Serum Metabolomics Reveals Metabolic Changes in Freestyle Wrestlers During Different Training Stages
Abstract
1. Introduction
2. Materials and Methods
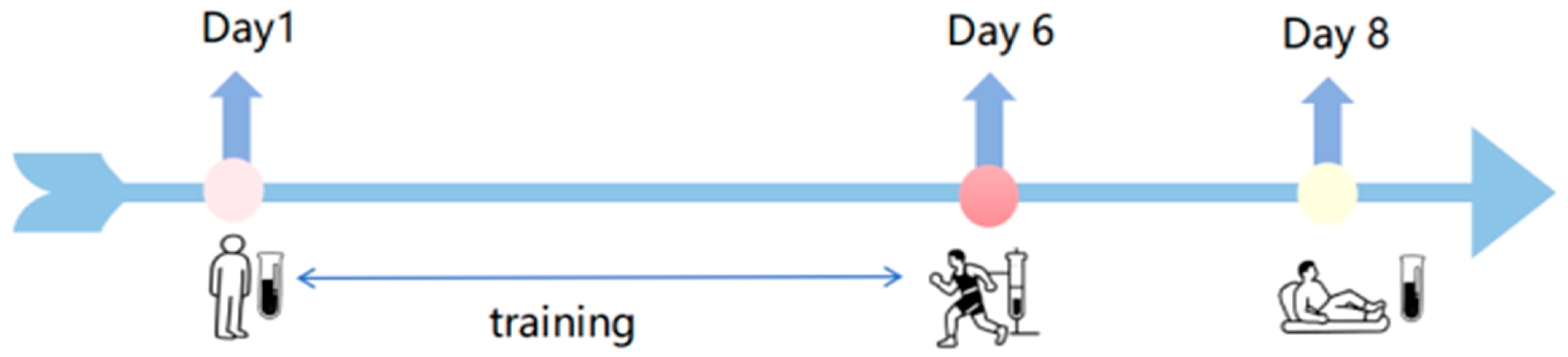
2.1. Participants and Ethical Statement
2.2. Sample Collection and Storage
2.3. Measurement of Biochemical Indicators
2.4. Metabolomics Analyses Methods
2.4.1. H-NMR Sample Preparation
2.4.2. Spectral Data Processing and Analyses
2.5. Statistical Analyses
3. Results
3.1. Results of Biochemical Indicator Tests
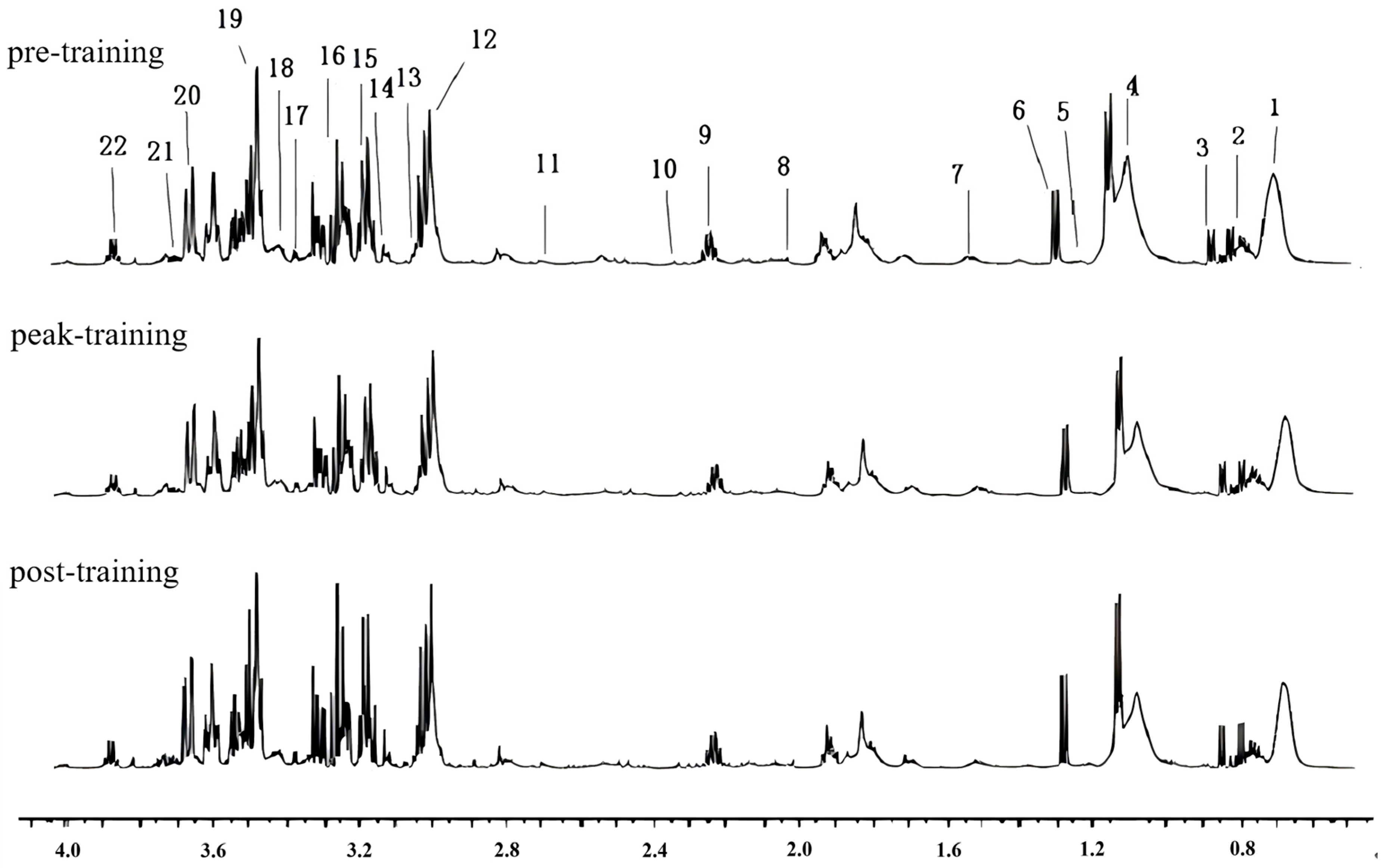
3.2. Results of Serum Metabolomics Detection
Assignment and Analyses of 1H-NMR Spectra
3.3. Formatting of Mathematical Components
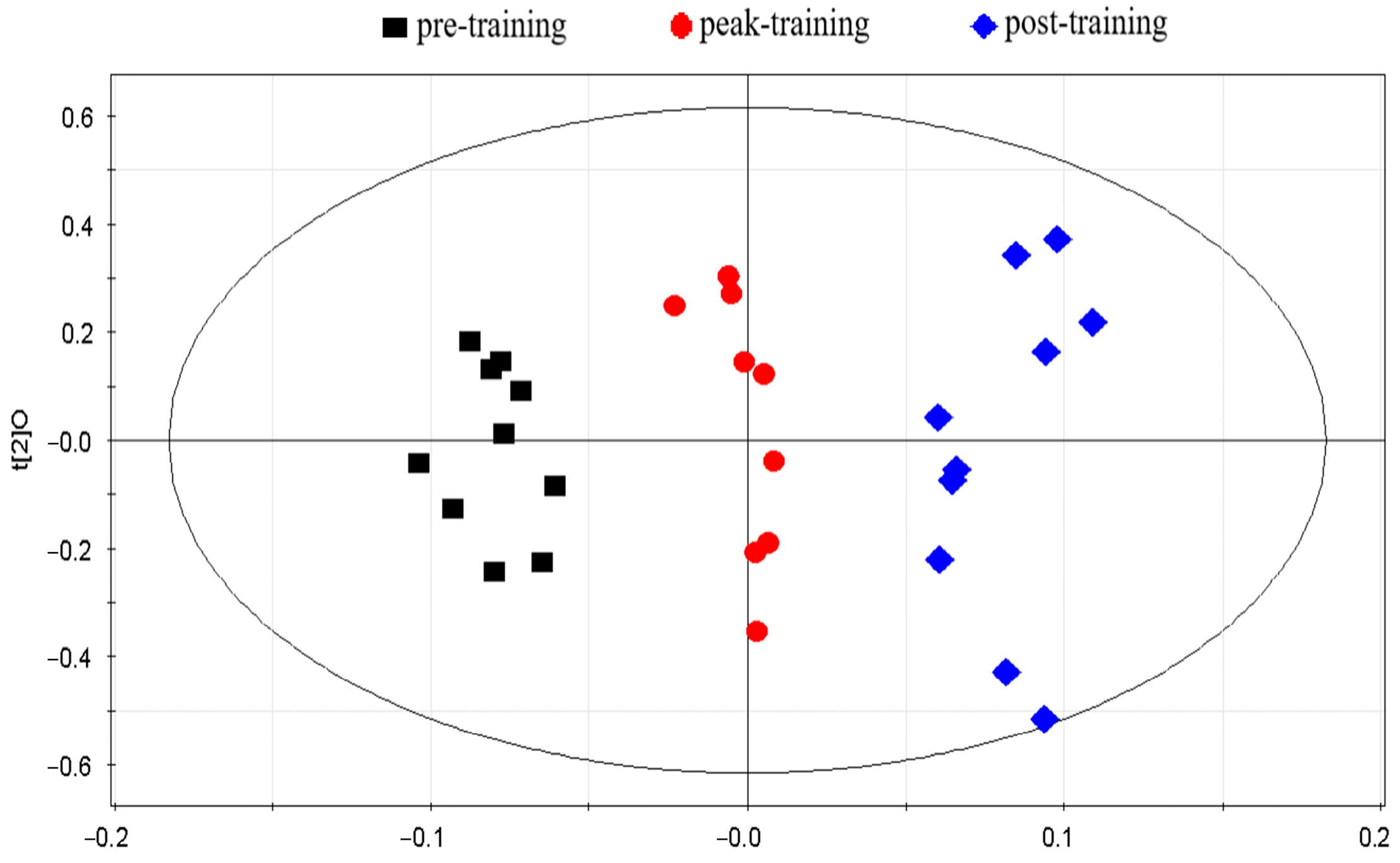
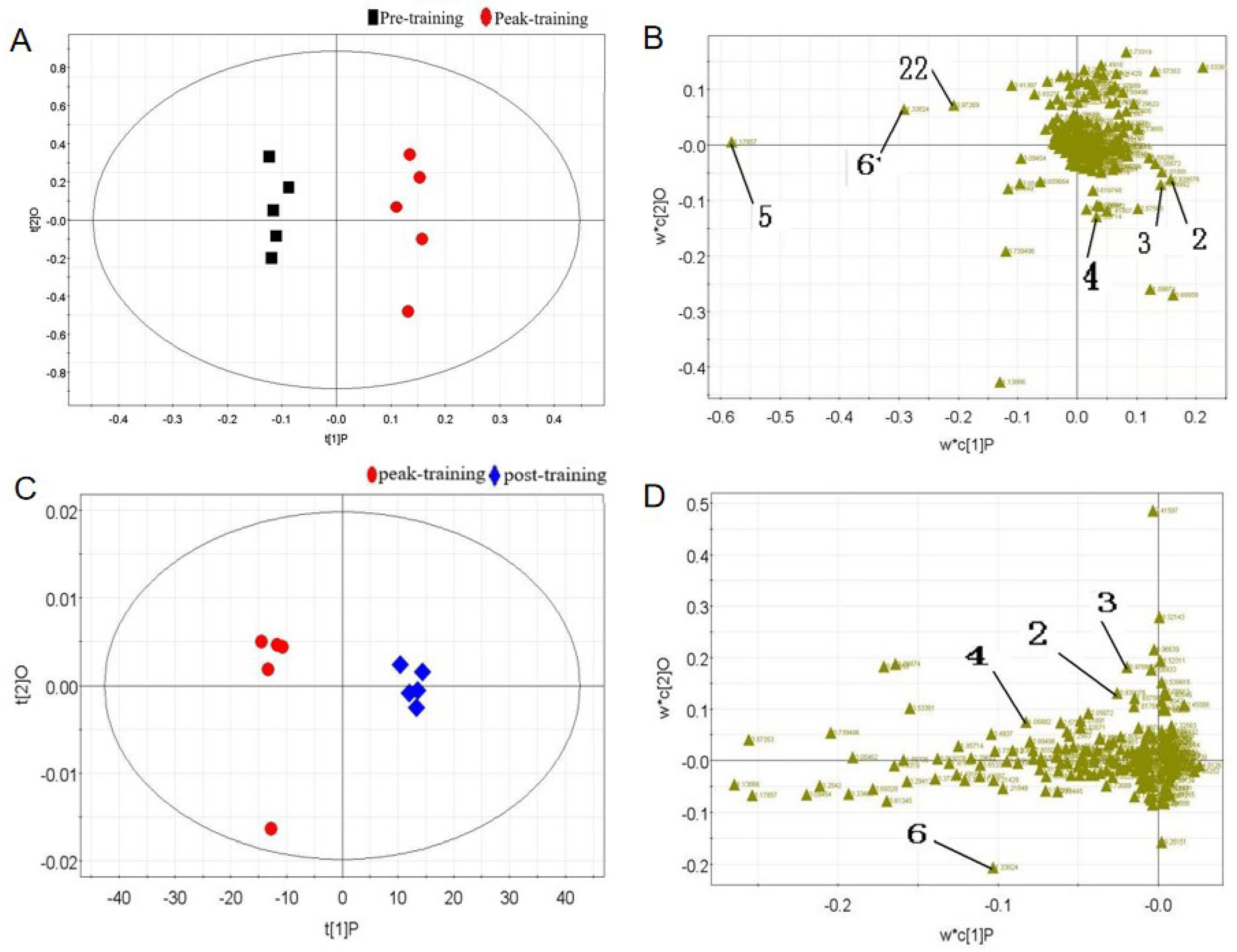
Results of Multivariate Statistical Analyses
4. Discussion
4.1. Training Load and Recovery Status Reflected by Conventional Biochemical Indicators
4.2. Sex-Specific Metabolic Response Patterns Revealed by Metabolomics
4.2.1. Metabolic Response Characteristics of Male Wrestlers
4.2.2. Metabolic Response Characteristics of Female Wrestlers
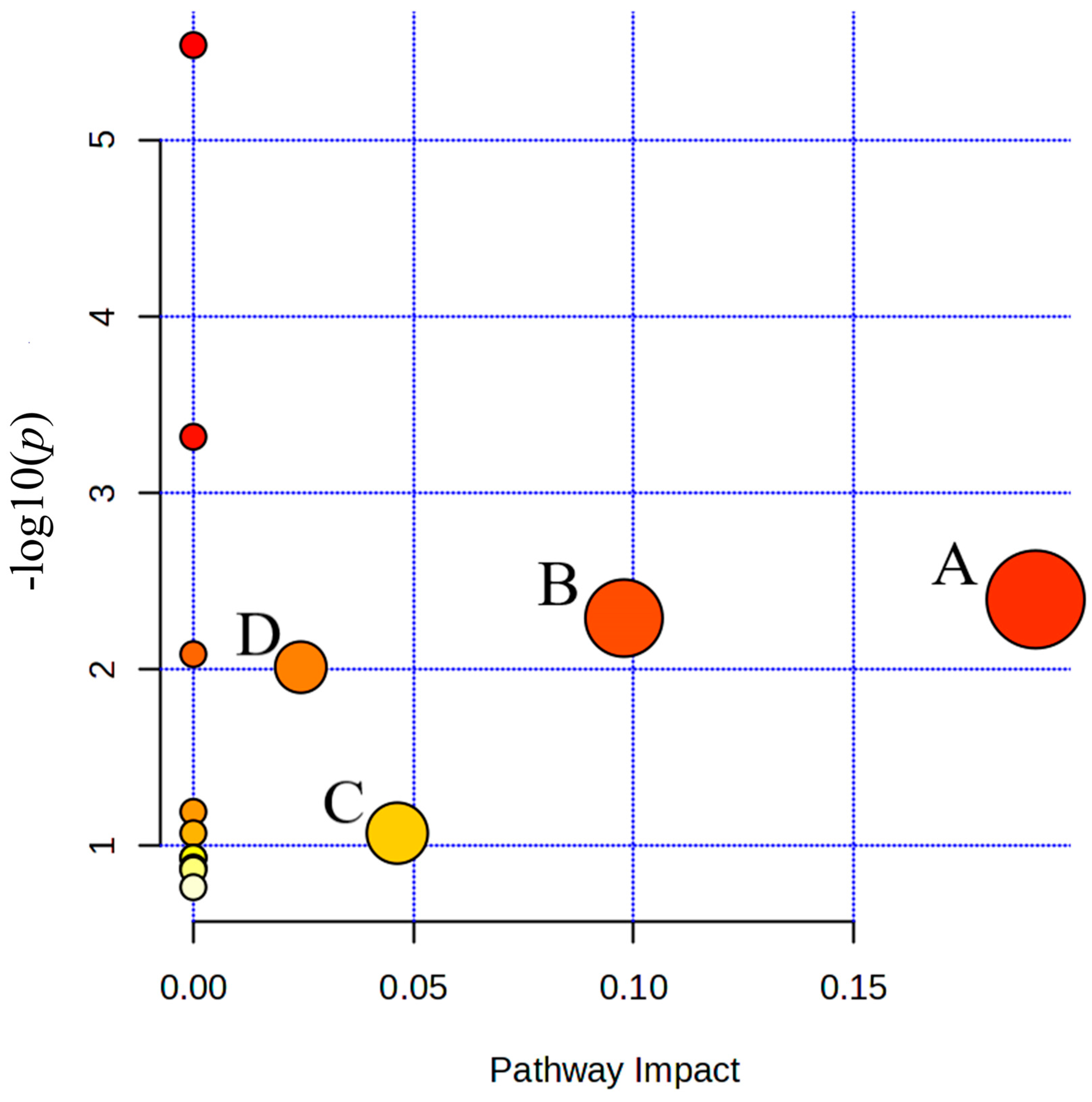
4.2.3. Pathway Perturbations and Training Adaptation
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.-H.; Feng, L.-S.; Zhang, H.-J.; Xu, K.-Y.; Chi, F.-T.; Tao, D.-L.; Liu, M.-Y.; Lucia, A.; Fleck, S.J. Physiological profile of elite Chinese female wrestlers. J. Strength Cond. Res. 2013, 27, 2374–2395. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, H.; Negra, Y.; Bouguezzi, R.; Mkaouer, B.; Franchini, E.; Julio, U.; Hachana, Y. Physical and Physiological Attributes of Wrestlers: An Update. J. Strength Cond. Res. 2017, 31, 1411–1442. [Google Scholar] [CrossRef] [PubMed]
- Franchini, E.; Brito, C.J.; Artioli, G.G. Weight loss in combat sports: Physiological, psychological and performance effects. J. Int. Soc. Sports Nutr. 2012, 9, 52. [Google Scholar] [CrossRef]
- Minetti, A.E.; Ardigò, L.P.; Saibene, F. Mechanical determinants of gradient walking energetics in man. J. Physiol. 1993, 472, 725–735. [Google Scholar] [CrossRef]
- Fogarasi, A.; Gonzalez, K.; Dalamaga, M.; Magkos, F. The Impact of the Rate of Weight Loss on Body Composition and Metabolism. Curr. Obes. Rep. 2022, 11, 33–44. [Google Scholar] [CrossRef]
- Hartmann, U.; Mester, J. Training and overtraining markers in selected sport events. Med. Sci. Sports Exerc. 2000, 32, 209–215. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Khoramipour, K.; Sandbakk, O.; Keshteli, A.H.; Gaeini, A.A.; Wishart, D.S.; Chamari, K. Metabolomics in Exercise and Sports: A Systematic Review. Sports Med. 2022, 52, 547–583. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, M.R.; Lawler, N.G.; Hoffman, N.J. Metabolomics and Lipidomics: Expanding the Molecular Landscape of Exercise Biology. Metabolites 2021, 11, 151. [Google Scholar] [CrossRef]
- Heaney, L.M.; Deighton, K.; Suzuki, T. Non-targeted metabolomics in sport and exercise science. J. Sports Sci. 2019, 37, 959–967. [Google Scholar] [CrossRef] [PubMed]
- MoTrPAC Study Group. Temporal dynamics of the multi-omic response to endurance exercise training. Nature 2024, 629, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Puigarnau, S.; Fernàndez, A.; Obis, E.; Jové, M.; Castañer, M.; Pamplona, R.; Portero-Otin, M.; Camerino, O. Metabolomics reveals that fittest trail runners show a better adaptation of bioenergetic pathways. J. Sci. Med. Sport 2022, 25, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.M.; Peterson, B.; Schranner, D.; Tahir, U.A.; Rienmuller, T.; Deng, S.; Keyes, M.J.; Katz, D.H.; Beltran, P.M.J.; Barber, J.L.; et al. Human plasma proteomic profiles indicative of cardiorespiratory fitness. Nat. Metab. 2021, 3, 786–797. [Google Scholar] [CrossRef]
- Saidi, K.; Abderrahman, A.B.; Hackney, A.C.; Bideau, B.; Zouita, S.; Granacher, U.; Zouhal, H. Hematology, Hormones, Inflammation, and Muscle Damage in Elite and Professional Soccer Players: A Systematic Review with Implications for Exercise. Sports Med. 2021, 51, 2607–2627. [Google Scholar] [CrossRef]
- Moore, E.; Fuller, J.T.; Buckley, J.D.; Saunders, S.; Halson, S.L.; Broatch, J.R.; Bellenger, C.R. Impact of Cold-Water Immersion Compared with Passive Recovery Following a Single Bout of Strenuous Exercise on Athletic Performance in Physically Active Participants: A Systematic Review with Meta-analysis and Meta-regression. Sports Med. 2022, 52, 1667–1688. [Google Scholar] [CrossRef]
- Urhausen, A.; Gabriel, H.; Kindermann, W. Blood hormones as markers of training stress and overtraining. Sports Med. 1995, 20, 251–276. [Google Scholar] [CrossRef]
- Bessa, A.; Nissenbaum, M.; Monteiro, A.; Gandra, P.G.; Nunes, L.S.; Bassini-Cameron, A.; Werneck-de-Castro, J.P.; de Macedo, D.V.; Cameron, L.C. High-intensity ultraendurance promotes early release of muscle injury markers. Br. J. Sports Med. 2008, 42, 889–893. [Google Scholar] [CrossRef]
- Emambokus, N.; Granger, A.; Messmer-Blust, A. Exercise Metabolism. Cell Metab. 2015, 22, 1. [Google Scholar] [CrossRef]
- Wu, L.; Qu, J.; Mou, L.; Liu, C. Apigenin improves testosterone synthesis by regulating endoplasmic reticulum stress. Biomed. Pharmacother. 2024, 177, 117075. [Google Scholar] [CrossRef]
- Kelava, I.; Chiaradia, I.; Pellegrini, L.; Kalinka, A.T.; Lancaster, M.A. Androgens increase excitatory neurogenic potential in human brain organoids. Nature 2022, 602, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Oura, M.; Son, B.K.; Song, Z.; Toyoshima, K.; Nanao-Hamai, M.; Ogawa, S.; Akishita, M. Testosterone/androgen receptor antagonizes immobility-induced muscle atrophy through Inhibition of myostatin transcription and inflammation in mice. Sci. Rep. 2025, 15, 10568. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, D.; Nolan, D.; Androulakis Korakakis, P.; Immonen, V.; Wolf, M.; Bell, L. Deloading Practices in Strength and Physique Sports: A Cross-sectional Survey. Sports Med. Open 2024, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Z.; Liu, J. Amino acids regulating skeletal muscle metabolism: Mechanisms of action, physical training dosage recommendations and adverse effects. Nutr. Metab. 2024, 21, 41. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Lopez-Maside, L.; Donapetry-Garcia, C.; Fernandez-Fernandez, C.; Sixto-Leal, C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 2017, 49, 1005–1028. [Google Scholar] [CrossRef]
- Gibala, M.J. Protein Metabolism and Endurance Exercise. Sports Med. 2007, 37, 337–340. [Google Scholar] [CrossRef]
- Shimomura, Y.; Honda, T.; Shiraki, M.; Murakami, T.; Sato, J.; Kobayashi, H.; Mawatari, K.; Obayashi, M.; Harris, R.A. Branched-chain amino acid catabolism in exercise and liver disease. J. Nutr. 2006, 136, 250s–253s. [Google Scholar] [CrossRef]
- Gawedzka, A.; Grandys, M.; Duda, K.; Zapart-Bukowska, J.; Zoladz, J.A.; Majerczak, J. Plasma BCAA concentrations during exercise of varied intensities in young healthy men-the impact of endurance training. PeerJ 2020, 8, e10491. [Google Scholar] [CrossRef]
- Luan, C.; Wang, Y.; Li, J.; Zhou, N.; Song, G.; Ni, Z.; Xu, C.; Tang, C.; Fu, P.; Wang, X.; et al. Branched-Chain Amino Acid Supplementation Enhances Substrate Metabolism, Exercise Efficiency and Reduces Post-Exercise Fatigue in Active Young Males. Nutrients 2025, 17, 1290. [Google Scholar] [CrossRef]
- Xu, M.; Hu, D.; Liu, X.; Li, Z.; Lu, L. Branched-Chain Amino Acids and Inflammation Management in Endurance Sports: Molecular Mechanisms and Practical Implications. Nutrients 2025, 17, 1335. [Google Scholar] [CrossRef]
- Davis, J.M.; Alderson, N.L.; Welsh, R.S. Serotonin and central nervous system fatigue: Nutritional considerations. Am. J. Clin. Nutr. 2000, 72, 573s–578s. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.R.; Cox, I.J.; Walker, D.G.; Cobbold, J.F.; Taylor-Robinson, S.D.; Marshall, S.E.; Orchard, T.R. Differences in gut microbial metabolism are responsible for reduced hippurate synthesis in Crohn’s disease. BMC Gastroenterol. 2010, 10, 108. [Google Scholar] [CrossRef]
- De Simone, G.; Balducci, C.; Forloni, G.; Pastorelli, R.; Brunelli, L. Hippuric acid: Could became a barometer for frailty and geriatric syndromes? Ageing Res. Rev. 2021, 72, 101466. [Google Scholar] [CrossRef]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 13670. [Google Scholar] [CrossRef]
- E, H.-W.; Kosmol, A.; Gajewski, J.J.B.o.S. Aerobic fitness of elite female and male wrestlers. Biol. Sport 2009, 26, 339–348. [Google Scholar] [CrossRef]
- Mansoori, S.; Ho, M.Y.; Ng, K.K.; Cheng, K.K. Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2025, 26, e13856. [Google Scholar] [CrossRef]
- Carter, S.L.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during endurance exercise in men and women after endurance training. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E898–E907. [Google Scholar] [CrossRef]
- Sanchez, B.N.; Volek, J.S.; Kraemer, W.J.; Saenz, C.; Maresh, C.M. Sex Differences in Energy Metabolism: A Female-Oriented Discussion. Sports Med. 2024, 54, 2033–2057. [Google Scholar] [CrossRef]
- Salem, A.; Ben Maaoui, K.; Jahrami, H.; AlMarzooqi, M.A.; Boukhris, O.; Messai, B.; Clark, C.C.T.; Glenn, J.M.; Ghazzaoui, H.A.; Bragazzi, N.L.; et al. Attenuating Muscle Damage Biomarkers and Muscle Soreness After an Exercise-Induced Muscle Damage with Branched-Chain Amino Acid (BCAA) Supplementation: A Systematic Review and Meta-analysis with Meta-regression. Sports Med. Open 2024, 10, 42. [Google Scholar] [CrossRef]
- Sousa, J.; Westhoff, P.; Methling, K.; Lalk, M. The Absence of Pyruvate Kinase Affects Glucose-Dependent Carbon Catabolite Repression in Bacillus subtilis. Metabolites 2019, 9, 216. [Google Scholar] [CrossRef]
- Olek, R.A.; Kujach, S.; Radak, Z. Current knowledge about pyruvate supplementation: A brief review. Sports Med. Health Sci. 2024, 6, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Enerback, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef]
- Mirzaei, B.; Curby, D.G.; Rahmani-Nia, F.; Moghadasi, M. Physiological profile of elite Iranian junior freestyle wrestlers. J. Strength Cond. Res. 2009, 23, 2339–2344. [Google Scholar] [CrossRef]
- San-Millan, I.; Stefanoni, D.; Martinez, J.L.; Hansen, K.C.; D’Alessandro, A.; Nemkov, T. Metabolomics of Endurance Capacity in World Tour Professional Cyclists. Front. Physiol. 2020, 11, 578. [Google Scholar] [CrossRef]
- Lamont, L.S.; McCullough, A.J.; Kalhan, S.C. Gender differences in the regulation of amino acid metabolism. J. Appl. Physiol. 2003, 95, 1259–1265. [Google Scholar] [CrossRef]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stuckler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabol. Off. J. Metabol. Soc. 2015, 11, 1815–1833. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef]
- Tam, R.; Mitchell, L.; Forsyth, A. Does Creatine Supplementation Enhance Performance in Active Females? A Systematic Review. Nutrients 2025, 17, 238. [Google Scholar] [CrossRef]
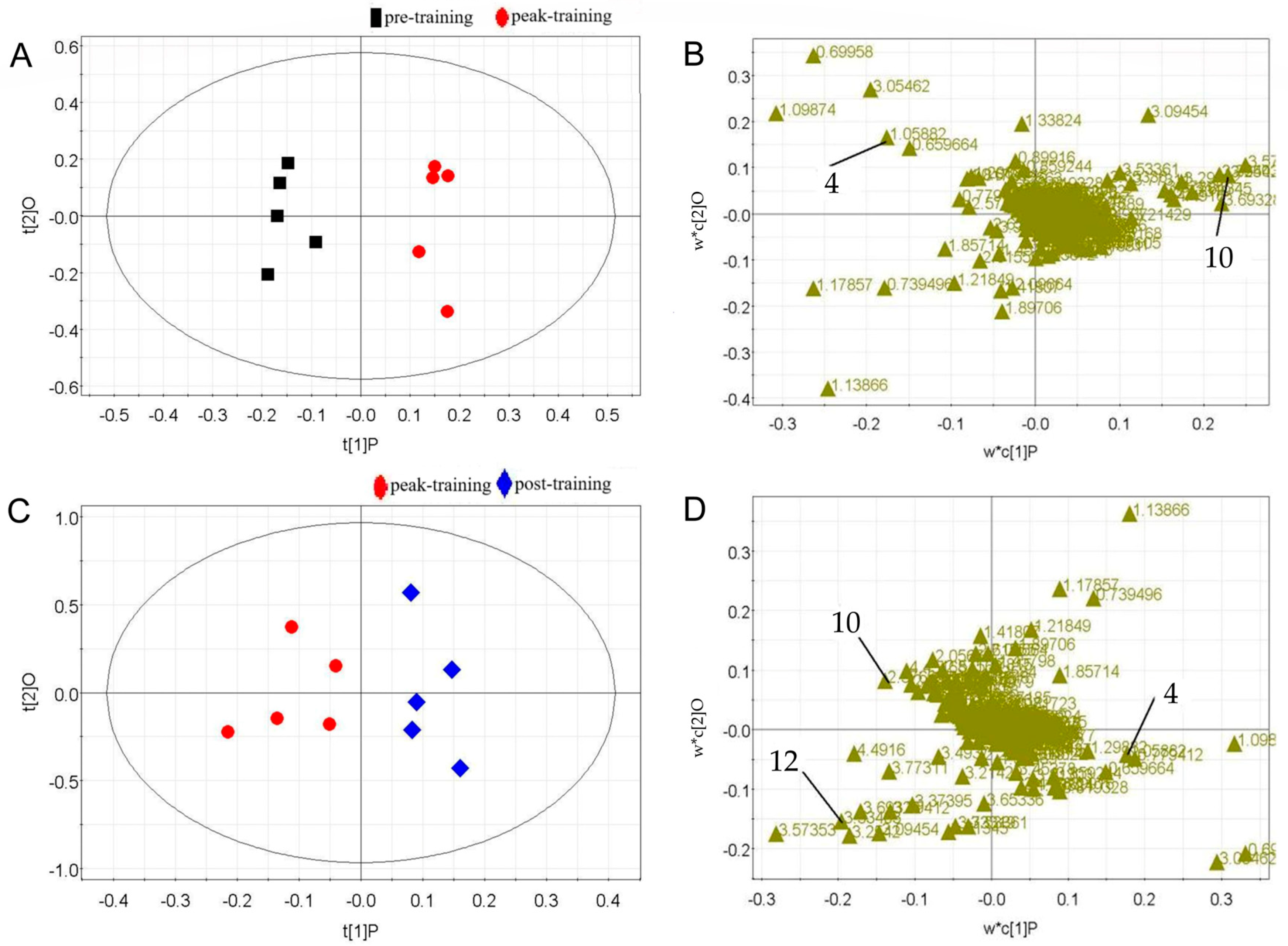
| Sex | Age (Years) | Height (cm) | Weight (kg) |
|---|---|---|---|
| Men | 20.40 ± 2.07 | 180.20 ± 3.83 | 88.60 ± 15.66 |
| Women | 19.60 ± 0.55 | 164.20 ± 4.02 | 64.40 ± 4.51 |
| Parameter | Male Wrestlers | Female Wrestlers | ||||
|---|---|---|---|---|---|---|
| Pre-Training | Peak Training | Post-Training | Pre-Training | Peak Training | Post-Training | |
| WBC (×109 g/L) | 5.40 ± 0.88 | 4.70 ± 0.42 | 5.04 ± 0.72 | 5.92 ± 1.76 | 5.14 ± 1.19 | 4.96 ± 1.07 |
| RBC (×1012 g/L) | 4.76 ± 0.29 | 4.48 ± 0.40 | 4.83 ± 0.37 | 4.09 ± 0.16 | 3.95 ± 0.19 | 4.14 ± 0.32 |
| Hb (g/L) | 155.80 ± 8.93 | 151.20 ± 9.15 | 160.20 ± 8.44 | 133.40 ± 6.35 | 126.80 ± 8.58 | 134.00 ± 11.77 |
| Hct (%) | 45.46 ± 1.40 | 43.02 ± 2.53 | 45.80 ± 2.08 | 38.46 ± 1.70 | 37.02 ± 1.77 | 38.96 ± 3.06 |
| MCV (Fl) | 95.60 ± 3.08 | 95.20 ± 3.30 | 95.06 ± 3.59 | 94.02 ± 3.62 | 93.68 ± 2.95 | 94.08 ± 3.06 |
| CK (U/L) | 183.60 ± 48.44 | 451.80 ± 95.70 * | 228.00 ± 48.84 # | 80.80 ± 14.51 | 230.00 ± 29.80 * | 98.40 ± 32.19 # |
| BU (mmol/L) | 5.10 ± 0.43 | 6.25 ± 0.30 * | 5.58 ± 0.83 | 4.87 ± 1.89 | 6.78 ± 1.66 | 5.27 ± 1.52 |
| T (ng/dL) | 666.60 ± 101.9 | 554.40 ± 135.07 | 580.40 ± 146.26 | 50.35 ± 12.94 | 34.17 ± 12.27 * | 36.26 ± 15.35 |
| No. | Compound Name | Chemical Shift (δ) | Peak Shape |
|---|---|---|---|
| 1 | Lipids | 0.89 | m |
| 2 | Leucine | 0.95 | d |
| 3 | Isoleucine | 0.99 | d |
| 4 | Valine | 1.04 | d |
| 5 | β-Hydroxybutyrate | 1.15 | dd |
| 6 | Lactic Acid | 1.32 | d |
| 7 | Alanine | 1.48 | d |
| 8 | N-Acetylglycoproteins | 2.04 | s |
| 9 | Acetoacetic Acid | 2.27 | s |
| 10 | Pyruvic Acid | 2.37 | s |
| 11 | Citric Acid | 2.65 | d |
| 12 | Creatine | 3.03 | s |
| 13 | Creatinine | 3.04 | t |
| 14 | Choline | 3.20 | s |
| 15 | Trimethylamine N-Oxide | 3.23 | m |
| 16 | Taurine | 3.26 | t |
| 17 | Proline | 3.41 | d |
| 18 | α-Glucose | 3.47 | d |
| 19 | Glycerol | 3.53 | s |
| 20 | β-Glucose | 3.71 | d |
| 21 | Glutamic Acid | 3.72 | t |
| 22 | Hippurate | 3.98 | d |
| No. | Metabolites | Chemical Shift (δ) | Mean ± SD | Peak-Training vs. Pre-Training | Post-Training vs. Peak-Training |
|---|---|---|---|---|---|
| 1 | Leucine | 0.95 (d) | 0.0009 ± 0.00054 | ↑ | ↓ |
| 2 | Isoleucine | 0.99 (d) | 0.0009 ± 0.00050 | ↑ | ↓ |
| 3 | Valine | 1.05 (d) | 0.0030 ± 0.00052 | ↑ | ↓ |
| 4 | β-hydroxybutyric acid | 1.17 (dd) | 0.0698 ± 0.00989 | ↓ | — |
| 5 | Lactic acid | 1.32 (d) | 0.0116 ± 0.0278 | ↑ | ↓ |
| 6 | Hippurate | 3.98 (d) | 0.0060 ± 0.00112 | ↓ | — |
| No. | Metabolites | Chemical Shift (δ) | Mean ± SD | Peak- Training vs. Pre-Training | Post-Training vs. Peak Training |
|---|---|---|---|---|---|
| 1 | Valine | 1.05 (d) | 0.0082 ± 0.00123 | ↓ | ↑ |
| 2 | Pyruvate | 2.37 (s) | 0.0016 ± 0.00040 | ↑ | ↓ |
| 3 | Creatine | 3.03 (s) | 0.0389 ± 0.00326 | — | ↑ |
| Metabolic Pathway | Total | Hits | p | −log(p) | Holm p | FDR | Impact |
|---|---|---|---|---|---|---|---|
| Pyruvate metabolism | 23 | 2 | 0.004014 | 2.3964 | 0.31309 | 0.10248 | 0.19137 |
| Glycolysis or Gluconeogenesis | 26 | 2 | 0.0051238 | 2.2904 | 0.39453 | 0.10248 | 0.09785 |
| Citrate cycle (TCA cycle) | 20 | 1 | 0.084848 | 1.0714 | 1.0 | 0.7542 | 0.04634 |
| Arginine and proline metabolism | 36 | 1 | 0.0097248 | 2.0121 | 0.72936 | 0.12966 | 0.02442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, X.; Liu, J.; Liu, Y.; Han, Y.; Zhang, W. Serum Metabolomics Reveals Metabolic Changes in Freestyle Wrestlers During Different Training Stages. Metabolites 2025, 15, 737. https://doi.org/10.3390/metabo15110737
Li X, Liu X, Liu J, Liu Y, Han Y, Zhang W. Serum Metabolomics Reveals Metabolic Changes in Freestyle Wrestlers During Different Training Stages. Metabolites. 2025; 15(11):737. https://doi.org/10.3390/metabo15110737
Chicago/Turabian StyleLi, Xiaonan, Xiangyu Liu, Jianxing Liu, Yinhai Liu, Yumei Han, and Wei Zhang. 2025. "Serum Metabolomics Reveals Metabolic Changes in Freestyle Wrestlers During Different Training Stages" Metabolites 15, no. 11: 737. https://doi.org/10.3390/metabo15110737
APA StyleLi, X., Liu, X., Liu, J., Liu, Y., Han, Y., & Zhang, W. (2025). Serum Metabolomics Reveals Metabolic Changes in Freestyle Wrestlers During Different Training Stages. Metabolites, 15(11), 737. https://doi.org/10.3390/metabo15110737







