Relation Between Inflammatory Parameters and Insulin Resistance Indices in Cows During Early Lactation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Blood Sampling and Metabolic Parameters Analysis
2.3. Statistical Analysis
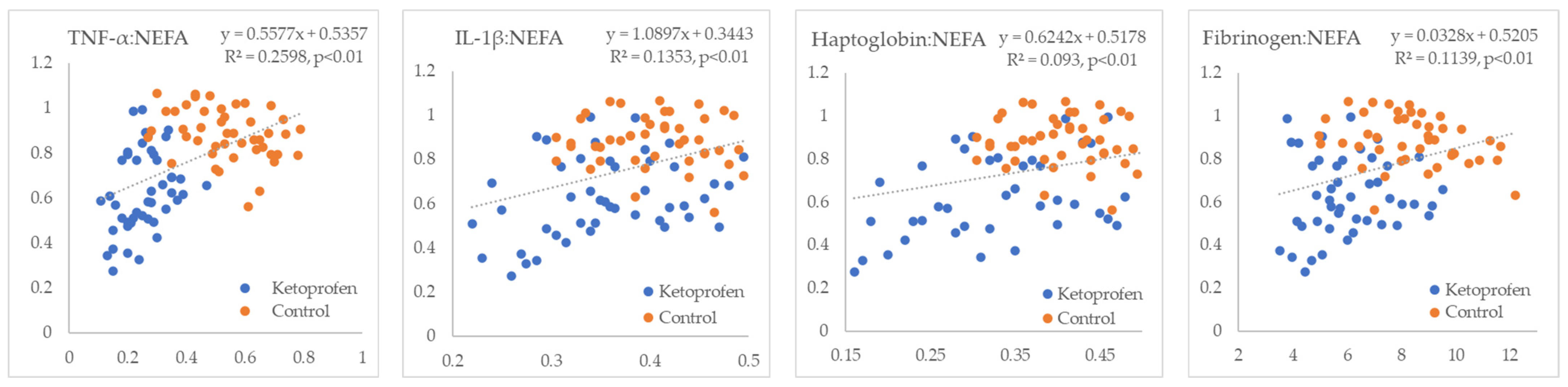
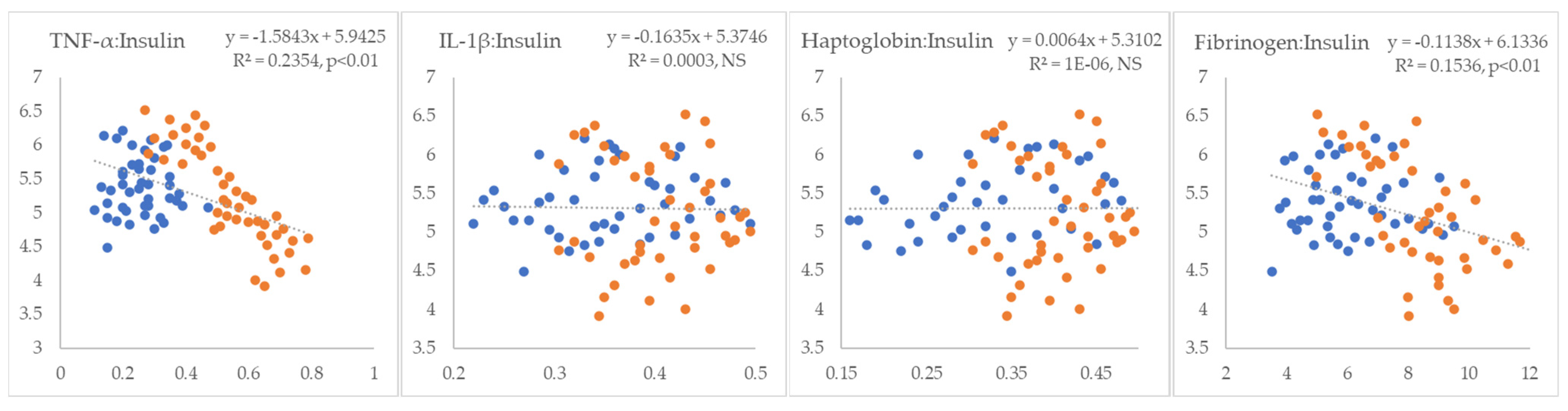
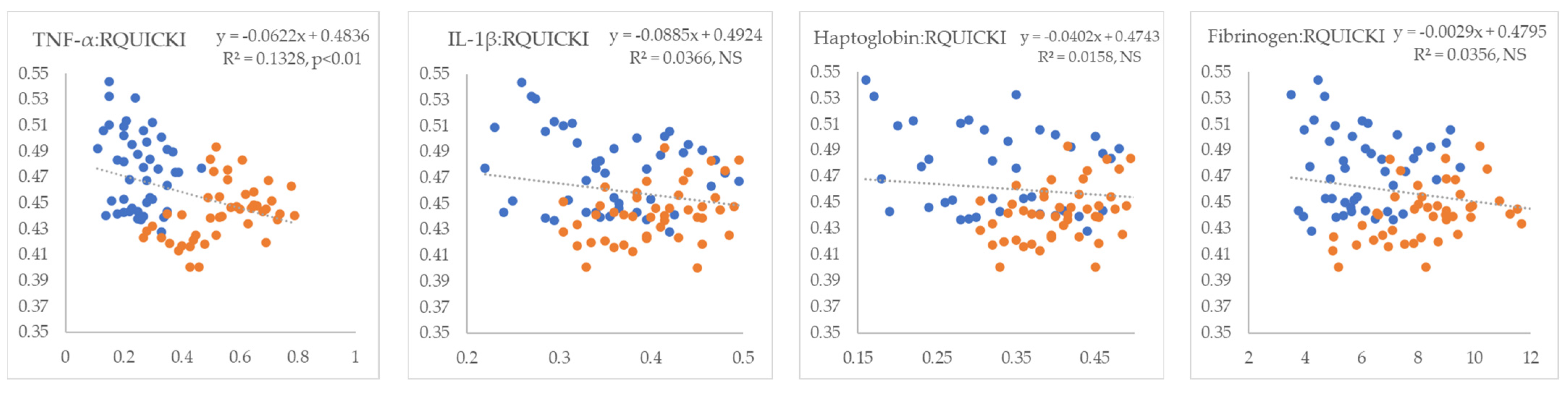
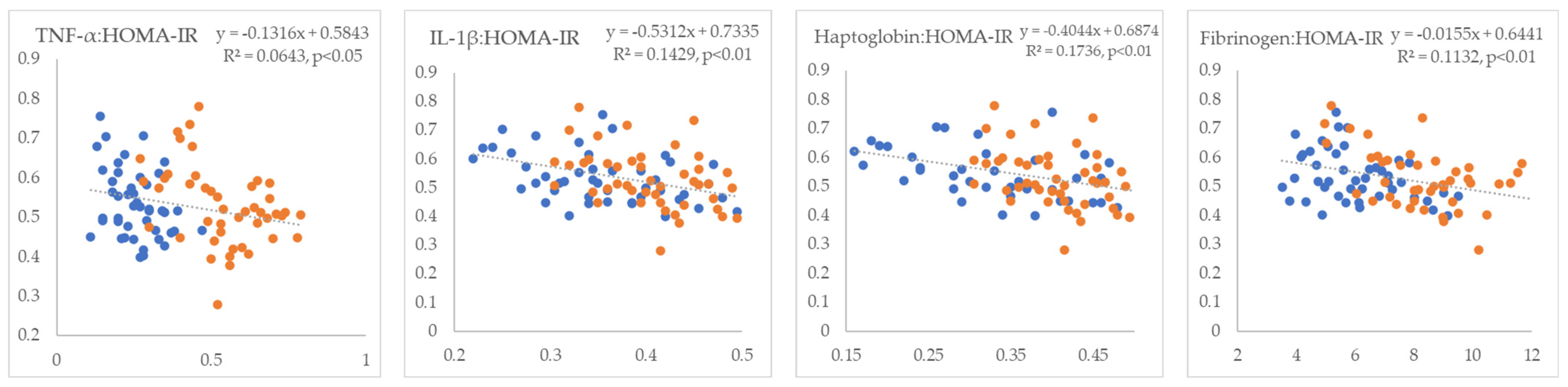
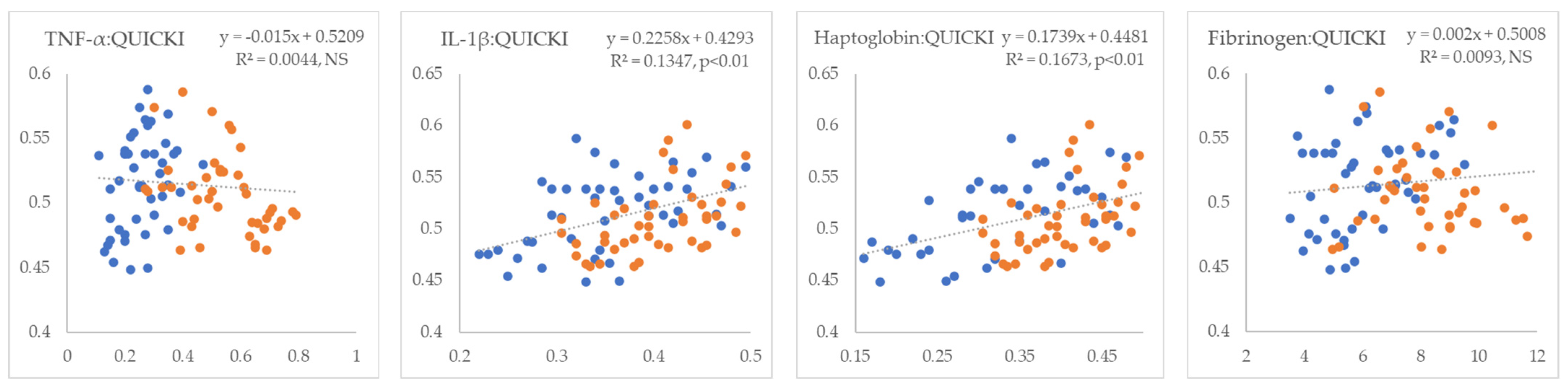
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IR | Insulin resistance |
| NEB | Negative Energy Balance |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-1β | Interleukin 1 Beta |
| NEFA | Non-Esterified Fatty Acids |
| RQUICKI | Revised Quantitative Insulin Sensitivity Check Index |
| Adipo-IR | Adipose Tissue Insulin Resistance Index |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| QUICKI | Quantitative Insulin Sensitivity Check Index |
| NSAID | Nonsteroidal Anti-Inflammatory Drugs |
| COX-1 or 2 | Cyclooxygenase-1 or 2 |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| TLR4 | Toll-Like Receptor 4 |
| ROS | Reactive Oxygen Species |
| DAMP | Damage-Associated Molecular Patterns |
| PI3K/AKT | Phosphoinositide 3-Kinase / Protein Kinase B (AKT) signaling pathway |
| IRS | Insulin Receptor Substrate |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
References
- Mezzetti, M.; Cattaneo, L.; Passamonti, M.M.; Lopreiato, V.; Minuti, A.; Trevisi, E. The transition period updated: A review of the new insights into the adaptation of dairy cows to the new lactation. Dairy 2021, 2, 617–636. [Google Scholar] [CrossRef]
- Krnjaić, S.; Cincović, M.; Djoković, R.; Belić, B.; Ježek, J.; Starič, J. The influence of energy balance, lipolysis and ketogenesis on metabolic adaptation in cows milked twice and three times daily. Metabolites 2022, 12, 1090. [Google Scholar] [CrossRef]
- Habel, J.; Chapoutot, P.; Koch, C.; Sundrum, A. Estimation of individual glucose reserves in high-yielding dairy cows. Dairy 2022, 3, 438–464. [Google Scholar] [CrossRef]
- Djoković, R.; Dosković, V.; Cincović, M.; Belić, B.; Fratrić, N.; Jašović, B.; Lalović, M. Estimation of Insulin Resistance in Healthy and Ketotic Cows during an Intravenous Glucose Tolerance Test. Pak. Vet. J. 2017, 37, 387–392. [Google Scholar]
- Delić, B.; Belić, B.; Cincović, M.R.; Djokovic, R.; Lakić, I. Metabolic adaptation in first week after calving and early prediction of ketosis type I and II in dairy cows. Large Anim. Rev. 2020, 26, 51–55. [Google Scholar]
- Cincović, M.; Kirovski, D.; Vujanac, I.; Belić, B.; Đoković, R. Relationship between the indexes of insulin resistance and metabolic status in dairy cows during early lactation. Acta Vet.-Beogr. 2017, 67, 57–70. [Google Scholar] [CrossRef]
- Youssef, M.A.; El-Ashker, M.R.; Younis, M.S. The effect of subclinical ketosis on indices of insulin sensitivity and selected metabolic variables in transition dairy cattle. Comp. Clin. Pathol. 2017, 26, 329–334. [Google Scholar] [CrossRef]
- Omidi, A.; Mohebbi-Fani, M.; Nazifi, S.; Mirzaei, A.; Seirafinia, M. The effects of post-partum drops in body condition on indices of energy metabolism in mid-lactation Holstein cows. Iran. J. Vet. Res. 2019, 20, 180. [Google Scholar]
- Guyot, H.; Detilleux, J.; Lebreton, P.; Garnier, C.; Bonvoisin, M.; Rollin, F.; Sandersen, C. Comparison of various indices of energy metabolism in recumbent and healthy dairy cows. PLoS ONE 2017, 12, e0169716. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M.; Jafari-Gharabaghlou, D.; Zarghami, N. An update on mode of action of metformin in modulation of meta-inflammation and inflammaging. Pharmacol. Rep. 2022, 74, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Blond, B.; Majkić, M.; Spasojević, J.; Hristov, S.; Radinović, M.; Nikolić, S.; Anđušić, L.; Čukić, A.; Došenović Marinković, M.; Vujanović, B.D.; et al. Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows. Metabolites 2024, 14, 104. [Google Scholar] [CrossRef]
- Petrović, M.Ž.; Cincović, M.; Starič, J.; Djoković, R.; Belić, B.; Radinović, M.; Majkić, M.; Ilić, Z.Ž. The Correlation between Extracellular Heat Shock Protein 70 and Lipid Metabolism in a Ruminant Model. Metabolites 2022, 12, 19. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of inflammatory cytokines, growth factors and adipokines in adipogenesis and insulin resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 2012, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Camell, C.D. Adipose tissue microenvironments during aging: Effects on stimulated lipolysis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2022, 1867, 159118. [Google Scholar] [CrossRef] [PubMed]
- Brodzki, P.; Marczuk, J.; Lisiecka, U.; Szczubiał, M.; Brodzki, A.; Gorzkoś, H.; Kulpa, K. Comparative evaluation of cytokine and acute-phase protein concentrations in sera of dairy cows with subclinical and clinical ketosis as a different view of the causes of the disease. Vet. World 2021, 14, 1572. [Google Scholar] [CrossRef] [PubMed]
- Seminara, J.A.; Seely, C.R.; McArt, J.A.A. Acute phase responses in clinically healthy multiparous Holsteins with and without calcium dysregulation during the early postpartum period. J. Dairy Sci. 2025, 108, 1930–1939. [Google Scholar] [CrossRef]
- Kumar, B.; Sahani, V.; Patil, S. Review on Ketoprofen (Anti-Inflammatory Drug). J. Res. Appl. Sci. Biotechnol. 2024, 3, 41–50. [Google Scholar] [CrossRef]
- Lora, I.; Massignani, M.; Stefani, A.; Gottardo, F. Potential benefits to dairy cow welfare of using a ceftiofur–ketoprofen combination drug for the treatment of inflammatory disease associated with pyrexia: A field clinical trial on acute puerperal metritis. Animals 2021, 11, 1597. [Google Scholar] [CrossRef]
- Barragan, A.A.; Hovingh, E.; Bas, S.; Lakritz, J.; Byler, L.; Ludwikowski, A.; Takitch, S.; Zug, J.; Hann, S. Effects of postpartum acetylsalicylic acid on metabolic status, health, and production in lactating dairy cattle. J. Dairy Sci. 2020, 103, 8443–8452. [Google Scholar] [CrossRef]
- Kovačević, Z.; Stojanović, D.; Cincović, M.; Belić, B.; Davidov, I.; Plavša, N.; Radinović, M. Association of metabolic and inflammatory markers with milk yield in postpartum dairy cows treated with ketoprofen. Pol. J. Vet. Sci. 2018, 21, 325–331. [Google Scholar] [CrossRef]
- National Research Council; Committee on Animal Nutrition; Subcommittee on Dairy Cattle Nutrition. Nutrient Requirements of Dairy Cattle: 2001; National Academies Press: Washington, DC, USA, 2001.
- Ryden, M.; Andersson, D.P.; Arner, P. Usefulness of surrogate markers to determine insulin action in fat cells. Int. J. Obes. 2020, 44, 2436–2443. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Brodzki, A.; Wawron, W.; Marczuk, J. Inflammatory cytokines and acute-phase proteins concentrations in the peripheral blood and uterus of cows that developed endometritis during early postpartum. Theriogenology 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Chitko-McKown, C.G.; Bierman, S.L.; Kuehn, L.A.; Bennett, G.L.; DeDonder, K.D.; Apley, M.D.; Harhay, G.P.; Clawson, M.L.; White, B.J.; Larson, R.L.; et al. Detection of bovine inflammatory cytokines IL-1β, IL-6, and TNF-α with a multiplex electrochemiluminescent assay platform. Vet. Immunol. Immunopathol. 2021, 237, 110274. [Google Scholar] [CrossRef]
- Peker, C.; Musal, B. Assessment of inflammatory cytokine concentrations during diagnosis and after treatment of postpartum dairy cows with clinical and subclinical endometritis. Large Anim. Rev. 2022, 28, 213–220. [Google Scholar]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute Phase Proteins in Ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Bradford, B.J.; Bobe, G.; Nafikov, R.A.; Lu, Y.; Young, J.W.; Beitz, D.C. Strong relationships between mediators of the acute phase response and fatty liver in dairy cows. Can. J. Anim. Sci. 2005, 85, 165–175. [Google Scholar] [CrossRef]
- Kováč, G.; Tóthová, C.; Nagy, O.; Seidel, H.; Konvičná, J. Acute phase proteins and their relation to energy metabolites in dairy cows during the pre-and postpartal period. Acta Vet. Brno 2009, 78, 441–447. [Google Scholar] [CrossRef]
- Nazifi, S.; Rezakhani, A.; Koohimoghadam, M.; Ansari-Lari, M.; Esmailnezhad, Z. Evaluation of serum haptoglobin in clinically healthy cattle and cattle with inflammatory diseases in Shiraz, a tropical area in Southern Iran. Bulg. J. Vet. Med. 2008, 11, 95–101. [Google Scholar]
- Milczak, A.; Abramowicz, B.; Szczepanik, M.; Madany, J.; Wrześniewska, K.; Buczek, K.; Staniec, M.; Żółkiewski, P.; Kurek, Ł. Could Fibrinogen Concentration Be a Useful Indicator of Cattle Herd Health Status? Approaches to Setting Reference Values. Agriculture 2023, 13, 1224. [Google Scholar] [CrossRef]
- Jeremejeva, J.; Orro, T.; Kask, K. Relationship between acute phase proteins and subsequent fertility of dairy cows after postpartum uterine inflammation and healthy cows. Vet. Med. Zoot 2015, 70, 37–41. [Google Scholar]
- Zhang, M.Q.; Heirbaut, S.; Jing, X.P.; Stefańska, B.; Vandaele, L.; De Neve, N.; Fievez, V. Systemic inflammation in early lactation and its relation to the cows’ oxidative and metabolic status, productive and reproductive performance, and activity. J. Dairy Sci. 2024, 107, 7121–7137. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Donalisio, C.; Barbero, R.; Cuniberti, B.; Vercelli, C.; Casalone, M.; Re, G. Effects of flunixin meglumine and ketoprofen on mediator production in ex vivo and in vitro models of inflammation in healthy dairy cows. J. Vet. Pharmacol. Tharapeutics 2013, 36, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Farney, J.K.; Mamedova, L.K.; Coetzee, J.F.; KuKanich, B.; Sordillo, L.M.; Stoakes, S.K.; Minton, J.E.; Hollis, L.C.; Bradford, B.J. Anti-inflammatory salicylate treatment alters the metabolic adaptations to lactation in dairy cattle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R110–R117. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Piccioli-Cappelli, F. Effects of acetyl-salicylate used in postcalving of dairy cows. Vet. Res. Commun. 2004, 28 (Suppl. 1), 217–219. [Google Scholar] [CrossRef]
- Dan, D.; Bruckmaier, R.M.; Wellnitz, O. Ketoprofen affects the mammary immune response in dairy cows in vivo and in vitro. J. Dairy Sci. 2018, 101, 11321–11329. [Google Scholar] [CrossRef]
- Gross, J.J. Dairy cow physiology and production limits. Anim. Front. Rev. Mag. Anim. Agric. 2023, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Martens, H. Invited Review: Increasing Milk Yield and Negative Energy Balance: A Gordian Knot for Dairy Cows? Animals 2023, 13, 3097. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Van Schyndel, S.J.; Spricigo, J.F.W.; Carvalho, M.R.; Mion, B.; Ribeiro, E.S.; LeBlanc, S.J. Effect of anti-inflammatory treatment on systemic inflammation, immune function, and endometrial health in postpartum dairy cows. Sci. Rep. 2020, 10, 5236. [Google Scholar] [CrossRef] [PubMed]
- Cincović, M.R.; Đoković, R.; Belić, B.; Lakić, I.; Stojanac, N.; Stevančević, O.; Staničkov, N. Insulin resistance in cows during the periparturient period. Acta Agric. Serbica 2018, 23, 233–245. [Google Scholar] [CrossRef]
- Tahapary, D.L.; Pratisthita, L.B.; Fitri, N.A.; Marcella, C.; Wafa, S.; Kurniawan, F.; Rizka, A.; Tarigan, T.J.E.; Saksono Harbuwono, S.; Purnamasari, D.; et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102581. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads Jr, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Collier, R.J.; Bauman, D.E. A 100-Year Review: Regulation of nutrient partitioning to support lactation. J. Dairy Sci. 2017, 100, 10353–10366. [Google Scholar] [CrossRef]
- Kuhla, B. Pro-inflammatory cytokines and hypothalamic inflammation: Implications for insufficient feed intake of transition dairy cows. Animal 2020, 14, s65–s77. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Ferrari, A.; Archetti, I.; Bertoni, G. Anti-inflammatory treatments in calving dairy cows: Effects on haematological and metabolic profiles. Ital. J. Anim. Sci. 2005, 4, 203–205. [Google Scholar] [CrossRef]
- De Zentella, P.M.; Vázquez-Meza, H.; Piña-Zentella, G.; Pimentel, L.; Piña, E. Non-steroidal anti-inflammatory drugs inhibit epinephrine- and cAMP-mediated lipolysis in isolated rat adipocytes. J. Pharm. Pharmacol. 2002, 54, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.R.; Mamedova, L.K.; Zachut, M.; Kra, G.; Häussler, S.; Vaughn, M.; Gonzales, J.; Bradford, B.J. Effects of sodium salicylate on glucose kinetics and insulin signaling in postpartum dairy cows. J. Dairy Sci. 2019, 102, 1617–1629. [Google Scholar] [CrossRef]
- Carpenter, A.J.; Ylioja, C.M.; Mamedova, L.K.; Olagaray, K.E.; Bradford, B.J. Effects of early postpartum sodium salicylate treatment on long-term milk, intake, and blood parameters of dairy cows. J. Dairy Sci. 2018, 101, 1437–1447. [Google Scholar] [CrossRef]
- McGuckin, M.M.; Giesy, S.L.; Overton, T.R.; Boisclair, Y.R. Inflammatory tone in liver and adipose tissue in dairy cows experiencing a healthy transition from late pregnancy to early lactation. J. Dairy Sci. 2023, 106, 8122–8132. [Google Scholar] [CrossRef]
- Trevisi, E.; Jahan, N.; Bertoni, G.; Ferrari, A.; Minuti, A. Pro-inflammatory cytokine profile in dairy cows: Consequences for new lactation. Ital. J. Anim. Sci. 2015, 14, 3862. [Google Scholar] [CrossRef]
- Dong, Z.; Zhuang, Q.; Ning, M.; Wu, S.; Lu, L.; Wan, X. Palmitic acid stimulates NLRP3 inflammasome activation through TLR4-NF-κB signal pathway in hepatic stellate cells. Ann. Transl. Med. 2020, 8, 168. [Google Scholar] [CrossRef]
- Sun, X.; Tang, Y.; Jiang, C.; Luo, S.; Jia, H.; Xu, Q.; Zhao, C.; Liang, Y.; Cao, Z.; Shao, G.; et al. Oxidative stress, NF-κB signaling, NLRP3 inflammasome, and caspase apoptotic pathways are activated in mammary gland of ketotic Holstein cows. J. Dairy Sci. 2021, 104, 849–861. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, W.M.; El-Bahr, S.M. Biomarkers of ketosis in dairy cows at postparturient period: Acute phase proteins and pro-inflammatory cytokines. Vet. Arh. 2017, 87, 431–440. [Google Scholar] [CrossRef]
- Petrović, K.; Stojanović, D.; Cincović, M.R.; Belić, B.; Lakić, I.; Đoković, R. Influence of niacin application on inflammatory parameters, non-esterified fatty acids and functional status of liver in cows during early lactation. Large Anim. Rev. 2021, 27, 17–21. [Google Scholar]
- Wan, B.N.; Zhou, S.G.; Wang, M.; Zhang, X.; Ji, G. Progress on haptoglobin and metabolic diseases. World J. Diabetes 2021, 12, 206. [Google Scholar] [CrossRef]
- Qiao, K.; Jiang, R.; Contreras, G.A.; Xie, L.; Pascottini, O.B.; Opsomer, G.; Dong, Q. The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows. Animals 2024, 14, 832. [Google Scholar] [CrossRef]


| Blood Parameters | Control | Ketoprofen | Treatment | Week | Treatment × Week | ||||
|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 2 | Week 0 | Week 1 | Week 2 | ||||
| TNF-α (ng/mL) | 0.38 ± 0.05 a | 0.54 ± 0.04 b | 0.69 ± 0.06 c | 0.35 ± 0.05 a | 0.31 ± 0.06 a | 0.21 ± 0.05 d | <0.01 | <0.01 | <0.05 |
| IL-1β (ng/mL) | 0.38 ± 0.04 a | 0.45 ± 0.05 b | 0.37 ± 0.04 a | 0.36 ± 0.04 a | 0.39 ± 0.04 a | 0.29 ± 0.03 c | <0.05 | <0.05 | NS |
| Haptoglobin (g/L) | 0.41 ± 0.12 a | 0.84 ± 0.11 b | 0.90 ± 0.09 c | 0.36 ± 0.08 a | 0.49 ± 0.8 a | 0.24 ± 0.09 d | <0.01 | <0.01 | <0.01 |
| Fibrinogen (g/L) | 6.61 ± 1.22 a | 8.69 ± 1.51 b | 9.86 ± 1.61 b | 5.50 ± 1.22 a | 7.53 ± 1.25 c | 5.08 ± 1.18 a | <0.05 | <0.05 | <0.05 |
| NEFA (mmol/L) | 0.94 ± 0.11 a | 0.82 ± 0.09 b | 0.73 ± 0.09 b | 0.92 ± 0.1 a | 0.61 ± 0.09 c | 0.51 ± 0.7 d | <0.01 | <0.01 | <0.01 |
| Glucose (mmol/L) | 2.29 ± 0.26 a | 2.00 ± 0.25 a | 2.55 ± 0.21 b | 2.16 ± 0.23 a | 2.04 ± 0.25 a | 2.64 ± 0.26 b | NS | <0.05 | NS |
| Insulin (mU/L) | 6.10 ± 0.51 a | 5.14 ± 0.43 b | 4.50 ± 0.48 c | 5.83 ± 0.41 a | 5.23 ± 0.43 b | 5.09 ± 0.55 b | NS | <0.05 | NS |
| RQUICKI | 0.48 ± 0.02 a | 0.46 ± 0.01 b | 0.45 ± 0.01 b | 0.45 ± 0.01 b | 0.49 ± 0.02 c | 0.49 ± 0.01 c | <0.01 | <0.01 | <0.05 |
| Adipo-IR | 5.51 ± 0.52 a | 4.40 ± 0.59 b | 3.81 ± 0.54 c | 4.99 ± 0.53 a | 3.17 ± 0.48 d | 2.35 ± 0.41 e | <0.01 | <0.01 | <0.01 |
| HOMA-IR | 0.61 ± 0.08 a | 0.46 ± 0.07 b | 0.51 ± 0.09 b | 0.57 ± 0.08 a | 0.47 ± 0.07 b | 0.60 ± 0.08 a | NS | <0.05 | NS |
| QUICKI | 0.55 ± 0.03 a | 0.53 ± 0.02 a | 0.52 ± 0.03 a | 0.53 ± 0.03 a | 0.55 ± 0.03 a | 0.54 ± 0.02 a | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cincović, M.; Stojanović, D.; Djoković, R.; Majkić, M.; Starič, J.; Petrović, M.; Kovačević, Z. Relation Between Inflammatory Parameters and Insulin Resistance Indices in Cows During Early Lactation. Metabolites 2025, 15, 751. https://doi.org/10.3390/metabo15110751
Cincović M, Stojanović D, Djoković R, Majkić M, Starič J, Petrović M, Kovačević Z. Relation Between Inflammatory Parameters and Insulin Resistance Indices in Cows During Early Lactation. Metabolites. 2025; 15(11):751. https://doi.org/10.3390/metabo15110751
Chicago/Turabian StyleCincović, Marko, Dragica Stojanović, Radojica Djoković, Mira Majkić, Jože Starič, Miloš Petrović, and Zorana Kovačević. 2025. "Relation Between Inflammatory Parameters and Insulin Resistance Indices in Cows During Early Lactation" Metabolites 15, no. 11: 751. https://doi.org/10.3390/metabo15110751
APA StyleCincović, M., Stojanović, D., Djoković, R., Majkić, M., Starič, J., Petrović, M., & Kovačević, Z. (2025). Relation Between Inflammatory Parameters and Insulin Resistance Indices in Cows During Early Lactation. Metabolites, 15(11), 751. https://doi.org/10.3390/metabo15110751










