Metatranscriptomics for Understanding the Microbiome in Food and Nutrition Science
Abstract
1. Introduction
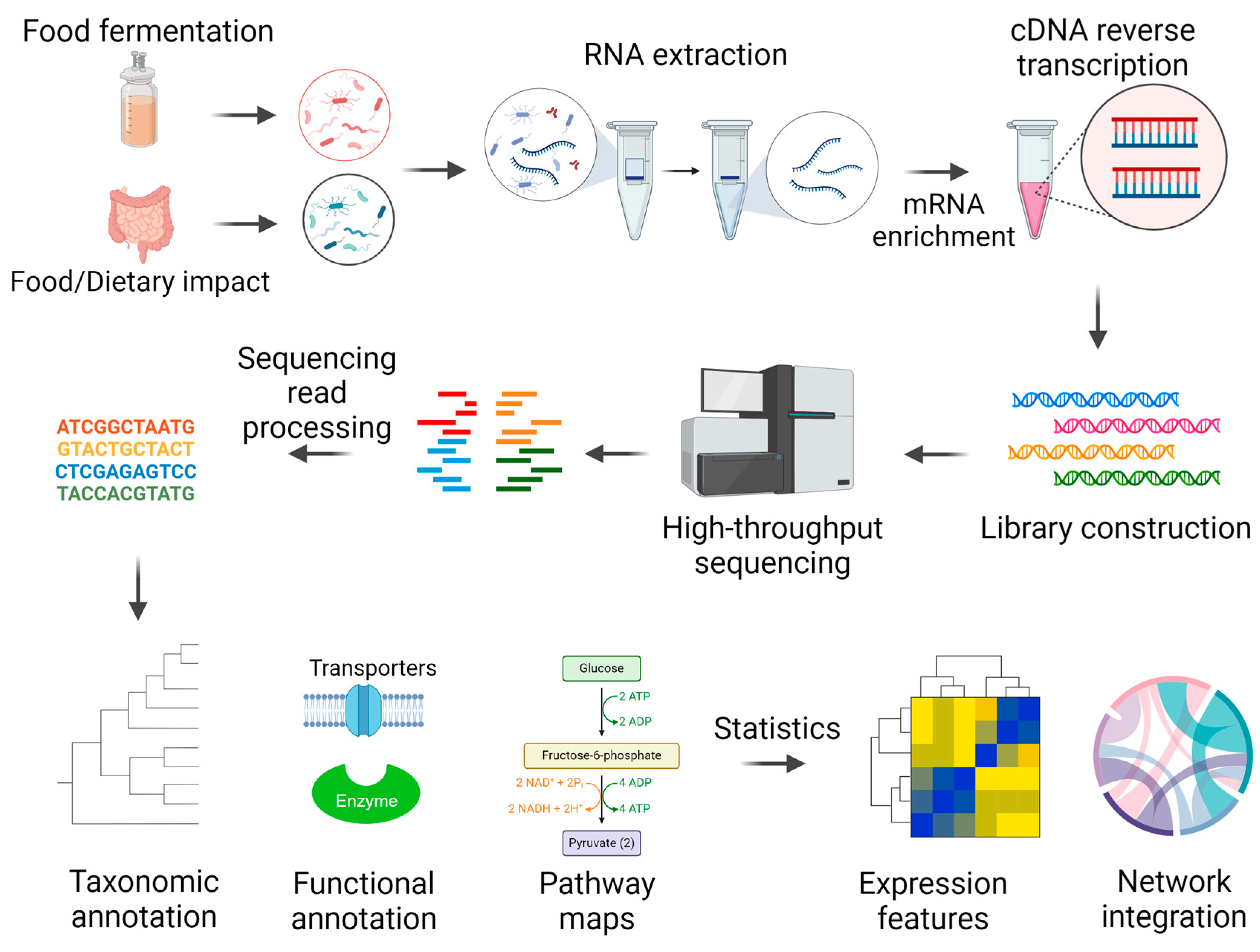
2. Metatranscriptomics Basics
3. Metatranscriptomics Methodology
3.1. RNA Extraction and mRNA Enrichment
3.2. Library Construction and Sequencing
3.3. Bioinformatics
4. Utilization of Metatranscriptomics in Food Science
4.1. Integrating Metatranscriptomics with Multi-Omics to Understand Food Fermentation
4.2. Metatranscriptomics-Guided Enzyme Discovery During Food Fermentation
4.3. Inter-Kingdom Impact Revealed by Metatranscriptomics
4.4. Fibre Degradation in Food Fermentation
4.5. Bioconversion of Food Ingredients
5. Utilization of Metatranscriptomics in Nutrition Science
5.1. Interaction Between Gut Microbiome and Fibres Revealed by Metatranscriptomics
5.2. Metatranscriptomics Identify Biomarkers of Dietary Style, Macronutrients, and Micronutrient Intake
5.3. Metatranscriptomics in Understanding Probiotic Action in the Gut
6. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, Z.; Zhu, W.; Wu, G. Amino Acids in Microbial Metabolism and Function. Adv. Exp. Med. Biol. 2022, 1354, 127–143. [Google Scholar]
- Jing, Y.; Mu, C.; Wang, H.; Shen, J.; Zoetendal, E.G.; Zhu, W. Amino acid utilization allows intestinal dominance of Lactobacillus amylovorus. ISME J. 2022, 16, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Patterson, A.D. The gut microbiome: An orchestrator of xenobiotic metabolism. Acta Pharm. Sin. B 2020, 10, 19–32. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Abellan-Schneyder, I.; Matchado, M.S.; Reitmeier, S.; Sommer, A.; Sewald, Z.; Baumbach, J.; List, M.; Neuhaus, K. Primer, Pipelines, Parameters: Issues in 16S rRNA Gene Sequencing. mSphere 2021, 6, 22. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef] [PubMed]
- Zoetendal, E.G.; Rajilic-Stojanovic, M.; de Vos, W.M. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut 2008, 57, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, W.; Xu, Y. Metaproteomics insights into traditional fermented foods and beverages. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2506–2529. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Banan-MwineDaliri, E.; Khan, I.; Wei, S.; Elahi, F.; Yeon, S.J.; Selvakumar, V.; Ofosu, F.K.; Rubab, M.; Ju, H.H.; et al. A review on the application of bioinformatics tools in food microbiome studies. Brief. Bioinform. 2022, 23, bbac007. [Google Scholar] [CrossRef]
- Ojala, T.; Kankuri, E.; Kankainen, M. Understanding human health through metatranscriptomics. Trends Mol. Med. 2023, 29, 376–389. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Lagishetty, V.; Hauer, M.C.; Labus, J.S.; Dong, T.S.; Toma, R.; Vuyisich, M.; Naliboff, B.D.; Lackner, J.M.; Gupta, A.; et al. Multi-omics profiles of the intestinal microbiome in irritable bowel syndrome and its bowel habit subtypes. Microbiome 2023, 11, 5. [Google Scholar] [CrossRef]
- Mehta, R.S.; Mayers, J.R.; Zhang, Y.; Bhosle, A.; Glasser, N.R.; Nguyen, L.H.; Ma, W.; Bae, S.; Branck, T.; Song, K.; et al. Gut microbial metabolism of 5-ASA diminishes its clinical efficacy in inflammatory bowel disease. Nat. Med. 2023, 29, 700–709. [Google Scholar] [CrossRef]
- Gallardo-Becerra, L.; Cornejo-Granados, F.; García-López, R.; Valdez-Lara, A.; Bikel, S.; Canizales-Quinteros, S.; López-Contreras, B.E.; Mendoza-Vargas, A.; Nielsen, H.; Ochoa-Leyva, A. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb. Cell Factories 2020, 19, 61. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; May, P.; Laczny, C.C.; Lebrun, L.A.; Bellora, C.; Krishna, A.; Wampach, L.; Schneider, J.G.; Hogan, A.; de Beaufort, C.; et al. Integrated multi-omics of the human gut microbiome in a case study of familial type 1 diabetes. Nat. Microbiol. 2016, 2, 16180. [Google Scholar]
- Mascardi, M.F.; Mazzini, F.N.; Suárez, B.; Ruda, V.M.; Marciano, S.; Casciato, P.; Narvaez, A.; Haddad, L.; Anders, M.; Orozco, F.; et al. Integrated analysis of the transcriptome and its interaction with the metabolome in metabolic associated fatty liver disease: Gut microbiome signatures, correlation networks, and effect of PNPLA3 genotype. Proteomics 2023, 23, e2200414. [Google Scholar] [CrossRef]
- Solbiati, J.; Frias-Lopez, J. Metatranscriptome of the Oral Microbiome in Health and Disease. J. Dent. Res. 2018, 97, 492–500. [Google Scholar] [CrossRef]
- Mann, A.E.; Chakraborty, B.; O’Connell, L.M.; Nascimento, M.M.; Burne, R.A.; Richards, V.P. Heterogeneous lineage-specific arginine deiminase expression within dental microbiome species. Microbiol. Spectr. 2024, 12, e0144523. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, C.; Lei, Z. Meta-omics insights in the microbial community profiling and functional characterization of fermented foods. Trends Food Sci. Technol. 2017, 65, 23–31. [Google Scholar] [CrossRef]
- He, S.; Wurtzel, O.; Singh, K.; Froula, J.L.; Yilmaz, S.; Tringe, S.G.; Wang, Z.; Chen, F.; Lindquist, E.A.; Sorek, R.; et al. Validation of two ribosomal RNA removal methods for microbial metatranscriptomics. Nat. Methods 2010, 7, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Huptas, C.; Neuhaus, K. Comparison of rRNA depletion methods for efficient bacterial mRNA sequencing. Sci. Rep. 2022, 12, 5765. [Google Scholar] [CrossRef]
- Alberti, A.; Belser, C.; Engelen, S.; Bertrand, L.; Orvain, C.; Brinas, L.; Cruaud, C.; Giraut, L.; Da Silva, C.; Firmo, C.; et al. Comparison of library preparation methods reveals their impact on interpretation of metatranscriptomic data. BMC Genom. 2014, 15, 912. [Google Scholar] [CrossRef]
- Barber, D.G.; Davies, C.A.; Hartley, I.P.; Tennant, R.K. Evaluation of commercial RNA extraction kits for long-read metatranscriptomics in soil. Microb. Genom. 2024, 10, 001298. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Heilig, H.G.; Molenaar, D.; Kajander, K.; Surakka, A.; Smidt, H.; de Vos, W.M. Development and application of the human intestinal tract chip, a phylogenetic microarray: Analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ. Microbiol. 2009, 11, 1736–1751. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, C.; He, X.; Su, Y.; Mao, S.; Zhang, J.; Smidt, H.; Zhu, W. Effects of dietary fibre source on microbiota composition in the large intestine of suckling piglets. FEMS Microbiol. Lett. 2016, 363, fnw138. [Google Scholar] [CrossRef]
- Westreich, S.T.; Treiber, M.L.; Mills, D.A.; Korf, I.; Lemay, D.G. SAMSA2: A standalone metatranscriptome analysis pipeline. BMC Bioinform. 2018, 19, 175. [Google Scholar] [CrossRef]
- Martinez, X.; Pozuelo, M.; Pascal, V.; Campos, D.; Gut, I.; Gut, M.; Azpiroz, F.; Guarner, F.; Manichanh, C. MetaTrans: An open-source pipeline for metatranscriptomics. Sci. Rep. 2016, 6, 26447. [Google Scholar] [CrossRef] [PubMed]
- Taj, B.; Adeolu, M.; Xiong, X.; Ang, J.; Nursimulu, N.; Parkinson, J. MetaPro: A scalable and reproducible data processing and analysis pipeline for metatranscriptomic investigation of microbial communities. Microbiome 2023, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef]
- Sequeira, J.C.; Rocha, M.; Madalena Alves, M.; Salvador, A.F. MOSCA: An Automated Pipeline for Integrated Metagenomics and Metatranscriptomics Data Analysis. In Practical Applications of Computational Biology and Bioinformatics, Proceedings of the 12th International Conference, Toledo, Spain, 20–22 June 2018; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Narayanasamy, S.; Jarosz, Y.; Muller, E.E.; Heintz-Buschart, A.; Herold, M.; Kaysen, A.; Laczny, C.C.; Pinel, N.; May, P.; Wilmes, P. IMP: A pipeline for reproducible reference-independent integrated metagenomic and metatranscriptomic analyses. Genome Biol. 2016, 17, 260. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.S.; Koh, A.Y.; Xie, Y.; Zhan, X. FMAP: Functional Mapping and Analysis Pipeline for metagenomics and metatranscriptomics studies. BMC Bioinform. 2016, 17, 420. [Google Scholar] [CrossRef] [PubMed]
- Leimena, M.M.; Ramiro-Garcia, J.; Davids, M.; van den Bogert, B.; Smidt, H.; Smid, E.J.; Boekhorst, J.; Zoetendal, E.G.; Schaap, P.J.; Kleerebezem, M. A comprehensive metatranscriptome analysis pipeline and its validation using human small intestine microbiota datasets. BMC Genom. 2013, 14, 530. [Google Scholar] [CrossRef]
- Shakya, M.; Lo, C.C.; Chain, P.S.G. Advances and Challenges in Metatranscriptomic Analysis. Front. Genet. 2019, 10, 904. [Google Scholar] [CrossRef]
- FastQC. A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 March 2025).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Leung, H.C.; Yiu, S.M.; Parkinson, J.; Chin, F.Y. IDBA-MT: De novo assembler for metatranscriptomic data generated from next-generation sequencing technology. J. Comput. Biol. 2013, 20, 540–550. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Truong, D.T.; Franzosa, E.A.; Tickle, T.L.; Scholz, M.; Weingart, G.; Pasolli, E.; Tett, A.; Huttenhower, C.; Segata, N. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 2015, 12, 902–903. [Google Scholar] [CrossRef]
- Menzel, P.; Ng, K.L.; Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 2016, 7, 11257. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Louw, N.L.; Lele, K.; Ye, R.; Edwards, C.B.; Wolfe, B.E. Microbiome Assembly in Fermented Foods. Annu. Rev. Microbiol. 2023, 77, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Y.; Hao, W.; Zhou, S.; Duan, C.; Li, Q.; Wei, J.; Liu, G. Exploring the Role of Active Functional Microbiota in Flavor Generation by Integrated Metatranscriptomics and Metabolomics during Niulanshan Baijiu Fermentation. Foods 2023, 12, 4140. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Hu, X.; Li, M.; Miao, J.; Du, J.; Wu, R. Composition and Metabolic Activities of the Bacterial Community in Shrimp Sauce at the Flavor-Forming Stage of Fermentation As Revealed by Metatranscriptome and 16S rRNA Gene Sequencings. J. Agric. Food Chem. 2016, 64, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Chun, B.H.; Kim, J.; Jeon, C.O. Identification of biogenic amine-producing microbes during fermentation of ganjang, a Korean traditional soy sauce, through metagenomic and metatranscriptomic analyses. Food Control 2021, 121, 107681. [Google Scholar] [CrossRef]
- Nam, Y.D.; Chang, H.W.; Kim, K.H.; Roh, S.W.; Bae, J.W. Metatranscriptome analysis of lactic acid bacteria during kimchi fermentation with genome-probing microarrays. Int. J. Food Microbiol. 2009, 130, 140–146. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Z.; Miyao, S.; Zhang, W. Unraveling the flavor profile and microbial roles during industrial Sichuan radish paocai fermentation by molecular sensory science and metatranscriptomics. Food Biosci. 2022, 48, 101815. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Guo, H.; Bai, J.; Xiao, Y.; Fu, Y.; Wu, Y.; Wan, H.; Huang, Y.; Gao, H. Metabolomics and metatranscriptomics reveal the influence mechanism of endogenous microbe (Staphylococcus succinus) inoculation on the flavor of fermented chili pepper. Int. J. Food Microbiol. 2023, 406, 110371. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Li, H.; Zhou, Z.; Wu, Z.; Zhang, W. Integrating metabolomics and metatranscriptomics to explore the formation pathway of aroma-active volatile phenolics and metabolic profile during industrial radish paocai fermentation. Food Res. Int. 2023, 167, 112719. [Google Scholar] [CrossRef]
- Qin, X.; Xiong, T.; Xiong, S.; Liu, Z.; Xie, M.; Guan, Q. Metatranscriptomics unravel the formation mechanism of key flavors during the natural fermentation of suansun, a Chinese traditional fermented bamboo shoot. Food Biosci. 2024, 57, 103436. [Google Scholar] [CrossRef]
- Xiong, S.; Xu, X.; Liu, Q.; Xu, Y.; Ren, H.; Xiong, T.; Xie, M. Integrated metatranscriptomics and metabolomics revealed the metabolic pathways of biogenic amines during Laotan Suancai fermentation. Food Biosci. 2024, 57, 103517. [Google Scholar] [CrossRef]
- Xiong, S.; Xu, X.; Zhang, L.; Du, T.; Huang, T.; Huang, J.; Ren, H.; Xiong, T.; Xie, M. Integrated metatranscriptomics and metabolomics reveal microbial succession and flavor formation mechanisms during the spontaneous fermentation of Laotan Suancai. Food Res. Int. 2024, 177, 113865. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Chen, Y.; Guo, J.; Li, C.; Zhang, J. Metatranscriptomic approach reveals the functional and enzyme dynamics of core microbes during noni fruit fermentation. Food Res. Int. 2021, 141, 109999. [Google Scholar] [CrossRef]
- Sangkaew, O.; Prombutara, P.; Roytrakul, S.; Yompakdee, C. Metatranscriptomics Reveals Sequential Expression of Genes Involved in the Production of Melanogenesis Inhibitors by the Defined Microbial Species in Fermented Unpolished Black Rice. Microbiol. Spectr. 2023, 11, e0313922. [Google Scholar] [CrossRef]
- Monnet, C.; Dugat-Bony, E.; Swennen, D.; Beckerich, J.M.; Irlinger, F.; Fraud, S.; Bonnarme, P. Investigation of the Activity of the Microorganisms in a Reblochon-Style Cheese by Metatranscriptomic Analysis. Front. Microbiol. 2016, 7, 536. [Google Scholar] [CrossRef]
- Duru, I.C.; Laine, P.; Andreevskaya, M.; Paulin, L.; Kananen, S.; Tynkkynen, S.; Auvinen, P.; Smolander, O.P. Metagenomic and metatranscriptomic analysis of the microbial community in Swiss-type Maasdam cheese during ripening. Int. J. Food Microbiol. 2018, 281, 10–22. [Google Scholar] [CrossRef]
- Song, Z.; Du, H.; Zhang, Y.; Xu, Y. Unraveling Core Functional Microbiota in Traditional Solid-State Fermentation by High-Throughput Amplicons and Metatranscriptomics Sequencing. Front. Microbiol. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Verce, M.; Schoonejans, J.; Hernandez Aguirre, C.; Molina-Bravo, R.; De Vuyst, L.; Weckx, S. A Combined Metagenomics and Metatranscriptomics Approach to Unravel Costa Rican Cocoa Box Fermentation Processes Reveals Yet Unreported Microbial Species and Functionalities. Front. Microbiol. 2021, 12, 641185. [Google Scholar] [CrossRef]
- An, F.; Li, M.; Zhao, Y.; Zhang, Y.; Mu, D.; Hu, X.; You, S.; Wu, J.; Wu, R. Metatranscriptome-based investigation of flavor-producing core microbiota in different fermentation stages of dajiang, a traditional fermented soybean paste of Northeast China. Food Chem. 2021, 343, 128509. [Google Scholar] [CrossRef]
- Chen, C.; Yao, W.; Yu, H.; Yuan, H.; Guo, W.; Huang, K.; Tian, H. Dynamics of microbial communities associated with flavor formation during sour juice fermentation and the milk fan drying process. J. Dairy Sci. 2023, 106, 7432–7446. [Google Scholar] [CrossRef]
- Lee, H.W.; Yoon, S.R.; Dang, Y.M.; Yun, J.H.; Jeong, H.; Kim, K.N.; Bae, J.W.; Ha, J.H. Metatranscriptomic and metataxonomic insights into the ultra-small microbiome of the Korean fermented vegetable, kimchi. Front. Microbiol. 2022, 13, 1026513. [Google Scholar] [CrossRef]
- Mota, V.T.; Delforno, T.P.; Ribeiro, J.C.; Zaiat, M.; Oliveira, V.M. Understanding microbiome dynamics and functional responses during acidogenic fermentation of sucrose and sugarcane vinasse through metatranscriptomic analysis. Environ. Res. 2024, 246, 118150. [Google Scholar] [CrossRef]
- Liu, X.F.; Liu, C.J.; Zeng, X.Q.; Zhang, H.Y.; Luo, Y.Y.; Li, X.R. Metagenomic and Metatranscriptomic Analysis of the Microbial Community Structure and Metabolic Potential of Fermented Soybean in Yunnan Province. Food Sci. Technol. 2020, 40, 18–25. [Google Scholar] [CrossRef]
- De Filippis, F.; Genovese, A.; Ferranti, P.; Gilbert, J.A.; Ercolini, D. Metatranscriptomics reveals temperature-driven functional changes in microbiome impacting cheese maturation rate. Sci. Rep. 2016, 6, 21871. [Google Scholar] [CrossRef]
- Kim, K.H.; Chun, B.H.; Baek, J.H.; Roh, S.W.; Lee, S.H.; Jeon, C.O. Genomic and metabolic features of Lactobacillus sakei as revealed by its pan-genome and the metatranscriptome of kimchi fermentation. Food Microbiol. 2020, 86, 103341. [Google Scholar] [CrossRef]
- Jeong, S.E.; Chun, B.H.; Kim, K.H.; Park, D.; Roh, S.W.; Lee, S.H.; Jeon, C.O. Genomic and metatranscriptomic analyses of Weissella koreensis reveal its metabolic and fermentative features during kimchi fermentation. Food Microbiol. 2018, 76, 1–10. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, B.; Zhou, Z.; Wu, Z.; Zhang, W. Cloning and characterization of phenolic acid decarboxylase responsible for aromatic volatile phenols production in Paocai based on metatranscriptomics. Food Biosci. 2023, 55, 102953. [Google Scholar] [CrossRef]
- Chai, K.F.; Ng, K.R.; Samarasiri, M.; Chen, W.N. Precision fermentation to advance fungal food fermentations. Curr. Opin. Food Sci. 2022, 47, 100881. [Google Scholar] [CrossRef]
- Scherlach, K.; Graupner, K.; Hertweck, C. Molecular bacteria-fungi interactions: Effects on environment, food, and medicine. Annu. Rev. Microbiol. 2013, 67, 375–397. [Google Scholar] [CrossRef]
- Maske, B.L.; de Melo Pereira, G.V.; da Silva Vale, A.; Marques Souza, D.S.; De Dea Lindner, J.; Soccol, C.R. Viruses in fermented foods: Are they good or bad? Two sides of the same coin. Food Microbiol. 2021, 98, 103794. [Google Scholar] [CrossRef]
- Paillet, T.; Dugat-Bony, E. Bacteriophage ecology of fermented foods: Anything new under the sun? Curr. Opin. Food Sci. 2021, 40, 102–111. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 2019, 25, 803–814.e805. [Google Scholar] [CrossRef]
- Kim, D.S.; Jung, J.Y.; Wang, Y.; Oh, H.J.; Choi, D.; Jeon, C.O.; Hahn, Y. Plant RNA virus sequences identified in kimchi by microbial metatranscriptome analysis. J. Microbiol. Biotechnol. 2014, 24, 979–986. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Wu, X.; Chen, B.; Fang, X.; Zhong, Q.; Liao, Z.; Wang, J.; Wang, L. The dietary intervention of synbiotic preparation promotes the bioconversion of soy isoflavones to equol and its metabolic mechanism. J. Funct. Foods 2023, 109, 105784. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Moraïs, S.; Winkler, S.; Zorea, A.; Levin, L.; Nagies, F.S.P.; Kapust, N.; Lamed, E.; Artan-Furman, A.; Bolam, D.N.; Yadav, M.P.; et al. Cryptic diversity of cellulose-degrading gut bacteria in industrialized humans. Science 2024, 383, eadj9223. [Google Scholar] [CrossRef]
- Kelly, W.J.; Mackie, R.I.; Attwood, G.T.; Janssen, P.H.; McAllister, T.A.; Leahy, S.C. Hydrogen and formate production and utilisation in the rumen and the human colon. Anim. Microbiome 2022, 4, 22. [Google Scholar] [CrossRef]
- Borewicz, K.; Hornung, B.; Gu, F.; van der Zaal, P.H.; Schols, H.A.; Schaap, P.J.; Smidt, H. Metatranscriptomic analysis indicates prebiotic effect of isomalto/malto-polysaccharides on human colonic microbiota in-vitro. Sci. Rep. 2024, 14, 18866. [Google Scholar] [CrossRef]
- Bendiks, Z.A.; Guice, J.; Coulon, D.; Raggio, A.M.; Page, R.C.; Carvajal-Aldaz, D.G.; Luo, M.; Welsh, D.A.; Marx, B.D.; Taylor, C.M.; et al. Resistant starch type 2 and whole grain maize flours enrich different intestinal bacteria and metatranscriptomes. J. Funct. Foods 2022, 90, 104982. [Google Scholar] [CrossRef]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef]
- Hoegenauer, C.; Hammer, H.F.; Mahnert, A.; Moissl-Eichinger, C. Methanogenic archaea in the human gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 805–813. [Google Scholar] [CrossRef]
- Xu, R.; Li, Q.; Wang, H.; Su, Y.; Zhu, W. Reduction of Redox Potential Exerts a Key Role in Modulating Gut Microbial Taxa and Function by Dietary Supplementation of Pectin in a Pig Model. Microbiol. Spectr. 2023, 11, e0328322. [Google Scholar] [CrossRef]
- Penumutchu, S.; Korry, B.J.; Hewlett, K.; Belenky, P. Fiber supplementation protects from antibiotic-induced gut microbiome dysbiosis by modulating gut redox potential. Nat. Commun. 2023, 14, 5161. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Wolter, M.; Grant, E.T.; Boudaud, M.; Steimle, A.; Pereira, G.V.; Martens, E.C.; Desai, M.S. Leveraging diet to engineer the gut microbiome. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 885–902. [Google Scholar] [CrossRef]
- Ramos, S.; Martín, M.Á. Impact of diet on gut microbiota. Curr. Opin. Food Sci. 2021, 37, 83–90. [Google Scholar] [CrossRef]
- Hugenholtz, F.; Davids, M.; Schwarz, J.; Müller, M.; Tomé, D.; Schaap, P.; Hooiveld, G.; Smidt, H.; Kleerebezem, M. Metatranscriptome analysis of the microbial fermentation of dietary milk proteins in the murine gut. PLoS ONE 2018, 13, e0194066. [Google Scholar] [CrossRef]
- Faits, T.; Walker, M.E.; Rodriguez-Morato, J.; Meng, H.; Gervis, J.E.; Galluccio, J.M.; Lichtenstein, A.H.; Johnson, W.E.; Matthan, N.R. Exploring changes in the human gut microbiota and microbial-derived metabolites in response to diets enriched in simple, refined, or unrefined carbohydrate-containing foods: A post hoc analysis of a randomized clinical trial. Am. J. Clin. Nutr. 2020, 112, 1631–1641. [Google Scholar] [CrossRef]
- Wang, F.; Wan, Y.; Yin, K.; Wei, Y.; Wang, B.; Yu, X.; Ni, Y.; Zheng, J.; Huang, T.; Song, M.; et al. Lower Circulating Branched-Chain Amino Acid Concentrations Among Vegetarians are Associated with Changes in Gut Microbial Composition and Function. Mol. Nutr. Food Res. 2019, 63, e1900612. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Sapp, P.A.; Kris-Etherton, P.M.; Arnesen, E.A.; Chen See, J.R.; Lamendella, R.; Petersen, K.S. Peanuts as a nighttime snack enrich butyrate-producing bacteria compared to an isocaloric lower-fat higher-carbohydrate snack in adults with elevated fasting glucose: A randomized crossover trial. Clin. Nutr. 2022, 41, 2169–2177. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, K.; Suliburska, J. Association between the gut microbiota and mineral metabolism. J. Sci. Food Agric. 2018, 98, 2449–2460. [Google Scholar] [CrossRef]
- Lin, D.; Medeiros, D.M. The microbiome as a major function of the gastrointestinal tract and its implication in micronutrient metabolism and chronic diseases. Nutr. Res. 2023, 112, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Wang, Y.; Yang, Q.; Jiang, X.; Zhao, R.; Chen, H.; Zhang, Y.; Ran, J.; Chen, W.; et al. Resistance role of Lactobacillus sp. and Lactococcus sp. to copper ions in healthy children’s intestinal microorganisms. J. Hazard. Mater. 2024, 469, 134059. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Zhu, W. Gut Microbiota: The Brain Peacekeeper. Front. Microbiol. 2016, 7, 345. [Google Scholar] [CrossRef]
- Mu, C.; Nikpoor, N.; Tompkins, T.A.; Rho, J.M.; Scantlebury, M.H.; Shearer, J. Probiotics counteract hepatic steatosis caused by ketogenic diet and upregulate AMPK signaling in a model of infantile epilepsy. EBioMedicine 2022, 76, 103838. [Google Scholar] [CrossRef]
- Mu, C.; Nikpoor, N.; Tompkins, T.A.; Choudhary, A.; Wang, M.; Marks, W.N.; Rho, J.M.; Scantlebury, M.H.; Shearer, J. Targeted gut microbiota manipulation attenuates seizures in a model of infantile spasms syndrome. JCI Insight 2022, 7, e158521. [Google Scholar] [CrossRef]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, N.; Feng, Y.; Zhou, M.; Li, H.; Zhang, X.; Ma, X. From probiotics to postbiotics: Concepts and applications. Anim. Res. One Health 2023, 1, 92–114. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Liu, W.; Zhang, H.; Sun, Z. Metagenomic and metatranscriptomic profiling of Lactobacillus casei Zhang in the human gut. NPJ Biofilms Microbiomes 2021, 7, 55. [Google Scholar] [CrossRef]
- Zaccaria, E.; Klaassen, T.; Alleleyn, A.M.E.; Boekhorst, J.; Chervaux, C.; Smokvina, T.; Troost, F.J.; Kleerebezem, M.L. rhamnosus CNCM I-3690 survival, adaptation, and small bowel microbiome impact in human. Gut Microbes 2023, 15, 2244720. [Google Scholar] [CrossRef]


| Food | Research Purpose | Approach | Functional Microorganisms | Major Functions | Reference |
|---|---|---|---|---|---|
| Fermented Bamboo shoot | Flavour formation | Metatranscriptomics Metabolomics | Lactococcus, Enterococcus, Leuconostoc, Lactiplantibacillus and Weissella | Lactic, acetic, malic and citric acid synthesis | [61] |
| Fermented Laotan Suancai | Flavour formation | Metatranscriptomics Metabolomics | Companilactobacillus alimentarius, Weissella cibaria, Lactiplantibacillus plantarum, and Loigolactobacillus coryniformis | Oxalic acid and lactic acid synthesis, Geranyl-PP metabolism | [63] |
| Fermented Laotan Suancai | Biogenic amine production | Metatranscriptomics Metabolomics | Lactobacillaceae Species and Tetragenococcus halophilus | Putrescine and histamine synthesis | [62] |
| Fermented Kimchi | Lactic acid bacteria succession | Metagenomic Metatranscriptomics | Lactobacillus graminis, Lactobacillus curvatus, Lactobacillus sakei subsp. carnosus, Weissella viridescens | Lactic acid synthesis | [57] |
| Fermented Unpolished Black Rice | Production of Melanogenesis Inhibitors | Metatranscriptomics | Saccharomyces cerevisiae, Saccharomycopsis fibuligera, Rhizopus oryzae, and Pediococcus pentosaceus | Species interaction leads to maximum melanogenesis inhibition activity | [65] |
| Reblochon-Style Cheese | Microbial activity during cheese ripening | Metatranscriptomics | Debaryomyces hansenii, Geotrichum candidum | Amino acid catabolism | [66] |
| Swiss-type Maasdam cheese | Microbial activity during cheese ripening | Metagenomic Metatranscriptomics | Lactococcus lactis | Upregulated vitamin biosynthesis and homolactic fermentation during cold room ripening | [67] |
| Soy sauce aroma type liquor | Flavour formation | Metatranscriptomics Amplicon sequencing | Stage 1: Schizosaccharomyces Stage 2: Lactobacillus | Stage 1: Ethanol production Stage 2: Lactic acid and acetic acid production | [68] |
| Fermented Costa Rican Cocoa Box | Microbial composition and activity | Metagenomics Metatranscriptomics | Limosilactobacillus fermentum, Liquorilactobacillus cacaonum, Lactiplantibacillus plantarum, Acetobacter pasteurianus, Acetobacter ghanensis, Hanseniaspora opuntiae and Saccharomyces cerevisiae | Utilization of glucose, fructose, and citric acid to produce ethanol, lactic acid, acetic acid, and mannitol | [69] |
| Fermented ganjang (Korean traditional soy sauce) | Biogenic amine production | Metagenomics Metatranscriptomics | Staphylococcus produces cadaverine; Tetragenococcus produces histamine; Lactobacillus and Halomonas produce putrescine; Tetragenococcus, Bacillus, and Enterococcus produce tyramine | Biogenic amine synthesis | [56] |
| Fermented dajiang (soybean Paste) | Flavour formation | Metatranscriptomics | Lactobacillus produces acetic acid and ethanol; Tetragenococcus produces aldehydes and ketones | Flavour metabolite synthesis | [70] |
| Fermented Sichuan radish paocai | Flavour formation | Metatranscriptomic | Lactobacillus, Debaryomyces | flavour metabolism, such as acetic acid and lactic acid | [58] |
| Fermented Sichuan radish paocai | Aromatic volatile phenol production | Metatranscriptomic | Lactobacillus vermolensis | Produce phenolic acid decarboxylase for the decarboxylation of p-coumaric acid, ferulic acid, and caffeic acid into 4-vinylphenol and 4-vinylguaiacol | [60] |
| Fermentation of sour juice and drying of milk fan | Flavour formation | Metatranscriptomic | Lactococcus, Rhodotorula, Candida, Cutaneotrichosporon, Yarrowia | Positive association with aroma-active compounds, including ethyl acetate, 2-heptanone, isovaleraldehyde, butyric acid, nonanal, and hexanal. | [71] |
| Fermented noni fruit | Odour formation | Metatranscriptomic | Acetobacter sp., Acetobacter aceti, and Gluconobacter sp. | Carbohydrate metabolism, acetic acid production | [64] |
| Kimchi | Ultra-small microbiome | Metatranscriptome Metataxonome | Lactobacillus, Leuconostoc, Weissella, Akkermansia | Lactic acid production, protein metabolism | [72] |
| Niulanshan Baijiu Fermentation | Flavour formation | Metatranscriptomics Metabolomics | Streptococcus, Lactobacillus, Pediococcus, Campylobacter, Yersinia, Weissella, Talaromyces, Aspergillus, Mixia, Rhizophagus, and Gloeophyllum | Produce volatile compounds, such as 3-methylbutanol, 2-methylpropanoate, 3-methylbutal | [54] |
| Fermented chilli pepper | Flavour formation | Metatranscriptomics Metabolomics | Staphylococcus, Lactobacillus | Esters, terpenes, alcohols, aspartic acid and glutamic acid production | [59] |
| Sugarcane vinasse | Vinasse metabolism | Metatranscriptomics | Pectinatus, Megasphaera, Clostridium, Pectinatus frisingensis | Acetate or propionate production | [73] |
| Fermented soybean | Microbial community | Metagenomic Metatranscriptomics | Providencia stuartii | Carbohydrates, protein, energy, and amino acid metabolism | [74] |
| Shrimp sauce | Flavour formation | Metatranscriptomics | Tetragenococcus halophilus | Citrate cycle and oxidative phosphorylation | [55] |
| Traditional Italian Caciocavallo Silano cheese | Cheese ripening | Metatranscriptomics | Lactobacillus casei, Lactobacillus buchneri | Proteolysis, lipolysis, and amino acid/lipid catabolism | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butowski, C.F.; Dixit, Y.; Reis, M.M.; Mu, C. Metatranscriptomics for Understanding the Microbiome in Food and Nutrition Science. Metabolites 2025, 15, 185. https://doi.org/10.3390/metabo15030185
Butowski CF, Dixit Y, Reis MM, Mu C. Metatranscriptomics for Understanding the Microbiome in Food and Nutrition Science. Metabolites. 2025; 15(3):185. https://doi.org/10.3390/metabo15030185
Chicago/Turabian StyleButowski, Christina F., Yash Dixit, Marlon M. Reis, and Chunlong Mu. 2025. "Metatranscriptomics for Understanding the Microbiome in Food and Nutrition Science" Metabolites 15, no. 3: 185. https://doi.org/10.3390/metabo15030185
APA StyleButowski, C. F., Dixit, Y., Reis, M. M., & Mu, C. (2025). Metatranscriptomics for Understanding the Microbiome in Food and Nutrition Science. Metabolites, 15(3), 185. https://doi.org/10.3390/metabo15030185






