Bacillus megaterium DSM 32963 Enhances Specialized Pro-Resolving Mediator Production from an n-3 PUFA Salt in a Dynamic Model of the Human Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Samples
2.2. SynΩ3
2.3. Ω3 Salt (n-3 PUFA Lysine Salt)
2.4. Fish Oil (SPM-Precursor-Enriched Fish Oil)
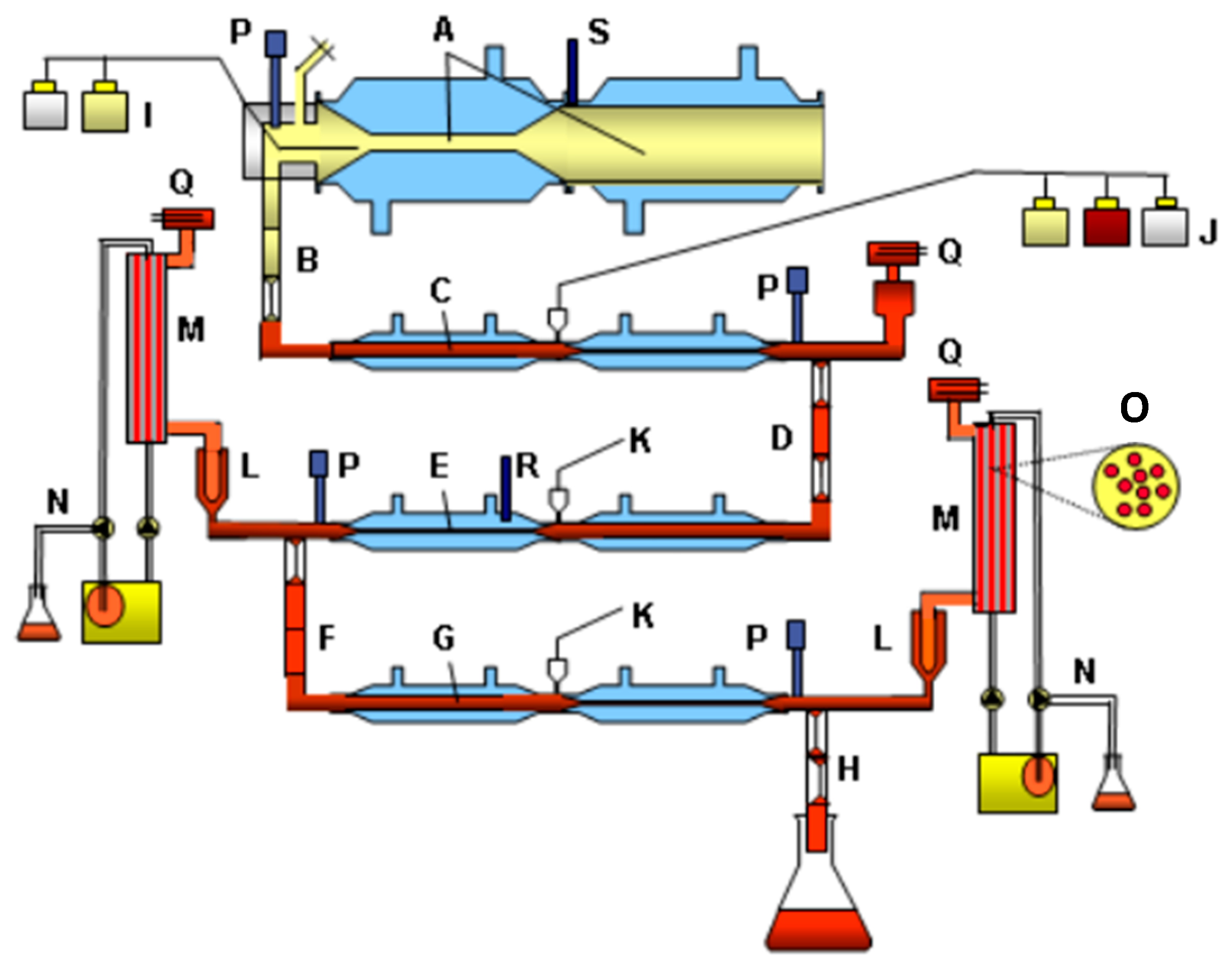
2.5. Set-Up of TIM-1
2.6. Set-Up of TIM-2
2.7. Quantification of Bacillus sp. CFU
2.8. Quantification of Bacillus megaterium DSM 32963 by qPCR
2.9. Analysis of Lipid Mediators in TIM-1 and TIM-2 Samples
2.10. Data Presentation and Statistical Analysis
3. Results
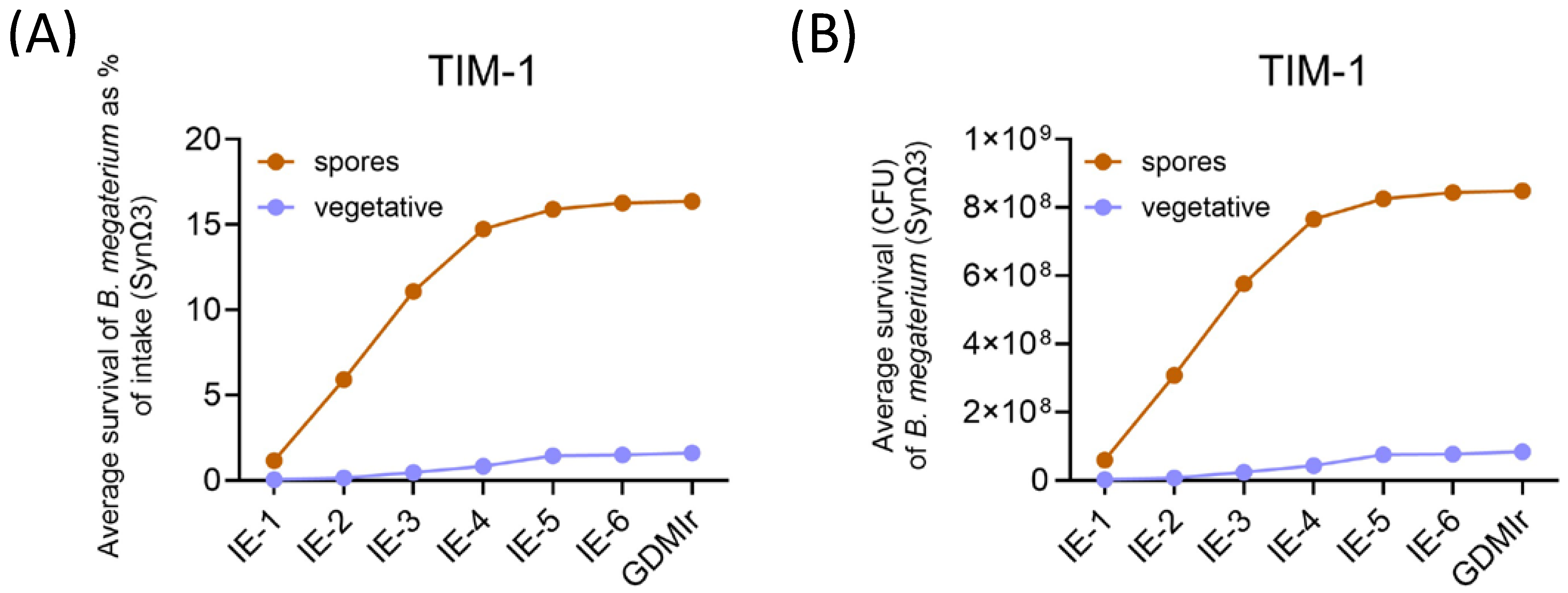
3.1. Germination and Survival of Bacillus megaterium DSM 32963 During Gastrointestinal Transit in TIM-1 and TIM-2
3.1.1. Determination of Viable Cells and Spores by Plate Counting
3.1.2. Determination of Bacillus megaterium DSM 32963 Count by Strain-Specific qPCR
3.2. Production of Lipid Mediators During Gastrointestinal Transit in TIM-1 and TIM-2
3.2.1. Production of SPM and Precursors in TIM-1
3.2.2. Production of SPM and Precursors in TIM-2
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frankenfeld, C.L. Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol. Nutr. Food Res. 2017, 61, 1500900. [Google Scholar] [CrossRef]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sanchez, S.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Lifelines Cohort, S.; Weersma, R.K.; Medema, M.H.; et al. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Orem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- Speckmann, B.; Ehring, E.; Hu, J.; Rodriguez Mateos, A. Exploring substrate-microbe interactions: A metabiotic approach toward developing targeted synbiotic compositions. Gut Microbes 2024, 16, 2305716. [Google Scholar] [CrossRef] [PubMed]
- Caffaratti, C.; Plazy, C.; Cunin, V.; Toussaint, B.; Le Gouellec, A. Bioengineering of Escherichia coli Nissle 1917 for Production and Excretion of Spermidine, a Key Metabolite in Human Health. Metabolites 2022, 12, 1061. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltran, D.; Luna, M.C.; Romo-Vaquero, M.; Garcia-Villalba, R.; Mira, A.; Espin, J.C.; Tomas-Barberan, F.A. Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Kleinbolting, J.; Borner, F.; Jordan, P.M.; Werz, O.; Pelzer, S.; Tom Dieck, H.; Wagner, T.; Schon, C. Synbiotic Compositions of Bacillus megaterium and Polyunsaturated Fatty Acid Salt Enable Self-Sufficient Production of Specialized Pro-Resolving Mediators. Nutrients 2022, 14, 2265. [Google Scholar] [CrossRef]
- Lopez-Vicario, C.; Rius, B.; Alcaraz-Quiles, J.; Garcia-Alonso, V.; Lopategi, A.; Titos, E.; Claria, J. Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol. 2016, 785, 133–143. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Ungaro, F.; Rubbino, F.; Danese, S.; D’Alessio, S. Actors and Factors in the Resolution of Intestinal Inflammation: Lipid Mediators As a New Approach to Therapy in Inflammatory Bowel Diseases. Front. Immunol. 2017, 8, 1331. [Google Scholar] [CrossRef] [PubMed]
- Pochard, C.; Coquenlorge, S.; Jaulin, J.; Cenac, N.; Vergnolle, N.; Meurette, G.; Freyssinet, M.; Neunlist, M.; Rolli-Derkinderen, M. Defects in 15-HETE Production and Control of Epithelial Permeability by Human Enteric Glial Cells From Patients With Crohn’s Disease. Gastroenterology 2016, 150, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; Tacconi, C.; Massimino, L.; Corsetto, P.A.; Correale, C.; Fonteyne, P.; Piontini, A.; Garzarelli, V.; Calcaterra, F.; Della Bella, S.; et al. MFSD2A Promotes Endothelial Generation of Inflammation-Resolving Lipid Mediators and Reduces Colitis in Mice. Gastroenterology 2017, 153, 1363–1377.e1366. [Google Scholar] [CrossRef]
- Coquenlorge, S.; Van Landeghem, L.; Jaulin, J.; Cenac, N.; Vergnolle, N.; Duchalais, E.; Neunlist, M.; Rolli-Derkinderen, M. The arachidonic acid metabolite 11beta-ProstaglandinF2alpha controls intestinal epithelial healing: Deficiency in patients with Crohn’s disease. Sci. Rep. 2016, 6, 25203. [Google Scholar] [CrossRef]
- Mangino, M.J.; Brounts, L.; Harms, B.; Heise, C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 2006, 79, 84–92. [Google Scholar] [CrossRef]
- Capdevila, J.H.; Wei, S.; Helvig, C.; Falck, J.R.; Belosludtsev, Y.; Truan, G.; Graham-Lorence, S.E.; Peterson, J.A. The highly stereoselective oxidation of polyunsaturated fatty acids by cytochrome P450BM-3. J. Biol. Chem. 1996, 271, 22663–22671. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 Monooxygenases in Biotechnology and Synthetic Biology. Trends Biotechnol. 2019, 37, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Wagner, T.; Jordan, P.M.; Werz, O.; Wilhelm, M.; Tom Dieck, H.; Schon, C. Synbiotic Bacillus megaterium DSM 32963 and n-3 PUFA Salt Composition Elevates Pro-Resolving Lipid Mediator Levels in Healthy Subjects: A Randomized Controlled Study. Nutrients 2024, 16, 1354. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Smeets-Peeters, M.; Bernalier, A.; Marol-Bonnin, S.; Havenaar, R.; Marteau, P.; Alric, M.; Fonty, G.; Huis in’t Veld, J.H. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl. Microbiol. Biotechnol. 1999, 53, 108–114. [Google Scholar] [CrossRef]
- Venema, K.; van den Abbeele, P. Experimental models of the gut microbiome. Best. Pract. Res. Clin. Gastroenterol. 2013, 27, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaer, A.E.; Regan, J.; Buddenbaum, N.; Tharwani, S.; Drawdy, C.; Behee, M.; Sergin, S.; Fenton, J.I.; Maddipati, K.R.; Kane, S.; et al. Enriched Marine Oil Supplement Increases Specific Plasma Specialized Pro-Resolving Mediators in Adults with Obesity. J. Nutr. 2022, 152, 1783–1791. [Google Scholar] [CrossRef]
- Keller, D.; Verbruggen, S.; Cash, H.; Farmer, S.; Venema, K. Spores of Bacillus coagulans GBI-30, 6086 show high germination, survival and enzyme activity in a dynamic, computer-controlled in vitro model of the gastrointestinal tract. Benef. Microbes 2019, 10, 77–87. [Google Scholar] [CrossRef]
- Aguirre, M.; Ramiro-Garcia, J.; Koenen, M.E.; Venema, K. To pool or not to pool? Impact of the use of individual and pooled fecal samples for in vitro fermentation studies. J. Microbiol. Methods 2014, 107, 1–7. [Google Scholar] [CrossRef]
- Werz, O.; Gerstmeier, J.; Libreros, S.; De la Rosa, X.; Werner, M.; Norris, P.C.; Chiang, N.; Serhan, C.N. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Sulciner, M.L. Resolution medicine in cancer, infection, pain and inflammation: Are we on track to address the next Pandemic? Cancer Metastasis Rev. 2023, 42, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Lichtenstein, A.H. Omega-3 Fatty Acids and Cardiovascular Disease: Summary of the 2016 Agency of Healthcare Research and Quality Evidence Review. Nutrients 2017, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R.V. An Update on Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Al-Thubiani, A.S.A.; Maher, Y.A.; Fathi, A.; Abourehab, M.A.S.; Alarjah, M.; Khan, M.S.A.; Al-Ghamdi, S.B. Identification and characterization of a novel antimicrobial peptide compound produced by Bacillus megaterium strain isolated from oral microflora. Saudi Pharm. J. 2018, 26, 1089–1097. [Google Scholar] [CrossRef]
- Pagac, M.P.; Gempeler, M.; Campiche, R. A New Generation of Postbiotics for Skin and Scalp: In Situ Production of Lipid Metabolites by Malassezia. Microorganisms 2024, 12, 1711. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gou, W.; Ma, Y.; Shuai, M.; Liang, X.; Fu, Y.; Zheng, J.S. The Short-Term Variation of Human Gut Mycobiome in Response to Dietary Intervention of Different Macronutrient Distributions. Nutrients 2023, 15, 2152. [Google Scholar] [CrossRef] [PubMed]
- Vance, R.E.; Hong, S.; Gronert, K.; Serhan, C.N.; Mekalanos, J.J. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 2004, 101, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.F.; Claudino, R.F.; Dutra, R.C.; Marcon, R.; Calixto, J.B. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J. Immunol. 2011, 187, 1957–1969. [Google Scholar] [CrossRef]
- Perna, E.; Aguilera-Lizarraga, J.; Florens, M.V.; Jain, P.; Theofanous, S.A.; Hanning, N.; De Man, J.G.; Berg, M.; De Winter, B.; Alpizar, Y.A.; et al. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 2021, 70, 1275–1286. [Google Scholar] [CrossRef]






| Blank | SynΩ3 | Ω3 Salt | Fish Oil |
|---|---|---|---|
| 150 g GES 1 | 150 g GES 1 | 150 g GES 1 | 150 g GES 1 |
| 150 g LB medium 2 | 150 g LB medium 2 | 150 g LB medium 2 | 150 g LB medium 2 |
| 300 mg n-3 PUFA lysine salt containing 83.3 mg EPA, 41.7 mg DHA, ~2.4 billion CFU Bacillus megaterium DSM 32963 (B4U™63) | 300 mg n-3 PUFA lysine salt containing 83.3 mg EPA, 41.7 mg DHA | 500 mg fish oil containing ~54 mg EPA, ~108 mg DHA | |
| 5 mL start residue | 5 mL start residue | 5 mL start residue | 5 mL start residue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speckmann, B.; Jordan, P.M.; Werz, O.; Hofstetter, R.K.; Ehring, E.; Vogel, M.-L.; Venema, K. Bacillus megaterium DSM 32963 Enhances Specialized Pro-Resolving Mediator Production from an n-3 PUFA Salt in a Dynamic Model of the Human Intestine. Metabolites 2025, 15, 105. https://doi.org/10.3390/metabo15020105
Speckmann B, Jordan PM, Werz O, Hofstetter RK, Ehring E, Vogel M-L, Venema K. Bacillus megaterium DSM 32963 Enhances Specialized Pro-Resolving Mediator Production from an n-3 PUFA Salt in a Dynamic Model of the Human Intestine. Metabolites. 2025; 15(2):105. https://doi.org/10.3390/metabo15020105
Chicago/Turabian StyleSpeckmann, Bodo, Paul M. Jordan, Oliver Werz, Robert K. Hofstetter, Ellen Ehring, Marie-Luise Vogel, and Koen Venema. 2025. "Bacillus megaterium DSM 32963 Enhances Specialized Pro-Resolving Mediator Production from an n-3 PUFA Salt in a Dynamic Model of the Human Intestine" Metabolites 15, no. 2: 105. https://doi.org/10.3390/metabo15020105
APA StyleSpeckmann, B., Jordan, P. M., Werz, O., Hofstetter, R. K., Ehring, E., Vogel, M.-L., & Venema, K. (2025). Bacillus megaterium DSM 32963 Enhances Specialized Pro-Resolving Mediator Production from an n-3 PUFA Salt in a Dynamic Model of the Human Intestine. Metabolites, 15(2), 105. https://doi.org/10.3390/metabo15020105









