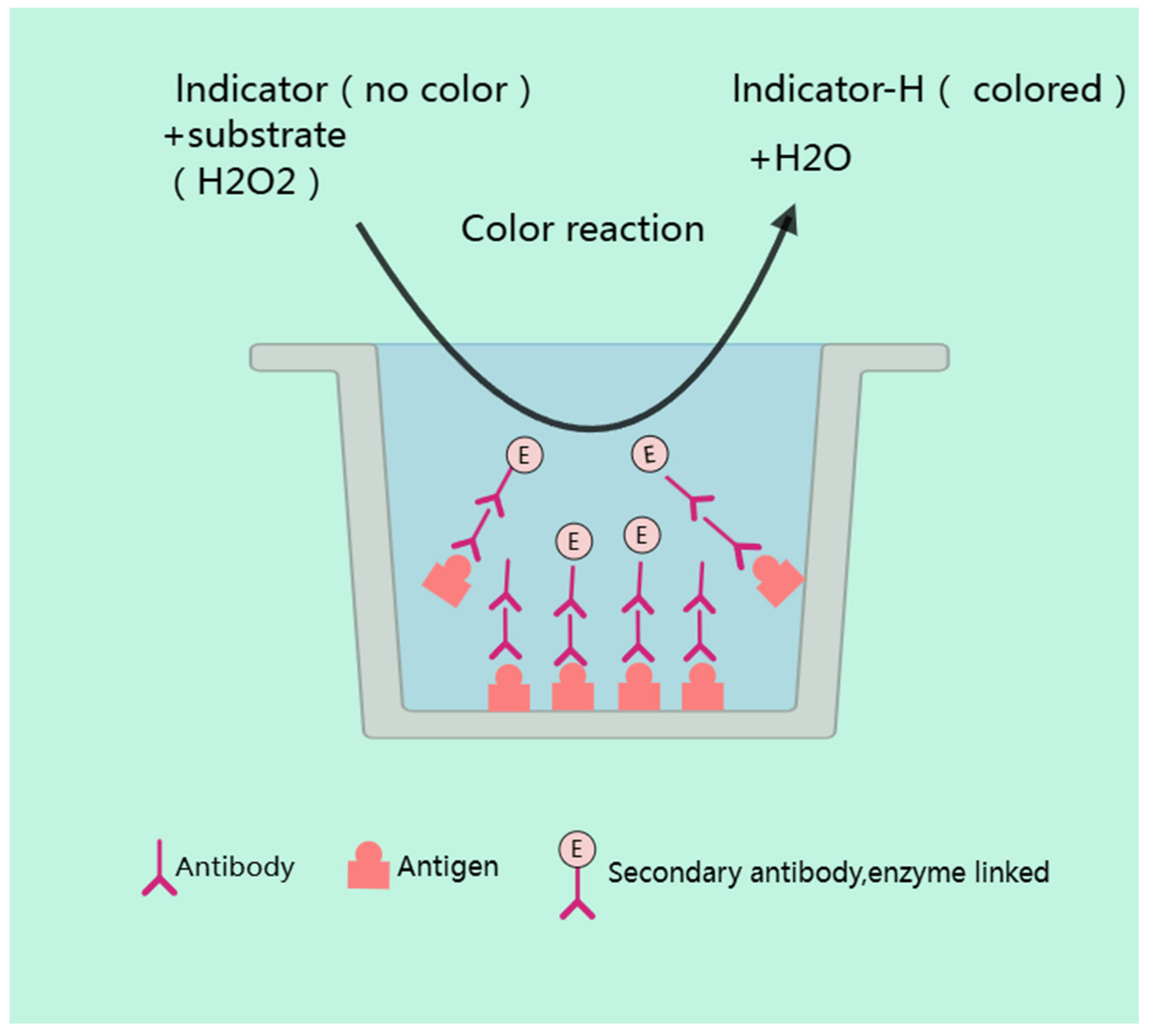
4.1.2. ELISA
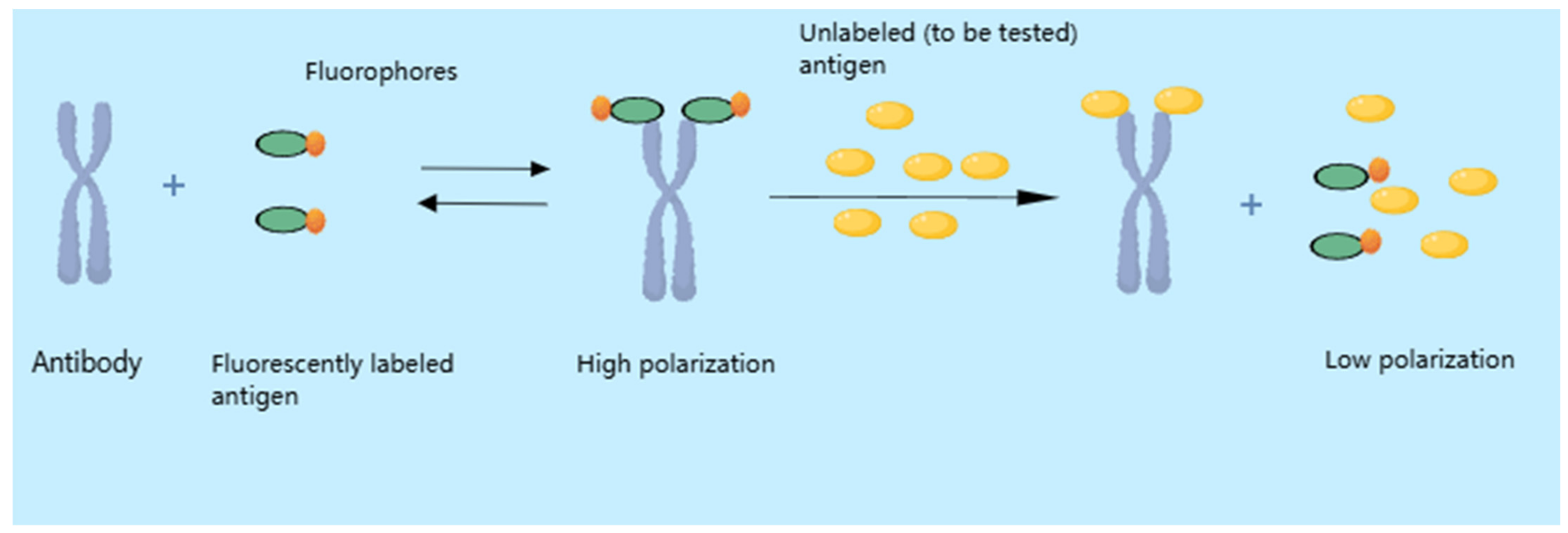
4.2. Fluorescence Polarization Immunoassay (FPIA)
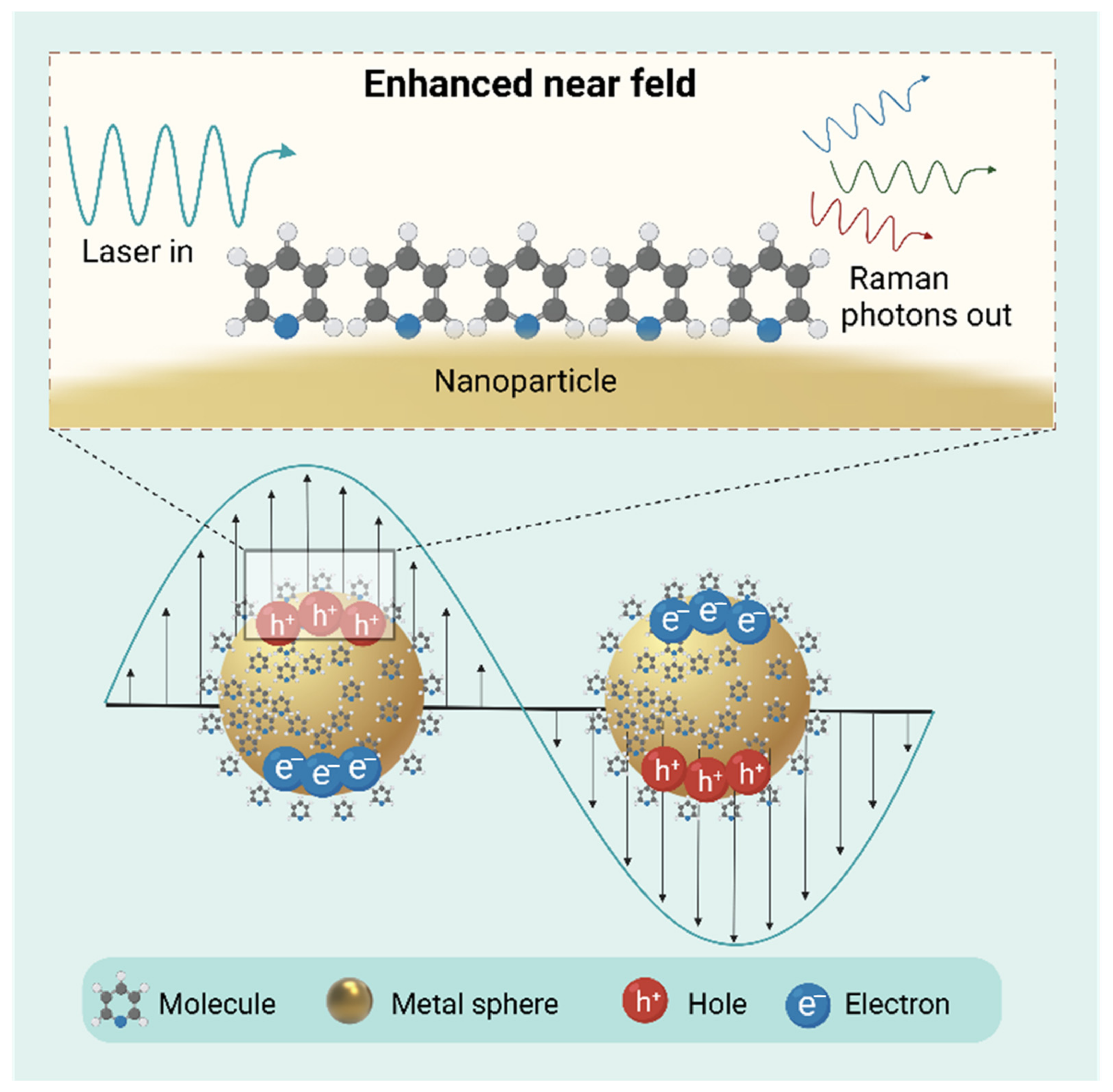
4.3. Surface-Enhanced Raman Scattering (SERS)
5. Comparative Analysis of Merits and Limitations in Contemporary Veterinary Drug Residue Analytical Techniques for Animal-Derived Food Products
6. Conclusions and Further Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Xie, K.; Lee, K. Veterinary drug residues in animal-derived foods: Sample preparation and analytical methods. Foods 2021, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Shikoray, L.; Mohamed, M.I.; Habib, I.; Matsumoto, T. Veterinary drug residues in the food chain as an emerging public health threat: Sources, analytical methods, health impacts, and preventive measures. Foods 2024, 13, 1629. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, R.; Ramadhanti, S.P.; Amatulloh, A.; Megantara, S.; Subra, L. Recent advances in the determination of veterinary drug residues in food. Foods 2023, 12, 3422. [Google Scholar] [CrossRef]
- Mesfin, Y.M.; Mitiku, B.A.; Tamrat Admasu, H. Veterinary drug residues in food products of animal origin and their public health consequences: A review. Vet. Med. Sci. 2024, 10, e70049. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Q.; Niu, B. Risk assessment of veterinary drug residues in meat products. Curr. Drug Metab. 2020, 21, 779–789. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Parmar, J.K.; Chaubey, K.K.; Gupta, V.; Bharath, M.N. Assessment of various veterinary drug residues in animal originated food products. Vet. World 2021, 14, 1650–1664. [Google Scholar] [CrossRef] [PubMed]
- Colopi, A.; Guida, E.; Cacciotti, S.; Fuda, S.; Lampitto, M.; Onorato, A.; Zucchi, A.; Balistreri, C.R.; Grimaldi, P.; Barchi, M. Dietary exposure to pesticide and veterinary drug residues and their effects on human fertility and embryo development: A global overview. Int. J. Mol. Sci. 2024, 25, 9116. [Google Scholar] [CrossRef]
- Chicoine, A.; Erdely, H.; Fattori, V.; Finnah, A.; Fletcher, S.; Lipp, M.; Sanders, P.; Scheid, S. Assessment of veterinary drug residues in food: Considerations when dealing with sub-optimal data. Regul. Toxicol. Pharmacol. 2020, 118, 104806. [Google Scholar] [CrossRef]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing veterinary drug residues in animal products: A review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef]
- Atta, A.H.; Atta, S.A.; Nasr, S.M.; Mouneir, S.M. Current perspective on veterinary drug and chemical residues in food of animal origin. Environ. Sci. Pollut. Res. Int. 2022, 29, 15282–15302. [Google Scholar] [CrossRef]
- Lozano, A.; Hernando, M.D.; Ucles, S.; Hakme, E.; Fernandez-Alba, A.R. Identification and measurement of veterinary drug residues in beehive products. Food Chem. 2019, 274, 61–70. [Google Scholar] [CrossRef]
- Xia, S.; Niu, B.; Chen, J.; Deng, X.; Chen, Q. Risk analysis of veterinary drug residues in aquatic products in the Yangtze river delta of China. J. Food Prot. 2021, 84, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tian, H.; Yang, F.; Fan, S.; Zhang, J.; Ma, J.; Ai, L.; Zhang, Y. Rapid determination of 103 common veterinary drug residues in milk and dairy products by ultra performance liquid chromatography tandem mass spectrometry. Front. Nutr. 2022, 9, 879518. [Google Scholar] [CrossRef]
- Li, F.; Lv, H.; Zhu, F.; Zhang, Q.; Xu, Q.; Ji, W. High throughput detection of veterinary drug residues in chicken and eggs. Food Chem. 2025, 463, 141267. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lee, D.J.; Jo, A.; Yun, S.H.; Eun, J.B.; Im, M.H.; Shim, J.H.; Abd El-Aty, A.M. Onsite/on-field analysis of pesticide and veterinary drug residues by a state-of-art technology: A review. J. Sep. Sci. 2021, 44, 2310–2327. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Liu, J.; Li, Y.; Yin, Z.; Zhang, Y.; Pi, F.; Sun, X. Sensitive techniques for poct sensing on the residues of pesticides and veterinary drugs in food. Bull. Environ. Contam. Toxicol. 2021, 107, 206–214. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X. Nanostructure-based surface-enhanced raman spectroscopy techniques for pesticide and veterinary drug residues screening. Bull. Environ. Contam. Toxicol. 2021, 107, 194–205. [Google Scholar] [CrossRef]
- Zhang, B.; Lang, Y.; Guo, B.; Cao, Z.; Cheng, J.; Cai, D.; Shentu, X.; Yu, X. Indirect competitive enzyme-linked immunosorbent assay based on broad-spectrum antibody for simultaneous determination of thirteen fluoroquinolone antibiotics in Rana catesbeianus. Foods 2023, 12, 2530. [Google Scholar] [CrossRef]
- Park, D.; Choi, Y.S.; Kim, J.Y.; Choi, J.D.; Moon, G.I. Determination of flunixin and 5-hydroxy flunixin residues in livestock and fishery products using liquid chromatography-tandem mass spectrometry (lc-ms/ms). Food Sci. Anim. Resour. 2024, 44, 873–884. [Google Scholar] [CrossRef]
- Gens, K.D.; Singer, R.S.; Dilworth, T.J.; Heil, E.L.; Beaudoin, A.L. Antimicrobials in animal agriculture in the united states: A multidisciplinary overview of regulation and utilization to foster collaboration: On behalf of the society of infectious diseases pharmacists. Open Forum Infect. Dis. 2022, 9, ofac542. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Wang, F.; He, P. Recent advances in immunoassays and biosensors for mycotoxins detection in feedstuffs and foods. J. Anim. Sci. Biotechnol. 2021, 12, 108. [Google Scholar] [CrossRef]
- Park, H.; Kim, E.; Lee, T.H.; Park, S.; Choi, J.D.; Moon, G. Multiclass method for the determination of anthelmintic and antiprotozoal drugs in livestock products by ultra-high-performance liquid chromatography-tandem mass spectrometry. Food Sci. Anim. Resour. 2023, 43, 914–937. [Google Scholar] [CrossRef]
- Beguiristain, I.; Alongi, A.; Rubies, A.; Granados, M. Analysis of corticosteroids in samples of animal origin using QuEChERS and ultrahigh-performance liquid chromatography coupled to high-resolution mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 449–457. [Google Scholar] [CrossRef]
- Okerman, L.; Noppe, H.; Cornet, V.; De Zutter, L. Microbiological detection of 10 quinolone antibiotic residues and its application to artificially contaminated poultry samples. Food Addit. Contam. 2007, 24, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Liao, G.Q.; Chen, L.; Qian, Y.Z.; Yan, X.; Qiu, J. Pesticide residues in animal-derived food: Current state and perspectives. Food Chem. 2024, 438, 137974. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhao, Z.; Lv, Y.K.; Shen, S.; Liang, S.X. Recent advances in porous organic frameworks for sample pretreatment of pesticide and veterinary drug residues: A review. Analyst 2021, 146, 7394–7417. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; E, Z.; Zhai, F.; Bing, X. Rapid multi-residue detection methods for pesticides and veterinary drugs. Molecules 2020, 25, 3590. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Peng, Y.; Liu, J. Nanomaterial-based fluorescent biosensors for veterinary drug detection in foods. J. Food Drug Anal. 2020, 28, 575–594. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, B. Liquid-liquid extraction (lle). In Separation and Purification Technologies in Biorefineries; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 61–78. [Google Scholar]
- Tshepho, R.; Dube, S.; Nindi, M.M. Ionic liquid-based dispersive liquid-liquid microextraction of anthelmintic drug residues in small-stock meat followed by LC-ESI-MS/MS detection. Food Sci. Nutr. 2023, 11, 6288–6302. [Google Scholar] [CrossRef]
- Araujo, P.; Iqbal, S.; Arno, A.; Espe, M.; Holen, E. Validation of a liquid-liquid extraction method to study the temporal production of d-series resolvins by head kidney cells from atlantic salmon (Salmon salar) exposed to docosahexaenoic acid. Molecules 2023, 28, 4728. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xie, Y.; Chen, Z.; Cao, M.; Lei, X.; Le, T. A comprehensive review on the pretreatment and detection methods of nitrofurans and their metabolites in animal-derived food and environmental samples. Food Chem. X 2024, 24, 101928. [Google Scholar] [CrossRef] [PubMed]
- Doria, S.; Yost, J.; Gagnon, Z. Free-flow biomolecular concentration and separation of proteins and nucleic acids using teíchophoresis. Talanta 2023, 255, 124198. [Google Scholar] [CrossRef]
- Kannouma, R.E.; Hammad, M.A.; Kamal, A.H.; Mansour, F.R. Miniaturization of liquid-liquid extraction; the barriers and the enablers. Microchem. J. 2022, 182, 107863. [Google Scholar] [CrossRef]
- Faraji, H. Advancements in overcoming challenges in dispersive liquid-liquid microextraction: An overview of advanced strategies. TrAC Trends Anal. Chem. 2024, 170, 117429. [Google Scholar] [CrossRef]
- Cantwell, F.F.; Losier, M. Chapter 11 Liquid—Liquid extraction. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 37, pp. 297–340. [Google Scholar]
- Sun, Q.; Zhang, S.; Chen, Q.; Cao, L. Salting-out assisted liquid-liquid extraction for the simple and rapid determination of veterinary antibiotic residues in aquatic products by HPLC-MS/MS. Food Chem. 2024, 460, 140775. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Ji, X.; Wu, H.; Yang, H.; Zhang, H.; Zhang, X.; Li, Z.; Ni, X.; Qian, M. Determination of veterinary drug/pesticide residues in livestock and poultry excrement using selective accelerated solvent extraction and magnetic material purification combined with ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1617, 460808. [Google Scholar] [CrossRef]
- Pang, M.; Xu, J.; Tang, Y.; Guo, Y.; Ding, H.; Wang, R.; Zhang, T.; Zhang, G.; Guo, X.; Dai, G.; et al. Combining gc-ms/mS with a lle-spe sample pretreatment step to simultaneously analyse enrofloxacin and ofloxacin residues in chicken tissues and pork. Food Chem. 2024, 456, 139972. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, X.; Su, S.; Guo, Z.; Wang, J.; Ding, L.; Liu, Y.; Zhu, J. A multi-class, multi-residue method for detection of veterinary drugs in multiple meat using a pass-through cleanup spe technique and uplc-ms/mS analysis. Food Anal. Methods 2018, 11, 2865–2884. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Cheng, R.; Mao, X.; Yu, J.; Liu, F.; Guo, L.; Luo, D.; Wan, Y. A dispersive solid-phase extraction method for the determination of Aristolochic acids in Houttuynia cordata based on MIL-101(Fe): An analytes-oriented adsorbent selection design. Food Chem. 2023, 407, 135074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Okoli, C.P. Development of a solid-phase extraction method based on biocompatible starch polyurethane polymers for gc-ms analysis of polybrominated diphenyl ethers in ambient water samples. Molecules 2022, 27, 3253. [Google Scholar] [CrossRef]
- Islas, G.; Ibarra, I.S.; Hernandez, P.; Miranda, J.M.; Cepeda, A. Dispersive solid phase extraction for the analysis of veterinary drugs applied to food samples: A review. Int. J. Anal. Chem. 2017, 2017, 8215271. [Google Scholar] [CrossRef] [PubMed]
- Brandi, J.; Siragusa, G.; Robotti, E.; Marengo, E.; Cecconi, D. Analysis of veterinary drugs and pesticides in food using liquid chromatography-mass spectrometry. TrAC Trends Anal. Chem. 2024, 179, 117888. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Shen, D.; Mao, J.; Cao, Y.; Zhang, K.; Peng, J.; Dong, F.; Wang, N.; He, K. A one-step solid-phase extraction with uhplc-ms/ms for fast and accurate determination of multi-class veterinary drugs in animal muscles. Food Chem. 2023, 428, 136712. [Google Scholar] [CrossRef]
- Mehl, A.; Hudel, L.; Bucker, M.; Morlock, G.E. Validated screening method for 81 multiclass veterinary drug residues in food via online-coupling high-throughput planar solid-phase extraction to high-performance liquid chromatography-orbitrap tandem mass spectrometry. J. Agric. Food Chem. 2022, 70, 10886–10898. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Lopez de Alda, M.J.; Barceló, D. Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography–mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J. Chromatogr. A 2007, 1152, 97–115. [Google Scholar] [CrossRef]
- Talib, N.; Mohd-Setapar, S.; Khamis, A. The benefits and limitations of methods development in solid phase extraction: Mini review. J. Teknol. (Sci. Eng.) 2014, 69, 69–72. [Google Scholar] [CrossRef]
- Zheng, M.; Tang, S.; Bao, Y.; Daniels, K.D.; How, Z.T.; El-Din, M.G.; Wang, J.; Tang, L. Fully-automated spe coupled to uhplc-ms/ms method for multiresidue analysis of 26 trace antibiotics in environmental waters: SPE optimization and method validation. Environ. Sci. Pollut. Res. Int. 2022, 29, 16973–16987. [Google Scholar] [CrossRef]
- Sharmeen, S.; Suh, K.; Kyei, I.; Jones, J.; Olupathage, H.; Campbell, A.; Hage, D.S. Immunoaffinity chromatography for protein purification and analysis. Curr. Protoc. 2023, 3, e867. [Google Scholar] [CrossRef]
- Ren, J.; Xiong, H.; Huang, C.; Ji, F.; Jia, L. An engineered peptide tag-specific nanobody for immunoaffinity chromatography application enabling efficient product recovery at mild conditions. J. Chromatogr. A 2022, 1676, 463274. [Google Scholar] [CrossRef] [PubMed]
- Poddar, S.; Sharmeen, S.; Hage, D.S. Affinity monolith chromatography: A review of general principles and recent developments. Electrophoresis 2021, 42, 2577–2598. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Leonard, P.; Darcy, E.; Sharma, S.; O’Kennedy, R. Immunoaffinity chromatography: Concepts and applications. Methods Mol. Biol. 2017, 1485, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A. Immunoaffinity chromatography. Mol. Biotechnol. 2002, 20, 41–47. [Google Scholar] [CrossRef]
- Moser, A.C.; Hage, D.S. Immunoaffinity chromatography: An introduction to applications and recent developments. Bioanalysis 2010, 2, 769–790. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, X.; Wang, Y.; Pan, Y.; Liu, Z.; Chen, D.; Sheng, F.; Yuan, Z. An immunoaffinity column for the selective purification of 3-methyl-quinoxaline-2-carboxylic acid from swine tissues and its determination by high-performance liquid chromatography with ultraviolet detection and a colloidal gold-based immunochromatographic assay. Food Chem. 2017, 237, 290–296. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Xi, C.; Wang, G.; Chen, D.; Ding, S. Determination of chloramphenicol and zeranols in pig muscle by immunoaffinity column clean-up and LC-MS/MS analysis. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1177–1186. [Google Scholar] [CrossRef]
- Nelson, M.A.; Reiter, W.S.; Hage, D.S. Chromatographic competitive binding immunoassays: A comparison of the sequential and simultaneous injection methods. Biomed. Chromatogr. 2003, 17, 188–200. [Google Scholar] [CrossRef]
- Thompson, N.E.; Burgess, R.R. Immunoaffinity chromatography: Advantages and limitations. In Analytical Separation Science; Wiley: Hoboken, NJ, USA, 2015; pp. 483–502. [Google Scholar] [CrossRef]
- Kavianpour, A.; Ashjari, M.; Hosseini, S.N.; Khatami, M. Quantitative assessment of LPS-HBsAg interaction by introducing a novel application of immunoaffinity chromatography. Prep. Biochem. Biotechnol. 2023, 53, 672–682. [Google Scholar] [CrossRef]
- Gonzalez-Curbelo, M.A.; Varela-Martinez, D.A.; Riano-Herrera, D.A. Pesticide-residue analysis in soils by the QuEChERS method: A Review. Molecules 2022, 27, 4323. [Google Scholar] [CrossRef]
- Kim, K.; Choi, Y.; Mok, S.; Moon, H.B.; Jeon, J. Optimization of the QuEChERS method for multi-residue analysis of pharmaceuticals and pesticides in aquaculture products. Food Chem. 2023, 399, 133958. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, J.; Tang, Y.; Kong, C.; Li, S.; Wang, S.; Ding, S.; Gu, L.; Shen, X.; Martin, A.A.; et al. Development and validation of rapid screening of 192 veterinary drug residues in aquatic products using HPLC-HRMS coupled with QuEChERS. Food Chem. X 2024, 22, 101504. [Google Scholar] [CrossRef]
- Wang, H.; Tian, H.; Ai, L.F.; Liang, S.X. Screening and quantification of 146 veterinary drug residues in beef and chicken using QuEChERS combined with high performance liquid chromatography-quadrupole orbitrap mass spectrometry. Food Chem. 2023, 408, 135207. [Google Scholar] [CrossRef]
- Mu, P.; Xu, N.; Chai, T.; Jia, Q.; Yin, Z.; Yang, S.; Qian, Y.; Qiu, J. Simultaneous determination of 14 antiviral drugs and relevant metabolites in chicken muscle by uplc-ms/ms after QuEChERS preparation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1023–1024, 17–23. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Assessment and validation of the p-QuEChERS sample preparation methodology for the analysis of >200 veterinary drugs in various animal-based food matrices. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2023, 40, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, J.; Yu, H.; Feng, R.; Zhang, J.; Zhou, H.; Ji, S. Simultaneous Screening of 172 Veterinary drugs by modified QuEChERS-LC-MS/MS in TCM galli gigerii endothelium corneum. J. Chromatogr. Sci. 2024, 62, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, J.; Gou, Y.; Chen, T.; Shen, X.; Wang, T.; Li, Y.; He, H.; Deng, H.; Hua, Y. Determination of veterinary drugs in foods of animal origin by QuEChERS coupled with ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). J. Chromatogr. A 2025, 1744, 465726. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Ye, K.; Xu, S.; Zhou, Q.; Wang, S.; Xu, Z.; Liu, Z. Advances in molecular imprinting technology for the extraction and detection of quercetin in plants. Polymers 2023, 15, 2107. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Z.; Liu, Z. Paper-based molecular-imprinting technology and its application. Biosensors 2022, 12, 595. [Google Scholar] [CrossRef]
- Song, X.; Zhou, T.; Li, J.; Su, Y.; Xie, J.; He, L. Determination of macrolide antibiotics residues in pork using molecularly imprinted dispersive solid-phase extraction coupled with LC-MS/MS. J. Sep. Sci. 2018, 41, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J.; Shi, S.; Hu, S.; Yuan, L. Determination of beta-agonist residues in animal-derived food by a liquid chromatography-tandem mass spectrometric method combined with molecularly imprinted stir bar sorptive extraction. J. Anal. Methods Chem. 2018, 2018, 9053561. [Google Scholar] [CrossRef]
- Elfadil, D.; Lamaoui, A.; Della Pelle, F.; Amine, A.; Compagnone, D. Molecularly Imprinted polymers combined with electrochemical sensors for food contaminants analysis. Molecules 2021, 26, 4607. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Xu, L.; Ullah, S.; Li, J.; Nie, J.; Ping, J.; Ying, Y. Advancements and greenification potential of magnetic molecularly imprinted polymers for chromatographic analysis of veterinary drug residues in milk. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13399. [Google Scholar] [CrossRef]
- Tang, Y.; Meng, H.; Wang, W.; Song, Y.; Wang, S.; Li, Z.; Wang, X.; Hu, X. Off-line magnetic Fe3O4@SiO2@MIPs-based solid phase dispersion extraction coupling with HPLC for the simultaneous determination of olaquindox and its metabolite in fish muscle and milk samples. Food Chem. X 2023, 17, 100611. [Google Scholar] [CrossRef]
- Norman, R.L.; Singh, R.; Muskett, F.W.; Parrott, E.L.; Rufini, A.; Langridge, J.I.; Runau, F.; Dennison, A.; Shaw, J.A.; Piletska, E.; et al. Mass spectrometric detection of KRAS protein mutations using molecular imprinting. Nanoscale 2021, 13, 20401–20411. [Google Scholar] [CrossRef]
- Li, D.; Luo, K.; Zhang, L.; Gao, J.; Liang, J.; Li, J.; Pan, H. Research and application of highly selective molecular imprinting technology in chiral separation analysis. Crit. Rev. Anal. Chem. 2023, 53, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, S.A.; Hamidi, S.; Siahi-Shadbad, M.R. Application of liquid-liquid extraction for the determination of antibiotics in the foodstuff: Recent trends and developments. Crit. Rev. Anal. Chem. 2022, 52, 327–342. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.Y.; Wang, X.D.; Lv, Y.F.; Wei, N. Application of QuEChERS for analysis of contaminants in dairy products: A review. J. Food Prot. 2025, 88, 100453. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, J.; Cao, Y.; Yao, T.; Wang, S.; Liu, J.; Suo, D.; Tian, J.; Jia, Z.; Li, Y.; et al. Quick and high-throughput quantification of 22 beta-agonists residues in animal-derived foods using enzymatic probe sonication. Food Chem. 2023, 408, 135262. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, H.; Zhang, Y. Recent progress on the detection of animal-derived food stimulants using mass spectrometry-based techniques. Front. Nutr. 2023, 10, 1226530. [Google Scholar] [CrossRef] [PubMed]
- Hajrulai-Musliu, Z.; Uzunov, R.; Jovanov, S.; Musliu, D.; Dimitrieska-Stojkovikj, E.; Stojanovska-Dimzoska, B.; Angeleska, A.; Stojkovski, V.; Sasanya, J.J. Multi-class/residue method for determination of veterinary drug residues, mycotoxins and pesticide in urine using LC-MS/MS technique. BMC Vet. Res. 2023, 19, 156. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Zhai, J.; Guo, J.; Li, Q.; Wang, C.F.; Chen, S. Microfluidic fluorescent platform for rapid and visual detection of veterinary drugs. RSC Adv. 2022, 12, 8485–8491. [Google Scholar] [CrossRef]
- Mondello, L.; Cordero, C.; Janssen, H.-G.; Synovec, R.E.; Zoccali, M.; Tranchida, P.Q. Comprehensive two-dimensional gas chromatography–mass spectrometry. Nat. Rev. Methods Primers 2025, 5, 7. [Google Scholar] [CrossRef]
- Brettell, T.A.; Lum, B.J. Analysis of drugs of abuse by gas chromatography-mass spectrometry (gc-ms). Methods Mol. Biol. 2018, 1810, 29–42. [Google Scholar] [CrossRef]
- Gruber, B.; David, F.; Sandra, P. Capillary gas chromatography-mass spectrometry: Current trends and perspectives. TrAC Trends Anal. Chem. 2020, 124, 115475. [Google Scholar] [CrossRef]
- Welke, J.E.; Hernandes, K.C.; Lago, L.O.; Silveira, R.D.; Marques, A.T.B.; Zini, C.A. Flavoromic analysis of wines using gas chromatography, mass spectrometry and sensory techniques. J. Chromatogr. A 2024, 1734, 465264. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Y.; Lu, Y.; Guo, Y.; Xie, K.; Guan, F.; Gao, P.; Zhu, Y.; Dong, Y.; Zhang, T.; et al. Qualitative and quantitative determination of decoquinate in chicken tissues by gas chromatography tandem mass spectrometry. Molecules 2023, 28, 3875. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Xie, X.; Diao, Z.; Xie, K.; Zhang, G.; Zhang, T.; Dai, G. Quantitative analysis of spectinomycin and lincomycin in poultry eggs by accelerated solvent extraction coupled with gas chromatography tandem mass spectrometry. Foods 2020, 9, 651. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, X.; Diao, Z.; Wang, Y.; Wang, B.; Xie, K.; Wang, X.; Zhang, P. Detection and determination of spectinomycin and lincomycin in poultry muscles and pork by ASE-SPE-GC–MS/MS. J. Food Compos. Anal. 2021, 101, 103979. [Google Scholar] [CrossRef]
- Yu, H.; Tao, Y.; Le, T.; Chen, D.; Ishsan, A.; Liu, Y.; Wang, Y.; Yuan, Z. Simultaneous determination of amitraz and its metabolite residue in food animal tissues by gas chromatography-electron capture detector and gas chromatography-mass spectrometry with accelerated solvent extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-J.; Schultz, A.W.; Wang, J.; Johnson, C.H.; Yannone, S.M.; Patti, G.J.; Siuzdak, G. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the metlin database. Nat. Protoc. 2013, 8, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Glish, G.L.; Burinsky, D.J. Hybrid mass spectrometers for tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2008, 19, 161–172. [Google Scholar] [CrossRef]
- Chernushevich, I.V.; Loboda, A.V.; Thomson, B.A. An introduction to quadrupole-time-of-flight mass spectrometry. J. Mass Spectrom. JMS 2001, 36, 849–865. [Google Scholar] [CrossRef]
- Allen, D.R.; McWhinney, B.C. Quadrupole Time-of-Flight Mass Spectrometry: A paradigm shift in toxicology screening applications. Clin. Biochem. Rev. 2019, 40, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Du, M.; Wei, K.; Dai, C.; Yang, R.Y.H.; Zhou, B.; Luo, Z.; Yang, X.; Yu, Y.; Lin, W.; et al. Study of Xuanhuang Pill in protecting against alcohol liver disease using ultra-performance liquid chromatography/time-of-flight mass spectrometry and network pharmacology. Front. Endocrinol. 2023, 14, 1175985. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, Q.; Li, J.; Qiu, G.; Wu, F.; Zhu, R.; Wang, X.; Su, M. A liquid chromatography-time-of-flight/mass spectrometry method for analysis of pesticides and transfer behavior in Radix Codonopsis and Angelica sinensis decoctions. Anal. Methods 2023, 15, 2121–2131. [Google Scholar] [CrossRef]
- Li, X.; Chi, Q.; Xia, S.; Pan, Y.; Chen, Y.; Wang, K. Untargeted multi-residue method for the simultaneous determination of 141 veterinary drugs and their metabolites in pork by high-performance liquid chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2020, 1634, 461671. [Google Scholar] [CrossRef] [PubMed]
- Vardali, S.C.; Samanidou, V.F.; Kotzamanis, Y.P. Development and validation of an ultra performance liquid chromatography-quadrupole time of flight-mass spectrometry (in MS(E) mode) method for the quantitative determination of 20 antimicrobial residues in edible muscle tissue of European sea bass. J. Chromatogr. A 2018, 1575, 40–48. [Google Scholar] [CrossRef]
- Choi, B.S.; Lee, D.U.; Kim, W.S.; Park, C.W.; Choe, W.J.; Moon, M.J. Simultaneous screening of 322 residual pesticides in fruits and vegetables using gc-ms/ms and deterministic health risk assessments. Foods 2023, 12, 3001. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Braga, P.A.C.; Reyes, F.G.R.; Bragotto, A.P.A. Exploring miniaturized sample preparation approaches combined with LC-QToF-MS for the analysis of sulfonamide antibiotic residues in meat- and/or egg-based baby foods. Food Chem. 2022, 366, 130587. [Google Scholar] [CrossRef] [PubMed]
- Stachniuk, A.; Kozub, A.; Czeczko, R.; Montowska, M.; Fornal, E. LC-QTOF-MS evaluation of rabbit-specific peptide markers for meat quantitation. J. Food Drug Anal. 2022, 30, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, S.B.; Akram, A.; Musharraf, S.G.; Wajidi, M.; Tabassum, N.; Nazir, N.; Shah, S.M.Z. The lc-qtof-ms/ms analysis of acid degradation products of rifaximin, an antibiotic. MethodsX 2022, 9, 101735. [Google Scholar] [CrossRef]
- Zhang, M.; Li, E.; Su, Y.; Zhang, Y.; Xie, J.; He, L. Quick Multi-Class Determination of Residues of Antimicrobial Veterinary Drugs in Animal Muscle by LC-MS/MS. Molecules 2018, 23, 1736. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Kanda, M.; Yoshikawa, S.; Nakajima, T.; Hayashi, H.; Matsushima, Y.; Ohba, Y.; Koike, H.; Nagano, C.; Otsuka, K.; et al. Single-laboratory Validation Study and Surveillance Using an Improved Multiresidue Analytical Method for Veterinary Drugs in Livestock Products by lc-ms/ms. Shokuhin Eiseigaku Zasshi 2023, 64, 53–60. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Yu, H.; Chen, Y.; Yuan, F.; Zhang, X.; Fang, S. Furazolidone and nitrofurazone metabolic studies in crucian carp by ultra-performance liquid chromatography Tandem Mass Spectrometry. J. Chromatogr. Sci. 2022, 60, 963–969. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Lee, C.H.; Wang, S.Y.; Chou, C.Y.; Yang, Y.J.; Kao, C.C.; Wu, H.Y.; Dong, Y.; Hung, W.Y.; Su, C.Y.; et al. Multiplexed antibody glycosylation profiling using dual enzyme digestion and liquid chromatography-triple quadrupole mass spectrometry method. Mol. Cell Proteom. 2024, 23, 100710. [Google Scholar] [CrossRef]
- Jung, Y.S.; Kim, D.B.; Nam, T.G.; Seo, D.; Yoo, M. Identification and quantification of multi-class veterinary drugs and their metabolites in beef using LC-MS/MS. Food Chem. 2022, 382, 132313. [Google Scholar] [CrossRef]
- Lehotay, S.J. Comparison of analyte identification criteria and other aspects in triple quadrupole tandem mass spectrometry: Case study using UHPLC-MS/MS for regulatory analysis of veterinary drug residues in liquid and powdered eggs. Anal. Bioanal. Chem. 2022, 414, 287–302. [Google Scholar] [CrossRef]
- Na, T.W.; Seo, H.J.; Jang, S.N.; Kim, H.; Yun, H.; Kim, H.; Ahn, J.; Cho, H.; Hong, S.H.; Kim, H.J.; et al. Multi-residue analytical method for detecting pesticides, veterinary drugs, and mycotoxins in feed using liquid- and gas chromatography coupled with mass spectrometry. J. Chromatogr. A 2022, 1676, 463257. [Google Scholar] [CrossRef]
- Wittenberg, J.B.; Simon, K.A.; Wong, J.W. Targeted multiresidue analysis of veterinary drugs in milk-based powders using liquid chromatography-tandem mass spectrometry (lc-ms/ms). J. Agric. Food Chem. 2017, 65, 7288–7293. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, A.; Bessaire, T.; Savoy, M.C.; Tarres, A.; Mujahid, C.; Beck, A.; Mottier, P.; Delatour, T. Screening of 154 veterinary drug residues in foods of animal origin using lc-ms/ms: First action 2020.04. J. AOAC Int. 2021, 104, 650–681. [Google Scholar] [CrossRef]
- Jang, S.; Seo, H.; Kim, H.; Kim, H.; Ahn, J.; Cho, H.; Hong, S.; Lee, S.; Na, T. Development of a quantitative method for detection of multiclass veterinary drugs in feed using modified quppe extraction and lc-ms/ms. Molecules 2022, 27, 4483. [Google Scholar] [CrossRef] [PubMed]
- Khadim, A.; Yaseen Jeelani, S.U.; Khan, M.N.; Kumari, S.; Raza, A.; Ali, A.; Zareena, B.; Zaki Shah, S.M.; Musharraf, S.G. Targeted analysis of veterinary drugs in food samples by developing a high-resolution tandem mass spectral library. J. Agric. Food Chem. 2023, 71, 12839–12848. [Google Scholar] [CrossRef] [PubMed]
- Al-Shakliah, N.S.; Kadi, A.A.; Aljohar, H.I.; AlRabiah, H.; Attwa, M.W. Profiling of in vivo, in vitro and reactive zorifertinib metabolites using liquid chromatography ion trap mass spectrometry. RSC Adv. 2022, 12, 20991–21003. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Meijer, T.; Mol, H.G.J.; van Ginkel, L.; Nielen, M.W.F. A global inter-laboratory study to assess acquisition modes for multi-compound confirmatory analysis of veterinary drugs using liquid chromatography coupled to triple quadrupole, time of flight and orbitrap mass spectrometry. Anal. Chim. Acta 2017, 962, 60–72. [Google Scholar] [CrossRef]
- Guo, Y.; Mao, R.; Zhang, Y.; Li, R.; Oduro, P.K.; Si, D.; Han, L.; Huang, Y.; Pan, G. An integrated strategy for the systematic chemical characterization of Salvianolate lyophilized injection using four scan modes based on the ultra-high performance liquid chromatography-triple quadrupole-linear ion trap mass spectrometry. J. Pharm. Biomed. Anal. 2022, 215, 114769. [Google Scholar] [CrossRef]
- Chen, G.Y.; Zhang, Q. Quantification of plasma oxylipins using solid-phase extraction and reversed-phase liquid chromatography-triple quadrupole mass spectrometry. Methods Mol. Biol. 2021, 2306, 171–186. [Google Scholar] [CrossRef]
- Kang, J.; Park, S.J.; Park, H.C.; Hossain, M.A.; Kim, M.A.; Son, S.W.; Lim, C.M.; Kim, T.W.; Cho, B.H. Multiresidue screening of veterinary drugs in meat, milk, egg, and fish using liquid chromatography coupled with ion trap time-of-flight mass spectrometry. Appl. Biochem. Biotechnol. 2017, 182, 635–652. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Perez-Quintanilla, D.; Sierra, I. Application of a hybrid ordered mesoporous silica as sorbent for solid-phase multi-residue extraction of veterinary drugs in meat by ultra-high-performance liquid chromatography coupled to ion-trap tandem mass spectrometry. J. Chromatogr. A 2016, 1459, 24–37. [Google Scholar] [CrossRef]
- Sabei, F.Y.; Khardali, I.; Al-Kasim, M.A.; Shaheen, E.S.; Oraiby, M.; Alamir, A.; David, B.; Alshahrani, S.; Jali, A.M.; Attafi, M.; et al. Disposition kinetics of cathinone and its metabolites after oral administration in rats. Curr. Drug Metab. 2024, 25, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, B.; Xu, L.; Xiong, L.; Guo, D.; Cui, W.; Xu, X.; Lin, Y. Quality consistency evaluation on three species of North Patrininae herba by high-performance liquid chromatography coupled with electrospray ion trap time-of-flight mass spectrometry and network pharmacology approaches. J. Sep. Sci. 2022, 45, 3852–3865. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, X.; Geng, Y.; Dai, J.; Tang, S.; Adams, E.; Chen, D.; Yuan, Y. Analysis of impurity profiling of arbekacin sulfate by ion-pair liquid chromatography coupled with pulsed electrochemical detection and online ion suppressor-ion trap-time off light mass spectrometry. J. Pharm. Biomed. Anal. 2022, 221, 115061. [Google Scholar] [CrossRef]
- Saade, J.; Biacchi, M.; Giorgetti, J.; Lechner, A.; Beck, A.; Leize-Wagner, E.; Francois, Y.N. Analysis of monoclonal antibody glycopeptides by capillary electrophoresis-mass spectrometry coupling (ce-ms). Methods Mol. Biol. 2021, 2271, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ferre, S.; Boccard, J.; Rudaz, S.; Gonzalez-Ruiz, V. Evaluation of prototype ce-ms interfaces. Methods Mol. Biol. 2022, 2531, 1–13. [Google Scholar] [CrossRef]
- Latosinska, A.; Siwy, J.; Mischak, H.; Frantzi, M. Peptidomics and proteomics based on ce-ms as a robust tool in clinical application: The past, the present, and the future. Electrophoresis 2019, 40, 2294–2308. [Google Scholar] [CrossRef]
- Fang, P.; Pan, J.Z.; Fang, Q. A robust and extendable sheath flow interface with minimal dead volume for coupling ce with esi-ms. Talanta 2018, 180, 376–382. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Marina, M.L. Applications of capillary electrophoresis-mass spectrometry to chiral analysis. Methods Mol. Biol. 2022, 2531, 211–225. [Google Scholar] [CrossRef]
- Blasco, C.; Pico, Y.; Andreu, V. Analytical method for simultaneous determination of pesticide and veterinary drug residues in milk by CE-MS. Electrophoresis 2009, 30, 1698–1707. [Google Scholar] [CrossRef]
- Seyfinejad, B.; Jouyban, A. Capillary electrophoresis-mass spectrometry in pharmaceutical and biomedical analyses. J. Pharm. Biomed. Anal. 2022, 221, 115059. [Google Scholar] [CrossRef]
- Shamsi, S.A.; Akter, F. Capillary electrophoresis mass spectrometry: Developments and applications for enantioselective analysis from 2011–2020. Molecules 2022, 27, 4126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, Y.; Zhang, L.; Qin, L.; Wen, H.; Fan, X.; Peng, D. Highly sensitive magnetic-nanoparticle-based immunochromatography assay for rapid detection of amantadine in chicken and eggs. Biosensors 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, X.; Yang, H.; Wen, H.; Han, S.; Pan, X.; Li, H.; Peng, D. A sensitive and specific monoclonal antibody based enzyme-linked immunosorbent assay for the rapid detection of pretilachlor in grains and the environment. Foods 2023, 13, 12. [Google Scholar] [CrossRef]
- Lee, M.J.; Aliya, S.; Lee, E.S.; Jeon, T.J.; Oh, M.H.; Huh, Y.S. Simple and rapid detection of ractopamine in pork with comparison of LSPR and LFIA sensors. J. Food Drug Anal. 2022, 30, 590–602. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Abu bakr Shabbir, M.; Cheng, G.; Yuan, Z. Current advances in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Calidonio, J.M.; Hamad-Schifferli, K. Biophysical and biochemical insights in the design of immunoassays. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130266. [Google Scholar] [CrossRef]
- Sun, B.; Chen, Z.; Feng, B.; Chen, S.; Feng, S.; Wang, Q.; Niu, X.; Zhang, Z.; Zheng, P.; Lin, M.; et al. Development of a colloidal gold-based immunochromatographic assay for rapid detection of nasal mucosal secretory IgA against SARS-CoV-2. Front. Microbiol. 2024, 15, 1386891. [Google Scholar] [CrossRef]
- Ziyang, H.; Shaoen, Z.; Qingzhou, C.; Hong, L.; Jianxin, S.; Kaiqiang, W.; Huiying, W.; Limin, C. Novel solid-phase extraction promotes simultaneous colloidal gold immunochromatographic assay of malachite green, leuco-malachite green, chloramphenicol, and semi-carbazone metabolites in aquatic products. Food Chem. 2025, 465, 142118. [Google Scholar] [CrossRef] [PubMed]
- Na, G.; Hu, X.; Yang, J.; Sun, Y.; Kwee, S.; Tang, L.; Xing, G.; Xing, Y.; Zhang, G. A rapid colloidal gold-based immunochromatographic strip assay for monitoring nitroxynil in milk. J. Sci. Food Agric. 2020, 100, 1860–1866. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Hu, X.; Xing, Y.; Kwee, S.; Na, G.; Zhang, G. Colloidal gold-based immunochromatographic strip assay for the rapid detection of diminazene in milk. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 1667–1677. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; Cai, X.; Cao, M.; Lu, Q.; Hu, D.; Chen, Q.; Xiong, X. A rapid colloidal gold immunochromatographic assay based on polyclonal antibodies against HtpsC protein for the detection of Streptococcus suis. Front. Microbiol. 2023, 14, 1294368. [Google Scholar] [CrossRef] [PubMed]
- Na, G.; Hu, X.; Yang, J.; Sun, Y.; Kwee, S.; Tang, L.; Xing, G.; Xing, Y.; Zhang, G. Colloidal gold-based immunochromatographic strip assay for the rapid detection of bacitracin zinc in milk. Food Chem. 2020, 327, 126879. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cao, Y.; Yan, M.; Sun, M.; Zhang, Q.; Wang, J.; Fu, G.; Liu, R.; Huang, Y.; Su, J. Development of a colloidal gold immunochromatographic assay for duck enteritis virus detection using monoclonal antibodies. Pathogens 2021, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Qian, C.; Jiang, H.; Yang, H. Development of an enzyme-linked immunosorbent assay for the determination of maduramicin in broiler chicken tissues. J. Agric. Food Chem. 2001, 49, 2697–2701. [Google Scholar] [CrossRef]
- Chen, R.; Shang, H.; Niu, X.; Huang, J.; Miao, Y.; Sha, Z.; Qin, L.; Huang, H.; Peng, D.; Zhu, R. Establishment and evaluation of an indirect ELISA for detection of antibodies to goat Klebsiella pneumonia. BMC Vet. Res. 2021, 17, 107. [Google Scholar] [CrossRef]
- Qin, L.; Xiao, J.; Yang, H.; Liang, J.; Li, L.; Wu, S.; Peng, D. Rapid immunoassays for the detection of quinoxalines and their metabolites residues in animal-derived foods: A review. Food Chem. 2024, 443, 138539. [Google Scholar] [CrossRef]
- Wernike, K.; Aebischer, A.; Michelitsch, A.; Hoffmann, D.; Freuling, C.; Balkema-Buschmann, A.; Graaf, A.; Muller, T.; Osterrieder, N.; Rissmann, M.; et al. Multi-species ELISA for the detection of antibodies against SARS-CoV-2 in animals. Transbound. Emerg. Dis. 2021, 68, 1779–1785. [Google Scholar] [CrossRef]
- Fu, B.; Gao, H.; Fang, C.; Cheng, G.; Wang, H.; Wang, Y.; Hao, H.; Wang, X.; Huang, L.; Peng, D. Development of a monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for screening of diethylstilbestrol in animal-derived foods. Heliyon 2024, 10, e39769. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Wu, S.; Lu, Y.; Yang, Y.; Qin, L.; Li, L.; Xiao, J.; Liang, J.; Si, Y.; et al. A sensitive and specific enzyme-linked immunosorbent assay for the detection of pymetrozine in vegetable, cereal, and meat. Food Chem. 2023, 418, 135949. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Yang, H. Development of a nucleocapsid protein-based blocking elisa for the detection of porcine deltacoronavirus antibodies. Viruses 2022, 14, 1815. [Google Scholar] [CrossRef]
- He, D.; Sun, M.; Jiang, X.; Zhang, S.; Wei, F.; Wu, B.; Diao, Y.; Tang, Y. Development of an indirect competitive ELISA method based on ORF2 detecting the antibodies of novel goose astrovirus. J. Virol. Methods 2023, 311, 114643. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Li, Z.; He, X.; Zhao, S.; Ma, Q.; Li, X.; Liu, J.; Liu, A.; Li, Y.; et al. A universal ELISA assay for detecting six strains of ovine Babesia species in China. Vet. Parasitol. 2021, 300, 109616. [Google Scholar] [CrossRef]
- Guo, L.; Liu, M.; Li, Q.; Dong, B.; Li, H.; Mari, G.M.; Liu, R.; Yu, W.; Yu, X.; Wang, Z.; et al. Synthesis and characterization of tracers and development of a fluorescence polarization immunoassay for amantadine with high sensitivity in chicken. J. Food Sci. 2021, 86, 4754–4767. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liang, D.; Xiong, J.; Wang, Z.; Zheng, P.; Zhang, H.; Ren, Z.; Jiang, H. Development of a sensitive and rapid fluorescence polarization immunoassay for high throughput screening eight glucocorticoids in beef. J. Pharm. Biomed. Anal. 2022, 214, 114719. [Google Scholar] [CrossRef]
- Mukhametova, L.I.; Eremin, S.A.; Arutyunyan, D.A.; Goryainova, O.S.; Ivanova, T.I.; Tillib, S.V. Fluorescence Polarization Immunoassay of Human Lactoferrin in Milk Using Small Single-Domain Antibodies. Biochemistry 2022, 87, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Mukhametova, L.I.; Zvereva, E.A.; Zherdev, A.V.; Eremin, S.A. A sensitive fluorescence polarization immunoassay for the rapid detection of okadaic acid in environmental waters. Biosensors 2023, 13, 477. [Google Scholar] [CrossRef]
- Smith, D.S.; Eremin, S.A. Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal. Bioanal. Chem. 2008, 391, 1499–1507. [Google Scholar] [CrossRef]
- Mukhametova, L.I.; Eremin, S.A. Fluorescence polarization assays for organic compounds in food safety. Front. Biosci. (Elite Ed.) 2024, 16, 4. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, H.; Zhang, Y.; Li, Q.; Li, P.; Mari, G.M.; Eremin, S.A.; Shen, J.; Wang, Z. A robust homogeneous fluorescence polarization immunoassay for rapid determination of erythromycin in milk. Foods 2023, 12, 1581. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, Y.; Li, P.; Li, Q.; Yu, W.; Wen, K.; Eremin, S.A.; Shen, J.; Yu, X.; Wang, Z. Dual-wavelength fluorescence polarization immunoassay for simultaneous detection of sulfonamides and antibacterial synergists in milk. Biosensors 2022, 12, 1053. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, M.; Wang, W.; Li, J.; Liang, X. Design, synthesis, and characterization of tracers and development of a fluorescence polarization immunoassay for rapid screening of 4,4′-dinitrocarbanilide in chicken muscle. Foods 2021, 10, 1822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, J.; Tao, Z.; Eremin, S.A.; Hua, X.; Wang, M. Development of fluorescence polarization immunoassay for imidacloprid in environmental and agricultural samples. Front. Chem. 2020, 8, 615594. [Google Scholar] [CrossRef]
- Chowdhury, J.; Ranc, V.; Sarkar, S. Editorial: Surface enhanced raman scattering: Theory and applications. Front. Chem. 2023, 11, 1176495. [Google Scholar] [CrossRef]
- Liu, H.L.; Zhan, K.; Wang, K.; Xia, X.H. Nanopore-based surface-enhanced Raman scattering technologies. Sci. Bull. 2022, 67, 1539–1541. [Google Scholar] [CrossRef]
- Tanwar, S.; Kim, J.H.; Bulte, J.W.M.; Barman, I. Surface-enhanced Raman scattering: An emerging tool for sensing cellular function. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1802. [Google Scholar] [CrossRef]
- Dhakal, S.; Chao, K.; Huang, Q.; Kim, M.; Schmidt, W.; Qin, J.; Broadhurst, C.L. A simple surface-enhanced raman spectroscopic method for on-site screening of tetracycline residue in whole milk. Sensors 2018, 18, 424. [Google Scholar] [CrossRef]
- Fan, R.; Tang, S.; Luo, S.; Liu, H.; Zhang, W.; Yang, C.; He, L.; Chen, Y. Duplex surface enhanced raman scattering-based lateral flow immunosensor for the low-level detection of antibiotic residues in milk. Molecules 2020, 25, 5249. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Jing, X.; Ma, W. SERS-active plasmonic metal NP-CsPbX(3) films for multiple veterinary drug residues detection. Food Chem. 2023, 412, 135420. [Google Scholar] [CrossRef]
- Xu, M.L.; Gao, Y.; Han, X.X.; Zhao, B. Innovative Application of sers in food quality and safety: A brief review of recent trends. Foods 2022, 11, 5249. [Google Scholar] [CrossRef]
- Wang, L.; Ma, P.; Chen, H.; Chang, M.; Lu, P.; Chen, N.; Yuan, Y.; Chen, N.; Zhang, X. Rapid determination of mixed pesticide residues on apple surfaces by surface-enhanced raman spectroscopy. Foods 2022, 11, 1089. [Google Scholar] [CrossRef]
- Lee, K.M.; Yarbrough, D.; Kozman, M.M.; Herrman, T.J.; Park, J.; Wang, R.; Kurouski, D. A rapid and convenient screening method for detection of restricted monensin, decoquinate, and lasalocid in animal feed by applying SERS and chemometrics. Food Chem. Toxicol. 2020, 144, 111633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lv, H.; Wang, J.; Yang, Z.; Ding, Y.; Zhao, B.; Tian, Y. An aptamer-based colorimetric/SERS dual-mode sensing strategy for the detection of sulfadimethoxine residues in animal-derived foods. Anal. Methods 2023, 15, 1047–1053. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, S.; Xie, Y.; Zhang, L.; Shao, Y.; Lin, J.; Wu, A. Advances in SERS detection method combined with microfluidic technology for bio-analytical applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 332, 125797. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, P.; Moorthy, N.; Rajpoot, V.S.; Rao, H.; Goswami, R.K.; Subash, P.; Khute, S.; Rao, K.S. Metabolite profiling, antimalarial potentials of Schleichera oleosa using LC-MS and GC-MS: In vitro, molecular docking and molecular dynamics. Front. Mol. Biosci. 2025, 12, 1543939. [Google Scholar] [CrossRef]
- Johnson, K.R.; Gao, Y.; Gregus, M.; Ivanov, A.R. On-capillary cell lysis enables top-down proteomic analysis of single mammalian cells by ce-ms/ms. Anal. Chem. 2022, 94, 14358–14367. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Rapacz, D.; Smolinska-Kempisty, K.; Wolska, J. Preparation and characterization of SPE column with smart green molecularly imprinted polymers materials for selective determination of S-metolachlor herbicide. Sci. Rep. 2025, 15, 3153. [Google Scholar] [CrossRef]
- Zad, N.; Tell, L.A.; Ampadi Ramachandran, R.; Xu, X.; Riviere, J.E.; Baynes, R.; Lin, Z.; Maunsell, F.; Davis, J.; Jaberi-Douraki, M. Development of machine learning algorithms to estimate maximum residue limits for veterinary medicines. Food Chem. Toxicol. 2023, 179, 113920. [Google Scholar] [CrossRef]
- Wu, P.Y.; Chou, W.C.; Wu, X.; Kamineni, V.N.; Kuchimanchi, Y.; Tell, L.A.; Maunsell, F.P.; Lin, Z. Development of machine learning-based quantitative structure-activity relationship models for predicting plasma half-lives of drugs in six common food animal species. Toxicol. Sci. 2025, 203, 52–66. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Improving the QuEChERS liquid/liquid extraction of analytes with widely varying physicochemical properties: Example of 201 veterinary drugs in milk. J. AOAC Int. 2022, 105, 1030–1042. [Google Scholar] [CrossRef]
- Fodor, A.; Cseppento, D.C.N.; Badea, G.E.; Petrehele, A.I.; Groze, A.; Tit, D.M.; Bungau, S.G. Colloidal gold immunochromatography and elisa traceability of tetracycline residues from raw milk to its dairy products. In Vivo 2023, 37, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Ge, L.; Li, J.; Qiu, G.; Wu, F.; Zhang, H.; Xu, F.; Zhu, R.; Qi, P.; Bai, R.; et al. Automated QuEChERS for the determination of 482 pesticide residues in Radix codonopsis by GC-Q-TOF/MS and LC-Q-TOF/MS. Anal. Methods 2021, 13, 5660–5669. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, S.; Chen, J.; Luo, W.; Kang, F.; Ren, Y.; Zhou, W. Development and application of a new QuEChERS method coupled with uplc-qtof-ms/ms for analysis of tiafenacil and its photolysis products in water. J. Agric. Food Chem. 2024, 72, 27007–27018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, H.; Chen, W.; Zhang, J. Nanomaterials used in fluorescence polarization based biosensors. Int. J. Mol. Sci. 2022, 23, 8625. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.W.; Jeon, H.J. Machine learning-driven innovations in microfluidics. Biosensors 2024, 14, 613. [Google Scholar] [CrossRef]
- Zhang, D.; He, S.; Wang, Z.; Yan, P.; Li, H.; Xu, X.; Zeng, Q.; Wang, N.; Chen, X. Recent advances in CRISPR/Cas systems-associated surface enhanced Raman spectroscopy biosensor towards nucleic acid detection. Med. Nov. Technol. Devices 2024, 24, 100336. [Google Scholar] [CrossRef]
| Method | LOD (μg/kg) | Accuracy (Recovery Rate) | Applicability | Advantages | Disadvantages | Feasibility | Cost-Effectiveness |
|---|---|---|---|---|---|---|---|
| LLE | 0.5–1.0 | 71.4–120% | Suitable for lipophilic drugs in aquatic products, milk, and tissues. | Excellent selectivity for lipophilic drugs. Simple operation with minimal equipment. Low cost. | High solvent consumption. Prone to emulsification. Limited extraction efficiency for polar analytes. | Simple but requires solvent management. | Low |
| SPE | 0.2–3.0 | 60–120% | Broad applicability for diverse matrices (honey, muscle, milk, and eggs). | Effective enrichment of trace residues. High selectivity with tailored adsorbents. Reduced solvent use. | High adsorbent costs. Complex optimization for matrix effects. Susceptible to column clogging. | Requires skilled optimization. | Moderate to high |
| IAC | 0.04–0.10 | 74.5–105% | Ideal for specific targets (e.g., chloramphenicol and β-agonists) in muscle/liver. | Exceptional specificity via antibody–antigen binding. High sensitivity for trace residues. High-throughput potential. | Antibody development is costly/time-consuming. Limited column lifespan. Cross-reactivity risks. | Antibody-dependent and storage-sensitive. | High |
| QuEChERS | 0.15–3.03 | 52.1–138.2% | Effective for high-fat matrices (beef and chicken) and multi-residue analysis. | Rapid and simple. Cost-effective with minimal solvents. Effective impurity removal. | Sorbent selectivity limitations. Residual matrix interference. Optimization challenges for diverse analytes. | Easy to implement with standard lab tools. | Low to moderate |
| MIT | 0.05–0.5 | 68.6–95.5% | Customizable for antibiotics (e.g., tetracyclines and β-agonists) in complex matrices. | Tailored specificity via imprinting. Reusable and stable. Adaptable to diverse targets. | Labor-intensive synthesis. Cross-reactivity with structural analogs. Requires confirmatory methods. | Specialized expertise needed for polymer design. | Moderate |
| Method | LOD (μg/kg) | Accuracy (Recovery Rate) | Applicability | Advantages | Disadvantages | Feasibility | Costing |
|---|---|---|---|---|---|---|---|
| GC-MS | 2.3–4.3 | 77.38–95.7% | Volatile/semi-volatile compounds. | High specificity for volatile analytes. Robust qualitative capabilities. Wide applicability for small molecules. | Requires derivatization for non-volatile compounds. Limited to thermally stable analytes. | Requires derivatization expertise. | Moderate to high |
| LC-QTOF-MS | 0.5 | More than 70% | High-resolution multi-residue screening. | Ultra-high resolution for accurate mass identification. Broad-spectrum detection. Rich structural data. | High equipment/maintenance costs. Demands advanced data analysis skills. | Requires high-end infrastructure. | Very high |
| LC-MS/MS | 0.02–82 | 70–120% | Gold standard for trace-level quantification. | High sensitivity and selectivity. Reliable for multi-residue analysis. Robust quantitative accuracy. | Expensive instrumentation. Complex sample preparation. Matrix effects require mitigation. | Skilled operation and maintenance needed. | High |
| LC-IT-MS | 0.01–18.75 | 63–122% | Multi-stage fragmentation for structural elucidation. | Mul-ti-stage mass for structural insights. Compact and cost-effective. | Slower scanning speeds. Moderate resolution limits complex mixture analysis. | Suitable for targeted analysis. | Moderate |
| CE-MS | 1–9 | More than 78% | Ionizable metabolites. | High separation efficiency. Minimal sample/reagent consumption. Fast analysis. | Poor reproducibility due to buffer/temperature sensitivity. | Technically demanding for calibration. | Moderate |
| GICA | 0.01–0.5 | 84.2–112.9% | Rapid on-site screening. | Equipment-free, rapid results. Low cost and user-friendly. | Qualitative/semi-quantitative only. Limited sensitivity for trace residues. Matrix interference risks. | Ideal for field testing. | Low |
| ELISA | 1.56–2.72 | 70.1–103.1% | High-throughput screening. | High throughput and specificity. Cost-effective for batch analysis. Minimal instrumentation. | Cross-reactivity with analogs. Enzyme activity affected by environmental factors. | Requires antibody development. | Low to moderate |
| FPIA | 0.01 | 78.6–107.77% | Homogeneous assays. | Rapid and homogeneous. Minimal sample pretreatment. Moderate sensitivity. | Limited by antibody/tracer availability. Matrix interference in complex samples. | Suitable for simple matrices. | Moderate |
| SERS | 0.01–0.015 | 88.8–111.3% | Ultra-trace detection. | Ultra-high sensitivity. Rapid and minimal pretreatment. Multiplexing potential. | Poor reproducibility due to nanoparticle variability. | Requires nanoparticle optimization. | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.; Tang, S.; Dai, C. Recent Advances in Pretreatment Methods and Detection Techniques for Veterinary Drug Residues in Animal-Derived Foods. Metabolites 2025, 15, 233. https://doi.org/10.3390/metabo15040233
Dai Q, Tang S, Dai C. Recent Advances in Pretreatment Methods and Detection Techniques for Veterinary Drug Residues in Animal-Derived Foods. Metabolites. 2025; 15(4):233. https://doi.org/10.3390/metabo15040233
Chicago/Turabian StyleDai, Qing, Shusheng Tang, and Chongshan Dai. 2025. "Recent Advances in Pretreatment Methods and Detection Techniques for Veterinary Drug Residues in Animal-Derived Foods" Metabolites 15, no. 4: 233. https://doi.org/10.3390/metabo15040233
APA StyleDai, Q., Tang, S., & Dai, C. (2025). Recent Advances in Pretreatment Methods and Detection Techniques for Veterinary Drug Residues in Animal-Derived Foods. Metabolites, 15(4), 233. https://doi.org/10.3390/metabo15040233






