Effect of Tryptophan Supplementation Levels on the Cecal Microbial Composition, Growth Performance, Immune Function and Antioxidant Capacity in Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Feeding, Management and Treatment
2.2. Sample Collection
2.3. Data Processing and Analyses
3. Results
3.1. Effects of Dietary Trp Levels on Growth Performance, Organ Development and Serum Biochemical Indicators in Broilers
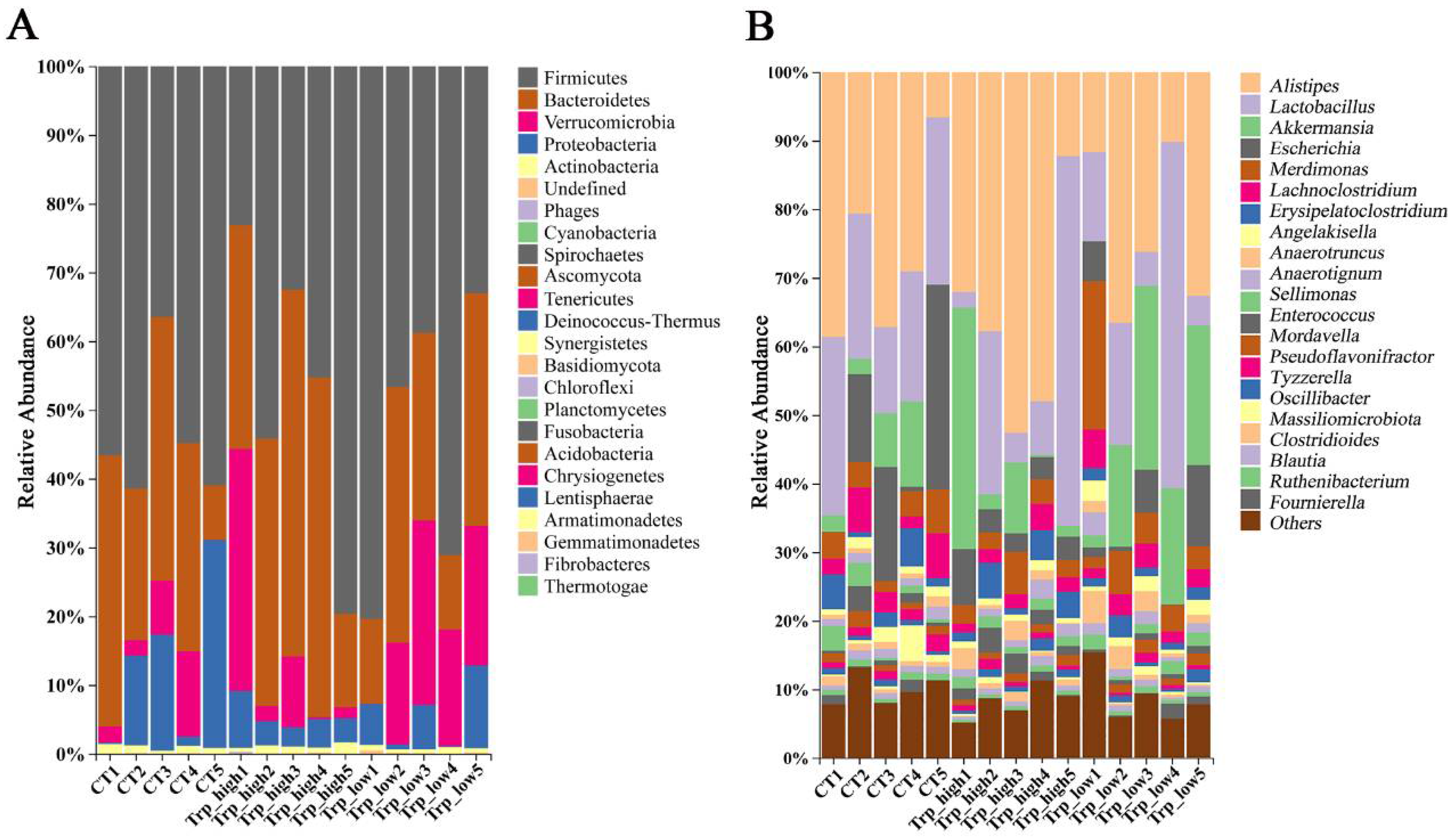
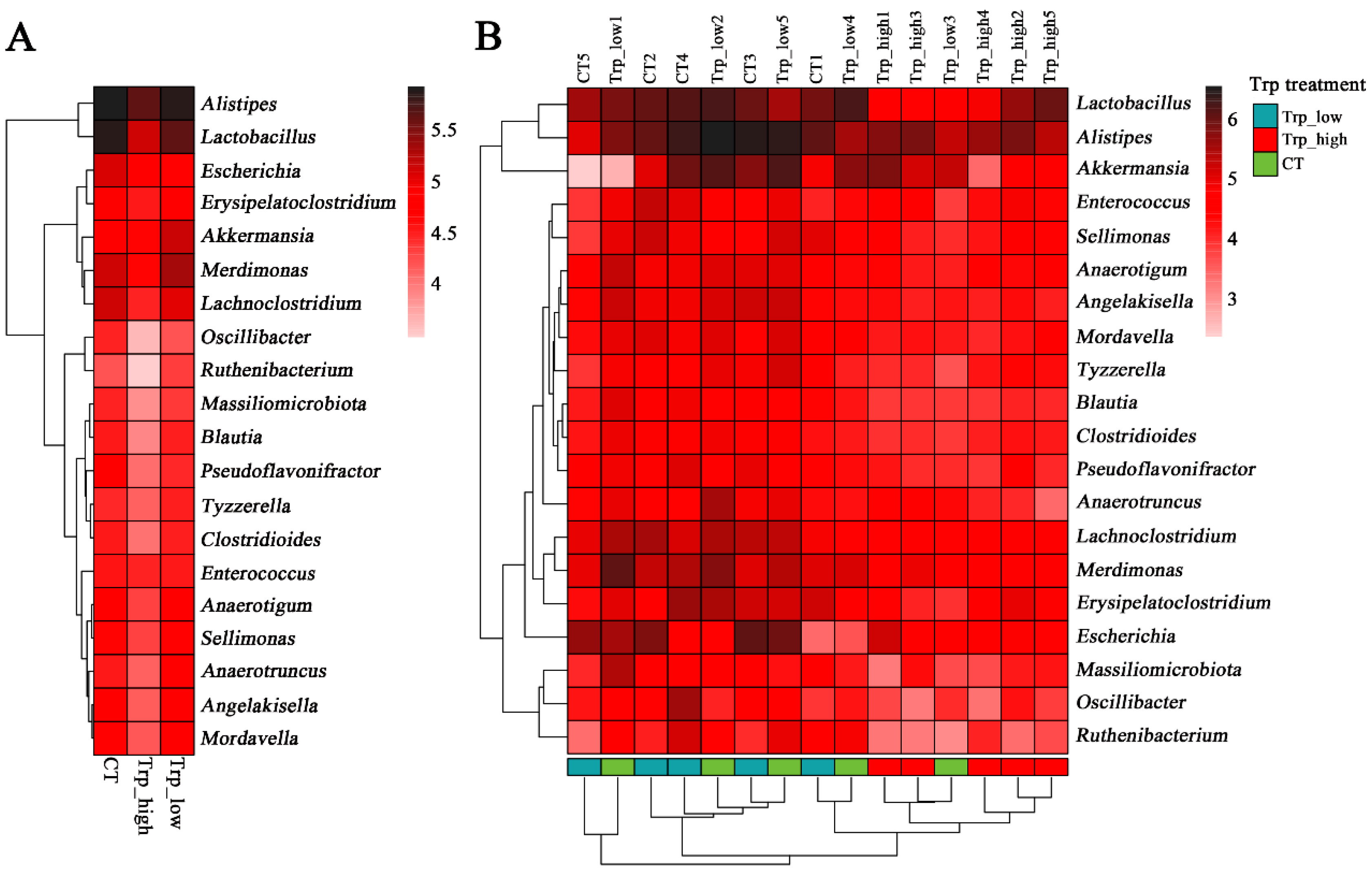
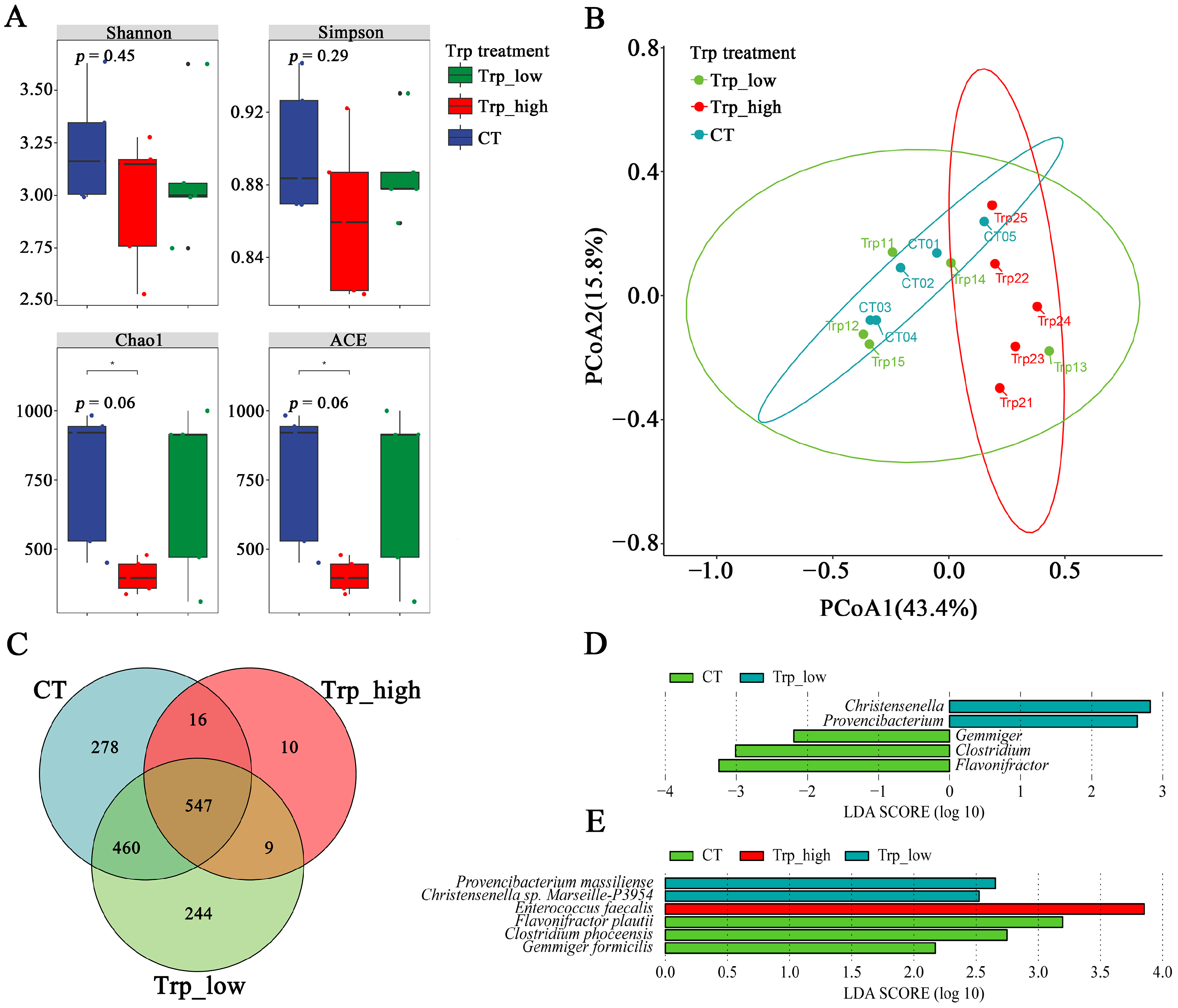
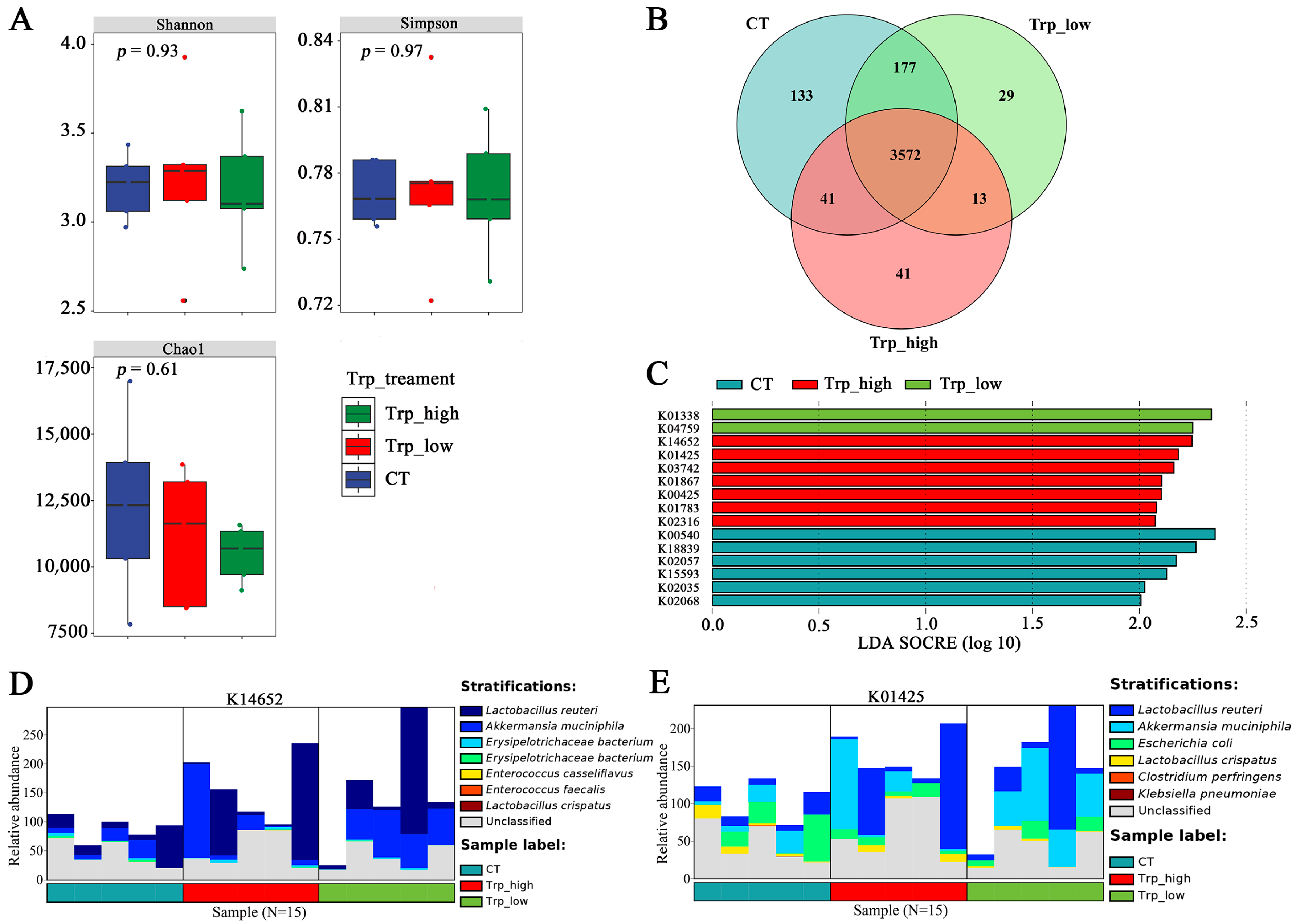
3.2. Effects of Dietary Trp Levels on Cecal Microbiota Composition and Diversity Analysis
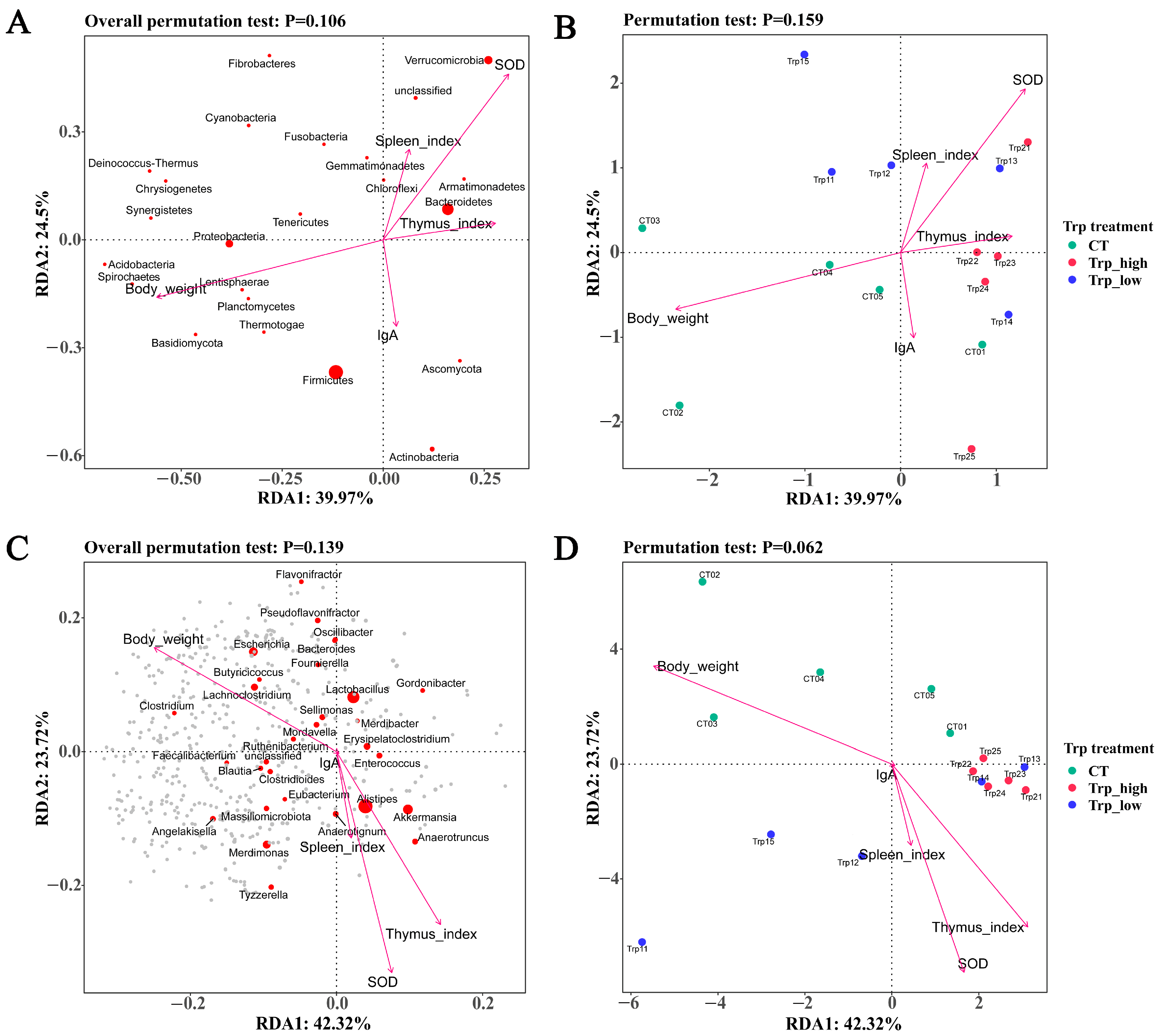
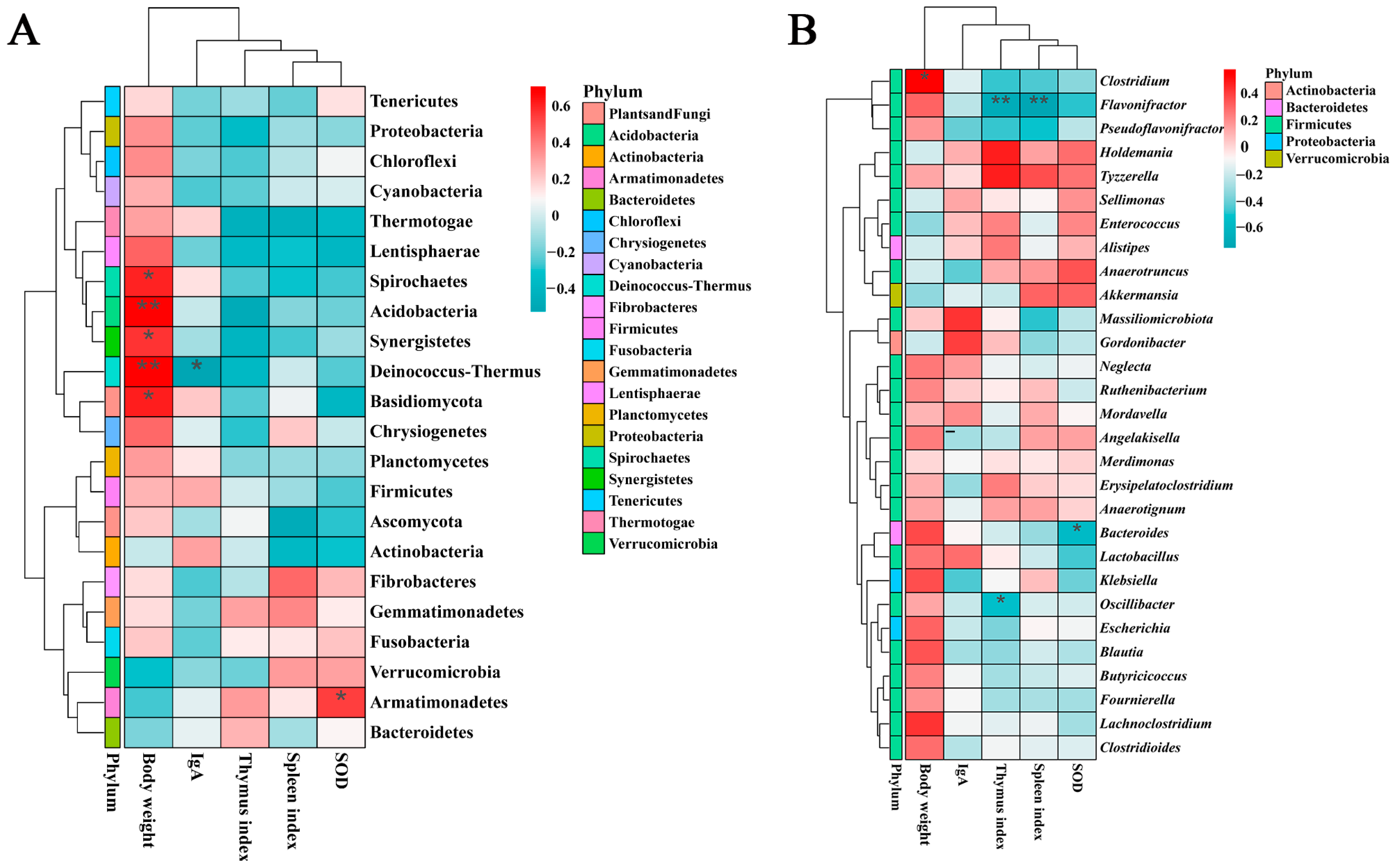
3.3. Correlation Analysis with Growth and Metabolism Indicators
3.4. Functional Analyses of Different Trp Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADFI | average daily feed intake |
| ADG | average daily gain |
| BW | body weight |
| BWG | body weight gain |
| FCR | feed conversion rate |
| GO | Gene Ontology |
| GSH-Px | glutathione peroxidase |
| IgA | immunoglobulin A |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KO | KEGG orthology |
| MDA | malondialdehyde |
| RDA | redundancy analysis |
| SOD | superoxide dismutase |
| T-AOC | total antioxidant capacity |
| Trp | tryptophan |
References
- Wang, B.; Min, Z.; Yuan, J. Apparent ileal digestible tryptophan requirements of 22- to 42-day-old broiler chicks. J. Appl. Poult. Res. 2016, 25, 54–61. [Google Scholar] [CrossRef]
- Fouad, A.M.; El-Senousey, H.K.; Ruan, D.; Wang, S.; Xia, W.; Zheng, C. Tryptophan in poultry nutrition: Impacts and mechanisms of action. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1146–1153. [Google Scholar] [CrossRef]
- Corzo, A.; Moran, E.T., Jr.; Hoehler, D.; Lemmell, A. Dietary tryptophan need of broiler males from forty-two to fifty-six days of age. Poult. Sci. 2005, 84, 226–231. [Google Scholar] [CrossRef]
- Bello, A.U.; Zulkifli, I.; Goh, Y.M.; Atta, A.E.; Abdoreza, S.F. Gut microbiota and transportation stress response affected by tryptophan supplementation in broiler chickens. Ital. J. Anim. Sci. 2018, 17, 107–113. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.M.; Zhang, L.S.; Zhang, Y.R.; Cai, S.M.; Yu, J.H.; Xia, Z.F. Effects of tryptophan supplementation on growth performance, antioxidative activity, and meat quality of ducks under high stocking density. Poult. Sci. 2015, 94, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Guo, Y.; Yang, Y. Effects of dietary L-tryptophan supplementation on intestinal response to chronic unpredictable stress in broilers. Amino Acids 2017, 49, 1227–1236. [Google Scholar] [CrossRef]
- Bai, M.; Liu, H.; Xu, K.; Oso, A.O.; Wu, X.; Liu, G.; Tossou, M.C.; Al-Dhabi, N.A.; Duraipandiyan, V.; Xi, Q.; et al. A review of the immunomodulatory role of dietary tryptophan in livestock and poultry. Amino Acids 2017, 49, 67–74. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Birkl, P.; Chow, J.; McBride, P.; Kjaer, J.B.; Kunze, W.; Forsythe, P.; Harlander-Matauschek, A. Effects of acute tryptophan depletion on repetitive behavior in laying hens. Front. Vet. Sci. 2019, 6, 230. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Bender, D.A. Effects of a dietary excess of leucine on the metabolism of tryptophan in the rat: A mechanism for the pellagragenic action of leucine. Br. J. Nutr. 1983, 50, 25–32. [Google Scholar] [CrossRef]
- Gao, K.; Pi, Y.; Mu, C.L.; Farzi, A.; Liu, Z.; Zhu, W.Y. Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: Aromatic amino acids linking the microbiota-brain axis. J. Neurochem. 2019, 149, 641–659. [Google Scholar] [CrossRef]
- Özoğul, F.; Kuley, E.; Özoğul, Y.; Özoğul, İ. The function of lactic acid bacteria on biogenic amines production by food-borne pathogens in arginine decarboxylase broth. Food Sci. Technol. Res. 2012, 18, 795–804. [Google Scholar] [CrossRef]
- Shishov, V.A.; Kirovskaia, T.A.; Kudrin, V.S.; Oleskin, A.V. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Prikl. Biokhim. Mikrobiol. 2009, 45, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Özoğul, F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. Eur. Food Res. Technol. 2004, 219, 465–469. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS ONE 2013, 8, e80604. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Modulation of immunity by tryptophan microbial metabolites. Front. Nutr. 2023, 10, 1209613. [Google Scholar] [CrossRef]
- Smith, N.K., Jr.; Waldroup, P.W. Estimation of the tryptophan requirement of male broiler chickens. Poult. Sci. 1988, 67, 1174–1177. [Google Scholar] [CrossRef]
- Mund, M.D.; Riaz, M.; Mirza, M.A.; Rahman, Z.U.; Mahmood, T.; Ahmad, F.; Ammar, A. Effect of dietary tryptophan supplementation on growth performance, immune response and anti-oxidant status of broiler chickens from 7 to 21 days. Vet. Med. Sci. 2020, 6, 48–53. [Google Scholar] [CrossRef]
- Goo, D.; Kim, J.H.; Park, G.H.; Delos Reyes, J.B.; Kil, D.Y. Effect of stocking density and dietary tryptophan on growth performance and intestinal barrier function in broiler chickens. Poult. Sci. 2019, 98, 4504–4508. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.P.; Pesti, G.M.; Edwards, H.M.; Bakalli, R. Tryptophan requirements of different broiler genotypes. Poult. Sci. 2001, 80, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Linh, N.T.; Guntoro, B.; Hoang Qui, N. Immunomodulatory, behavioral, and nutritional response of tryptophan application on poultry. Vet. World 2021, 14, 2244–2250. [Google Scholar] [CrossRef]
- Chen, J.; Jing, H.; Liu, H.; Zhu, X.; Yang, G. Interaction between dietary digestible tryptophan and soy oligosaccharides in broiler chickens: Effects on caecal skatole level and microflora. Anim. Biosci. 2023, 36, 471–483. [Google Scholar] [CrossRef]
- Fan, G.; Li, W.; Liu, H.; Chen, Q.; Zhang, S.; Zhang, B. Effects of dietary tryptophan supplementation on growth performance, intestinal health, and AhR/CYP1A1 signaling pathway in broiler chickens challenged with Clostridium perfringens. Poult. Sci. 2025, 104, 105955. [Google Scholar] [CrossRef]
- Baker, D.H.; Batal, A.B.; Parr, T.M.; Augspurger, N.R.; Parsons, C.M. Ideal ratio (relative to lysine) of tryptophan, threonine, isoleucine, and valine for chicks during the second and third weeks posthatch. Poult. Sci. 2002, 81, 485–494. [Google Scholar] [CrossRef]
- Leibowitz, S.F.; Alexander, J.T. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol. Psychiatry 1998, 44, 851–864. [Google Scholar] [CrossRef]
- Coşkun, S.; Ozer, C.; Gönül, B.; Take, G.; Erdoğan, D. The effect of repeated tryptophan administration on body weight, food intake, brain lipid peroxidation and serotonin immunoreactivity in mice. Mol. Cell. Biochem. 2006, 286, 133–138. [Google Scholar] [CrossRef]
- Xie, K.; Feng, X.; Zhu, S.; Liang, J.; Mo, Y.; Feng, X.; Ye, S.; Zhou, Y.; Shu, G.; Wang, S.; et al. Effects of tryptophan supplementation in diets with different protein levels on the production performance of broilers. Animals 2024, 14, 1838. [Google Scholar] [CrossRef]
- Khattak, F.; Helmbrecht, A. Effect of different levels of tryptophan on productive performance, egg quality, blood biochemistry, and caecal microbiota of hens housed in enriched colony cages under commercial stocking density. Poult. Sci. 2019, 98, 2094–2104. [Google Scholar] [CrossRef]
- Saraf, M.K.; Piccolo, B.D.; Bowlin, A.K.; Mercer, K.E.; LeRoith, T.; Chintapalli, S.V.; Shankar, K.; Badger, T.M.; Yeruva, L.A.-O. Formula diet driven microbiota shifts tryptophan metabolism from serotonin to tryptamine in neonatal porcine colon. Microbiome 2017, 5, 77. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Gao, B.; Wang, H.J.; He, J.G.; Chen, H.S.; Hu, Z.L.; Long, L.H.; Chen, J.G.; Wang, F. Melatonin ameliorates depressive-like behaviors in ovariectomized mice by improving tryptophan metabolism via inhibition of gut microbe Alistipes Inops. Adv. Sci. 2024, 11, e2309473. [Google Scholar] [CrossRef]
- Gu, Z.; Pei, W.; Shen, Y.; Wang, L.; Zhu, J.; Zhang, Y.; Fan, S.; Wu, Q.; Li, L.; Zhang, Z. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 2021, 12, 10184–10195. [Google Scholar] [CrossRef]
- Yusufu, I.; Ding, K.; Smith, K.; Wankhade, U.D.; Sahay, B.; Patterson, G.T.; Pacholczyk, R.; Adusumilli, S.; Hamrick, M.W.; Hill, W.D.; et al. A tryptophan-deficient diet induces gut microbiota dysbiosis and increases systemic inflammation in aged mice. Int. J. Mol. Sci. 2021, 22, 5005. [Google Scholar] [CrossRef]
- Khanipour, S.; Mehri, M.; Bagherzadeh-Kasmani, F.; Maghsoudi, A.; Assadi Soumeh, E. Excess dietary tryptophan mitigates aflatoxicosis in growing quails. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1462–1473. [Google Scholar] [CrossRef]
- Kankova, Z.; Drozdova, A.; Klobetzova, Z.; Lichovnikova, M.; Zeman, M. Development and reactivity of the immune system of Japanese quail lines divergently selected for the shape of the growth curve. Br. Poult. Sci. 2019, 60, 700–707. [Google Scholar] [CrossRef]
- Klasing, K.C.; Laurin, D.E.; Peng, R.K.; Fry, D.M. Immunologically mediated growth depression in chicks: Influence of feed intake, corticosterone and interleukin-1. J. Nutr. 1987, 117, 1629–1637. [Google Scholar] [CrossRef]
- Edmonds, M.S.; Baker, D.H. Comparative effects of individual amino acid excesses when added to a corn-soybean meal diet: Effects on growth and dietary choice in the chick. J. Anim. Sci. 1987, 65, 699–705. [Google Scholar] [CrossRef]
- Li, C.; Deng, L.; Pu, M.; Ye, X.; Lu, Q. Coptisine alleviates colitis through modulating gut microbiota and inhibiting TXNIP/NLRP3 inflammasome. J. Ethnopharmacol. 2024, 335, 118680. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, X.; Liu, L.; Voglmeir, J.; Zhong, X.; Yu, Q. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium. Vet. Res. 2020, 51, 34. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, D.; Nian, Y.; Li, C. Casein-fed mice showed faster recovery from DSS-induced colitis than chicken-protein-fed mice. Food Funct. 2021, 12, 5806–5820. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Yang, Y.; Gong, W.; Sun, X.; Yang, L.; Zhang, Q.; Jin, M. Akkermansia muciniphila improves host defense against influenza virus infection. Front. Microbiol. 2020, 11, 586476. [Google Scholar] [CrossRef]
- Hasani, A.; Ebrahimzadeh, S.; Hemmati, F.; Khabbaz, A.; Hasani, A.; Gholizadeh, P. The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis. J. Med. Microbiol. 2021, 70, 001435. [Google Scholar] [CrossRef]
- Lundberg, R.; Scharch, C.; Sandvang, D. The link between broiler flock heterogeneity and cecal microbiome composition. Anim. Microbiome 2021, 3, 54–67. [Google Scholar] [CrossRef]
- Shehata, A.A.; Tarabees, R.; Basiouni, S.; ElSayed, M.S.; Gaballah, A.; Krueger, M. Effect of a potential probiotic candidate Enterococcus faecalis-1 on growth performance, intestinal microbiota, and immune response of commercial broiler chickens. Probiotics Antimicrob. Proteins 2020, 12, 451–460. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Wang, Y.; Yuan, J.; Wang, Y.; Zeng, Y.; Zhang, H.; Yang, H.; Ma, Q.; Shi, D.; et al. Effects of 3-indoleacrylic acid on alleviating lipopolysaccharide-induced liver inflammatory damage in laying hens. Poult. Sci. 2025, 104, 105307. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, W.; Liang, Y.; Sun, L.; Yin, Y.; Zhang, W. Anti-Inflammatory and Anti-Oxidative Activity of Indole-3-Acetic Acid Involves Induction of HO-1 and Neutralization of Free Radicals in RAW264.7 Cells. Int. J. Mol. Sci. 2020, 21, 1579. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
| Item | Composition, % |
|---|---|
| Ingredients | |
| Corn | 60.0 |
| Soybean meal | 30.0 |
| Fish meal | 2.0 |
| Soybean oil | 3.0 |
| Premix 1 | 5.0 |
| Total | 100 |
| Energy and nutrient composition 2 | |
| Metabolizable energy (calculated) 3 | 12.22 |
| Crude protein (calculated) | 21.50 |
| Calcium (calculated) | 1.00 |
| Available phosphorus (calculated) | 0.45 |
| Methionine (measured) | 0.46 |
| Lysine (measured) | 1.15 |
| Methionine + cysteine (measured) | 0.91 |
| Tryptophan (measured) | 0.23 |
| Item 1 | Trp Level, % | p-Value | ||
|---|---|---|---|---|
| 0.23 | 0.23 + 0.06 | 0.23 + 0.12 | ||
| BW at day 1 (g) | 46.54 ± 0.73 | 46.79 ± 0.52 | 46.67 ± 0.72 | 0.845 |
| BW at day 21 (g) | 734.37 ± 23.83 a | 719.57 ± 11.31 a | 657.87 ± 25.93 b | 0.010 |
| BWG (g) | 687.84 ± 24.12 a | 672.77 ± 10.95 a | 611.21 ± 25.97 b | 0.010 |
| ADFI (g/d) | 44.86 ± 1.37 a | 43.29 ± 0.86 a | 39.44 ± 0.57 b | 0.001 |
| ADG (g/d) | 33.40 ± 1.07 a | 32.02 ± 0.55 a | 29.09 ± 1.25 b | 0.005 |
| FCR (g/g) | 1.34 ± 0.01 | 1.35 ± 0.02 | 1.36 ± 0.07 | 0.917 |
| Item | Trp Level, % | p-Value | ||
|---|---|---|---|---|
| 0.23 | 0.23 + 0.06 | 0.23 + 0.12 | ||
| Thymus index (g/kg) | 3.85 ± 0.42 b | 4.43 ± 0.62 ab | 4.80 ± 0.66 a | 0.011 |
| Liver index (g/kg) | 28.04 ± 1.39 a | 28.08 ± 3.69 a | 24.90 ± 1.66 b | 0.026 |
| Bursa of Fabricius index (g/kg) | 2.62 ± 0.52 ab | 2.97 ± 0.10 a | 2.28 ± 0.54 b | 0.016 |
| Spleen index (g/kg) | 0.76 ± 0.23 | 0.91 ± 0.17 | 0.81 ± 0.18 | 0.303 |
| Item 1 | Trp Level, % | p-Value | ||
|---|---|---|---|---|
| 0.23 | 0.23 + 0.06 | 0.23 + 0.12 | ||
| GSH-Px (U/mL) 1 | 112.09 ± 3.54 | 111.58 ± 13.16 | 105.69 ± 9.02 | 0.219 |
| SOD (U/mL) 1 | 214.63 ± 20.74 b | 290.95 ± 52.00 a | 303.96 ± 41.27 a | 0.022 |
| T-AOC (U/mL) 1 | 6.82 ± 1.38 | 6.27 ± 1.04 | 6.51 ± 1.56 | 0.718 |
| MDA (mmol/L) 1 | 7.03 ± 0.63 | 6.59 ± 0.94 | 6.09 ± 0.78 | 0.084 |
| IgG (μg/mL) 1 | 17.18 ± 3.19 | 18.87 ± 4.15 | 18.79 ± 4.72 | 0.648 |
| IgA (μg/mL) 1 | 62.82 ± 8.75 | 65.51 ± 12.90 | 67.54 ± 9.97 | 0.680 |
| IgM (ng/mL) 1 | 405.23 ± 80.20 | 396.17 ± 108.67 | 396.88 ± 83.39 | 0.976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Fu, C.; Gao, Q.; Zhang, H.; Shi, T.; Li, G.; Wang, Y.; Shang, Y. Effect of Tryptophan Supplementation Levels on the Cecal Microbial Composition, Growth Performance, Immune Function and Antioxidant Capacity in Broilers. Metabolites 2025, 15, 736. https://doi.org/10.3390/metabo15110736
Liu X, Fu C, Gao Q, Zhang H, Shi T, Li G, Wang Y, Shang Y. Effect of Tryptophan Supplementation Levels on the Cecal Microbial Composition, Growth Performance, Immune Function and Antioxidant Capacity in Broilers. Metabolites. 2025; 15(11):736. https://doi.org/10.3390/metabo15110736
Chicago/Turabian StyleLiu, Xuelan, Chunyan Fu, Qingtao Gao, Heng Zhang, Tianhong Shi, Guiming Li, Yunchao Wang, and Yan Shang. 2025. "Effect of Tryptophan Supplementation Levels on the Cecal Microbial Composition, Growth Performance, Immune Function and Antioxidant Capacity in Broilers" Metabolites 15, no. 11: 736. https://doi.org/10.3390/metabo15110736
APA StyleLiu, X., Fu, C., Gao, Q., Zhang, H., Shi, T., Li, G., Wang, Y., & Shang, Y. (2025). Effect of Tryptophan Supplementation Levels on the Cecal Microbial Composition, Growth Performance, Immune Function and Antioxidant Capacity in Broilers. Metabolites, 15(11), 736. https://doi.org/10.3390/metabo15110736







