Hepatocyte-Specific ApoJ Knockout Improves Metabolic Profiles in the Liver of Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedure
2.2. UHPLC-MS/MS Analysis
2.3. KEGG Enrichment Analysis
2.4. Multivariate Statistical Analysis
3. Results
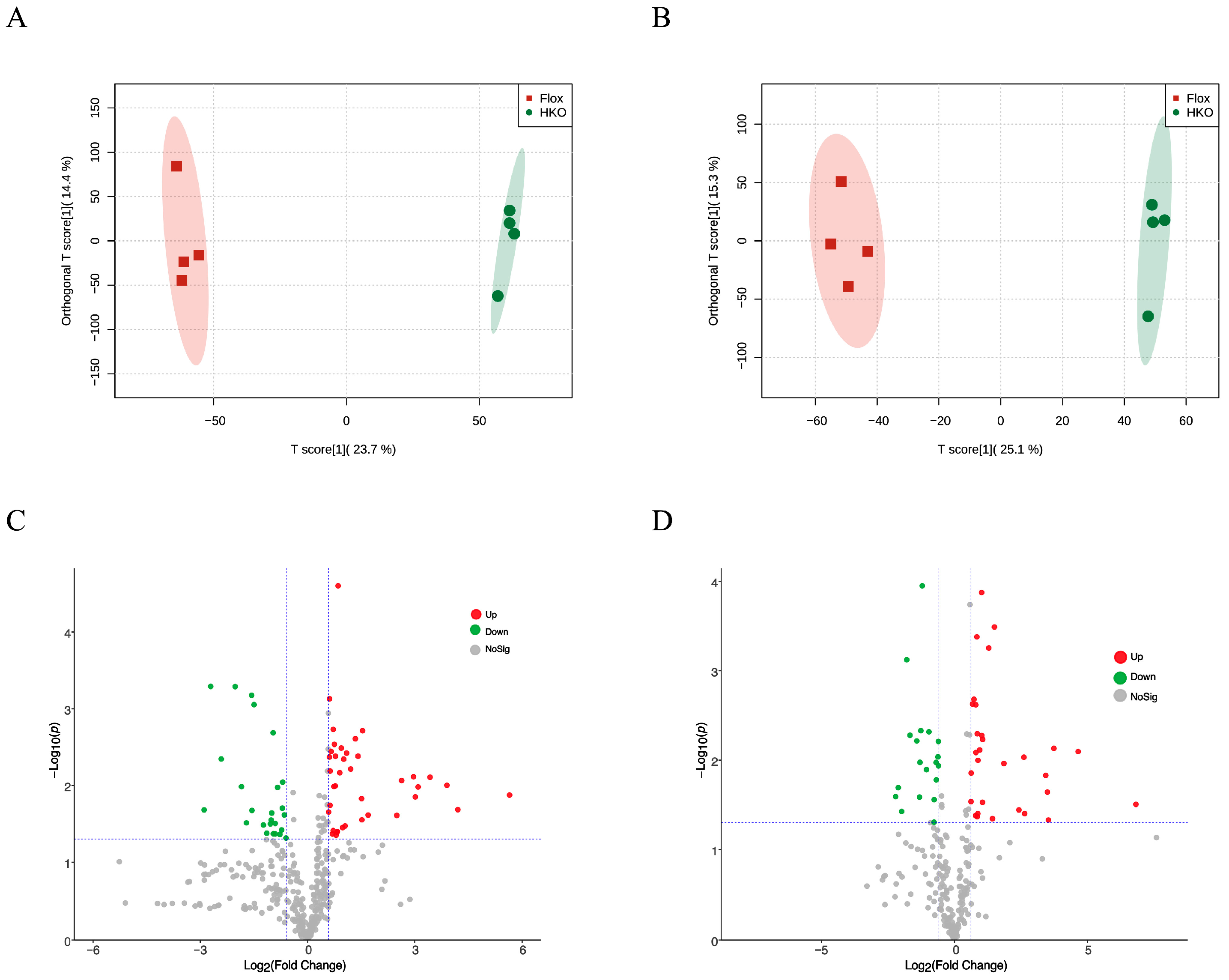
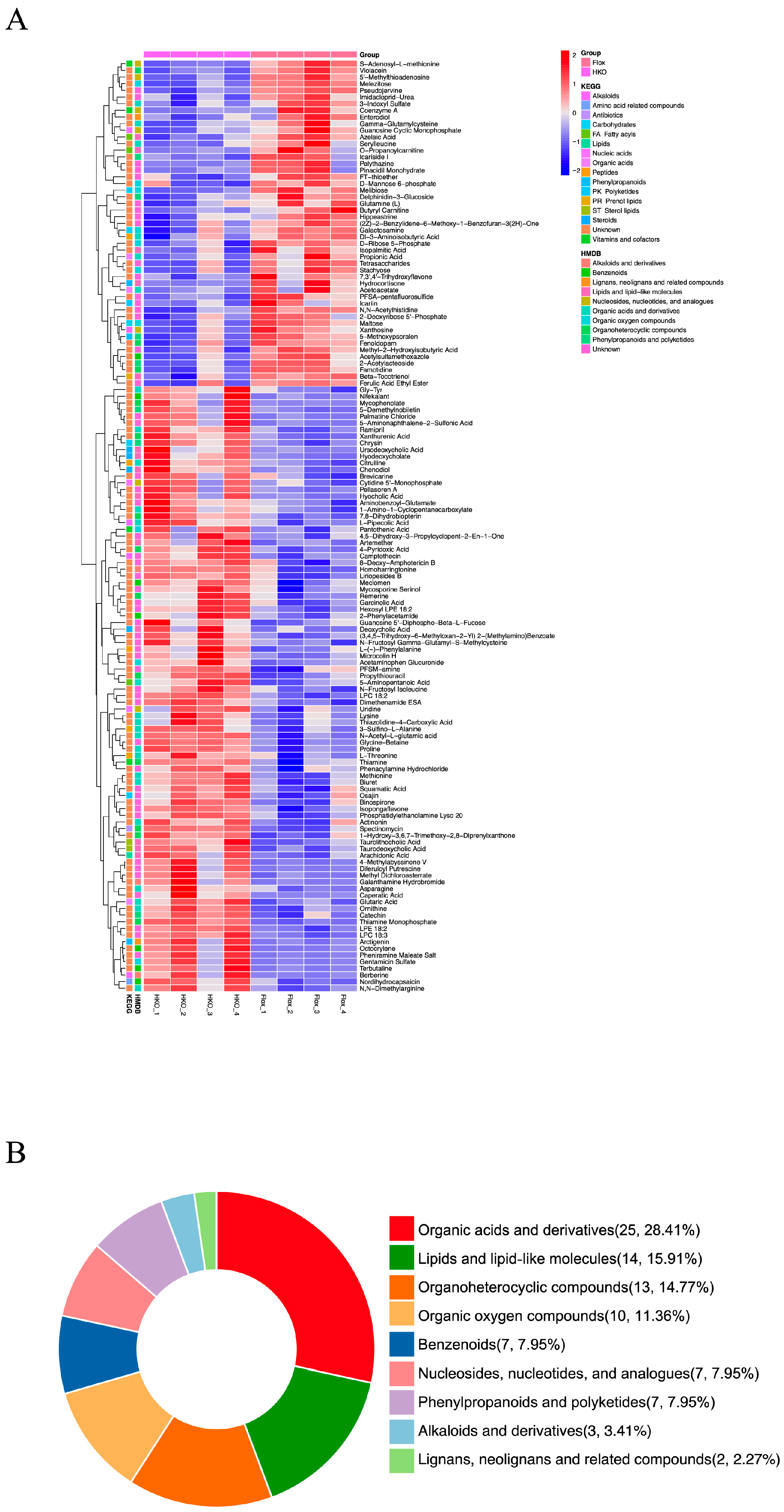
3.1. Hepatocyte-Specific ApoJ Knockout Alters Hepatic Metabolism in Diabetic Mice
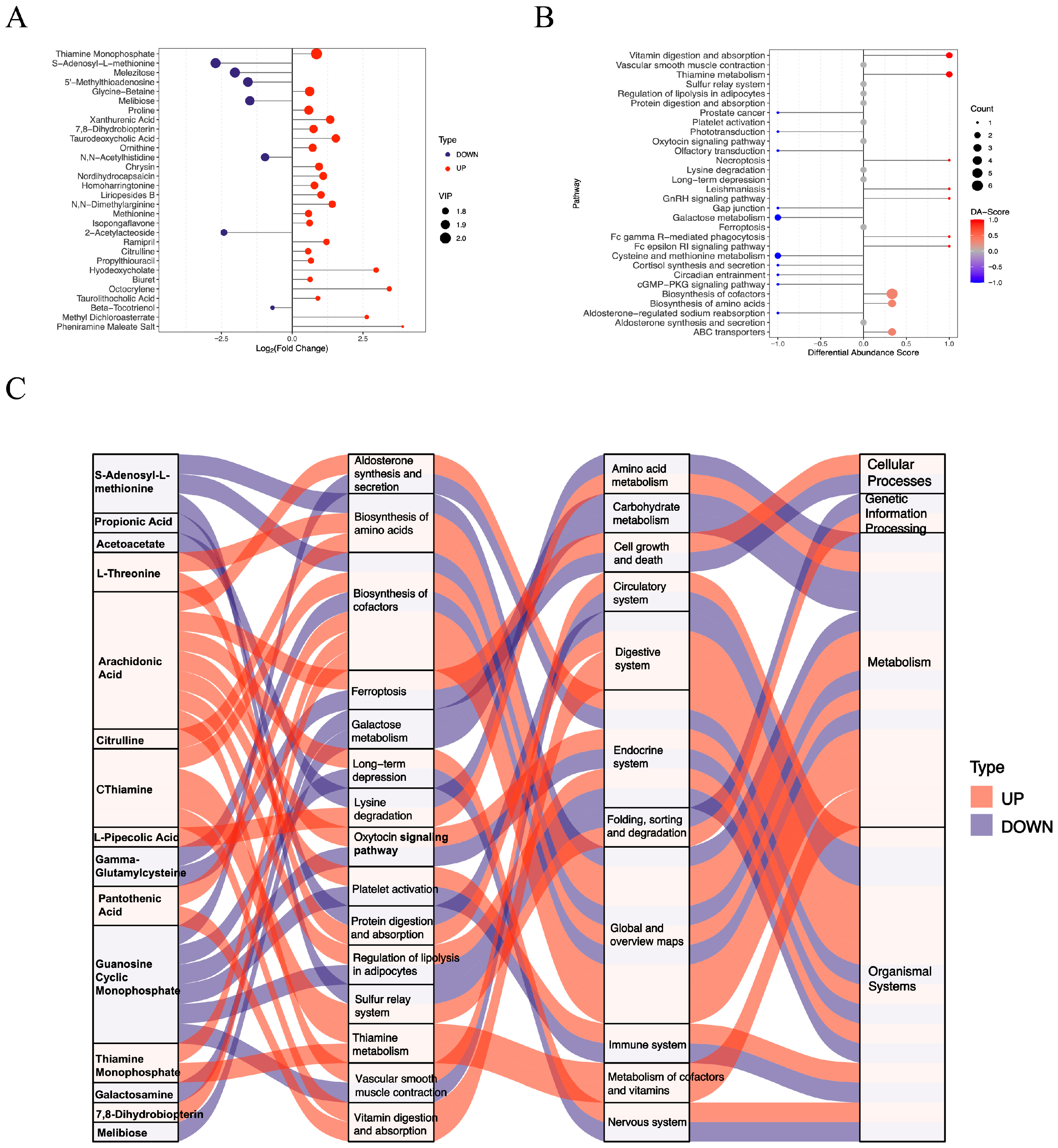
3.2. Functional Assessment of Differentially Abundant Positive Metabolites
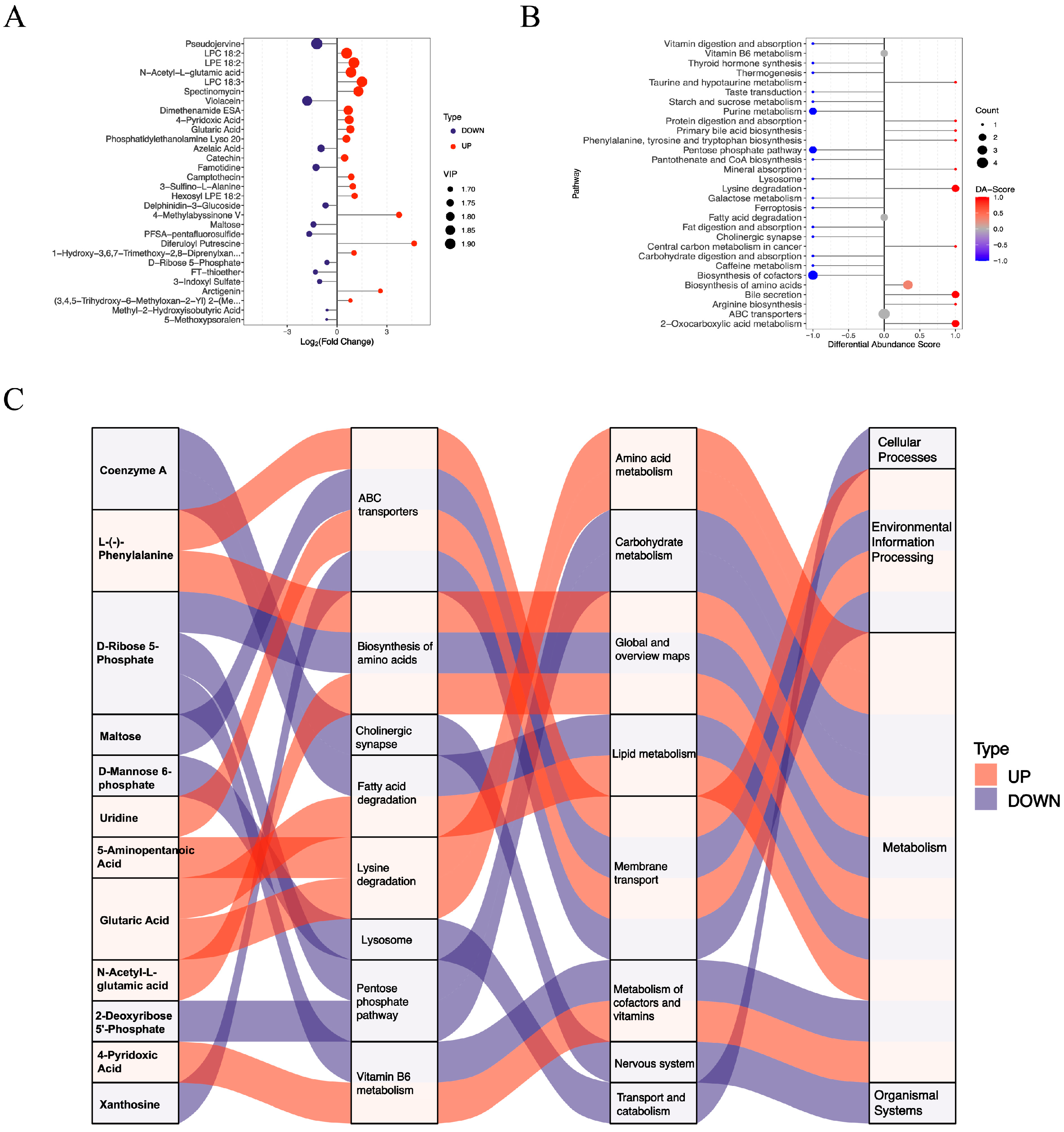
3.3. Functional Assessment of Differentially Abundant Negative Metabolites
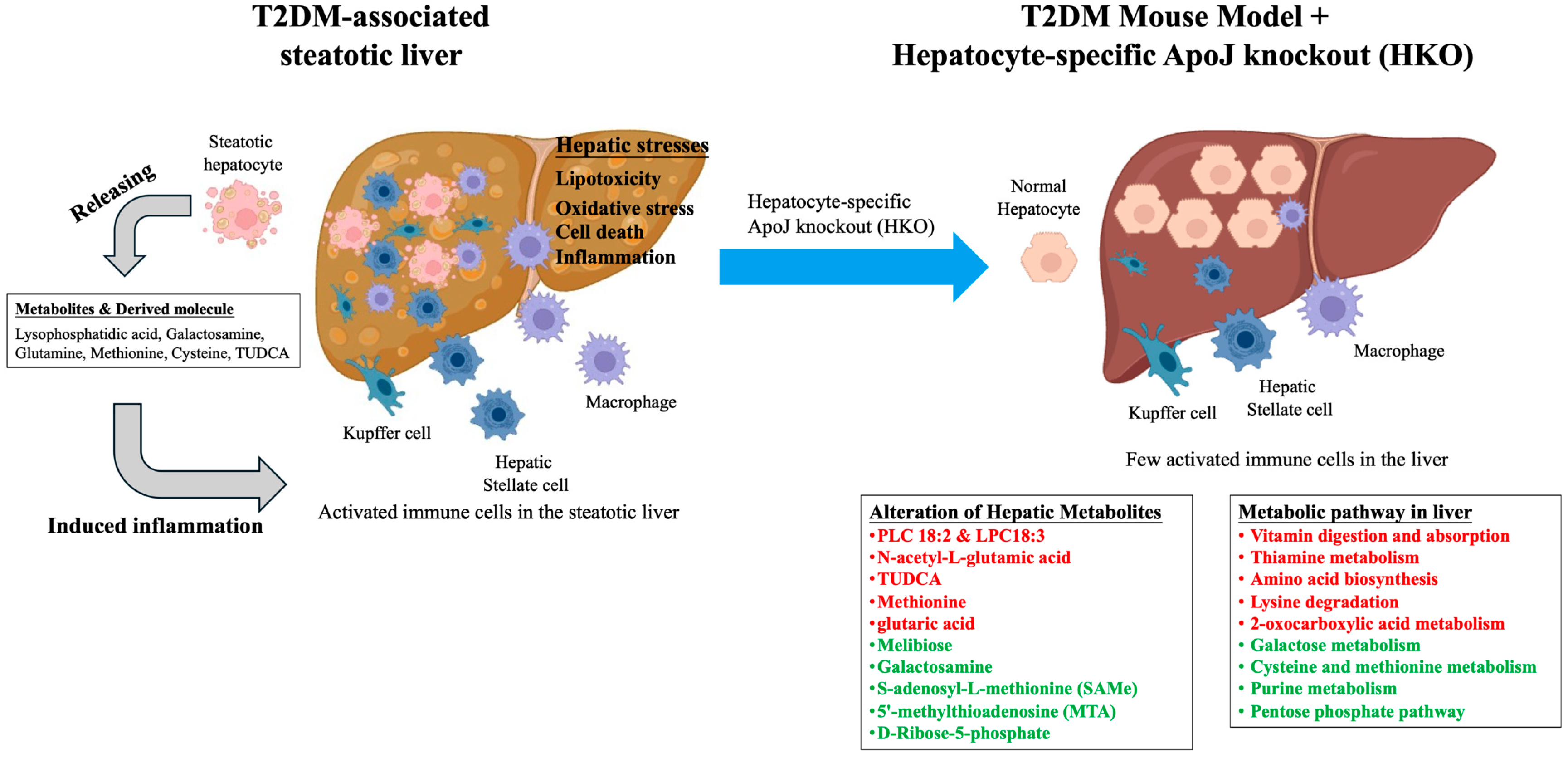
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, L.; An, Y.; Liu, H.; Jiang, J.; Liu, W.; Zhou, Y.; Shi, M.; Dai, W.; Lv, Y.; Zhao, Y.; et al. Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: A systematic review and meta-analysis. BMC Med. 2024, 22, 101. [Google Scholar] [CrossRef]
- Praharaj, P.P.; Patra, S.; Panigrahi, D.P.; Patra, S.K.; Bhutia, S.K. Clusterin as modulator of carcinogenesis: A potential avenue for targeted cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188500. [Google Scholar] [CrossRef]
- Seo, J.A.; Kang, M.C.; Yang, W.M.; Hwang, W.M.; Kim, S.S.; Hong, S.H.; Heo, J.I.; Vijyakumar, A.; Pereira de Moura, L.; Uner, A.; et al. Apolipoprotein J is a hepatokine regulating muscle glucose metabolism and insulin sensitivity. Nat. Commun. 2020, 11, 2024. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, G. Apolipoproteins, as the carrier proteins for lipids, are involved in the development of breast cancer. Clin. Transl. Oncol. 2020, 22, 1952–1962. [Google Scholar] [CrossRef]
- Sun, H.Y.; Chen, T.Y.; Tan, Y.C.; Wang, C.H.; Young, K.C. Sterol O-acyltransferase 2 chaperoned by apolipoprotein J facilitates hepatic lipid accumulation following viral and nutrient stresses. Commun. Biol. 2021, 4, 564. [Google Scholar] [CrossRef]
- Duan, S.; Qin, N.; Pi, J.; Sun, P.; Gao, Y.; Liu, L.; Li, Z.; Li, Y.; Shi, L.; Gao, Q.; et al. Antagonizing apolipoprotein J chaperone promotes proteasomal degradation of mTOR and relieves hepatic lipid deposition. Hepatology 2023, 78, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yang, H.; Li, M. Emerging Roles of Lysophosphatidic Acid in Macrophages and Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 12524. [Google Scholar] [CrossRef]
- Rahman, N.; Kuramochi, M.; Izawa, T.; Kuwamura, M.; Yamate, J. Characterization of Immature Myofibroblasts of Stellate Cell or Mesenchymal Cell Origin in D-Galactosamine-Induced Liver Injury in Rats. Vet. Pathol. 2021, 58, 80–90. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.; Chen, S.; Wu, G.; Bazer, F.W.; Zhou, B.; Tan, B.; Zhu, G.; Deng, J.; Yin, Y. Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes. Adv. Nutr. 2019, 10, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Liu, J.; Lai, J.Y.; Xu, H.S.; Fan, L.J.; Zou, Y.H.; Zhou, Q.; Yue, Z.Q.; Gan, J.H. Methionine restriction promotes the polarization of macrophages towards M1 and the immunotherapy effect of PD-L1/PD-1 blockades by inhibiting the secretion of MIF by gastric carcinoma cells. Transl. Oncol. 2025, 51, 102181. [Google Scholar] [CrossRef] [PubMed]
- Miki, S.; Suzuki, J.I.; Takashima, M.; Ishida, M.; Kokubo, H.; Yoshizumi, M. S-1-Propenylcysteine promotes IL-10-induced M2c macrophage polarization through prolonged activation of IL-10R/STAT3 signaling. Sci. Rep. 2021, 11, 22469. [Google Scholar] [CrossRef]
- Han, G.H.; Kim, S.J.; Ko, W.K.; Lee, D.; Han, I.B.; Sheen, S.H.; Hong, J.B.; Sohn, S. Transplantation of tauroursodeoxycholic acid-inducing M2-phenotype macrophages promotes an anti-neuroinflammatory effect and functional recovery after spinal cord injury in rats. Cell Prolif. 2021, 54, e13050. [Google Scholar] [CrossRef]
- Barranco-Altirriba, M.; Alonso, N.; Weber, R.J.M.; Lloyd, G.R.; Hernandez, M.; Yanes, O.; Capellades, J.; Jankevics, A.; Winder, C.; Falguera, M.; et al. Lipidome characterisation and sex-specific differences in type 1 and type 2 diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 109. [Google Scholar] [CrossRef]
- Bellot, P.; Moia, M.N.; Reis, B.Z.; Pedrosa, L.F.C.; Tasic, L.; Barbosa, F., Jr.; Sena-Evangelista, K.C.M. Are Phosphatidylcholine and Lysophosphatidylcholine Body Levels Potentially Reliable Biomarkers in Obesity? A Review of Human Studies. Mol. Nutr. Food Res. 2023, 67, e2200568. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bai, L.; Wang, W.; Song, Y.; Zhao, W.; Li, Q.; Wu, Q. LC-MS-Based Lipidomic Analysis of Serum Samples from Patients with Type 2 Diabetes Mellitus (T2DM). Dis. Markers 2022, 2022, 5559470. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Dong, T.; Li, J.; Liu, Y.; Zhou, S.; Wei, X.; Hua, H.; Tang, K.; Zhang, X.; Wang, Y.; Wu, Z.; et al. Roles of immune dysregulation in MASLD. Biomed. Pharmacother. 2024, 170, 116069. [Google Scholar] [CrossRef]
- OuYang, Y.N.; Jin, Y.X.; Zhao, X.R.; Chen, M.; Yang, P.F.; Zheng, X.W.; Zeng, L.; Chen, L.; Tian, Z. Revealing metabolic pathways relevant to prediabetes based on metabolomics profiling analysis. Biochem. Biophys. Res. Commun. 2020, 533, 188–194. [Google Scholar] [CrossRef]
- Capelo-Diz, A.; Lachiondo-Ortega, S.; Fernandez-Ramos, D.; Canas-Martin, J.; Goikoetxea-Usandizaga, N.; Serrano-Macia, M.; Gonzalez-Rellan, M.J.; Mosca, L.; Blazquez-Vicens, J.; Tinahones-Ruano, A.; et al. Hepatic levels of S-adenosylmethionine regulate the adaptive response to fasting. Cell Metab. 2023, 35, 1373–1389.e8. [Google Scholar] [CrossRef]
- Quinn, C.; Rico, M.C.; Merali, C.; Merali, S. Dysregulation of S-adenosylmethionine Metabolism in Nonalcoholic Steatohepatitis Leads to Polyamine Flux and Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 1986. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Wang, Y.; Wang, N.; Xu, J.; Liang, X.; Xiong, J.; Lu, S.; Zhou, P.; Liu, J.; et al. Betaine-homocysteine methyltransferase promotes adipocyte commitment and insulin resistance via p38 MAPK/Smad signaling. Obesity 2023, 31, 1569–1583. [Google Scholar] [CrossRef]
- Tenorio, M.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Argaev Frenkel, L.; Rozenfeld, H.; Rozenberg, K.; Sampson, S.R.; Rosenzweig, T. N-Acetyl-l-Cysteine Supplement in Early Life or Adulthood Reduces Progression of Diabetes in Nonobese Diabetic Mice. Curr. Dev. Nutr. 2019, 3, nzy097. [Google Scholar] [CrossRef]
- Fan, N.; Zhang, Y.; Zou, S. Methylthioadenosine phosphorylase deficiency in tumors: A compelling therapeutic target. Front. Cell Dev. Biol. 2023, 11, 1173356. [Google Scholar] [CrossRef]
- Varadaiah, Y.G.C.; Sivanesan, S.; Nayak, S.B.; Thirumalarao, K.R. Purine metabolites can indicate diabetes progression. Arch. Physiol. Biochem. 2022, 128, 87–91. [Google Scholar] [CrossRef]
- Martinez-Sanchez, F.D.; Vargas-Abonce, V.P.; Guerrero-Castillo, A.P.; Santos-Villavicencio, M.L.; Eseiza-Acevedo, J.; Meza-Arana, C.E.; Gulias-Herrero, A.; Gomez-Samano, M.A. Serum Uric Acid concentration is associated with insulin resistance and impaired insulin secretion in adults at risk for Type 2 Diabetes. Prim. Care Diabetes 2021, 15, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Bronczek, G.A.; Vettorazzi, J.F.; Soares, G.M.; Kurauti, M.A.; Santos, C.; Bonfim, M.F.; Carneiro, E.M.; Balbo, S.L.; Boschero, A.C.; Costa Junior, J.M. The Bile Acid TUDCA Improves Beta-Cell Mass and Reduces Insulin Degradation in Mice With Early-Stage of Type-1 Diabetes. Front. Physiol. 2019, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Freitas, I.N.; da Silva, J.A., Jr.; de Oliveira, K.M.; Lourenconi Alves, B.; Dos Reis Araujo, T.; Camporez, J.P.; Carneiro, E.M.; Davel, A.P. Insights by which TUDCA is a potential therapy against adiposity. Front. Endocrinol. 2023, 14, 1090039. [Google Scholar] [CrossRef]
- Kathirvel, E.; Morgan, K.; Malysheva, O.V.; Caudill, M.A.; Morgan, T.R. Betaine for the prevention and treatment of insulin resistance and fatty liver in a high-fat dietary model of insulin resistance in C57BL mice. Front. Nutr. 2024, 11, 1409972. [Google Scholar] [CrossRef]
- Szczepanska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Bronowicka-Szydelko, A.; Gostomska-Pampuch, K.; Szkudlarek, J.; Bartys, A.; Bieg, T.; Gamian, E.; Kochman, A.; Picur, B.; Pietkiewicz, J.; et al. The melibiose-derived glycation product mimics a unique epitope present in human and animal tissues. Sci. Rep. 2021, 11, 2940. [Google Scholar] [CrossRef] [PubMed]
- Abdella, F.I.A.; Alardan, D.; Alshammari, N.S.; Alrashdi, A.A.; Abdelhedi, O.; Hamden, K. Protective effects of the bacterial pigment violacein against gastric ulceration: Inhibition of acid-related enzymes, activation of protective pathways, and reduction of inflammation and oxidative stress. Biomed. Pharmacother. 2025, 191, 118544. [Google Scholar] [CrossRef] [PubMed]
- Bozic, I.; Lavrnja, I. Thiamine and benfotiamine: Focus on their therapeutic potential. Heliyon 2023, 9, e21839. [Google Scholar] [CrossRef]
- Ziegler, D.; Reiners, K.; Strom, A.; Obeid, R. Association between diabetes and thiamine status—A systematic review and meta-analysis. Metabolism 2023, 144, 155565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-T.; Li, X.-M.; Pi, J.; Hsu, Y.-T.; Chi, L.-C.; Sun, H.-Y. Hepatocyte-Specific ApoJ Knockout Improves Metabolic Profiles in the Liver of Diabetic Mice. Metabolites 2025, 15, 761. https://doi.org/10.3390/metabo15120761
Wang S-T, Li X-M, Pi J, Hsu Y-T, Chi L-C, Sun H-Y. Hepatocyte-Specific ApoJ Knockout Improves Metabolic Profiles in the Liver of Diabetic Mice. Metabolites. 2025; 15(12):761. https://doi.org/10.3390/metabo15120761
Chicago/Turabian StyleWang, Sin-Tian, Xing-Min Li, Jiayi Pi, Yu-Ting Hsu, Li-Chi Chi, and Hung-Yu Sun. 2025. "Hepatocyte-Specific ApoJ Knockout Improves Metabolic Profiles in the Liver of Diabetic Mice" Metabolites 15, no. 12: 761. https://doi.org/10.3390/metabo15120761
APA StyleWang, S.-T., Li, X.-M., Pi, J., Hsu, Y.-T., Chi, L.-C., & Sun, H.-Y. (2025). Hepatocyte-Specific ApoJ Knockout Improves Metabolic Profiles in the Liver of Diabetic Mice. Metabolites, 15(12), 761. https://doi.org/10.3390/metabo15120761





