Endoplasmic Reticulum Stress and Its Role in Metabolic Reprogramming of Cancer
Abstract
1. Introduction
1.1. Metabolic Reprogramming in Cancer: A Focus on Metabolomics and Metabolites
1.2. Glucose and Main Amino Acids
1.2.1. Glucose
1.2.2. Glutamine
1.2.3. Alanine
1.2.4. Arginine
1.2.5. Carnitine
1.2.6. Lipids
Fatty Acids
Phospholipids
Sterols
Ketone Bodies
1.2.7. Nucleic Acids and Folates
DNA and RNA
Purines
Folic Acid
2. ER Stress and Unfolded Protein Response Activation
2.1. IRE1
2.2. ATF6
2.3. PERK
3. The Role of ER Stress in Cancer
3.1. GRP78
- Honokiol: A natural biphenolic compound derived from Magnolia spp., honokiol has been reported to downregulate GRP78, inducing ER stress-mediated apoptosis in breast cancer and glioblastoma models [226].
- Metformin: Widely used for type 2 diabetes, metformin has demonstrated anti-cancer effects by inhibiting GRP78 and sensitizing tumor cells to chemotherapy. In colorectal and ovarian cancer models, metformin downregulates GRP78 and disrupts its interaction with AKT, enhancing drug-induced cytotoxicity [227]. Moreover, metformin enhanced the radiosensitivity of cancer stem cells in vitro and significantly improved the response of experimental tumors to irradiation, suggesting that this anti-diabetic drug could be beneficial in increasing the effectiveness of cancer radiotherapy [228].
- HA15: A novel thiazole benzenesulfonamide compound, HA15 selectively targets GRP78 and induces ER stress-mediated apoptosis in melanoma and pancreatic cancer cells. Preclinical studies have shown promising efficacy in overcoming drug resistance and improving tumor regression [229].
- Itraconazole: An antifungal agent, itraconazole inhibits GRP78 and mTOR signaling, leading to reduced proliferation in prostate and lung cancer models [230].
- Novel strategies, including monoclonal antibodies and small-molecule inhibitors, are being developed to enhance specificity while minimizing off-target effects [231].
3.2. IRE1
- 4μ8C and STF-083010: these small-molecule inhibitors specifically block IRE1’s RNase activity, preventing XBP1 splicing and impairing tumor cell survival. Preclinical studies have shown that 4μ8C enhances the efficacy of chemotherapy in multiple myeloma and TNBC models [235].
- MKC-3946: potent IRE1 inhibitor that sensitizes pancreatic cancer cells to gemcitabine, reducing tumor burden in preclinical models [236].
- Toyocamycin: these natural compounds inhibit IRE1-mediated XBP1 splicing, leading to ER stress accumulation and apoptosis in leukemia and lymphoma cells [237].
3.3. ATF6
- Ceapins (Ceapin-A7, Ceapin-272): selective inhibitors that block ATF6 activation by preventing its trafficking to the Golgi. These compounds sensitize breast and prostate cancer cells to chemotherapy by disrupting the ATF6-mediated stress response [243].
- Bortezomib: a proteasome inhibitor approved for multiple myeloma, bortezomib reduces ATF6 signaling by impairing ER stress resolution, leading to increased apoptosis in leukemia and solid tumors [244].
- Hydroxychloroquine (HCQ): an autophagy inhibitor that synergizes with ATF6 depletion, leading to reduced integrin expression and tempering tumor growth and metastasis. Combining HCQ with ATF6 inhibitors may enhance radiosensitivity by disrupting tumor cell survival mechanisms [245].
3.4. PERK
- GSK2606414 and GSK2656157: selective PERK inhibitors that suppress tumor growth in pancreatic, glioblastoma, and lung cancer models. However, systemic toxicity has limited their clinical application [252].
- ISRIB (integrated stress response inhibitor): a small molecule that restores protein synthesis by blocking eIF2α phosphorylation, reducing stress adaptation in colorectal and prostate cancer cells [253].
- Salubrinal: Initially developed as an eIF2α phosphatase inhibitor, salubrinal enhances ER stress and sensitizes tumor cells to chemotherapy in breast and liver cancer models [254].
- Metformin: Known for its metabolic effects, metformin inhibits PERK-Nrf2 signaling, reducing oxidative stress tolerance in hepatocellular cancer [255].
- Bortezomib: a proteasome inhibitor that induces excessive ER stress, leading to PERK-mediated apoptosis in multiple myeloma and leukemia [256].
4. The Interplay Between ER Stress and Cancer Metabolism
4.1. Glucose
4.2. Fatty Acids
4.3. Amino Acids
4.4. Nucleic Acids
5. ER Stress-Induced Metabolic Reprogramming and Its Impact on the Tumor Microenvironment and Cancer Resistance
5.1. ER Stress-Driven Metabolic Reprogramming Promotes Cancer Stemness and EMT
5.2. ER Stress-Associated Metabolic Reprogramming and Cancer Cell Resistance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The Cancer Genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Di, L.; Li, J.; Fan, W.; Liu, Y.; Guo, W.; Liu, W.; Liu, L.; Li, Q.; Chen, L.; et al. A Body Map of Somatic Mutagenesis in Morphologically Normal Human Tissues. Nature 2021, 597, 398–403. [Google Scholar] [CrossRef]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a Disease of Old Age: Changing Mutational and Microenvironmental Landscapes. Br. J. Cancer 2020, 122, 943–952. [Google Scholar] [CrossRef]
- Fowler, J.C.; Jones, P.H. Somatic Mutation: What Shapes the Mutational Landscape of Normal Epithelia? Cancer Discov. 2022, 12, 1642–1655. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor Initiation and Early Tumorigenesis: Molecular Mechanisms and Interventional Targets. Sig. Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Vasseur, S.; Guillaumond, F. Lipids in Cancer: A Global View of the Contribution of Lipid Pathways to Metastatic Formation and Treatment Resistance. Oncogenesis 2022, 11, 46. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The Reverse Warburg Effect: Aerobic Glycolysis in Cancer Associated Fibroblasts and the Tumor Stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 Gradients in Solid Tumors In Vivo: High-Resolution Measurements Reveal a Lack of Correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Smallbone, K.; Gatenby, R.A.; Gillies, R.J.; Maini, P.K.; Gavaghan, D.J. Metabolic Changes during Carcinogenesis: Potential Impact on Invasiveness. J. Theor. Biol. 2007, 244, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Trimmer, C.; Lin, Z.; Whitaker-Menezes, D.; Chiavarina, B.; Zhou, J.; Wang, C.; Pavlides, S.; Martinez-Cantarin, M.P.; Capozza, F.; et al. Autophagy in Cancer Associated Fibroblasts Promotes Tumor Cell Survival: Role of Hypoxia, HIF1 Induction and NFκB Activation in the Tumor Stromal Microenvironment. Cell Cycle 2010, 9, 3515–3533. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, T.; Marini, A.; Giannoni, E.; Taddei, M.L.; Gandellini, P.; De Donatis, A.; Lanciotti, M.; Serni, S.; Cirri, P.; Chiarugi, P. Reciprocal Metabolic Reprogramming through Lactate Shuttle Coordinately Influences Tumor-Stroma Interplay. Cancer Res. 2012, 72, 5130–5140. [Google Scholar] [CrossRef]
- Yang, L. Tumor Microenvironment and Metabolism. Int. J. Mol. Sci. 2017, 18, 2729. [Google Scholar] [CrossRef]
- Cairns, R.; Papandreou, I.; Denko, N. Overcoming Physiologic Barriers to Cancer Treatment by Molecularly Targeting the Tumor Microenvironment. Mol. Cancer Res. 2006, 4, 61–70. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. The Impact of the Endoplasmic Reticulum Protein-Folding Environment on Cancer Development. Nat. Rev. Cancer 2014, 14, 581–597. [Google Scholar] [CrossRef]
- Wang, M.; Law, M.E.; Castellano, R.K.; Law, B.K. The Unfolded Protein Response as a Target for Anticancer Therapeutics. Crit. Rev. Oncol. Hematol. 2018, 127, 66–79. [Google Scholar] [CrossRef]
- Shajahan-Haq, A.; Cook, K.; Schwartz-Roberts, J.; Eltayeb, A.; Demas, D.; Warri, A.; Hilakivi-Clarke, L.; Clarke, R. Glutamine Metabolism and the Unfolded Protein Response in MYC-Driven Breast Cancer. Cancer Metab. 2014, 2 (Suppl. 1), P66. [Google Scholar] [CrossRef]
- Gong, T.; Zheng, C.; Ou, X.; Zheng, J.; Yu, J.; Chen, S.; Duan, Y.; Liu, W. Glutamine Metabolism in Cancers: Targeting the Oxidative Homeostasis. Front. Oncol. 2022, 12, 994672. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Kaufman, R.J. A Trip to the ER: Coping with Stress. Trends Cell Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Soh, L.J.; Lee, S.Y.; Roebuck, M.M.; Wong, P.F. Unravelling the Interplay between ER Stress, UPR and the cGAS-STING Pathway: Implications for Osteoarthritis Pathogenesis and Treatment Strategy. Life Sci. 2024, 357, 123112. [Google Scholar] [CrossRef]
- Beger, R. A Review of Applications of Metabolomics in Cancer. Metabolites 2013, 3, 552–574. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishiumi, S.; Ikeda, A.; Yoshie, T.; Sakai, A.; Matsubara, A.; Izumi, Y.; Tsumura, H.; Tsuda, M.; Nishisaki, H.; et al. A Novel Serum Metabolomics-Based Diagnostic Approach to Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 571–579. [Google Scholar] [CrossRef]
- Ikeda, A.; Nishiumi, S.; Shinohara, M.; Yoshie, T.; Hatano, N.; Okuno, T.; Bamba, T.; Fukusaki, E.; Takenawa, T.; Azuma, T.; et al. Serum Metabolomics as a Novel Diagnostic Approach for Gastrointestinal Cancer. Biomed. Chromatogr. 2012, 26, 548–558. [Google Scholar] [CrossRef]
- Odunsi, K.; Wollman, R.M.; Ambrosone, C.B.; Hutson, A.; McCann, S.E.; Tammela, J.; Geisler, J.P.; Miller, G.; Sellers, T.; Cliby, W.; et al. Detection of Epithelial Ovarian Cancer Using 1H-NMR-Based Metabonomics. Int. J. Cancer 2005, 113, 782–788. [Google Scholar] [CrossRef]
- Osl, M.; Dreiseitl, S.; Pfeifer, B.; Weinberger, K.; Klocker, H.; Bartsch, G.; Schäfer, G.; Tilg, B.; Graber, A.; Baumgartner, C. A New Rule-Based Algorithm for Identifying Metabolic Markers in Prostate Cancer Using Tandem Mass Spectrometry. Bioinformatics 2008, 24, 2908–2914. [Google Scholar] [CrossRef]
- Gao, P.; Tchernyshyov, I.; Chang, T.-C.; Lee, Y.-S.; Kita, K.; Ochi, T.; Zeller, K.I.; De Marzo, A.M.; Van Eyk, J.E.; Mendell, J.T.; et al. C-Myc Suppression of miR-23a/b Enhances Mitochondrial Glutaminase Expression and Glutamine Metabolism. Nature 2009, 458, 762–765. [Google Scholar] [CrossRef]
- Wang, J.; Yu, L.-F.; Shen, P.; Wang, S.-F. Analysis of serum metabonome of patients with breast cancer by gas chromatography-mass spectrometry. Zhejiang Da Xue Xue Bao Yi Xue Ban 2009, 38, 478–484. [Google Scholar] [CrossRef]
- Beger, R.D.; Schnackenberg, L.K.; Holland, R.D.; Li, D.; Dragan, Y. Metabonomic Models of Human Pancreatic Cancer Using 1D Proton NMR Spectra of Lipids in Plasma. Metabolomics 2006, 2, 125–134. [Google Scholar] [CrossRef]
- Yan, S.-K.; Wei, B.-J.; Lin, Z.-Y.; Yang, Y.; Zhou, Z.-T.; Zhang, W.-D. A Metabonomic Approach to the Diagnosis of Oral Squamous Cell Carcinoma, Oral Lichen Planus and Oral Leukoplakia. Oral Oncol. 2008, 44, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Coates, J.M.; Bowles, T.L.; McNerney, G.P.; Sutcliffe, J.; Jung, J.U.; Gandour-Edwards, R.; Chuang, F.Y.S.; Bold, R.J.; Kung, H.-J. Arginine Deiminase as a Novel Therapy for Prostate Cancer Induces Autophagy and Caspase-Independent Apoptosis. Cancer Res. 2009, 69, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Chung, B.C.; Kim, Y.; Lee, K.; Lee, D. Combining Tissue Transcriptomics and Urine Metabolomics for Breast Cancer Biomarker Identification. Bioinformatics 2009, 25, 3151–3157. [Google Scholar] [CrossRef]
- Ganti, S.; Weiss, R.H. Urine Metabolomics for Kidney Cancer Detection and Biomarker Discovery. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 551–557. [Google Scholar] [CrossRef]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled Volatile Organic Compounds in Patients with Non-Small Cell Lung Cancer: Cross Sectional and Nested Short-Term Follow-up Study. Respir. Res. 2005, 6, 71. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.S.; Tietje, O.; Wong, C. Prediction of Breast Cancer Using Volatile Biomarkers in the Breath. Breast Cancer Res. Treat. 2006, 99, 19–21. [Google Scholar] [CrossRef]
- Wishart, D.S. Applications of Metabolomics in Drug Discovery and Development. Drugs R D 2008, 9, 307–322. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Metabolomics and Health: From Nutritional Crops and Plant-Based Pharmaceuticals to Profiling of Human Biofluids. Cell Mol. Life Sci. 2021, 78, 6487–6503. [Google Scholar] [CrossRef]
- Prodhan, M.A.I.; McClain, C.; Zhang, X. Comprehensive Two-Dimensional Gas Chromatography Mass Spectrometry-Based Metabolomics. Adv. Exp. Med. Biol. 2021, 1280, 57–67. [Google Scholar] [CrossRef]
- Vestuto, V.; Conte, M.; Vietri, M.; Mensitieri, F.; Santoro, V.; Di Muro, A.; Alfieri, M.; Moros, M.; Miranda, M.R.; Amante, C.; et al. Multiomic Profiling and Neuroprotective Bioactivity of Salvia Hairy Root-Derived Extracellular Vesicles in a Cellular Model of Parkinson’s Disease. Int. J. Nanomed. 2024, 19, 9373–9393. [Google Scholar] [CrossRef]
- Vacca, M.; Sommella, E.M.; Liso, M.; Verna, G.; Scarano, A.; Sila, A.; Curlo, M.; Mastronardi, M.; Petroni, K.; Tonelli, C.; et al. Anthocyanins from Purple Corn Affect Gut Microbiota and Metabolome in Inflammatory Bowel Disease Patients under Infliximab Infusion: The SiCURA Pilot Study. Food Sci. Hum. Wellness 2024, 13, 3536–3543. [Google Scholar] [CrossRef]
- Marino, C.; Pagano, I.; Castaldo, G.; Grimaldi, M.; D’Elia, M.; Santoro, A.; Conte, A.; Molettieri, P.; Parisella, C.; Buonocore, M.; et al. Supplementing Low-Sodium Bicarbonate-Calcic (Lete)® Water: Effects in Women on Bone and Systemic Metabolism. Metabolites 2023, 13, 1109. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, M.; Marino, C.; Buonocore, M.; Santoro, A.; Sommella, E.; Merciai, F.; Salviati, E.; De Rosa, A.; Nuzzo, T.; Errico, F.; et al. Prenatal and Early Postnatal Cerebral d-Aspartate Depletion Influences l-Amino Acid Pathways, Bioenergetic Processes, and Developmental Brain Metabolism. J. Proteome Res. 2021, 20, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Advances in Metabolite Identification. Bioanalysis 2011, 3, 1769–1782. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Park, J.; Rai, P.; Zhai, R.G. Subcellular Compartmentalization of NAD+ and Its Role in Cancer: A SereNADe of Metabolic Melodies. Pharmacol. Ther. 2019, 200, 27–41. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Gao, Y.; Qi, Y.; Du, J. GLUT1: A Promising Drug Target for Cancer Treatment. Anti-Cancer Drugs 2021, 32, 345–364. [Google Scholar] [CrossRef]
- Benny, S.; Mishra, R.; Manojkumar, M.K.; Aneesh, T.P. From Warburg effect to Reverse Warburg effect: The new horizons of anti-cancer therapy. Med. Hypotheses 2020, 144, 110216. [Google Scholar] [CrossRef]
- Tran, Q.; Lee, H.; Park, J.; Kim, S.H.; Park, J. Targeting Cancer Metabolism—Revisiting the Warburg Effect. Toxicol. Res. 2016, 32, 177–193. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Migneco, G.; Whitaker-Menezes, D.; Chiavarina, B.; Castello-Cros, R.; Pavlides, S.; Pestell, R.G.; Fatatis, A.; Flomenberg, N.; Tsirigos, A.; Howell, A.; et al. Glycolytic Cancer Associated Fibroblasts Promote Breast Cancer Tumor Growth, without a Measurable Increase in Angiogenesis: Evidence for Stromal-Epithelial Metabolic Coupling. Cell Cycle 2010, 9, 2412–2422. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Esposito, A.; Curigliano, G. Tumor-Stroma Crosstalk: Targeting Stroma in Breast Cancer. Curr. Opin. Oncol. 2014, 26, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Soga, T. Cancer Metabolism: Key Players in Metabolic Reprogramming. Cancer Sci. 2013, 104, 275–281. [Google Scholar] [CrossRef]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef]
- Liang, L.; Li, W.; Li, X.; Jin, X.; Liao, Q.; Li, Y.; Zhou, Y. ’Reverse Warburg effect’ of cancer associated fibroblasts (Review). Int. J. Oncol. 2022, 60, 67. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Arcucci, A.; Ruocco, M.R.; Granato, G.; Sacco, A.M.; Montagnani, S. Cancer: An oxidative crosstalk between solid tumor cells and cancer associated fibroblasts. Biomed. Res. Int. 2016, 2016, 4502846. [Google Scholar] [CrossRef]
- Pértega-Gomes, N.; Vizcaíno, J.R.; Attig, J.; Jurmeister, S.; Lopes, C.; Baltazar, F. A lactate shuttle system between tumour and stromal cells is associated with poor prognosis in prostate cancer. BMC Cancer 2014, 14, 352. [Google Scholar] [CrossRef]
- Chelakkot, C.; Chelakkot, V.S.; Shin, Y.; Song, K. Modulating Glycolysis to Improve Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 2606. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Abd El-Aleam, R.H.; Sayed, A.M.; Taha, M.N.; George, R.F.; Georgey, H.H.; Abdel-Rahman, H.M. Design and synthesis of novel benzimidazole derivatives as potential Pseudomonas aeruginosa anti-biofilm agents inhibiting LasR: Evidence from comprehensive molecular dynamics simulation and in vitro investigation. Eur. J. Med. Chem. 2022, 241, 114629. [Google Scholar] [CrossRef]
- Carbone, D.; Vestuto, V.; Ferraro, M.R.; Ciaglia, T.; Pecoraro, C.; Sommella, E.; Cascioferro, S.; Salviati, E.; Novi, S.; Tecce, M.F.; et al. Metabolomics-Assisted Discovery of a New Anticancer GLS-1 Inhibitor Chemotype from a Nortopsentin-Inspired Library: From Phenotype Screening to Target Identification. Eur. J. Med. Chem. 2022, 234, 114233. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T.Q. The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Fendt, S.M.; Frezza, C.; Erez, A. Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer Discov. 2020, 10, 1797–1807. [Google Scholar] [CrossRef]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef]
- Flier, J.S.; Mueckler, M.M.; Usher, P.; Lodish, H.F. Elevated levels of glucose transport and transporter messenger RNA are induced by Ras or Src oncogenes. Science 1987, 235, 1492–1495. [Google Scholar] [CrossRef]
- Gaglio, D.; Metallo, C.M.; Gameiro, P.A.; Hiller, K.; Danna, L.S.; Balestrieri, C.; Alberghina, L.; Stephanopoulos, G.; Chiaradonna, F. Oncogenic K-Ras Decouples Glucose and Glutamine Metabolism to Support Cancer Cell Growth. Mol. Syst. Biol. 2011, 7, 523. [Google Scholar] [CrossRef]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.S.; Chandel, N.S. Mitochondrial Metabolism and ROS Generation Are Essential for Kras-Mediated Tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef]
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-Chang, N.; et al. Glutamine Supports Pancreatic Cancer Growth through a KRAS-Regulated Metabolic Pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, T.; Arsenian Henriksson, M. Impact of MYC in Regulation of Tumor Cell Metabolism. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2015, 1849, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Thompson, C.B. Tumor Suppressors and Cell Metabolism: A Recipe for Cancer Growth. Genes Dev. 2009, 23, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Dolde, C.; Lewis, B.C.; Wu, C.-S.; Dang, G.; Jungmann, R.A.; Dalla-Favera, R.; Dang, C.V. C-Myc Transactivation of LDH-A: Implications for Tumor Metabolism and Growth. Proc. Natl. Acad. Sci. USA 1997, 94, 6658–6663. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Zeller, K.I.; Potter, J.J.; Wonsey, D.R.; O’Donnell, K.A.; Kim, J.; Yustein, J.T.; Lee, L.A.; Dang, C.V. Myc Stimulates Nuclearly Encoded Mitochondrial Genes and Mitochondrial Biogenesis. Mol. Cell. Biol. 2005, 25, 6225–6234. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial Metabolism and Cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic Pathways Promoting Cancer Cell Survival and Growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef]
- Camarda, R.; Williams, J.; Goga, A. In Vivo Reprogramming of Cancer Metabolism by MYC. Front. Cell Dev. Biol. 2017, 5, 35. [Google Scholar] [CrossRef]
- Benjamin, D.I.; Cravatt, B.F.; Nomura, D.K. Global Profiling Strategies for Mapping Dysregulated Metabolic Pathways in Cancer. Cell Metab. 2012, 16, 565–577. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Garcia, R.; Lafita-Navarro, M.C.; Hao, Y.H.; Perez-Castro, L.; Nogueira, P.; Solmonson, A.; Mender, I.; Kilgore, J.A.; Fang, S.; et al. Tryptophan fuels MYC-dependent liver tumorigenesis through indole 3-pyruvate synthesis. Nat Commun. 2024, 15, 4266. [Google Scholar] [CrossRef]
- Sousa, C.M.; Biancur, D.E.; Wang, X.; Halbrook, C.J.; Sherman, M.H.; Zhang, L.; Kremer, D.; Hwang, R.F.; Witkiewicz, A.K.; Ying, H.; et al. Pancreatic Stellate Cells Support Tumor Metabolism through Autophagic Alanine Secretion. Nature 2016, 536, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Hosios, A.M.; Vander Heiden, M.G. The Redox Requirements of Proliferating Mammalian Cells. J. Biol. Chem. 2018, 293, 7490–7498. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Parker, S.J.; Amendola, C.R.; Hollinshead, K.E.R.; Yu, Q.; Yamamoto, K.; Encarnación-Rosado, J.; Rose, R.E.; LaRue, M.M.; Sohn, A.S.W.; Biancur, D.E.; et al. Selective Alanine Transporter Utilization Creates a Targetable Metabolic Niche in Pancreatic Cancer. Cancer Discov. 2020, 10, 1018–1037. [Google Scholar] [CrossRef]
- Chan, A.W.; Mercier, P.; Schiller, D.; Bailey, R.; Robbins, S.; Eurich, D.T.; Sawyer, M.B.; Broadhurst, D. 1H-NMR Urinary Metabolomic Profiling for Diagnosis of Gastric Cancer. Br. J. Cancer 2016, 114, 59–62. [Google Scholar] [CrossRef]
- An, Y.J.; Cho, H.R.; Kim, T.M.; Keam, B.; Kim, J.W.; Wen, H.; Park, C.-K.; Lee, S.-H.; Im, S.-A.; Kim, J.E.; et al. An NMR Metabolomics Approach for the Diagnosis of Leptomeningeal Carcinomatosis in Lung Adenocarcinoma Cancer Patients: NMR Metabolomics Approach. Int. J. Cancer 2015, 136, 162–171. [Google Scholar] [CrossRef]
- Buas, M.F.; Gu, H.; Djukovic, D.; Zhu, J.; Drescher, C.W.; Urban, N.; Raftery, D.; Li, C.I. Identification of Novel Candidate Plasma Metabolite Biomarkers for Distinguishing Serous Ovarian Carcinoma and Benign Serous Ovarian Tumors. Gynecol. Oncol. 2016, 140, 138–144. [Google Scholar] [CrossRef]
- Armitage, E.G.; Southam, A.D. Monitoring Cancer Prognosis, Diagnosis and Treatment Efficacy Using Metabolomics and Lipidomics. Metabolomics 2016, 12, 146. [Google Scholar] [CrossRef]
- Chen, C.-L.; Hsu, S.-C.; Ann, D.K.; Yen, Y.; Kung, H.-J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Marton, L.J. Targeting Polyamine Metabolism and Function in Cancer and Other Hyperproliferative Diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-D.; Lai, M.-W.; Yeh, C.-T. Unlocking the Potential of Arginine Deprivation Therapy: Recent Breakthroughs and Promising Future for Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 10668. [Google Scholar] [CrossRef] [PubMed]
- Husson, A.; Brasse-Lagnel, C.; Fairand, A.; Renouf, S.; Lavoinne, A. Argininosuccinate Synthetase from the Urea Cycle to the Citrulline-NO Cycle. Eur. J. Biochem. 2003, 270, 1887–1899. [Google Scholar] [CrossRef]
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer 2018, 18, 634–645. [Google Scholar] [CrossRef]
- Feun, L.; You, M.; Wu, C.J.; Kuo, M.T.; Wangpaichitr, M.; Spector, S.; Savaraj, N. Arginine deprivation as a targeted therapy for cancer. Curr. Pharm. Des. 2008, 14, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, V.L.; Pinzon-Guzman, C.; Barbul, A. Arginine—Dual roles as an onconutrient and immunonutrient. J. Surg. Oncol. 2017, 115, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sainz, R.M.; Lombo, F.; Mayo, J.C. Radical decisions in cancer: Redox control of cell growth and death. Cancers 2012, 4, 442–474. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-associated macrophages: A potential target for cancer therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef]
- Nathan, C.F.; Hibbs, J.B., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991, 3, 65–70. [Google Scholar] [CrossRef]
- Felley-Bosco, E. Role of nitric oxide in genotoxicity: Implication for carcinogenesis. Cancer Metastasis Rev. 1998, 17, 25–37. [Google Scholar] [CrossRef]
- Török, N.J.; Higuchi, H.; Bronk, S.; Gores, G.J. Nitric oxide inhibits apoptosis downstream of cytochrome C release by nitrosylating caspase 9. Cancer Res. 2002, 62, 1648–1653. [Google Scholar] [PubMed]
- Jaiswal, M.; LaRusso, N.F.; Shapiro, R.A.; Billiar, T.R.; Gores, G.J. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology 2001, 120, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Lind, D.S. Arginine and cancer. J. Nutr. 2004, 134, 2837S–2841S. [Google Scholar] [CrossRef] [PubMed]
- Farahzadi, R.; Hejazi, M.S.; Molavi, O.; Pishgahzadeh, E.; Montazersaheb, S.; Jafari, S. Clinical significance of carnitine in the treatment of cancer: From traffic to the regulation. Oxid. Med. Cell. Longev. 2023, 2023, 9328344. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Fong, M.Y.; McDunn, J.; Kakar, S.S. Identification of metabolites in the normal ovary and their transformation in primary and metastatic ovarian cancer. PLoS ONE 2011, 6, e19963. [Google Scholar] [CrossRef]
- Turkoglu, O.; Zeb, A.; Graham, S.; Szyperski, T.; Szender, J.B.; Odunsi, K.; Bahado-Singh, R. Metabolomics of biomarker discovery in ovarian cancer: A systematic review of the current literature. Metabolomics 2016, 12, 60. [Google Scholar] [CrossRef]
- Console, L.; Scalise, M.; Mazza, T.; Pochini, L.; Galluccio, M.; Giangregorio, N.; Tonazzi, A.; Indiveri, C. Carnitine traffic in cells: Link with cancer. Front. Cell Dev. Biol. 2020, 8, 583850. [Google Scholar] [CrossRef]
- Park, J.K.; Coffey, N.J.; Limoges, A.; Le, A. The heterogeneity of lipid metabolism in cancer. Adv. Exp. Med. Biol. 2018, 1063, 33–55. [Google Scholar] [CrossRef]
- Zara, V.; Assalve, G.; Ferramosca, A. Multiple roles played by the mitochondrial citrate carrier in cellular metabolism and physiology. Cell Mol. Life Sci. 2022, 79, 428. [Google Scholar] [CrossRef]
- Han, Q.; Chen, C.A.; Yang, W.; Liang, D.; Lv, H.W.; Lv, G.S.; Zong, Q.N.; Wang, H.Y. ATP-citrate lyase regulates stemness and metastasis in hepatocellular carcinoma via the Wnt/β-catenin signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Meng, X.; Zhou, Y.; Yu, J.; Li, Q.; Liao, Z.; Gu, Y.; Han, J.; Linghu, S.; Jiao, Z.; et al. Long noncoding RNA CTD-2245E15.3 promotes anabolic enzymes ACC1 and PC to support non-small cell lung cancer growth. Cancer Res. 2021, 81, 3509–3524. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Xue, H.; Zhou, L.; Dong, L.H.; Wei, D.P.; Li, H. Mechanism of fatty acid synthase in drug tolerance related to epithelial-mesenchymal transition of breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7617–7623. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Condello, S.; Thomes-Pepin, J.; Ma, X.; Xia, Y.; Hurley, T.D.; Matei, D.; Cheng, J.X. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell 2017, 20, 303–314.e5. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.; Brooks, A.; Guo, B.; Miskimins, K.W.; Qian, S.Y. Knockdown Delta-5-Desaturase Promotes the Formation of a Novel Free Radical Byproduct from COX-Catalyzed ω-6 Peroxidation to Induce Apoptosis and Sensitize Pancreatic Cancer Cells to Chemotherapy Drugs. Free Radic. Biol. Med. 2016, 97, 342–350. [Google Scholar] [CrossRef]
- Vriens, K.; Christen, S.; Parik, S.; Broekaert, D.; Yoshinaga, K.; Talebi, A.; Dehairs, J.; Escalona-Noguero, C.; Schmieder, R.; Cornfield, T.; et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 2019, 566, 403–406. [Google Scholar] [CrossRef]
- Tanaka, K.; Kandori, S.; Sakka, S.; Nitta, S.; Tanuma, K.; Shiga, M.; Nagumo, Y.; Negoro, H.; Kojima, T.; Mathis, B.J.; et al. ELOVL2 promotes cancer progression by inhibiting cell apoptosis in renal cell carcinoma. Oncol. Rep. 2022, 47, 23. [Google Scholar] [CrossRef]
- Hofmanová, J.; Slavík, J.; Ciganek, M.; Ovesná, P.; Tylichová, Z.; Karasová, M.; Zapletal, O.; Straková, N.; Procházková, J.; Bouchal, J.; et al. Complex alterations of fatty acid metabolism and phospholipidome uncovered in isolated colon cancer epithelial cells. Int. J. Mol. Sci. 2021, 22, 6650. [Google Scholar] [CrossRef]
- Su, Y.C.; Feng, Y.H.; Wu, H.T.; Huang, Y.S.; Tung, C.L.; Wu, P.; Chang, C.J.; Shiau, A.L.; Wu, C.L. Elovl6 is a negative clinical predictor for liver cancer and knockdown of Elovl6 reduces murine liver cancer progression. Sci. Rep. 2018, 8, 6586. [Google Scholar] [CrossRef]
- Tamura, K.; Makino, A.; Hullin-Matsuda, F.; Kobayashi, T.; Furihata, M.; Chung, S.; Ashida, S.; Miki, T.; Fujioka, T.; Shuin, T.; et al. Novel lipogenic enzyme ELOVL7 is involved in prostate cancer growth through saturated long-chain fatty acid metabolism. Cancer Res. 2009, 69, 8133–8140. [Google Scholar] [CrossRef]
- Das, M.; Giannoudis, A.; Sharma, V. The role of CPT1A as a biomarker of breast cancer progression: A bioinformatic approach. Sci. Rep. 2022, 12, 16441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Liu, J.; Ma, Y.; Ye, Q.; Yan, X.; Ding, L. MicroRNA-377-3p inhibits hepatocellular carcinoma growth and metastasis through negative regulation of CPT1C-mediated fatty acid oxidation. Cancer Metab. 2022, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Wang, Z.; Huang, T.; Xue, S.; Jiang, C.; Qiu, G.; Yuan, K. Fatty acids in cancer: Metabolic functions and potential treatment. MedComm—Oncol. 2023, 2, e25. [Google Scholar] [CrossRef]
- Samudio, I.; Harmancey, R.; Fiegl, M.; Kantarjian, H.; Konopleva, M.; Korchin, B.; Kaluarachchi, K.; Bornmann, W.; Duvvuri, S.; Taegtmeyer, H.; et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Investig. 2010, 120, 142–156. [Google Scholar] [CrossRef]
- Yang, L.; Cui, X.; Zhang, N.; Li, M.; Bai, Y.; Han, X.; Shi, Y.; Liu, H. Comprehensive Lipid Profiling of Plasma in Patients with Benign Breast Tumor and Breast Cancer Reveals Novel Biomarkers. Anal. Bioanal. Chem. 2015, 407, 5065–5077. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Jia, W. The Influence of Gut Microbiota on Drug Metabolism and Toxicity. Expert Opin. Drug Metab. Toxicol. 2016, 12, 31–40. [Google Scholar] [CrossRef]
- Buas, M.F.; Drescher, C.W.; Urban, N.; Li, C.I.; Bettcher, L.; Hait, N.C.; Moysich, K.B.; Odunsi, K.; Raftery, D.; Yan, L. Quantitative Global Lipidomics Analysis of Patients with Ovarian Cancer versus Benign Adnexal Mass. Sci. Rep. 2021, 11, 18156. [Google Scholar] [CrossRef]
- Masoodi, M.; Gastaldelli, A.; Hyötyläinen, T.; Arretxe, E.; Alonso, C.; Gaggini, M.; Brosnan, J.; Anstee, Q.M.; Millet, O.; Ortiz, P.; et al. Metabolomics and Lipidomics in NAFLD: Biomarkers and Non-invasive Diagnostic Tests. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 835–856. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The Critical Role of Phosphatidylcholine and Phosphatidylethanolamine Metabolism in Health and Disease. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Vethakanraj, H.S.; Chandrasekaran, N.; Sekar, A.K. Acid Ceramidase, a Double-Edged Sword in Cancer Aggression: A Minireview. Curr. Cancer Drug Targets 2020, 23. [Google Scholar] [CrossRef]
- Piazzesi, A.; Afsar, S.Y.; van Echten-Deckert, G. Sphingolipid Metabolism in the Development and Progression of Cancer: One Cancer’s Help Is Another’s Hindrance. Mol. Oncol. 2021, 15, 3256–3279. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Guo, J.; Zhang, X.; Feng, S.; Di, W.; Wang, Y.; Zhu, H. The Remodeling Roles of Lipid Metabolism in Colorectal Cancer Cells and Immune Microenvironment. Oncol. Res. 2023, 30, 231–242. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liu, Y.; Yan, F.; Zeng, Y.; Song, Y.; Fang, H.; Song, D.; Wang, X. Lysophosphatidylcholine Inhibits Lung Cancer Cell Proliferation by Regulating Fatty Acid Metabolism Enzyme Long-Chain Acyl-Coenzyme A Synthase 5. Clin. Transl. Med. 2023, 13, e1180. [Google Scholar] [CrossRef]
- Colquhoun, A.; Curi, R. Metabolic Fate and Effects of Saturated and Unsaturated Fatty Acids in Hep2 Human Larynx Tumor Cells. Biochem. Mol. Biol. Int. 1997, 41, 597–607. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Tabas, I. Role of Cholesterol and Lipid Organization in Disease. Nature 2005, 438, 612–621. [Google Scholar] [CrossRef]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid Metabolic Reprogramming in Cancer Cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Simons, K.; Sampaio, J.L. Membrane Organization and Lipid Rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef]
- Simons, K.; Gerl, M.J. Revitalizing Membrane Rafts: New Tools and Insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef]
- Xia, W.; Wang, H.; Zhou, X.; Wang, Y.; Xue, L.; Cao, B.; Song, J. The Role of Cholesterol Metabolism in Tumor Therapy, from Bench to Bed. Front. Pharmacol. 2023, 14, 928821. [Google Scholar] [CrossRef]
- Jin, X.; Demere, Z.; Nair, K.; Ali, A.; Ferraro, G.B.; Natoli, T.; Deik, A.; Petronio, L.; Tang, A.A.; Zhu, C.; et al. Publisher Correction: A Metastasis Map of Human Cancer Cell Lines. Nature 2021, 599, E7. [Google Scholar] [CrossRef]
- Ferraro, G.B.; Ali, A.; Luengo, A.; Kodack, D.P.; Deik, A.; Abbott, K.L.; Bezwada, D.; Blanc, L.; Prideaux, B.; Jin, X.; et al. Fatty Acid Synthesis Is Required for Breast Cancer Brain Metastasis. Nat. Cancer 2021, 2, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, U.; Ganai, R.A.; Macha, M.A.; Hamid, A.; Zargar, M.A.; Bhat, A.A.; Nasser, M.W.; Haris, M.; Batra, S.K.; Alshehri, B.; et al. The Tumor Microenvironment as Driver of Stemness and Therapeutic Resistance in Breast Cancer: New Challenges and Therapeutic Opportunities. Cell Oncol. 2021, 44, 1209–1229. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty Acid Synthase: A Druggable Driver of Breast Cancer Brain Metastasis. Expert Opin. Ther. Targets 2022, 26, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lin, X.; Wang, G. Targeting SREBP-1-Mediated Lipogenesis as Potential Strategies for Cancer. Front. Oncol. 2022, 12, 952371. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a Metabolic Disease: Implications for Novel Therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone Bodies as Signaling Metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Valencia, T.; Kim, J.Y.; Abu-Baker, S.; Moscat-Pardos, J.; Ahn, C.S.; Reina-Campos, M.; Duran, A.; Castilla, E.A.; Metallo, C.M.; Diaz-Meco, M.T.; et al. Metabolic Reprogramming of Stromal Fibroblasts through p62-mTORC1 Signaling Promotes Inflammation and Tumorigenesis. Cancer Cell 2014, 26, 121–135. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Borregaard, N. Neutrophil Extracellular Traps—The Dark Side of Neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef]
- Barber, G.N. STING-Dependent Cytosolic DNA Sensing Pathways. Trends Immunol. 2014, 35, 88–93. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Pekarek, L.; Torres-Carranza, D.; Fraile-Martinez, O.; García-Montero, C.; Pekarek, T.; Saez, M.A.; Rueda-Correa, F.; Pimentel-Martinez, C.; Guijarro, L.G.; Diaz-Pedrero, R.; et al. An Overview of the Role of MicroRNAs on Carcinogenesis: A Focus on Cell Cycle, Angiogenesis and Metastasis. Int. J. Mol. Sci. 2023, 24, 7268. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Xing, Z.; Zhou, J.; Cui, C.; Liu, C.; Liu, Y.; Li, Z. Retinoic Acid-Inducible Gene-I Like Receptor Pathway in Cancer: Modification and Treatment. Front. Immunol. 2023, 14, 1227041. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, X.; Han, S.; Liang, Q.; Ma, X.; Rong, P.; Wang, W.; Li, W. The cGAS/STING Pathway: A Novel Target for Cancer Therapy. Front. Immunol. 2022, 12, 795401. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of Uric Acid Metabolism and Excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Pedley, A.M.; Benkovic, S.J. A New View into the Regulation of Purine Metabolism: The Purinosome. Trends Biochem. Sci. 2017, 42, 141–154. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Huang, X.; Deng, J.; Li, T.; Yin, Y. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef]
- Saugstad, O. Hypoxanthine as a Measurement of Hypoxia. Pediatr. Res. 1975, 9, 158–161. [Google Scholar] [CrossRef]
- Lundgren, K.; Nordenskjöld, B.; Landberg, G. Hypoxia, Snail and Incomplete Epithelial-Mesenchymal Transition in Breast Cancer. Br. J. Cancer 2009, 101, 1769–1781. [Google Scholar] [CrossRef]
- Shakartalla, S.B.; Ashmawy, N.S.; Semreen, M.H.; Fayed, B.; Al Shareef, Z.M.; Jayakumar, M.N.; Ibrahim, S.; Rahmani, M.; Hamdy, R.; Soliman, S.S. M. 1H-NMR Metabolomics Analysis Identifies Hypoxanthine as a Novel Metastasis-Associated Metabolite in Breast Cancer. Sci. Rep. 2024, 14, 253. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Fisher, E.A.; Breslow, J.L. Overexpression of PCSK9 Accelerates the Degradation of the LDLR in a Post-Endoplasmic Reticulum Compartment. Proc. Natl. Acad. Sci. USA 2005, 102, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic Acid Food Fortification-Its History, Effect, Concerns, and Future Directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.M. B-Group Vitamins: Chemoprevention? Adv. Clin. Exp. Med. 2016, 25, 561–568. [Google Scholar] [CrossRef]
- Liew, S.C. Folic Acid and Diseases-Supplement It or Not? Rev. Assoc. Med. Bras. 2016, 62, 90–100. [Google Scholar] [CrossRef]
- Rai, V. Folate Pathway Gene MTHFR C677T Polymorphism and Risk of Lung Cancer in Asian Populations. Asian Pac. J. Cancer Prev. 2014, 15, 9259–9264. [Google Scholar] [CrossRef]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; et al. Targeting Folate Receptor Alpha for Cancer Treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef]
- Stanisławska-Sachadyn, A.; Borzyszkowska, J.; Krzemiński, M.; Janowicz, A.; Dziadziuszko, R.; Jassem, J.; Rzyman, W.; Limon, J. Folate/Homocysteine Metabolism and Lung Cancer Risk among Smokers. PLoS ONE 2019, 14, e0214462. [Google Scholar] [CrossRef]
- Rycyna, K.J.; Bacich, D.J.; O’Keefe, D.S. Opposing Roles of Folate in Prostate Cancer. Urology 2013, 82, 1197–1203. [Google Scholar] [CrossRef]
- Jan, C.H.; Williams, C.C.; Weissman, J.S. Principles of ER Cotranslational Translocation Revealed by Proximity-Specific Ribosome Profiling. Science 2014, 346, 1257521. [Google Scholar] [CrossRef]
- Reid, D.W.; Nicchitta, C.V. Diversity and Selectivity in mRNA Translation on the Endoplasmic Reticulum. Nat. Rev. Mol. Cell Biol. 2015, 16, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kaufman, R.J. The Role of ER Stress in Lipid Metabolism and Lipotoxicity. J. Lipid Res. 2016, 57, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Vasic, V.; Denkert, N.; Schmidt, C.C.; Riedel, D.; Stein, A.; Meinecke, M. Hrd1 Forms the Retrotranslocation Pore Regulated by Auto-Ubiquitination and Binding of Misfolded Proteins. Nat. Cell Biol. 2020, 22, 274–281. [Google Scholar] [CrossRef]
- Christianson, J.C.; Ye, Y. Cleaning Up in the Endoplasmic Reticulum: Ubiquitin in Charge. Nat. Struct. Mol. Biol. 2014, 21, 325–335. [Google Scholar] [CrossRef]
- Li, A.; Song, N.J.; Riesenberg, B.P.; Li, Z. The Emerging Roles of Endoplasmic Reticulum Stress in Balancing Immunity and Tolerance in Health and Diseases: Mechanisms and Opportunities. Front. Immunol. 2020, 10, 3154. [Google Scholar] [CrossRef]
- Luethy, J.D.; Holbrook, N.J. Activation of the gadd153 Promoter by Genotoxic Agents: A Rapid and Specific Response to DNA Damage. Cancer Res. 1992, 52, 5–10. [Google Scholar] [PubMed]
- Wang, X.; Eno, C.O.; Altman, B.J.; Zhu, Y.; Zhao, G.; Olberding, K.E.; Rathmell, J.C.; Li, C. ER Stress Modulates Cellular Metabolism. Biochem. J. 2011, 435, 285–296. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Oyang, L.; Cui, S.; Li, S.; Li, J.; Liu, L.; Li, Y.; Peng, M.; Tan, S.; et al. Endoplasmic Reticulum Stress-A Key Guardian in Cancer. Cell Death Discov. 2024, 10, 343. [Google Scholar] [CrossRef]
- Vannuvel, K.; Renard, P.; Raes, M.; Arnould, T. Functional and Morphological Impact of ER Stress on Mitochondria. J. Cell Physiol. 2013, 228, 1802–1818. [Google Scholar] [CrossRef]
- Liang, D.; Khoonkari, M.; Avril, T.; Chevet, E.; Kruyt, F.A.E. The Unfolded Protein Response as Regulator of Cancer Stemness and Differentiation: Mechanisms and Implications for Cancer Therapy. Biochem. Pharmacol. 2021, 192, 114737. [Google Scholar] [CrossRef]
- Miranda, M.R.; Vestuto, V.; Amodio, G.; Manfra, M.; Pepe, G.; Campiglia, P. Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential. Life 2024, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The Unfolded Protein Response: Controlling Cell Fate Decisions under ER Stress and Beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Madden, E.; Logue, S.E.; Healy, S.J.; Manie, S.; Samali, A. The Role of the Unfolded Protein Response in Cancer Progression: From Oncogenesis to Chemoresistance. Biol. Cell 2019, 111, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Novi, S.; Vestuto, V.; Campiglia, P.; Tecce, N.; Bertamino, A.; Tecce, M.F. Anti-Angiogenic Effects of Natural Compounds in Diet-Associated Hepatic Inflammation. Nutrients 2023, 15, 2748. [Google Scholar] [CrossRef]
- Siwecka, N.; Galita, G.; Granek, Z.; Wiese, W.; Majsterek, I.; Rozpędek-Kamińska, W. IRE1/JNK Is the Leading UPR Pathway in 6-OHDA-Induced Degeneration of Differentiated SH- SY5Y Cells. Int. J. Mol. Sci. 2024, 25, 7679. [Google Scholar] [CrossRef]
- Yan, M.M.; Ni, J.D.; Song, D.; Ding, M.; Huang, J. Interplay Between Unfolded Protein Response and Autophagy Promotes Tumor Drug Resistance. Oncol. Lett. 2015, 10, 1959–1969. [Google Scholar] [CrossRef]
- Ni, L.; Yang, L.; Lin, Y. Recent Progress of Endoplasmic Reticulum Stress in the Mechanism of Atherosclerosis. Front. Cardiovasc. Med. 2024, 11, 1413441. [Google Scholar] [CrossRef]
- Li, W.; Jin, K.; Luo, J.; Xu, W.; Wu, Y.; Zhou, J.; Wang, Y.; Xu, R.; Jiao, L.; Wang, T.; et al. NF-κB and Its Crosstalk with Endoplasmic Reticulum Stress in Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 988266. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic Reticulum Stress Signalling—From Basic Mechanisms to Clinical Applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Kroeger, H.; Grimsey, N.; Paxman, R.; Chiang, W.C.; Plate, L.; Jones, Y.; Shaw, P.X.; Trejo, J.; Tsang, S.H.; Powers, E.; et al. The Unfolded Protein Response Regulator ATF6 Promotes Mesodermal Differentiation. Sci. Signal. 2018, 11, eaan5785. [Google Scholar] [CrossRef]
- Wu, D.; Robinson, G.; Xie, J. Unfolded Protein Response Factor ATF6 Augments T Helper Cell Responses and Promotes Mixed Granulocytic Airway Inflammation. Mucosal Immunol. 2024, 16, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, K.R.; McMillan, J.D.; Kumar, A. Emerging Roles of ER Stress and Unfolded Protein Response Pathways in Skeletal Muscle Health and Disease. J. Cell Physiol. 2018, 233, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hillary, R.F.; FitzGerald, U. A Lifetime of Stress: ATF6 in Development and Homeostasis. J. Biomed. Sci. 2018, 25, 48. [Google Scholar] [CrossRef]
- Sharma, R.B.; Snyder, J.T.; Alonso, L.C. Atf6α Impacts Cell Number by Influencing Survival, Death and Proliferation. Mol. Metab. 2019, 27, S69–S80. [Google Scholar] [CrossRef]
- Pagliara, V.; Amodio, G.; Vestuto, V.; Franceschelli, S.; Russo, N.A.; Cirillo, V.; Mottola, G.; Remondelli, P.; Moltedo, O. Myogenesis in C2C12 Cells Requires Phosphorylation of ATF6α by p38 MAPK. Biomedicines 2023, 11, 1457. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, Y.; Zhao, N.; Guan, G.; Wang, J. Protein Kinase R-like ER Kinase and Its Role in Endoplasmic Reticulum Stress-Decided Cell Fate. Cell Death Dis. 2015, 6, e1822. [Google Scholar] [CrossRef]
- Bravo, R.; Parra, V.; Gatica, D.; Rodriguez, A.E.; Torrealba, N.; Paredes, F.; Wang, Z.V.; Zorzano, A.; Hill, J.A.; Jaimovich, E.; et al. Endoplasmic Reticulum and the Unfolded Protein Response: Dynamics and Metabolic Integration. Int. Rev. Cell Mol. Biol. 2013, 301, 215–290. [Google Scholar] [CrossRef]
- Rusiñol, A.E.; Cui, Z.; Chen, M.H.; Vance, J.E. A Unique Mitochondria-Associated Membrane Fraction from Rat Liver Has a High Capacity for Lipid Synthesis and Contains Pre-Golgi Secretory Proteins Including Nascent Lipoproteins. J. Biol. Chem. 1994, 269, 27494–27502. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Phospholipid Synthesis in a Membrane Fraction Associated with Mitochondria. J. Biol. Chem. 1990, 265, 7248–7256. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T. Microdomains with High Ca2+ Close to IP3- Sensitive Channels That Are Sensed by Neighboring Mitochondria. Science 1993, 262, 744–747. [Google Scholar] [CrossRef]
- Lynes, E.M.; Bui, M.; Yap, M.C.; Benson, M.D.; Schneider, B.; Ellgaard, L.; Berthiaume, L.G.; Simmen, T. Palmitoylated TMX and Calnexin Target to the Mitochondria-Associated Membrane. EMBO J. 2012, 31, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Gilady, S.Y.; Bui, M.; Lynes, E.M.; Benson, M.D.; Watts, R.; Vance, J.E.; Simmen, T. Ero1alpha Requires Oxidizing and Normoxic Conditions to Localize to the Mitochondria-Associated Membrane (MAM). Cell Stress Chaperones 2010, 15, 619–629. [Google Scholar] [CrossRef]
- Myhill, N.; Lynes, E.M.; Nanji, J.A.; Blagoveshchenskaya, A.D.; Fei, H.; Simmen, K.; Cooper, T.J.; Thomas, G.; Simmen, T. The Subcellular Distribution of Calnexin Is Mediated by PACS-2. Mol. Biol. Cell 2008, 19, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nishitoh, H. Stress Responses from the Endoplasmic Reticulum in Cancer. Front. Oncol. 2015, 5, 93. [Google Scholar] [CrossRef]
- Healy, S.J.; Gorman, A.M.; Mousavi-Shafaei, P.; Gupta, S.; Samali, A. Targeting the Endoplasmic Reticulum-Stress Response as an Anticancer Strategy. Eur. J. Pharmacol. 2009, 625, 234–246. [Google Scholar] [CrossRef]
- Ma, Y.; Hendershot, L.M. The Role of the Unfolded Protein Response in Tumour Development: Friend or Foe? Nat. Rev. Cancer 2004, 4, 966–977. [Google Scholar] [CrossRef]
- Moenner, M.; Pluquet, O.; Bouchecareilh, M.; Chevet, E. Integrated Endoplasmic Reticulum Stress Responses in Cancer. Cancer Res. 2007, 67, 10631–10634. [Google Scholar] [CrossRef]
- Dejeans, N.; Manié, S.; Hetz, C.; Bard, F.; Hupp, T.; Agostinis, P.; Samali, A.; Chevet, E. Addicted to Secrete—Novel Concepts and Targets in Cancer Therapy. Trends Mol. Med. 2014, 20, 242–250. [Google Scholar] [CrossRef]
- Chen, X.; Iliopoulos, D.; Zhang, Q.; Tang, Q.; Greenblatt, M.B.; Hatziapostolou, M.; Lim, E.; Tam, W.L.; Ni, M.; Chen, Y.; et al. XBP1 Promotes Triple-Negative Breast Cancer by Controlling the HIF1α Pathway. Nature 2014, 508, 103–107. [Google Scholar] [CrossRef]
- Croft, A.; Tay, K.H.; Boyd, S.C.; Guo, S.T.; Jiang, C.C.; Lai, F.; Tseng, H.Y.; Jin, L.; Rizos, H.; Hersey, P.; et al. Oncogenic Activation of MEK/ERK Primes Melanoma Cells for Adaptation to Endoplasmic Reticulum Stress. J. Investig. Dermatol. 2014, 134, 488–497. [Google Scholar] [CrossRef]
- Hart, L.S.; Cunningham, J.T.; Datta, T.; Dey, S.; Tameire, F.; Lehman, S.L.; Qiu, B.; Zhang, H.; Cerniglia, G.; Bi, M.; et al. ER Stress- Mediated Autophagy Promotes Myc-Dependent Transformation and Tumor Growth. J. Clin. Investig. 2012, 122, 4621–4634. [Google Scholar] [CrossRef] [PubMed]
- Blazanin, N.; Son, J.; Craig-Lucas, A.B.; John, C.L.; Breech, K.J.; Podolsky, M.A.; Glick, A.B. ER Stress and Distinct Outputs of the IRE1α RNase Control Proliferation and Senescence in Response to Oncogenic Ras. Proc. Natl. Acad. Sci. USA 2017, 114, 9900–9905. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jiang, M.; Chen, W.; Zhao, T.; Wei, Y. Cancer and ER Stress: Mutual Crosstalk between Autophagy, Oxidative Stress and Inflammatory Response. Biomed. Pharmacother. 2019, 118, 109249. [Google Scholar] [CrossRef]
- Ma, X.H.; Piao, S.F.; Dey, S.; McAfee, Q.; Karakousis, G.; Villanueva, J.; Hart, L.S.; Levi, S.; Hu, J.; Zhang, G.; et al. Targeting ER Stress-Induced Autophagy Overcomes BRAF Inhibitor Resistance in Melanoma. J. Clin. Investig. 2014, 124, 1406–1417. [Google Scholar] [CrossRef]
- Ding, W.X.; Ni, H.M.; Gao, W.; Hou, Y.F.; Melan, M.A.; Chen, X.; Stolz, D.B.; Shao, Z.M.; Yin, X.M. Differential Effects of Endoplasmic Reticulum Stress-Induced Autophagy on Cell Survival. J. Biol. Chem. 2007, 282, 4702–4710. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z. Glucose-Regulated Protein 78: A Critical Link Between Tumor Microenvironment and Cancer Hallmarks. Biochim. Biophys. Acta 2012, 1826, 13–22. [Google Scholar] [CrossRef]
- Zhuang, L.; Scolyer, R.A.; Lee, C.S.; McCarthy, S.W.; Cooper, W.A.; Zhang, X.D.; Thompson, J.F.; Hersey, P. Expression of Glucose-Regulated Stress Protein GRP78 Is Related to Progression of Melanoma. Histopathology 2009, 54, 462–470. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chae, S.W.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Cancer. J. Cancer Prev. 2014, 19, 75–88. [Google Scholar] [CrossRef]
- Kosakowska-Cholody, T.; Lin, J.; Srideshikan, S.; Scheffer, L.; Tarasova, N.I.; Acharya, J.K. HKH40A Downregulates GRP78/BiP Expression in Cancer Cells. Cell Death Dis. 2014, 5, e1240. [Google Scholar] [CrossRef]
- Lin, M.; Mo, Y.; Li, C.M.; Liu, Y.Z.; Feng, X.P. GRP78 as a Potential Therapeutic Target in Cancer Treatment: An Updated Review of Its Role in Chemoradiotherapy Resistance of Cancer Cells. Med. Oncol. 2025, 42, 49. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tsung, K.; Attenello, F.J. Characterizing Cell Stress and GRP78 in Glioma to Enhance Tumor Treatment. Front. Oncol. 2020, 10, 608911. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Lee, A.S. GRP78/BiP Inhibits Endoplasmic Reticulum BIK and Protects Human Breast Cancer Cells against Estrogen Starvation-Induced Apoptosis. Cancer Res. 2007, 67, 3734–3740. [Google Scholar] [CrossRef]
- Kim, K.W.; Moretti, L.; Mitchell, L.; Jung, D.K.; Lu, B. Endoplasmic Reticulum Stress Mediates Radiation-Induced Autophagy by PERK-eIF2α in Caspase-3/7-Deficient Cells. Oncogene 2010, 29, 3241–3251. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Buckley, C.E.; O’Brien, R.M.; Nugent, T.S.; Donlon, N.E.; O’Connell, F.; Reynolds, J.V.; Hafeez, A.; O’Ríordáin, D.S.; Hannon, R.A.; Neary, P.; et al. Metformin Is a Metabolic Modulator and Radiosensitiser in Rectal Cancer. Front. Oncol. 2023, 13, 1216911. [Google Scholar] [CrossRef]
- Song, C.W.; Lee, H.; Dings, R.P.; Williams, B.; Powers, J.; Santos, T.D.; Choi, B.H.; Park, H.J. Metformin Kills and Radiosensitizes Cancer Cells and Preferentially Kills Cancer Stem Cells. Sci. Rep. 2012, 2, 362. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Y.; Lian, X. Targeted Inhibition of GRP78 by HA15 Promotes Apoptosis of Lung Cancer Cells Accompanied by ER Stress and Autophagy. Biol. Open 2020, 9, bio053298. [Google Scholar] [CrossRef]
- Pantziarka, P.; Sukhatme, V.; Bouche, G.; Meheus, L.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)-Itraconazole as an Anti-Cancer Agent. Ecancermedicalscience 2015, 9, 521. [Google Scholar] [CrossRef]
- Amaresan, R.; Gopal, U. Cell Surface GRP78: A Potential Mechanism of Therapeutic Resistant Tumors. Cancer Cell Int. 2023, 23, 100. [Google Scholar] [CrossRef]
- Xue, Z.; He, Y.; Ye, K.; Gu, Z.; Mao, Y.; Qi, L. A Conserved Structural Determinant Located at the Interdomain Region of Mammalian Inositol-Requiring Enzyme 1alpha. J. Biol. Chem. 2011, 286, 30859–30866. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Wang, L.; Wang, E.S.; Perera, B.G.; Igbaria, A.; Morita, S.; Prado, K.; Thamsen, M.; Caswell, D.; Macias, H.; et al. Allosteric Inhibition of the IRE1 RNase Preserves Cell Viability and Function during Endoplasmic Reticulum Stress. Cell 2014, 158, 534–548. [Google Scholar] [CrossRef]
- Clarke, H.J.; Chambers, J.E.; Liniker, E.; Marciniak, S.J. Endoplasmic Reticulum Stress in Malignancy. Cancer Cell 2014, 25, 563–573. [Google Scholar] [CrossRef]
- Jiang, D.; Niwa, M.; Koong, A.C. Targeting the IRE1α-XBP1 Branch of the Unfolded Protein Response in Human Diseases. Semin. Cancer Biol. 2015, 33, 48–56. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Zhang, G.; Ji, G.; Xu, H. The Role and Therapeutic Implication of Endoplasmic Reticulum Stress in Inflammatory Cancer Transformation. Am. J. Cancer Res. 2022, 12, 2277–2292. [Google Scholar]
- Ri, M.; Tashiro, E.; Oikawa, D.; Shinjo, S.; Tokuda, M.; Yokouchi, Y.; Narita, T.; Masaki, A.; Ito, A.; Ding, J.; et al. Identification of Toyocamycin, an Agent Cytotoxic for Multiple Myeloma Cells, as a Potent Inhibitor of ER Stress-Induced XBP1 mRNA Splicing. Blood Cancer J. 2012, 2, e79. [Google Scholar] [CrossRef]
- Chen, X.L.; Fu, J.P.; Shi, J.; Wan, P.; Cao, H.; Tang, Z.M. CXC195 Induces Apoptosis and Endoplasmic Reticulum Stress in Human Hepatocellular Carcinoma Cells by Inhibiting the PI3K/Akt/mTOR Signaling Pathway. Mol. Med. Rep. 2015, 12, 8229–8236. [Google Scholar]
- Raymundo, D.P.; Doultsinos, D.; Guillory, X.; Carlesso, A.; Eriksson, L.A.; Chevet, E. Pharmacological Targeting of IRE1 in Cancer. Trends Cancer 2020, 6, 1018–1030. [Google Scholar] [CrossRef]
- Vandewynckel, Y.P.; Laukens, D.; Geerts, A.; Bogaerts, E.; Paridaens, A.; Verhelst, X.; Janssens, S.; Heindryckx, F.; Van Vlierberghe, H. The Paradox of the Unfolded Protein Response in Cancer. Anticancer Res. 2013, 33, 4683–4694. [Google Scholar]
- Chang, K.C.; Chen, P.C.; Chen, Y.P.; Chang, Y.; Su, I.J. Dominant Expression of Survival Signals of Endoplasmic Reticulum Stress Response in Hodgkin Lymphoma. Cancer Sci. 2011, 102, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Schewe, D.M.; Aguirre-Ghiso, J.A. ATF6α-Rheb-mTOR Signaling Promotes Survival of Dormant Tumor Cells In Vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 10519–10524. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.M.; Garri, C.; Cain, E.L.; Ang, K.K.; Wilson, C.G.; Chen, S.; Hearn, B.R.; Jaishankar, P.; Aranda-Diaz, A.; Arkin, M.R.; et al. Ceapins Are a New Class of Unfolded Protein Response Inhibitors, Selectively Targeting the ATF6α Branch. Elife 2016, 5, e11878. [Google Scholar] [CrossRef] [PubMed]
- Nikesitch, N.; Lee, J.M.; Ling, S.; Roberts, T.L. Endoplasmic Reticulum Stress in the Development of Multiple Myeloma and Drug Resistance. Clin. Transl. Immunol. 2018, 7, e1007. [Google Scholar] [CrossRef]
- Macke, A.J.; Pachikov, A.N.; Divita, T.E.; Morris, M.E.; LaGrange, C.A.; Holzapfel, M.S.; Kubyshkin, A.V.; Zyablitskaya, E.Y.; Makalish, T.P.; Eremenko, S.N.; et al. Targeting the ATF6-Mediated ER Stress Response and Autophagy Blocks Integrin-Driven Prostate Cancer Progression. Mol. Cancer Res. 2023, 21, 958–974. [Google Scholar] [CrossRef]
- Luo, B.; Lee, A.S. The Critical Roles of Endoplasmic Reticulum Chaperones and Unfolded Protein Response in Tumorigenesis and Anticancer Therapies. Oncogene 2013, 32, 805–818. [Google Scholar] [CrossRef]
- Sequeira, S.J.; Ranganathan, A.C.; Adam, A.P.; Iglesias, B.V.; Farias, E.F.; Aguirre-Ghiso, J.A. Inhibition of Proliferation by PERK Regulates Mammary Acinar Morphogenesis and Tumor Formation. PLoS ONE 2007, 2, e615. [Google Scholar] [CrossRef]
- Perkins, D.J.; Barber, G.N. Defects in Translational Regulation Mediated by the Alpha Subunit of Eukaryotic Initiation Factor 2 Inhibit Antiviral Activity and Facilitate the Malignant Transformation of Human Fibroblasts. Mol. Cell. Biol. 2004, 24, 2025–2040. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP Induces Death by Promoting Protein Synthesis and Oxidation in the Stressed Endoplasmic Reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Fawcett, T.W.; Martindale, J.L.; Guyton, K.Z.; Hai, T.; Holbrook, N.J. Complexes Containing Activating Transcription Factor (ATF)/cAMP-Responsive-Element-Binding Protein (CREB) Interact with the CCAAT/Enhancer-Binding Protein (C/EBP)-ATF Composite Site to Regulate Gadd153 Expression during the Stress Response. Biochem. J. 1999, 339, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bagratuni, T.; Patseas, D.; Mavrianou-Koutsoukou, N.; Liacos, C.I.; Sklirou, A.D.; Rousakis, P.; Gavriatopoulou, M.; Terpos, E.; Tsitsilonis, O.E.; Trougakos, I.P.; et al. Characterization of a PERK Kinase Inhibitor with Anti-Myeloma Activity. Cancers 2020, 12, 2864. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Walter, P. The Integrated Stress Response: From Mechanism to Disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, J.H.; Shin, J.I.; Jeong, M.; Cho, J.; Lee, K. Salubrinal-Mediated Upregulation of eIF2α Phosphorylation Increases Doxorubicin Sensitivity in MCF-7/ADR Cells. Mol. Cells 2016, 39, 129–135. [Google Scholar] [CrossRef]
- Cai, L.; Jin, X.; Zhang, J.; Li, L.; Zhao, J. Metformin Suppresses Nrf2-Mediated Chemoresistance in Hepatocellular Carcinoma Cells by Increasing Glycolysis. Aging 2020, 12, 17582–17600. [Google Scholar] [CrossRef]
- Obeng, E.A.; Carlson, L.M.; Gutman, D.M.; Harrington, W.J., Jr.; Lee, K.P.; Boise, L.H. Proteasome Inhibitors Induce a Terminal Unfolded Protein Response in Multiple Myeloma Cells. Blood 2006, 107, 4907–4916. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic Reticulum Stress Signals in the Tumour and Its Microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Denzel, M.S.; Antebi, A. Hexosamine Pathway and ER Protein Quality Control. Curr. Opin. Cell Biol. 2015, 33, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Domblides, C.; Lartigue, L.; Faustin, B. Metabolic Stress in the Immune Function of T Cells, Macrophages and Dendritic Cells. Cells 2018, 7, 68. [Google Scholar] [CrossRef]
- Braakman, I.; Bulleid, N.J. Protein Folding and Modification in the Mammalian Endoplasmic Reticulum. Annu. Rev. Biochem. 2011, 80, 71–99. [Google Scholar] [CrossRef]
- Moore, C.E.; Omikorede, O.; Gomez, E.; Willars, G.B.; Herbert, T.P. PERK Activation at Low Glucose Concentration Is Mediated by SERCA Pump Inhibition and Confers Preemptive Cytoprotection to Pancreatic β-cells. Mol. Endocrinol. 2011, 25, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.T.; Abdulhadi, M.A.; Alkubaisy, S.A.; Thejeel, S.H.; Essa, I.M.; Merza, M.S.; Zabibah, R.S.; Al-Tamimi, R. The Role of Endoplasmic Reticulum Stress in Promoting Aerobic Glycolysis in Cancer Cells: An Overview. Pathol. Res. Pract. 2023, 251, 154905. [Google Scholar] [CrossRef]
- Araki, K.; Iemura, S.; Kamiya, Y.; Ron, D.; Kato, K.; Natsume, T.; Nagata, K. Ero1-α and PDIs Constitute a Hierarchical Electron Transfer Network of Endoplasmic Reticulum Oxidoreductases. J. Cell Biol. 2013, 202, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.P.; Weissman, J.S. The FAD- and O2-Dependent Reaction Cycle of Ero1-Mediated Oxidative Protein Folding in the Endoplasmic Reticulum. Mol. Cell 2002, 10, 983–994. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, G.; Gao, S.; Chen, Y.; Jin, C.; Wang, Z.; Yang, Y.; Ma, Z.; Zhang, W.; Feng, X. Expression of ERO1L in Gastric Cancer and Its Association with Patient Prognosis. Exp. Ther. Med. 2017, 14, 2298–2302. [Google Scholar] [CrossRef]
- Zito, E. ERO1: A Protein Disulfide Oxidase and H2O2 Producer. Free Radic. Biol. Med. 2015, 83, 299–304. [Google Scholar] [CrossRef]
- Urra, H.; Aravena, R.; González-Johnson, L.; Hetz, C. The UPRising Connection Between Endoplasmic Reticulum Stress and the Tumor Microenvironment. Trends Cancer 2024, 10, 1161–1173. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of Oxygen Homeostasis by Hypoxia-Inducible Factor 1. Physiology 2009, 24, 97–106. [Google Scholar] [CrossRef]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Han, F.; Xu, Q.; Zhao, J.; Xiong, P.; Liu, J. ERO1L Promotes Pancreatic Cancer Cell Progression through Activating the Wnt/β-Catenin Pathway. J. Cell Biochem. 2018, 119, 8996–9005. [Google Scholar] [CrossRef]
- Yang, S.; Yang, C.; Yu, F.; Ding, W.; Hu, Y.; Cheng, F.; Zhang, F.; Guan, B.; Wang, X.; Lu, L.; et al. Endoplasmic Reticulum Resident Oxidase ERO1-L alpha Promotes Hepatocellular Carcinoma Metastasis and Angiogenesis through the S1PR1/STAT3/VEGF-A Pathway. Cell Death Dis. 2018, 9, 1105. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Lin, C.; Liu, W.; Huo, Y.; Yang, M.; Jiang, S.H.; Sun, Y.; Hua, R. Endoplasmic Reticulum Stress- Dependent Expression of ERO1L Promotes Aerobic Glycolysis in Pancreatic Cancer. Theranostics 2020, 10, 8400–8414. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Tomida, A.; Sato, S.; Tsukumo, Y.; Yun, J.; Yamori, T.; Hayakawa, Y.; Tsuruo, T.; Shin-ya, K. Effect on Tumor Cells of Blocking Survival Response to Glucose Deprivation. J. Natl. Cancer Inst. 2004, 96, 1300–1310. [Google Scholar] [CrossRef]
- Pandey, S.; Singh, R.; Habib, N.; Tripathi, R.M.; Kushwaha, R.; Mahdi, A.A. Regulation of Hypoxia-Dependent Reprogramming of Cancer Metabolism: Role of HIF-1 and Its Potential Therapeutic Implications in Leukemia. Asian Pac. J. Cancer Prev. 2024, 25, 1121–1134. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal Integration in the Endoplasmic Reticulum Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W.; Gao, Y.; Zhang, Y.; Zhao, Y.; Hao, S.; Ni, T. The Role of Tumor Metabolic Reprogramming in Tumor Immunity. Int. J. Mol. Sci. 2023, 24, 17422. [Google Scholar] [CrossRef]
- Xie, H.; Tang, C.H.; Song, J.H.; Mancuso, A.; Del Valle, J.R.; Cao, J.; Xiang, Y.; Dang, C.V.; Lan, R.; Sanchez, D.J.; et al. IRE1α RNase- Dependent Lipid Homeostasis Promotes Survival in Myc-Transformed Cancers. J. Clin. Investig. 2018, 128, 1300–1316. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Lu, Y.; Song, J.; Huang, M.; Zhang, J.; Huang, Y. The Role of Stearoyl- Coenzyme A Desaturase 1 in Clear Cell Renal Cell Carcinoma. Tumour Biol. 2016, 37, 479–489. [Google Scholar] [CrossRef]
- Almanza, A.; Mnich, K.; Blomme, A.; Robinson, C.M.; Rodriguez-Blanco, G.; Kierszniowska, S.; McGrath, E.P.; Le Gallo, M.; Pilalis, E.; Swinnen, J.V.; et al. Regulated IRE1α-Dependent Decay (RIDD)-Mediated Reprogramming of Lipid Metabolism in Cancer. Nat. Commun. 2022, 13, 2493. [Google Scholar] [CrossRef]
- Bobrovnikova-Marjon, E.; Hatzivassiliou, G.; Grigoriadou, C.; Romero, M.; Cavener, D.R.; Thompson, C.B.; Diehl, J.A. PERK-Dependent Regulation of Lipogenesis during Mouse Mammary Gland Development and Adipocyte Differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 16314–16319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Bai, B.; Shang, C.; Fang, J.; Cong, J.; Li, W.; Li, S.; Song, G.; Liu, Z.; et al. Effects of Apoptin-Induced Endoplasmic Reticulum Stress on Lipid Metabolism, Migration, and Invasion of HepG-2 Cells. Front. Oncol. 2021, 11, 614082. [Google Scholar] [CrossRef] [PubMed]
- Borradaile, N.M.; Han, X.; Harp, J.D.; Gale, S.E.; Ory, D.S.; Schaffer, J.E. Disruption of Endoplasmic Reticulum Structure and Integrity in Lipotoxic Cell Death. J. Lipid Res. 2006, 47, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Volmer, R.; van der Ploeg, K.; Ron, D. Membrane Lipid Saturation Activates Endoplasmic Reticulum Unfolded Protein Response Transducers through Their Transmembrane Domains. Proc. Natl. Acad. Sci. USA 2013, 110, 4628–4633. [Google Scholar] [CrossRef]
- Kitai, Y.; Ariyama, H.; Kono, N.; Oikawa, D.; Iwawaki, T.; Arai, H. Membrane Lipid Saturation Activates IRE1α without Inducing Clustering. Genes Cells 2013, 18, 798–809. [Google Scholar] [CrossRef]
- Ariyama, H.; Kono, N.; Matsuda, S.; Inoue, T.; Arai, H. Decrease in Membrane Phospholipid Unsaturation Induces Unfolded Protein Response. J. Biol. Chem. 2010, 285, 22027–22035. [Google Scholar] [CrossRef]
- Williams, K.J.; Argus, J.P.; Zhu, Y.; Wilks, M.Q.; Marbois, B.N.; York, A.G.; Kidani, Y.; Pourzia, A.L.; Akhavan, D.; Lisiero, D.N.; et al. An Essential Requirement for the SCAP/SREBP Signaling Axis to Protect Cancer Cells from Lipotoxicity. Cancer Res. 2013, 73, 2850–2862. [Google Scholar] [CrossRef]
- Rios-Marco, P.; Martin-Fernandez, M.; Soria-Bretones, I.; Rios, A.; Carrasco, M.P.; Marco, C. Alkylphospholipids Deregulate Cholesterol Metabolism and Induce Cell-Cycle Arrest and Autophagy in U-87 MG Glioblastoma Cells. Biochim. Biophys. Acta 2013, 1831, 1322–1334. [Google Scholar] [CrossRef]
- Rios-Marco, P.; Rios, A.; Jimenez-Lopez, J.M.; Carrasco, M.P.; Marco, C. Cholesterol Homeostasis and Autophagic Flux in Perifosine-Treated Human Hepatoblastoma HepG2 and Glioblastoma U-87 MG Cell Lines. Biochem. Pharmacol. 2015, 96, 10–19. [Google Scholar] [CrossRef]
- Sbiera, S.; Leich, E.; Liebisch, G.; Sbiera, I.; Schirbel, A.; Wiemer, L.; Matysik, S.; Eckhardt, C.; Gardill, F.; Gehl, A.; et al. Mitotane Inhibits Sterol-O-Acyl Transferase 1 Triggering Lipid-Mediated Endoplasmic Reticulum Stress and Apoptosis in Adrenocortical Carcinoma Cells. Endocrinology 2015, 156, 3895–3908. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, R. ER Stress and Hepatic Lipid Metabolism. Front. Genet. 2014, 5, 112. [Google Scholar] [CrossRef]
- Monaco, M.E. Fatty Acid Metabolism in Breast Cancer Subtypes. Oncotarget 2017, 8, 29487–29500. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid Metabolism in Cancer Progression and Therapeutic Strategies. MedComm 2020, 2, 27–59. [Google Scholar] [CrossRef]
- Jin, H.; Xia, B.; Wang, J.; Qi, S.; Jing, W.; Deng, K.; Yang, J. A Novel Lipid Metabolism and Endoplasmic Reticulum Stress-Related Risk Model for Predicting Immune Infiltration and Prognosis in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 13854. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of Fatty Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The Integrated Stress Response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Stechmiller, J.K.; Childress, B.; Cowan, L. Arginine Supplementation and Wound Healing. Nutr. Clin. Pract. 2005, 20, 52–61. [Google Scholar] [CrossRef]
- Field, G.C.; Pavlyk, I.; Szlosarek, P.W. Bench-to-Bedside Studies of Arginine Deprivation in Cancer. Molecules 2023, 28, 2150. [Google Scholar] [CrossRef]
- Feun, L.G.; Kuo, M.T.; Savaraj, N. Arginine Deprivation in Cancer Therapy. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 78–82. [Google Scholar] [CrossRef]
- Miraki-Moud, F.; Ghazaly, E.; Ariza-McNaughton, L.; Hodby, K.A.; Clear, A.; Anjos-Afonso, F.; Liapis, K.; Grantham, M.; Sohrabi, F.; Cavenagh, J.; et al. Arginine Deprivation Using Pegylated Arginine Deiminase Has Activity Against Primary Acute Myeloid Leukemia Cells in Vivo. Blood 2015, 125, 4060–4068. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.R.; Liu, X.; Chu, C.Y.; Shen, L.J.; Xu, J.; Gaur, S.; Forman, H.J.; Zhang, H.; Zheng, S.; et al. Arginine Starvation Impairs Mitochondrial Respiratory Function in ASS1-Deficient Breast Cancer Cells. Sci. Signal. 2014, 7, ra31. [Google Scholar] [CrossRef]
- Holeček, M. Origin and Roles of Alanine and Glutamine in Gluconeogenesis in the Liver, Kidneys, and Small Intestine under Physiological and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 7037. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Fahrmann, J.F.; Vykoukal, J.V.; Dennison, J.B.; Hanash, S.M. Targeting Metabolic Vulnerabilities of Cancer: Small Molecule Inhibitors in Clinic. Cancer Rep. 2019, 2, e1131. [Google Scholar] [CrossRef]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009, 136, 521–534. [Google Scholar] [CrossRef]
- Alhmoud, J.F.; Woolley, J.F.; Al Moustafa, A.E.; Malki, M.I. DNA Damage/Repair Management in Cancers. Cancers 2020, 12, 1050. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER Stress-Induced Cell Death Mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Hetz, C. RNA Metabolism: Putting the Brake on the UPR. EMBO Rep. 2015, 16, 545–546. [Google Scholar] [CrossRef][Green Version]
- Goyal, H.; Parwani, S.; Kaur, J. Deciphering the Nexus Between Long Non-Coding RNAs and Endoplasmic Reticulum Stress in Hepatocellular Carcinoma: Biomarker Discovery and Therapeutic Horizons. Cell Death Discov. 2024, 10, 451. [Google Scholar] [CrossRef]
- Mu, W.; Zhi, Y.; Zhou, J.; Wang, C.; Chai, K.; Fan, Z.; Lv, G.E. Endoplasmic Reticulum Stress and Quality Control in Relation to Cisplatin Resistance in Tumor Cells. Front. Pharmacol. 2024, 15, 1419468. [Google Scholar] [CrossRef]
- Kishino, A.; Hayashi, K.; Hidai, C.; Masuda, T.; Nomura, Y.; Oshima, T. XBP1-FoxO1 Interaction Regulates ER Stress-Induced Autophagy in Auditory Cells. Sci. Rep. 2017, 7, 4442. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Croce, C.M. MicroRNA and ER Stress in Cancer. Semin. Cancer Biol. 2021, 75, 3–14. [Google Scholar] [CrossRef]
- Fu, J.; Imani, S.; Wu, M.Y.; Wu, R.C. MicroRNA-34 Family in Cancers: Role, Mechanism, and Therapeutic Potential. Cancers 2023, 15, 4723. [Google Scholar] [CrossRef]
- Happel, C.; Ganguly, A.; Tagle, D.A. Extracellular RNAs as Potential Biomarkers for Cancer. J. Cancer Metastasis Treat. 2020, 6, 32. [Google Scholar] [CrossRef]
- Mahadevan, N.R.; Zanetti, M. Tumor stress inside out: Cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment. J. Immunol. 2011, 187, 4403–4409. [Google Scholar] [CrossRef]
- Ramirez, M.U.; Hernandez, S.R.; Soto-Pantoja, D.R.; Cook, K.L. Endoplasmic Reticulum Stress Pathway, the Unfolded Protein Response, Modulates Immune Function in the Tumor Microenvironment to Impact Tumor Progression and Therapeutic Response. Int. J. Mol. Sci. 2019, 21, 169. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Mehla, K.; Singh, P.K. Metabolic Regulation of Macrophage Polarization in Cancer. Trends Cancer 2019, 5, 822–834. [Google Scholar] [CrossRef]
- Cook, K.L.; Soto-Pantoja, D.R.; Clarke, P.A.; Cruz, M.I.; Zwart, A.; Wärri, A.; Hilakivi-Clarke, L.; Roberts, D.D.; Clarke, R. Endoplasmic Reticulum Stress Protein GRP78 Modulates Lipid Metabolism to Control Drug Sensitivity and Antitumor Immunity in Breast Cancer. Cancer Res. 2016, 76, 5657–5670. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Zeng, W.; Huang, L.; Pang, Q.; Nie, L.; Mu, J.; Yuan, F.; Feng, B. ER stress triggers MCP-1 expression through SET7/9-induced histone methylation in the kidneys of db/db mice. Am. J. Physiol. Renal Physiol. 2014, 306, F916–F925. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Wilson, A.S.; Clear, K.Y.; Westwood, B.; Triozzi, P.L.; Cook, K.L. Unfolded protein response signaling impacts macrophage polarity to modulate breast cancer cell clearance and melanoma immune checkpoint therapy responsiveness. Oncotarget 2017, 8, 80545–80559. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Everts, B.; Ivanova, Y.; O’Sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’Neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Tyurin, V.A.; Veglia, F.; Condamine, T.; Amoscato, A.; Mohammadyani, D.; Johnson, J.J.; Zhang, L.M.; Klein-Seetharaman, J.; Celis, E.; et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J. Immunol. 2014, 192, 2920–2931. [Google Scholar] [CrossRef]
- Lee, A.H.; Scapa, E.F.; Cohen, D.E.; Glimcher, L.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 2008, 320, 1492–1496. [Google Scholar] [CrossRef]
- Sriburi, R.; Jackowski, S.; Mori, K.; Brewer, J.W. XBP1: A link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 2004, 167, 35–41. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Silberman, P.C.; Rutkowski, M.R.; Chopra, S.; Perales-Puchalt, A.; Song, M.; Zhang, S.; Bettigole, S.E.; Gupta, D.; Holcomb, K.; et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015, 161, 1527–1538. [Google Scholar] [CrossRef]
- Chevet, E.; Hetz, C.; Samali, A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Discov. 2015, 5, 586–597. [Google Scholar] [CrossRef]
- Fang, P.; Xiang, L.; Huang, S.; Jin, L.; Zhou, G.; Zhuge, L.; Li, J.; Fan, H.; Zhou, L.; Pan, C.; et al. IRE1α-XBP1 signaling pathway regulates IL-6 expression and promotes progression of hepatocellular carcinoma. Oncol. Lett. 2018, 16, 4729–4736. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X. IRE1α-XBP1 pathway promotes melanoma progression by regulating IL-6/STAT3 signaling. J. Transl. Med. 2017, 15, 42. [Google Scholar] [CrossRef]
- Scharping, N.E.; Menk, A.V.; Moreci, R.S.; Whetstone, R.D.; Dadey, R.E.; Watkins, S.C.; Ferris, R.L.; Delgoffe, G.M. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity 2016, 45, 374–388. [Google Scholar] [CrossRef]
- Di Conza, G.; Ho, P.C.; Cubillos-Ruiz, J.R.; Huang, S.C. Control of immune cell function by the unfolded protein response. Nat. Rev. Immunol. 2023, 23, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.; Rodvold, J.J.; Xian, S.; Searles, S.C.; Lew, A.; Iwawaki, T.; Almanza, G.; Waller, T.C.; Lin, J.; Jepsen, K.; et al. IRE1α regulates macrophage polarization, PD-L1 expression, and tumor survival. PLoS Biol. 2020, 18, e3000687. [Google Scholar] [CrossRef]
- Daniel, Y.; Lelou, E.; Aninat, C.; Corlu, A.; Cabillic, F. Interplay between Metabolism Reprogramming and Epithelial-to-Mesenchymal Transition in Cancer Stem Cells. Cancers 2021, 13, 1973. [Google Scholar] [CrossRef]
- Nishimura, K.; Fukuda, A.; Hisatake, K. Mechanisms of the Metabolic Shift during Somatic Cell Reprogramming. Int. J. Mol. Sci. 2019, 20, 2254. [Google Scholar] [CrossRef]
- Peng, F.; Wang, J.H.; Fan, W.J.; Meng, Y.T.; Li, M.M.; Li, T.T.; Cui, B.; Wang, H.F.; Zhao, Y.; An, F.; et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene 2018, 37, 1062–1074. [Google Scholar] [CrossRef]
- Krebs, A.M.; Mitschke, J.; Lasierra Losada, M.; Schmalhofer, O.; Boerries, M.; Busch, H.; Boettcher, M.; Mougiakakos, D.; Reichardt, W.; Bronsert, P.; et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 2017, 19, 518–529. [Google Scholar] [CrossRef]
- Lu, Y.J.; Geng, Z.J.; Sun, X.Y.; Li, Y.H.; Fu, X.B.; Zhao, X.Y.; Wei, B. Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol. Cell Biochem. 2015, 408, 1–13. [Google Scholar] [CrossRef]
- Loriot, C.; Burnichon, N.; Gadessaud, N.; Vescovo, L.; Amar, L.; Libé, R.; Bertherat, J.; Plouin, P.F.; Jeunemaitre, X.; Gimenez-Roqueplo, A.P.; et al. Epithelial to mesenchymal transition is activated in metastatic pheochromocytomas and paragangliomas caused by SDHB gene mutations. J. Clin. Endocrinol. Metab. 2012, 97, E954–E962. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.M.; Wang, Y.H.; Feng, M.X.; Liu, X.J.; Zhang, Y.L.; Huang, S.; Wu, Z.; Xue, F.; Qin, W.X.; et al. Monoamine oxidase A suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J. Hepatol. 2014, 60, 1225–1234. [Google Scholar] [CrossRef]
- Huan, H.B.; Wen, X.D.; Chen, X.J.; Wu, L.; Wu, L.L.; Zhang, L.; Yang, D.P.; Zhang, X.; Bie, P.; Qian, C.; et al. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells. Brain Behav. Immun. 2017, 59, 118–134. [Google Scholar] [CrossRef]
- Siu, M.K.Y.; Jiang, Y.X.; Wang, J.J.; Leung, T.H.Y.; Han, C.Y.; Tsang, B.K.; Cheung, A.N.Y.; Ngan, H.Y.S.; Chan, K.K.L. Hexokinase 2 Regulates Ovarian Cancer Cell Migration, Invasion and Stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 Signaling Cascades. Cancers 2019, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Shi, X.; Zhang, W.; Li, D.; Hu, L.; Yang, J.; Zhao, J.; Wei, S.; Wei, X.; Ruan, X.; et al. LDHA induces EMT gene transcription and regulates autophagy to promote the metastasis and tumorigenesis of papillary thyroid carcinoma. Cell Death Dis. 2021, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeon, H.M.; Ju, M.K.; Jeong, E.K.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Dlx-2 and glutaminase upregulate epithelial-mesenchymal transition and glycolytic switch. Oncotarget 2016, 7, 7925–7939. [Google Scholar] [CrossRef] [PubMed]
- Morandi, A.; Taddei, M.L.; Chiarugi, P.; Giannoni, E. Targeting the Metabolic Reprogramming That Controls Epithelial-to-Mesenchymal Transition in Aggressive Tumors. Front. Oncol. 2017, 7, 40. [Google Scholar] [CrossRef]
- Dalmau, N.; Jaumot, J.; Tauler, R.; Bedia, C. Epithelial-to-mesenchymal transition involves triacylglycerol accumulation in DU145 prostate cancer cells. Mol. Biosyst. 2015, 11, 3397–3406. [Google Scholar] [CrossRef]
- Hung, C.M.; Kuo, D.H.; Chou, C.H.; Su, Y.C.; Ho, C.T.; Way, T.D. Osthole suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition via repression of the c-Met/Akt/mTOR pathway in human breast cancer cells. J. Agric. Food Chem. 2011, 59, 9683–9690. [Google Scholar] [CrossRef]
- Morishima, M.; Harada, N.; Hara, S.; Sano, A.; Seno, H.; Takahashi, A.; Morita, Y.; Nakaya, Y. Monoamine Oxidase A Activity and Norepinephrine Level in Hippocampus Determine Hyperwheel Running in SPORTS Rats. Neuropsychopharmacology 2006, 31, 2627–2638. [Google Scholar] [CrossRef]
- Yamazaki, K.; Masugi, Y.; Sakamoto, M. Molecular Pathogenesis of Hepatocellular Carcinoma: Altering Transforming Growth Factor-β Signaling in Hepatocarcinogenesis. Dig. Dis. 2011, 29, 284–288. [Google Scholar] [CrossRef]
- Albo, D.; Akay, C.L.; Marshall, C.L.; Wilks, J.A.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Ayala, G.E. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 2011, 117, 4834–4845. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Katsuno, Y.; Lamouille, S.; Derynck, R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr. Opin. Oncol. 2013, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.J.; Sasser, A.K.; Axel, A.E.; Vesuna, F.; Raman, V.; Ramirez, N.; Oberyszyn, T.M.; Hall, B.M. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009, 28, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef]
- Luo, W.; Semenza, G.L. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol. Metab. 2012, 23, 560–566. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Frezza, C. Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. FEBS J. 2017, 284, 3132–3144. [Google Scholar] [CrossRef]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef]
- Tavernier, Q.; Legras, A.; Didelot, A.; Normand, C.; Gibault, L.; Badoual, C.; Le Pimpec-Barthes, F.; Puig, P.L.; Blons, H.; Pallet, N. High expression of spliced X-Box Binding Protein 1 in lung tumors is associated with cancer aggressiveness and epithelial-to-mesenchymal transition. Sci. Rep. 2020, 10, 10188. [Google Scholar] [CrossRef]
- Feng, Y.X.; Sokol, E.S.; Del Vecchio, C.A.; Sanduja, S.; Claessen, J.H.; Proia, T.A.; Jin, D.X.; Reinhardt, F.; Ploegh, H.L.; Wang, Q.; et al. Epithelial-to-mesenchymal transition activates PERK-eIF2α and sensitizes cells to endoplasmic reticulum stress. Cancer Discov. 2014, 4, 702–715. [Google Scholar] [CrossRef]
- Mujcic, H.; Nagelkerke, A.; Rouschop, K.M.; Chung, S.; Chaudary, N.; Span, P.N.; Clarke, B.; Milosevic, M.; Sykes, J.; Hill, R.P.; et al. Hypoxic activation of the PERK/eIF2α arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin. Cancer Res. 2013, 19, 6126–6137. [Google Scholar] [CrossRef] [PubMed]
- Zeindl-Eberhart, E.; Brandl, L.; Liebmann, S.; Ormanns, S.; Scheel, S.K.; Brabletz, T.; Kirchner, T.; Jung, A. Epithelial-mesenchymal transition induces endoplasmic-reticulum-stress response in human colorectal tumor cells. PLoS ONE 2014, 9, e87386. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Beverly, L.J. Regulation of VCP/p97 demonstrates the critical balance between cell death and epithelial-mesenchymal transition (EMT) downstream of ER stress. Oncotarget 2015, 6, 17725–17737. [Google Scholar] [CrossRef]
- Wang, H.; Mi, K. Emerging roles of endoplasmic reticulum stress in the cellular plasticity of cancer cells. Front. Oncol. 2023, 13, 1110881. [Google Scholar] [CrossRef]
- Peñaranda Fajardo, N.M.; Meijer, C.; Kruyt, F.A. The Endoplasmic Reticulum Stress/Unfolded Protein Response in Gliomagenesis, Tumor Progression and as a Therapeutic Target in Glioblastoma. Biochem. Pharmacol. 2016, 118, 1–8. [Google Scholar] [CrossRef]
- Mao, X.; Yu, C.; Yin, F.; Xu, W.; Pan, Y.; Yang, B.; Huang, T.; Chen, S.; Luo, W.; Su, T.; et al. IRE1α-XBP1 regulates PDK1-dependent induction of epithelial-mesenchymal transition in non-small cell lung cancer cells. Exp. Cell Res. 2022, 421, 113376. [Google Scholar] [CrossRef]
- Rouschop, K.M.; Dubois, L.J.; Keulers, T.G.; van den Beucken, T.; Lambin, P.; Bussink, J.; van der Kogel, A.J.; Koritzinsky, M.; Wouters, B.G. PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc. Natl. Acad. Sci. USA 2013, 110, 4622–4627. [Google Scholar] [CrossRef]
- Bobrovnikova-Marjon, E.; Grigoriadou, C.; Pytel, D.; Zhang, F.; Ye, J.; Koumenis, C.; Cavener, D.; Diehl, J.A. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene 2010, 29, 3881–3895. [Google Scholar] [CrossRef]
- Del Vecchio, C.A.; Feng, Y.; Sokol, E.S.; Tillman, E.J.; Sanduja, S.; Reinhardt, F.; Gupta, P.B. De-differentiation confers multidrug resistance via noncanonical PERK-Nrf2 signaling. PLoS Biol. 2014, 12, e1001945. [Google Scholar] [CrossRef]
- Bu, Y.; Diehl, J.A. PERK Integrates Oncogenic Signaling and Cell Survival During Cancer Development. J. Cell Physiol. 2016, 231, 2088–2096. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gui, F.; Zhang, Z.; Chen, Z.; Zhang, T.; Hu, Y.; Wei, H.; Fu, Y.; Chen, X.; Wu, Z. IRE1α-XBP1s axis regulates SREBP1-dependent MRP1 expression to promote chemoresistance in non-small cell lung cancer cells. Thorac. Cancer 2024, 15, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.; Song, Q.; Zhang, Q.; Zhang, X.; Liu, X.; Wu, Z.; Xu, X.; Xu, Y.; Yan, Y.; et al. Transmissible ER stress between macrophages and tumor cells configures tumor microenvironment. Cell Mol. Life Sci. 2022, 79, 403. [Google Scholar] [CrossRef]
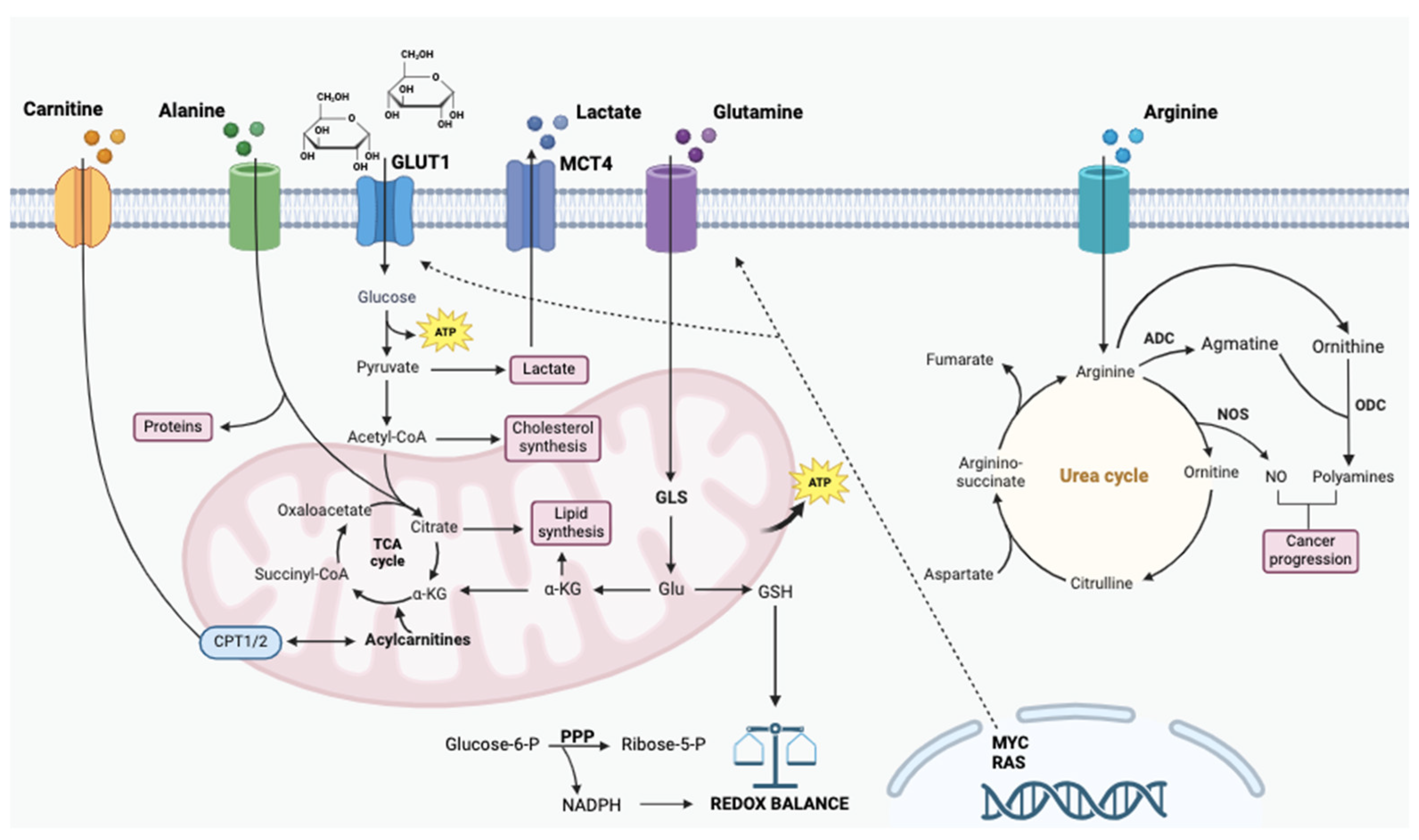

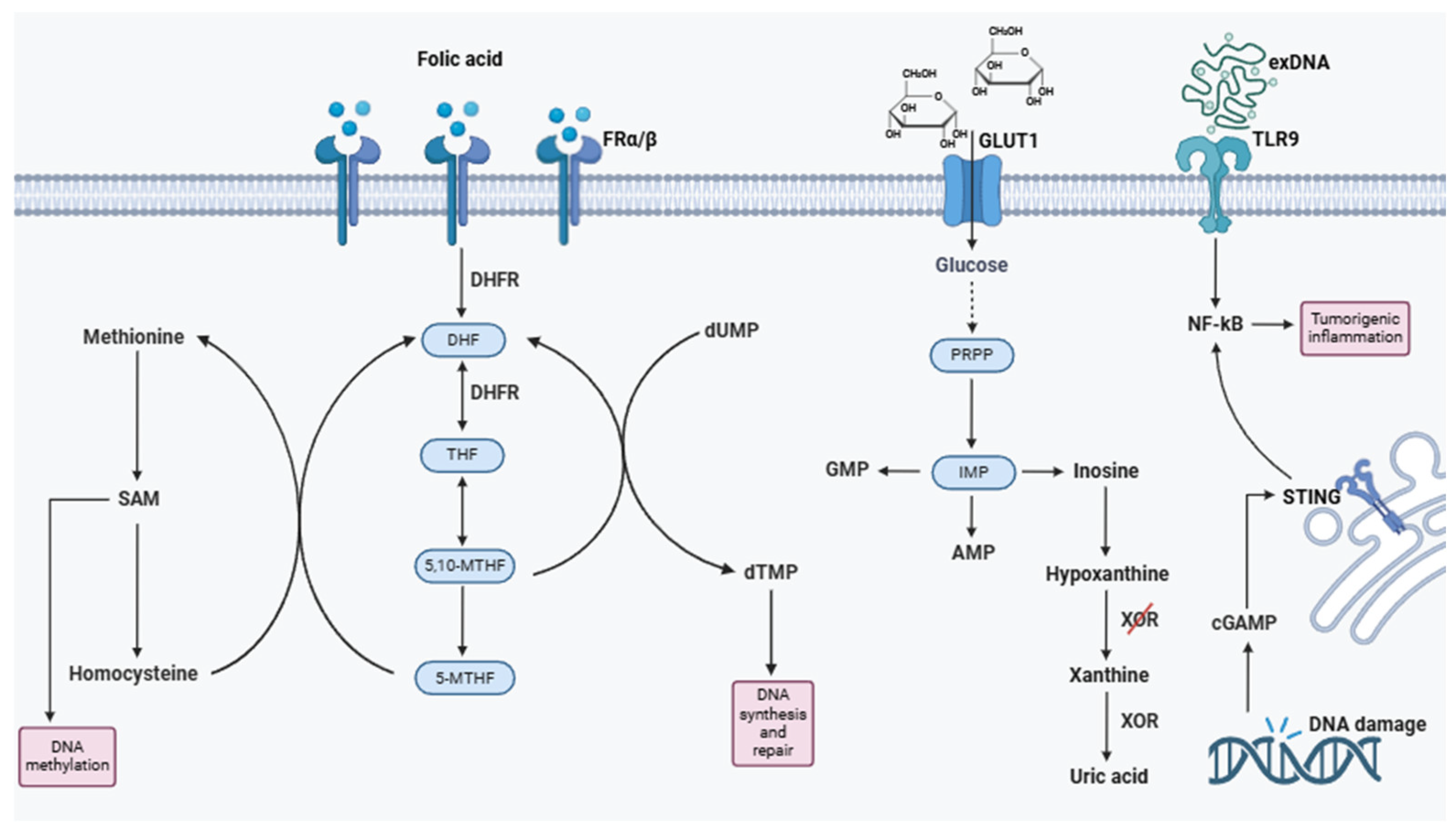
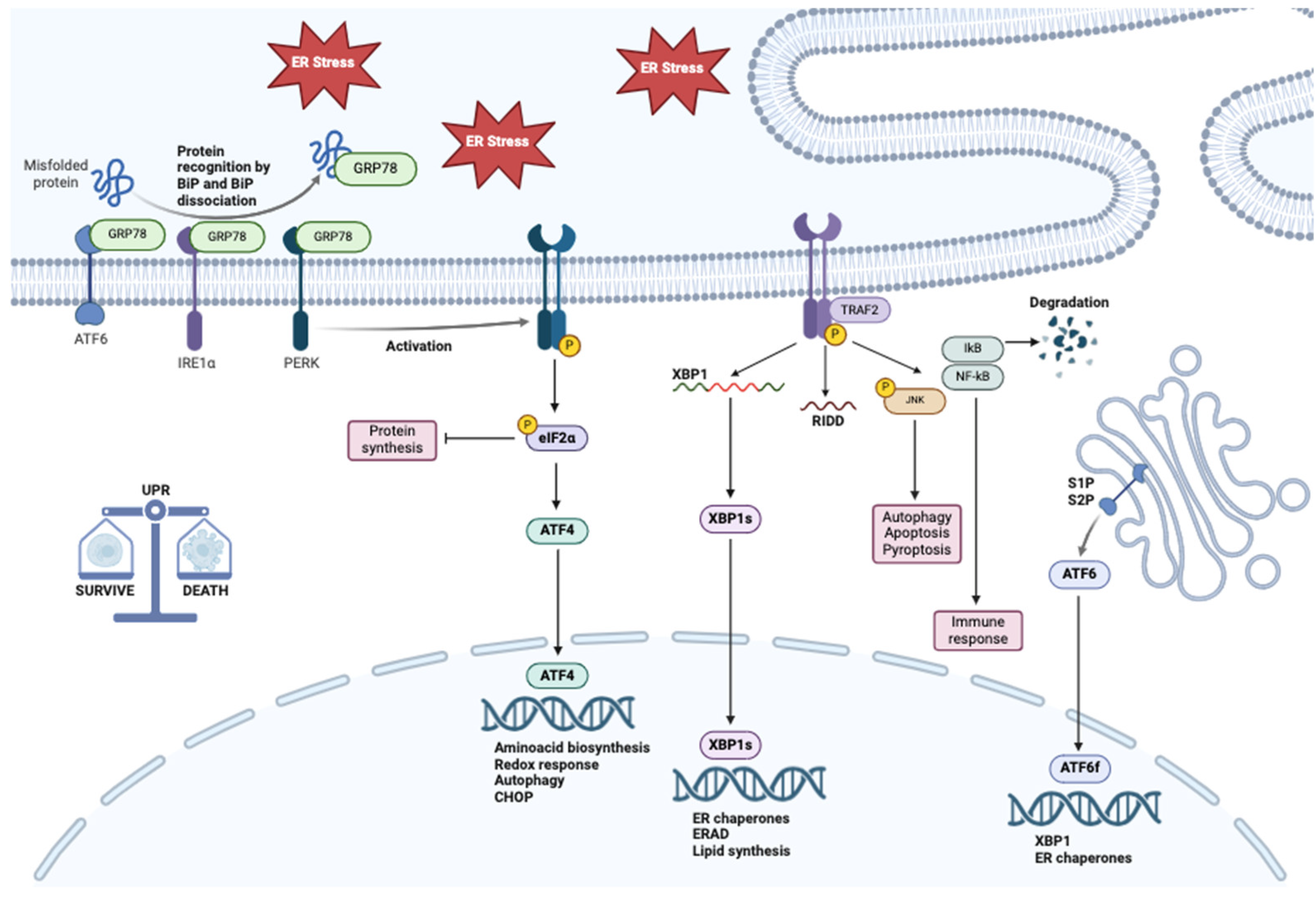
| Metabolic Change | Biomarkers | References |
|---|---|---|
| Increased glycolysis (Warburg effect) | Lactate, Pyruvate, GLUT1 | [48,54,55] |
| Altered glutamine metabolism (glutaminolysis) | Glutamine, Glutamate, α-ketoglutarate | [61,62,63,64,65,66] |
| Lipid remodeling (phospholipid synthesis) | Phosphatidylcholine, Sphingomyelin | [125,126,127,128] |
| Increased oxidative stress response | Glutathione, NADPH | [57,66] |
| Altered amino acid metabolism | Alanine, Arginine, Carnitine | [80,81,82,83,84,89,90,91,92,93,94,95,96] |
| Increased nucleotide biosynthesis | Purines (IMP, AMP, GMP), Hypoxanthine | [155,156,157,158,159,160] |
| Increased ketone body metabolism | β-hydroxybutyrate, Acetoacetate | [145,146,147,148] |
| Altered folate metabolism | 5-MTHF, Homocysteine | [162,163,164,165,166,167,168,169] |
| Cholesterol and sterol metabolism | Cholesterol, SREBP1, PCSK9 | [135,136,137,138,139,140,141,142,143,144] |
| Metabolites | Pathway | Role in Cancer | Role in Ers | References |
|---|---|---|---|---|
| Glucose | Aerobic glycolysis | Warburg effect facilitates rapid tumor growth. ATP production. | Increased aerobic glycolysis in cancer cells leads to higher levels of ROS, promoting reticulum stress. | [260,262,268] |
| Glucose deprivation | Tumor cells are resistant to glucose deprivation, maintaining survival through metabolic adaptations. | Glucose scarcity: Impairs ATP production, affecting protein folding and ER function. Impairs SERCA activity, disrupting calcium homeostasis and contributing to ER stress. | [261,273] | |
| Endoplasmic reticulum oxidoreductase 1 alpha (ERO1) | Overexpression of ERO1 enhances tumor growth, metastasis, and immune evasion. | ERO1 overactivation leads to chronic UPR activation, supporting cell survival and metabolic reprogramming. | [263,265,266] | |
| Hexosamine biosynthetic pathway (HBP) | UDP-GlcNAc is crucial for protein glycosylation and cell surface signaling. | Glucose deficiency disrupts the HBP, impairing N-linked glycosylation and protein folding in the ER. | [257,258,259] | |
| Fatty Acids | ||||
| Phospholipids | Enhanced mitochondrial activity | Proliferation of tumor cells. | The accumulation of phospholipids in cell membranes can impair ER function by activating ER stress. | [129,283,284,291] |
| Acetyl-CoA | β-oxidation of fatty acids | Alterations in β-oxidation of fatty acids are implicated in supporting tumor growth. | Alteration in β-oxidation of fatty acids and accumulation of intermediate metabolites such as acetyl-CoA may contribute to ER stress. | [295] |
| Saturated fatty acids Cholesterol | SREBP1, PI3K/Akt, mTOR | Contribute to lipotoxicity in cancer cells by damaging cell structures. | Excess saturated fatty acids (such as palmitate) induce ER stress, accumulating misfolded proteins and activating the UPR. | [294,295] |
| SCD1 (stearoyl-CoA desaturase 1) | SREBP1, PI3K/Akt, mTOR | SCD1 promotes the synthesis of unsaturated fatty acids, increasing tumor proliferation and invasiveness. | SCD1 regulates lipid metabolism in the ER, contributing to lipid homeostasis and ER stress. | [278,279] |
| Free cholesterol (FC) | SREBP2, PI3K/Akt, mTOR | FC accumulation in tumor cells promotes invasion and metastasis. | FC overload causes ER stress, activating markers such as CHOP. | [288,289] |
| Phosphatidylserine (PS) Fosfatidilcolina (PC) | Sphingolipid metabolism, Choline metabolism, SREBP1 | PS and PC contribute to membrane integrity and invasiveness of cancer cells. | Altered PS and PC in membranes can reduce ER stability, contributing to stress. | [283] |
| Amino Acids | ||||
| Alanine | TCA cycle | Enhance cancer cell growth because it acts as an anaplerotic substrate, replenishing the TCA cycle in cancer cells. | Helps to sustain cellular energy levels and maintains a balance between oxidative and reductive reactions essential for folding in the ERs. | [80,82,303,304] |
| Nucleotide production | Enhance cancer cell growth. | Contributes to tumor progression by promoting metabolic flexibility. | [80,303,304,305] | |
| Glucose–alanine cycle | It serves as a nitrogen carrier and a gluconeogenic substrate, important in the metabolic reprogramming of cancer cells. | Elevated levels of alanine can support energy metabolism, which can help alleviate oxidative stress and mitigate ER stress. | [81,304] | |
| Arginine | Polyamine synthesis | Rapid proliferation. | Arginine depletion can exacerbate ER stress in tumor cells. | [91,297] |
| NO synthase | NO promotes cancer development, suppresses T-cell activation, and induces genomic instability interfering with the DNA repair mechanism. | Arginine-deprived tumor cells may be more susceptible to apoptosis and may exhibit increased sensitivity to chemotherapeutic agents that further induce ER stress. | [97,99,298] | |
| Nucleic acids | ExDNA | Promote tumor cell adhesion, migration, and metastasis. exDNA induces pro-inflammatory cytokines and enhances tumor-promoting inflammation. | Persistent or unresolved DNA damage can exacerbate ER stress, triggering an amplified UPR. | [149,150,307] |
| ExRNA | Regulate gene expression cells, influencing angiogenesis, immune evasion, and metastasis. | Abnormal or defective RNA transcripts can place additional stress on the ER’s ability to properly fold proteins, further triggering the UPR. | [151,308] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarrella, S.; Miranda, M.R.; Covelli, V.; Restivo, I.; Novi, S.; Pepe, G.; Tesoriere, L.; Rodriquez, M.; Bertamino, A.; Campiglia, P.; et al. Endoplasmic Reticulum Stress and Its Role in Metabolic Reprogramming of Cancer. Metabolites 2025, 15, 221. https://doi.org/10.3390/metabo15040221
Zarrella S, Miranda MR, Covelli V, Restivo I, Novi S, Pepe G, Tesoriere L, Rodriquez M, Bertamino A, Campiglia P, et al. Endoplasmic Reticulum Stress and Its Role in Metabolic Reprogramming of Cancer. Metabolites. 2025; 15(4):221. https://doi.org/10.3390/metabo15040221
Chicago/Turabian StyleZarrella, Salvatore, Maria Rosaria Miranda, Verdiana Covelli, Ignazio Restivo, Sara Novi, Giacomo Pepe, Luisa Tesoriere, Manuela Rodriquez, Alessia Bertamino, Pietro Campiglia, and et al. 2025. "Endoplasmic Reticulum Stress and Its Role in Metabolic Reprogramming of Cancer" Metabolites 15, no. 4: 221. https://doi.org/10.3390/metabo15040221
APA StyleZarrella, S., Miranda, M. R., Covelli, V., Restivo, I., Novi, S., Pepe, G., Tesoriere, L., Rodriquez, M., Bertamino, A., Campiglia, P., Tecce, M. F., & Vestuto, V. (2025). Endoplasmic Reticulum Stress and Its Role in Metabolic Reprogramming of Cancer. Metabolites, 15(4), 221. https://doi.org/10.3390/metabo15040221













