Metabolomic Profile of Weight Gain of People Living with HIV Treated with Integrase Strand Transfer Inhibitor Regimens Reveals Dysregulated Lipid Metabolism and Mitochondrial Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Body Composition Measurements
2.3. Blood Sampling and Data Collection
2.4. Metabolite Extraction for Untargeted Metabolomics
2.5. Untargeted Metabolomics
2.6. Statistical Analysis of Clinical Dataset
2.7. Metadata Variables—Biplot
2.8. Multivariate Analysis
2.9. Metabolite Classification and Filtering
2.10. Heatmaps
2.11. Volcano Plots
2.12. Enrichment Pathway
3. Results
3.1. Characteristic of the Study Population
| Characteristics | General (N = 66) | Controls (N = 38) | Cases (N= 28) | p |
|---|---|---|---|---|
| Age (years) | 27.74 ± 7.09 | 26.84 ± 6.49 | 28.96 ± 7.78 | 0.261 |
| Body weight (kg) | 68.04 ± 11.52 | 67.48 ± 9.46 | 67.30 ± 13.63 | 0.661 |
| BMI (kg/m2) | 23.36 ± 3.40 | 23.50 ± 2.81 | 23.16 ± 4.3 | 0.691 |
| HIV RNA VL (copies/mL) | 35,879 [8740–101,317] | 21,614 [4228–50,314] | 68,258 [33,149–233,295] | 0.001 |
| Log VL | 4.41 ± 0.93 | 4.18 ± 0.92 | 4.74 ± 085 | 0.678 |
| T CD4+ (cel/μL) | 279.23 ± 155.75 | 298.90 ± 174.96 | 252.53 ± 123.16 | 0.269 |
| T CD8+ (cel/μL) | 665.74 ± 570.66 | 561.32 ± 447.621 | 807.45 ± 687.92 | 0.436 |
| HIV stage CDC | ||||
| 1 | 7.6% (5/66) | 10.5% (4/38) | 3.6% (1/28) | |
| 2 | 60% (40/66) | 65.8% (25/38) | 53.6% (15/28) | 0.193 |
| 3 | 31.8% (21/66) | 23.7% (9/38) | 42.9% (12/38) | |
| INSTI | ||||
| BIC | 48.48% (32/66) | 50% (19/38) | 53.57% (15/28) | 0.808 |
| DTG | 51.51% (34/66) | 50% (19/38) | 46.42% (13/28) | |
| Insulin (µUI/mL), median [IQR] | 12.69 [10.34–17.8) | 12.38 [9.83–18.27] | 12.89 [11.01–16.65] | 0.683 |
| HOMA-IR, median [IQR] | 1.2 [0.7–2.02] | 1.1 [0.6–2.32] | 1.3 [0.92–1.17] | 0.399 |
| Muscle mass (%), median [IQR] | 35.85 [34.52–39.05] | 36.55 [34.7–39.05] | 35.5 [34.5–38.8] | 0.590 |
| Body fat (%) | 21.24 ± 4.12 | 20.91 ± 3.88 | 21.69 ± 4.46 | 0.460 |
| Body water (%) | 50.21 ± 3.73 | 50.32 ± 3.60 | 50.05 ± 3.94 | 0.451 |
| Bone (kg) | 2.86 ± 0.35 | 2.79 ± 0.28 | 2.96 ± 0.41 | 0.065 |
| SAT (cm) | 1.73 ± 0.80 | 1.80 ± 0.83 | 1.64 ± 0.77 | 0.423 |
| VAT (cm) | 3.73 ± 0.87 | 3.70 ± 0.83 | 3.76 ± 0.94 | 0.801 |
| Liver stiffness (kPa) | 4.69 ± 0.93 | 4.6 ± 0.93 | 4.82 ± 0.93 | 0.681 |
| TGO (AST) (UI/L) | 30.27 ± 6.31 | 30.13 ± 8.45 | 28.35 ± 10.37 | 0.377 |
| TGP (ALT) (UI/L) | 29.37 ± 9.285 | 30.42 ± 5.51 | 30.07 ± 7.35 | 0.474 |
| GGT (UI/L) median [IQR] | 40 [31.5–45.75] | 43.5 [33–56] | 33 [29.6–42.33] | 0.012 |
| PA (UI/L) | 69 [56–89] | 75.2 [56–88.14] | 65.2 [52–84.12] | 0.259 |
| TC (mg/dL) | 169.25 ± 14.61 | 164.05 ± 12.79 | 176.32 ± 14.12 | 0.436 |
| TG (mg/dL) | 146.43 ± 13.26 | 141.07 ± 12.55 | 153.71 ± 10.60 | 0.078 |
| HDL (mg/dL), median (IQR) | 23 [19–32] | 23.14 [19.12–32.4] | 24.25 [20.5–28.8] | 0.607 |
| Creatinine mg/dL, median (IQR) | 0.89 (0.78–0.98) | 0.89 (0.78–0.98) | 0.92 (0.81–0.98) | 0.927 |
| Characteristics | General (N = 66) | Controls (N = 38) | Cases (N = 28) | p |
|---|---|---|---|---|
| Body weight (kg) | 70.7 ± 12.7 | 67.48 ± 9.46 | 75.16 ± 15.20 | 0.023 |
| T CD4+ (cel/μL) | 754 [613–932] | 772.50 [636.05–956.50] | 668.15 [566.75–859.50] | 0.102 |
| T CD8+ (cel/μL) | 1103 [867–1444] | 1129 [903–1445] | 1092 [829–1444] | 0.456 |
| TCD4+/TCD8+ ratio | 0.64 [0.49–0.87] | 0.61 [0.55–0.88] | 0.67 [0.49–0.85] | 0.886 |
| Insulin (µUI/mL) | 12.64 [9.66–16.75] | 11.61 [8.16–15.53] | 13.57 [11.28–24.38] | 0.179 |
| HOMA-IR > 2.6 | 25 (37.87%) | 10 (15.15%) | 15 (22.72%) | 0.049 |
| Muscle mass (%) | 37.55 [34.85–41.18] | 38.85 [35.32–41.37] | 35.85 [34.3–38.55] | 0.073 |
| Body fat (%) | 21.79 ± 4.76 | 21.23 ± 3.91 | 22.52 ± 5.71 | 0.308 |
| Body water (%) | 49.90 [46.80–53.75] | 51.80 [48.30–53.88] | 47.55 [46.08–50.80] | 0.053 |
| Bone (kg) | 2.86 ± 0.35 | 2.78 ± 0.28 | 2.95 ± 0.41 | 0.065 |
| SAT (cm) | 1.85 [1.40–2.50] | 1.75 [1.30–2.58] | 2.05 [1.55–2.50 | 0.583 |
| VAT (cm) | 3.60 [3.02–4.38] | 3.40 [2.90–3.88] | 3.85 [3.27–4.72] | 0.009 |
| Liver stiffness (kPa) | 4.50 [4.00–4.90] | 4.7 [4.0–5.32] | 4.3 [3.82–4.67] | 0.034 |
| TGO (AST) (UI/L) | 36 [30–40] | 35 [27–41] | 37 [35–40] | 0.216 |
| TGP (ALT) (UI/L) | 32.86 ± 10.15 | 33.68 ± 10.57 | 31.75 ± 9.60 | 0.441 |
| GGT (UI/L) | 24 [21–26] | 24.5 [21.0–26.0] | 24.5 [22.5–26.0] | 0.583 |
| PA (UI/L) | 64.50 [61–72] | 64.50 [61.00–72.00] | 66.50 [61.00–72.00 | 0.995 |
| TC (mg/dL) | 135 [89–158] | 135.00 [89.25–157.00] | 135.50 [89.00–177.50] | 0.617 |
| TG (mg/dL) | 149.50 [141.25–159.50] | 145.00 [136.50–152.00] | 162.50 [148.25–171.25] | 0.004 |
| HDL (mg/dL) | 41 [29–45] | 34 [28–45] | 42 [31–44] | 0.580 |
| Creatinine mg/dL | 0.89 (0.78–0.98) | 0.89 (0.78–0.98) | 0.92 (0.81–0.98) | 0.927 |
3.2. Factors Associated with Weight Gain
| Characteristics | Controls (N = 38) | Cases (N = 28) | OR (95% CI) | p |
|---|---|---|---|---|
| HOMA-IR > 2.6 | 10 (15.15%) | 15 (22.72%) | 3.23 (1.14–9.10) | 0.023 |
| VAT ≥ 4 cm | 12 (18.18%) | 19 (28.78%) | 4.50 (1.60–13.03) | 0.004 |
| TG ≥ 150 mg/dL | 12 (18.18%) | 18 (27.27%) | 3.90 (1.38–10.94) | 0.008 |
| Baseline HIV RNA VL > 50,000 copies/mL | 9 (13.63%) | 20 (30.30%) | 8.05 (2.65–24.43) | 0.0002 |
| Baseline HIV RNA VL > 100,000 copies/mL | 6 (9.09%) | 11 (16.66%) | 3.45 (1.08–10.96) | 0.035 |
| Characteristics | aOR (95% CI) | p |
|---|---|---|
| HOMA-IR > 2.6 | 1.90 (0.51–7.07) | 0.334 |
| VAT ≥ 4 cm | 5.77 (1.52–21.87) | 0.010 |
| TG ≥ 150 mg/dL | 3.82 (1.048–13.98) | 0.042 |
| Baseline HIV RNA VL ≥ 50,000 copies/mL | 6.58 (1.83–23.58) | 0.004 |
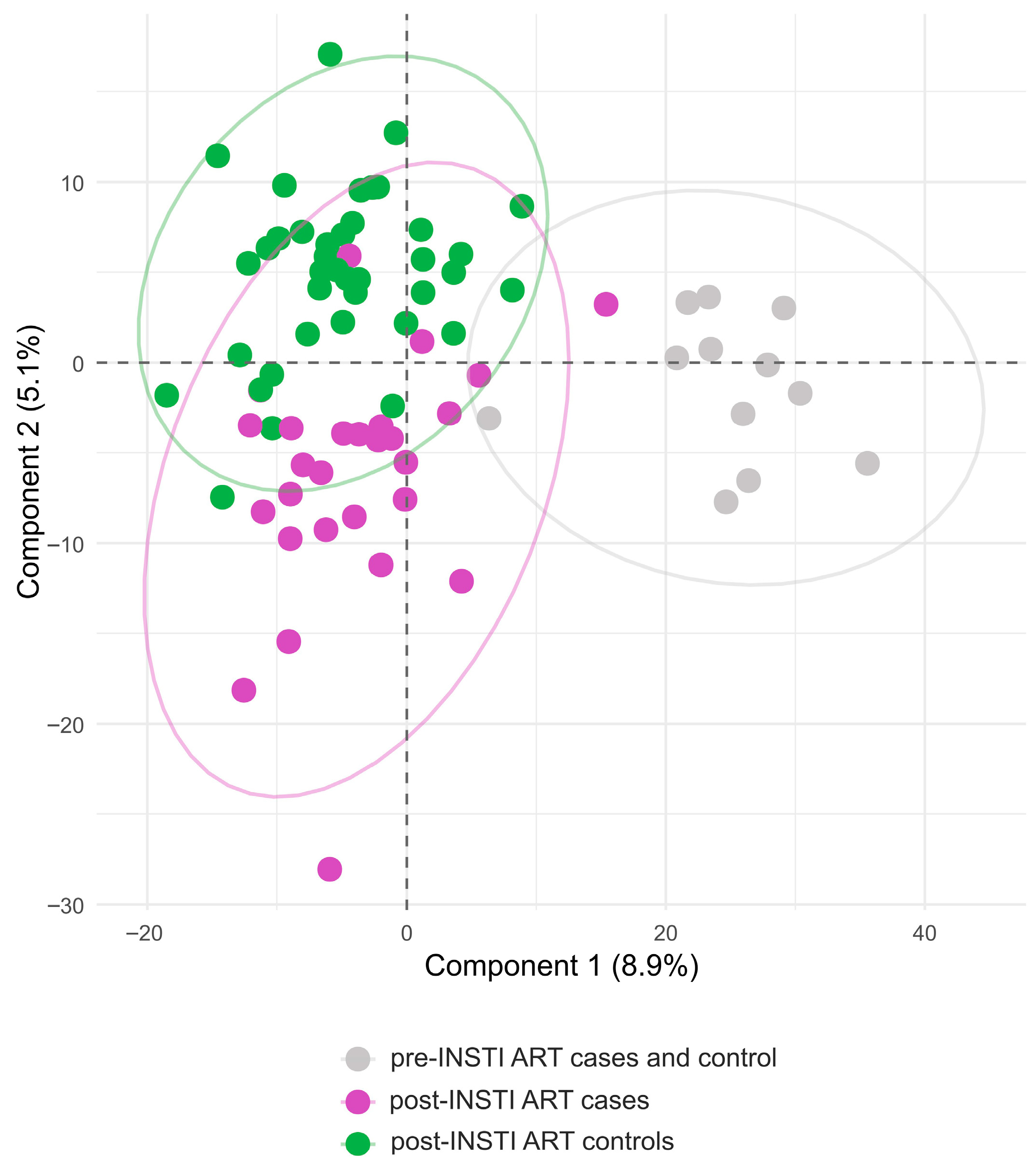
3.3. Plasma Metabolites in the Cases and Controls Groups Pre- and Post- INSTI-ART Are Separated in Distinct Clusters
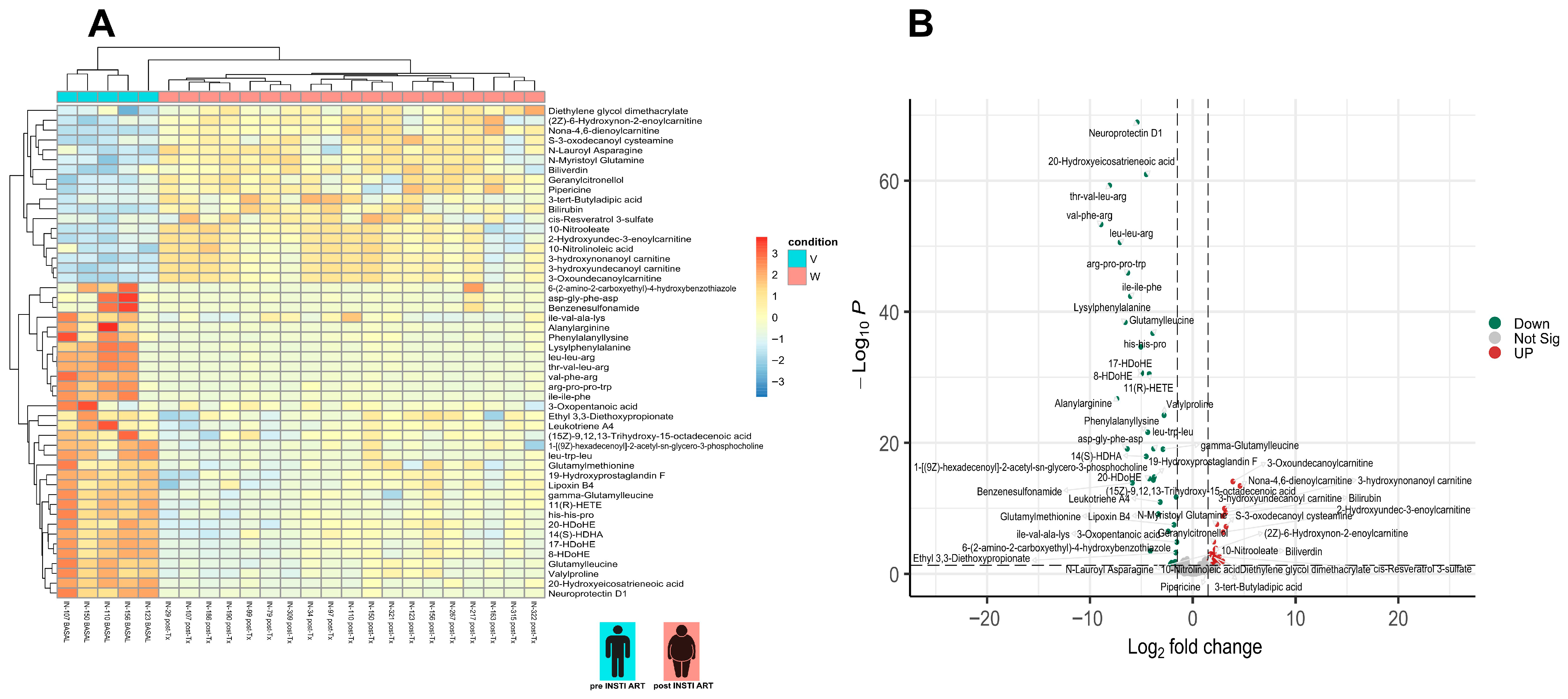
3.4. Differential Metabolite Analysis
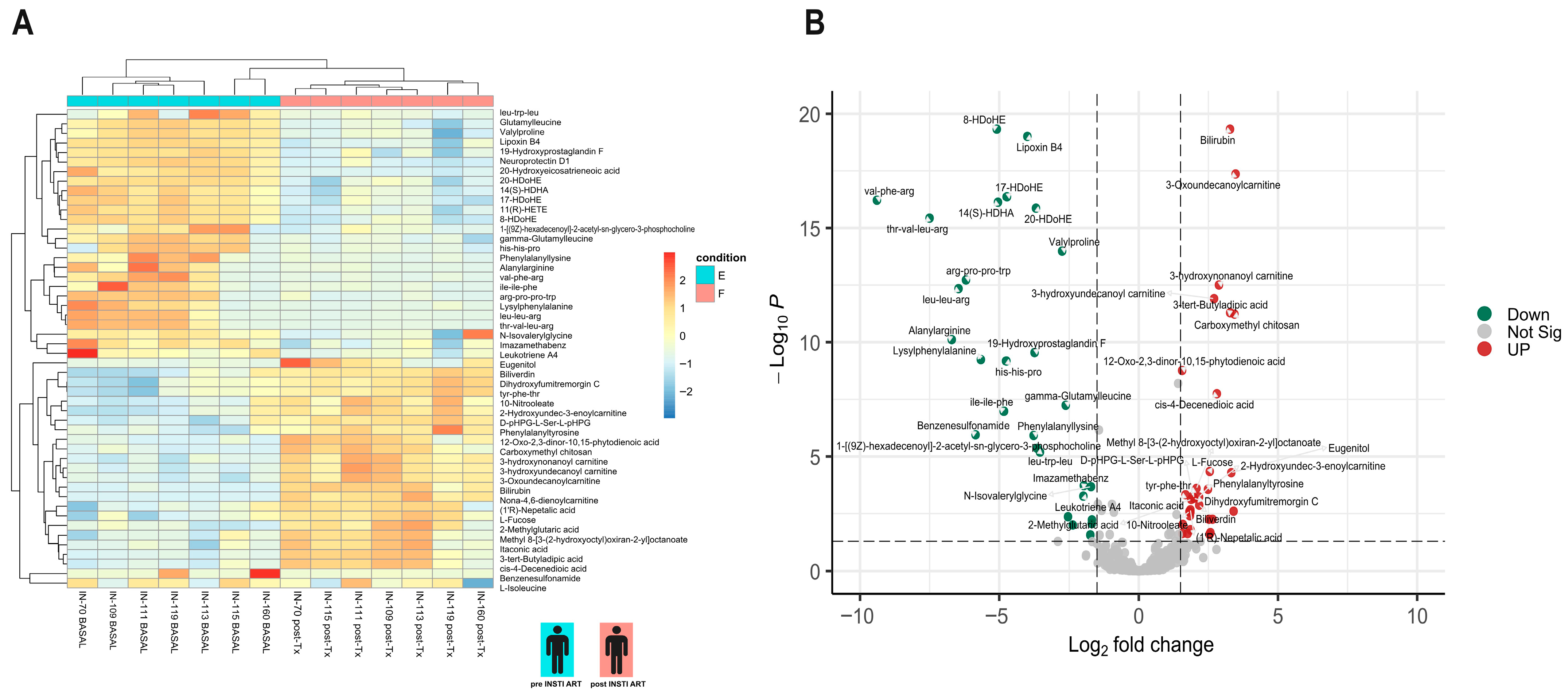
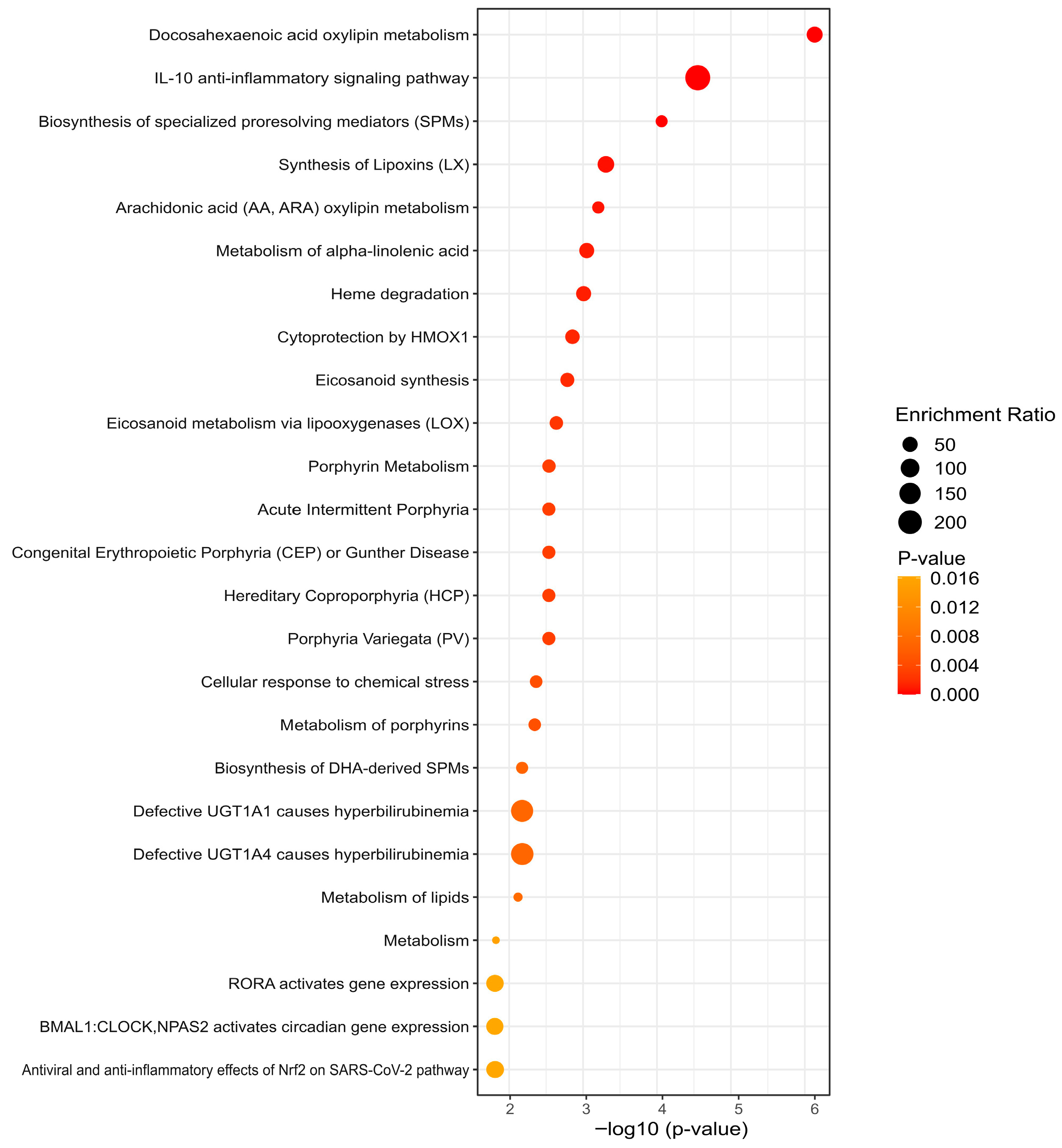
3.5. KEGG Analysis Reveals Divergent Pathway Signatures
4. Discussion
5. Conclusions
5.1. Study Strengths
5.2. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Antiretroviral therapy |
| BCAA | Branched-chain amino acid |
| BIC/TAF/FTC | Bictegravir/tenofovir alafenamide/emtricitabine |
| DHA | Docosahexanoic acid |
| DTG/ABC/3TC | Dolutegravir/abacavir/lamivudine |
| GGT | Gamma-glutamyl transpeptidase |
| INSTI | Integrase strand transfer inhibitors |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| HEV | Hepatitis E Virus |
| HIV | Human Immunodeficiency Virus |
| HMDB | Human Metabolome Database |
| HOMA-IR | Homeostasis Model Assessment for Insulin Resistance |
| LC-MS/MS | Liquid chromatography–mass spectrometry |
| OR | Odds ratio |
| PCA | Principal Component Analysis |
| PWH | People living with HIV |
| RORA | Retinoic Acid-related Orphan Receptor alpha |
| SAT | Subcutaneous adipocyte tissue |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TGP or ALT | Alanine aminotransferase |
| TGO or AST | Aspartate aminotransferase |
| TG | Triglycerides |
| USG | Ultrasonography |
| VAT | Visceral adipose tissue |
| VL | Viral load |
References
- Kumar, S.; Samaras, K. The Impact of Weight Gain During HIV Treatment on Risk of Pre-Diabetes, Diabetes Mellitus, Cardiovascular Disease, and Mortality. Front. Endocrinol. 2018, 9, 705. [Google Scholar] [CrossRef]
- Parra-Rodriguez, L.; Sahrmann, J.M.; Butler, A.M.; Olsen, M.A.; Powderly, W.G.; O’Halloran, J.A. Antiretroviral Therapy and Cardiovascular Risk in People with HIV in the United States-An Updated Analysis. Open Forum Infect. Dis. 2024, 11, ofae485. [Google Scholar] [CrossRef]
- Ursenbach, A.; Sireyjol, A.; Delpierre, C.; Duvivier, C.; Hocqueloux, L.; Rey, D.; Dat’AIDS Study Group. Increased Incidence of Diabetes in People Living with HIV Treated with First-Line Integrase Strand Transfer Inhibitors: A French Multicentre Retrospective Study. HIV Med. 2025, 26, 166–172. [Google Scholar] [CrossRef]
- Venter, W.D.F.; Sokhela, S.; Simmons, B.; Moorhouse, M.; Fairlie, L.; Mashabane, N.; Serenata, C.; Akpomiemie, G.; Masenya, M.; Qavi, A.; et al. Dolutegravir with Emtricitabine and Tenofovir Alafenamide or Tenofovir Disoproxil Fumarate versus Efavirenz, Emtricitabine, and Tenofovir Disoproxil Fumarate for Initial Treatment of HIV-1 Infection (ADVANCE): Week 96 Results from a Randomised, Phase 3, Non-Inferiority Trial. Lancet HIV 2020, 7, e666–e676. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.; Blanco, J.-R.; Muriel, A.; Pérez-Elías, M.J.; Rubio-Martín, R.; Berenguer, J.; Peraire, J.; Bernal, E.; Martínez, O.J.; Serrano-Villar, S.; et al. Weight Changes after Antiretroviral Therapy Initiation in CoRIS (Spain): A Prospective Multicentre Cohort Study. J. Int. AIDS Soc. 2021, 24, e25732. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, K.; Jenkins, C.A.; Rebeiro, P.F.; Palella, F.; Moore, R.D.; Altoff, K.N.; Gill, J.; Rabkin, C.S.; Gange, S.J.; Horberg, M.A.; et al. Weight Gain among Treatment-Naïve Persons with HIV Starting Integrase Inhibitors Compared to Non-Nucleoside Reverse Transcriptase Inhibitors or Protease Inhibitors in a Large Observational Cohort in the United States and Canada. J. Int. AIDS Soc. 2020, 23, e25484. [Google Scholar] [CrossRef] [PubMed]
- Bailin, S.S.; Gabriel, C.L.; Fan, R.; Ye, F.; Nair, S.; Terry, J.G.; Carr, J.J.; Silver, H.; Wanjalla, C.N.; Mashayekhi, M.; et al. Relationship of Subcutaneous Adipose Tissue Inflammation-Related Gene Expression with Ectopic Lipid Deposition in Persons with HIV. J. Acquir. Immune Defic. Syndr. 2022, 90, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Mata Marín, J.A.; Rodríguez Evaristo, M.S.; Cano Díaz, A.L.; Salinas Velázquez, G.E.; Triana González, S.; Chaparro Sánchez, A.; Pompa Mera, E.; Meneses Cisneros, B.; Gaytán Martínez, J.E.; MICTLAN study group. Incidence of Metabolic Syndrome in People with HIV Who Start Dolutegravir Based-Regimen Compared with Bictegravir Based-Regimen after 48 Weeks. AIDS 2025, 39, 1731–1738. [Google Scholar] [CrossRef]
- Gorwood, J.; Bourgeois, C.; Pourcher, V.; Pourcher, G.; Charlotte, F.; Mantecon, M.; Rose, C.; Morichon, R.; Atlan, M.; Le Grand, R.; et al. The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Clin. Infect. Dis. 2020, 71, e549–e560. [Google Scholar] [CrossRef]
- Ngono Ayissi, K.; Gorwood, J.; Le Pelletier, L.; Bourgeois, C.; Beaupère, C.; Auclair, M.; Foresti, R.; Motterlini, R.; Atlan, M.; Barrail-Tran, A.; et al. Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes. Cells 2022, 11, 1841. [Google Scholar] [CrossRef]
- Bailin, S.S.; Ma, S.; Perry, A.S.; Terry, J.G.; Carr, J.J.; Nair, S.; Silver, H.J.; Shi, M.; Mashayekhi, M.; Kropski, J.A.; et al. The Primacy of Adipose Tissue Gene Expression and Plasma Lipidome in Cardiometabolic Disease in Persons with HIV. J. Infect. Dis. 2025, 231, e407–e418. [Google Scholar] [CrossRef]
- Incidence of Metabolic Syndrome in People Living with HIV Without Experience to ART Who Start Dolutegravir-Based Regimen Compared with Bictegravir-Based Regimen. Available online: https://clinicaltrials.gov/study/NCT06629480 (accessed on 12 September 2025).
- Thabane, L.; Ma, J.; Chu, R.; Cheng, J.; Ismaila, A.; Rios, L.P.; Robson, R.; Thabane, M.; Giangregorio, L.; Goldsmith, C.H. A tutorial on pilot studies: The what, why and how. BMC Med. Res. Methodol. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Del Angel-Pablo, A.D.; Juárez-Martín, A.I.; Pérez-Rubio, G.; Ambrocio-Ortiz, E.; López-Flores, L.A.; Camarena, A.E.; Falfán-Valencia, R. HLA Allele and Haplotype Frequencies in Three Urban Mexican Populations: Genetic Diversity for the Approach of Genomic Medicine. Diagnostics 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Campos, O.; Gómez-Velasco, D.V.; Bello-Chavolla, O.Y.; Cruz-Bautista, I.; Melgarejo-Hernandez, M.A.; Muñoz-Hernandez, L.; Guillén, L.E.; Garduño-Garcia, J.D.J.; Alvirde, U.; Ono-Yoshikawa, Y.; et al. Development and Validation of a Predictive Model for Incident Type 2 Diabetes in Middle-Aged Mexican Adults: The Metabolic Syndrome Cohort. BMC Endocr. Disord. 2019, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R. J. 2016, 8, 474. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R Package Version 1.0.7; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 12 September 2025).
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R Package for ’Omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer: New York, NY, USA, 2016. Available online: https://ggplot2.tidyverse.org (accessed on 12 September 2025).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.5-7; R Foundation for Statistical Computing: Vienna, Austria, 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 September 2025).
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Martinez Arbizu, P. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis, R package Version 0.4; GitHub Repository: San Francisco, CA, USA, 2020. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 12 September 2025).
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminform 2016, 8, 61. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps, R Package Version 1.0.12; R Foundation for Statistical Computing: Vienna, Austria, 2025. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 12 September 2025).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling, R Package Version 1.8.0; Bioconductor: Seattle, WA, USA, 2025. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 12 September 2025).
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Hill, A.; Tovar Sanchez, T.; Delaporte, E.; Sokhela, S.; Simmons, B.; Kouanfack, C.; Mccann, K.; Levi, J.; Fairhead, C.; Venter, F. Low CD4 Counts Predict Excessive Weight Gains during First-Line Treatment for HIV. J. Antimicrob. Chemother. 2024, 79, 2369–2378. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Wu, K.; Lake, J.E.; Samuels, D.C.; Bares, S.H.; Tassiopoulos, K.; Koethe, J.R.; Brown, T.T.; Leonard, M.; Benson, C.A.; et al. Mitochondrial DNA Haplogroups and Weight Gain Following Switch to Integrase Strand Transfer Inhibitor-Based Antiretroviral Therapy. AIDS 2021, 35, 439–445. [Google Scholar] [CrossRef]
- Leonard, M.A.; Cindi, Z.; Bradford, Y.; Bourgi, K.; Koethe, J.; Turner, M.; Norwood, J.; Woodward, B.; Erdem, H.; Basham, R.; et al. Efavirenz Pharmacogenetics and Weight Gain Following Switch to Integrase Inhibitor-Containing Regimens. Clin. Infect. Dis. 2021, 73, e2153–e2163. [Google Scholar] [CrossRef]
- Grabar, S.; Potard, V.; Piroth, L.; Abgrall, S.; Bernard, L.; Allavena, C.; Caby, F.; De Truchis, P.; Duvivier, C.; Enel, P.; et al. Striking differences in weight gain after cART initiation depending on early or advanced presentation: Results from the ANRS CO4 FHDH cohort. J. Antimicrob. Chemother. 2023, 78, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Odubela, O.O.; Peer, N.; Odunukwe, N.N.; Musa, A.Z.; Salako, B.L.; Kengne, A.P. Weight changes among antiretroviral thera-py-naïve people living with human immunodeficiency virus in Lagos, Nigeria. Front. Public Health 2025, 13, 1545676. [Google Scholar] [CrossRef] [PubMed]
- Garofoli, N.; Flandre, P.; Vincensini, J.P.; Richier, Q.; Couture, P.; Lacombe, K.; Pialoux, G.; Meynard, J.L. Impact on weight of a Doravirine switch in people living with HIV. HIV Med. 2025; in press. [Google Scholar]
- Orkin, C.; Molina, J.M.; Cahn, P.; Lombaard, J.; Supparatpinyo, K.; Kumar, S.; Campbell, H.; Wan, H.; Teal, V.; Jin Xu, Z.; et al. Safety and efficacy of doravirine as first-line therapy in adults with HIV-1: Week 192 results from the open-label extensions of the DRIVE-FORWARD and DRIVE-AHEAD phase 3 trials. Lancet HIV 2024, 11, e75–e85. [Google Scholar] [CrossRef]
- Gianotti, N.; Muccini, C.; Galli, L.; Poli, A.; Spagnuolo, V.; Andolina, A.; Galizzi, N.; Ripa, M.; Messina, E.; Piatti, P.M.; et al. Homeostatic Model Assessment for Insulin Resistance Index Trajectories in HIV-Infected Patients Treated with Different First-Line Antiretroviral Regimens. J. Med. Virol. 2019, 91, 1937–1943. [Google Scholar] [CrossRef]
- Dias da Costa, S.; Bichança, E.; Cruz, F.; Guimarães, A.R.; Piñeiro, C.; Santos-Sousa, H.; Serrão, R.; Freitas, P. Diabetes Mellitus in Patients with HIV Naïve to Antiretroviral Therapy Initiating Integrase Inhibitors Therapy. Sci. Rep. 2025, 15, 20439. [Google Scholar] [CrossRef]
- Engin, A.B. Mechanism of Obesity-Related Lipotoxicity and Clinical Perspective. Adv. Exp. Med. Biol. 2024, 1460, 131–166. [Google Scholar]
- Abdul-Ghani, M.A.; Muller, F.L.; Liu, Y.; Chavez, A.O.; Balas, B.; Zuo, P.; Chang, Z.; Tripathy, D.; Jani, R.; Molina-Carrion, M.; et al. Deleterious Action of FA Metabolites on ATP Synthesis: Possible Link between Lipotoxicity, Mitochondrial Dysfunction, and Insulin Resistance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E678–E685. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Gutaj, P.; Plewa, S.; Spasenenko, I.; Olasińska-Wiśniewska, A.; Krasińska, B.; Tykarski, A.; Krasińska-Plachta, A.; Pilaczyńska-Szcześniak, Ł.; Krasiński, Z.; et al. Obesity and Acylcarnitine Derivates Interplay with Coronary Artery Disease. Sci. Rep. 2025, 15, 15676. [Google Scholar] [CrossRef]
- Wajner, M.; Amaral, A.U. Mitochondrial Dysfunction in Fatty Acid Oxidation Disorders: Insights from Human and Animal Studies. Biosci. Rep. 2015, 36, e00281. [Google Scholar] [CrossRef]
- Guerra, I.M.S.; Ferreira, H.B.; Melo, T.; Rocha, H.; Moreira, S.; Diogo, L.; Domingues, M.R.; Moreira, A.S.P. Mitochondrial Fatty Acid β-Oxidation Disorders: From Disease to Lipidomic Studies-A Critical Review. Int. J. Mol. Sci. 2022, 23, 13933. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.A.; Mentz, R.J.; Nguyen, M.; Green, J.B.; Truby, L.K.; Ilkayeva, O.; Newgard, C.B.; Buse, J.B.; Sourij, H.; Sjöström, C.D.; et al. Mitochondrial Metabolites Predict Adverse Cardiovascular Events in Individuals with Diabetes. JCI Insight 2023, 8, e168563. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Saavedra, D.; Stanford, K.I. Lipid Mediators in Cardiovascular Physiology and Disease. In Cardiovascular Signaling in Health and Disease; Parinandi, N.L., Hund, T.J., Eds.; Springer: Cham, Switzerland, 2022; ISBN 9783031083082. [Google Scholar]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Docosahexaenoic acid,22:6n-3: Its Roles in the Structure and Function of the Brain. Int. J. Dev. Neurosci. 2019, 79, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, J.; Zhu, J.; Sun, X.; He, D.; Li, J.; Cheng, Z.; Zhang, X.; Xu, Y.; Chen, Q.; et al. Gamma-Glutamyl-Leucine Levels Are Causally Associated with Elevated Cardio-Metabolic Risks. Front. Nutr. 2022, 9, 936220. [Google Scholar] [CrossRef]
- Javed, K.; Fairweather, S.J. Amino Acid Transporters in the Regulation of Insulin Secretion and Signalling. Biochem. Soc. Trans. 2019, 47, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Amino Acid Transporters as Modulators of Glucose Homeostasis. Trends Endocrinol. Metab. 2022, 33, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Úbeda, M.; Dash, S.N. Predictors of Powerhouse: A Perspective of Mitochondrial Biomarkers in Type 2 Diabetes. Front. Endocrinol. 2025, 16, 1595557. [Google Scholar] [CrossRef]
- Kohler, J.J.; Lewis, W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ. Mol. Mutagen. 2007, 48, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Walker, U.A.; Bickel, M.; Lütke Volksbeck, S.I.; Ketelsen, U.P.; Schöfer, H.; Setzer, B.; Venhoff, N.; Rickerts, V.; Staszewski, S. Evidence of nucleoside analogue reverse transcriptase inhibitor--associated genetic and structural defects of mitochondria in adipose tissue of HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2002, 29, 117–121. [Google Scholar] [CrossRef]
- Milic, J.; Calza, S.; Cantergiani, S.; Albertini, M.; Gallerani, A.; Menozzi, M.; Barp, N.; Todisco, V.; Renzetti, S.; Motta, F.; et al. Sarcopenic Obesity Phenotypes in Patients with HIV: Implications for Cardiovascular Prevention and Rehabilitation. Can. J. Cardiol. 2023, 39, S359–S367. [Google Scholar] [CrossRef]
- Ortega, R.; Hueso, L.; Benito, E.; Ortega, J.; Civera, M.; Sanz, M.-J.; Real, J.T.; Piqueras, L. The Nuclear Retinoid-Related Orphan Receptor RORα Controls Adipose Tissue Inflammation in Patients with Morbid Obesity and Diabetes. Int. J. Obes. 2021, 45, 1369–1381. [Google Scholar] [CrossRef]
- Ouellette, A.; Allain, E.P.; Almaghraby, A.; Bouhamdani, D.; Ben Amor, M. A Novel RORA Genetic Variant Associated with Early-Onset Obesity and Insomnia. Eur. J. Med. Genet. 2025, 76, 105028. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ascencio-Anastacio, A.M.; Larios-Serrato, V.; Mata-Marín, J.A.; Rodríguez Evaristo, M.; Núñez-Armendáriz, M.; Cano-Díaz, A.L.; Chaparro-Sánchez, A.; Salinas-Velázquez, G.E.; Maldonado-Rodríguez, A.; Torres, J.; et al. Metabolomic Profile of Weight Gain of People Living with HIV Treated with Integrase Strand Transfer Inhibitor Regimens Reveals Dysregulated Lipid Metabolism and Mitochondrial Dysfunction. Metabolites 2025, 15, 695. https://doi.org/10.3390/metabo15110695
Ascencio-Anastacio AM, Larios-Serrato V, Mata-Marín JA, Rodríguez Evaristo M, Núñez-Armendáriz M, Cano-Díaz AL, Chaparro-Sánchez A, Salinas-Velázquez GE, Maldonado-Rodríguez A, Torres J, et al. Metabolomic Profile of Weight Gain of People Living with HIV Treated with Integrase Strand Transfer Inhibitor Regimens Reveals Dysregulated Lipid Metabolism and Mitochondrial Dysfunction. Metabolites. 2025; 15(11):695. https://doi.org/10.3390/metabo15110695
Chicago/Turabian StyleAscencio-Anastacio, Ana Miriam, Violeta Larios-Serrato, José Antonio Mata-Marín, Mara Rodríguez Evaristo, Mireya Núñez-Armendáriz, Ana Luz Cano-Díaz, Alberto Chaparro-Sánchez, Gloria Elizabeth Salinas-Velázquez, Angélica Maldonado-Rodríguez, Javier Torres, and et al. 2025. "Metabolomic Profile of Weight Gain of People Living with HIV Treated with Integrase Strand Transfer Inhibitor Regimens Reveals Dysregulated Lipid Metabolism and Mitochondrial Dysfunction" Metabolites 15, no. 11: 695. https://doi.org/10.3390/metabo15110695
APA StyleAscencio-Anastacio, A. M., Larios-Serrato, V., Mata-Marín, J. A., Rodríguez Evaristo, M., Núñez-Armendáriz, M., Cano-Díaz, A. L., Chaparro-Sánchez, A., Salinas-Velázquez, G. E., Maldonado-Rodríguez, A., Torres, J., García-Flores, M. M., Martínez-Valencia, Z. E., Arroyo-Sánchez, B. I., Olin-Sandoval, V., Minauro, F., Gaytán-Martínez, J. E., & Pompa-Mera, E. N. (2025). Metabolomic Profile of Weight Gain of People Living with HIV Treated with Integrase Strand Transfer Inhibitor Regimens Reveals Dysregulated Lipid Metabolism and Mitochondrial Dysfunction. Metabolites, 15(11), 695. https://doi.org/10.3390/metabo15110695





