Metabolic Signatures of Four Polygonatum Rhizoma Species Mapped Using Untargeted Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.3. Processing and Analysis of UPLC-MS/MS Test Data
2.4. Metabolite Correlation Network Construction
3. Results
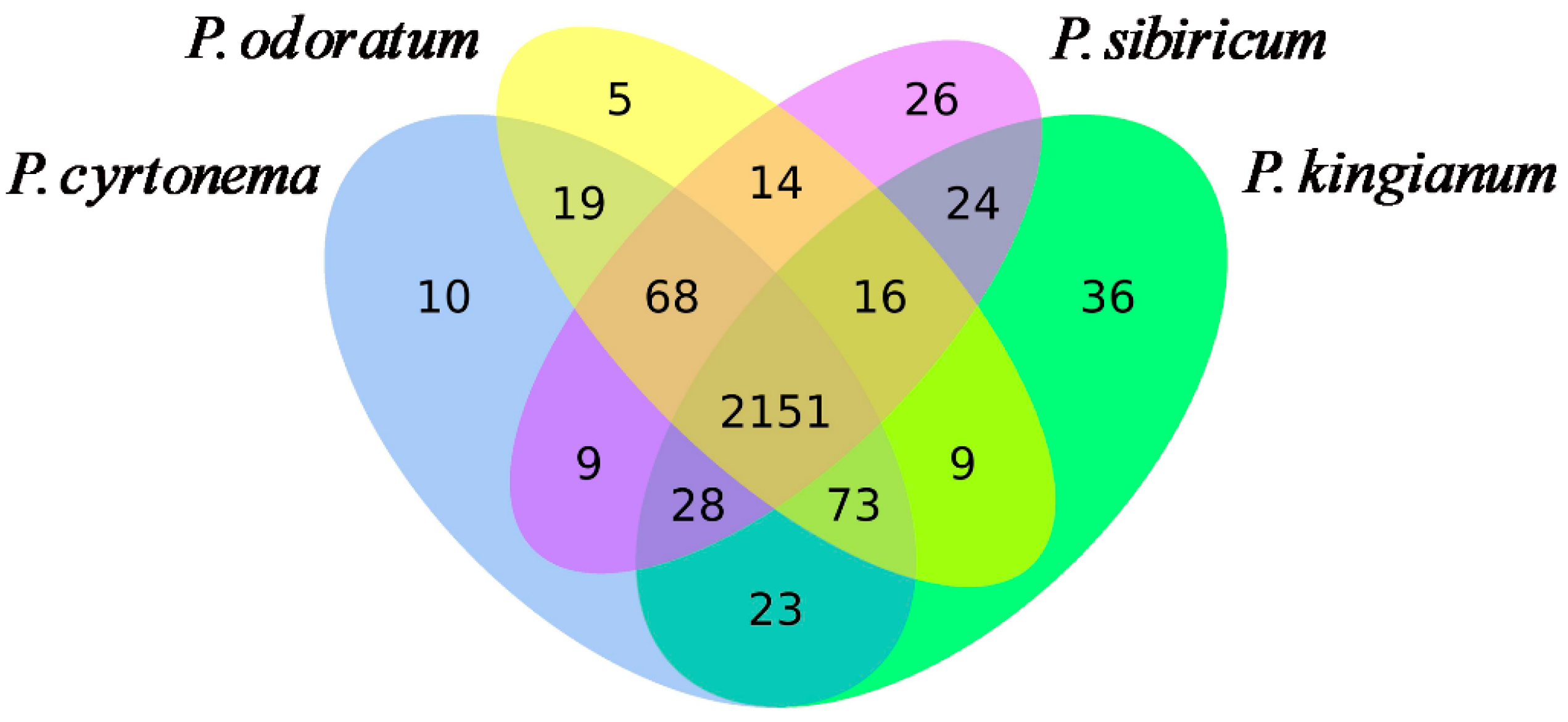
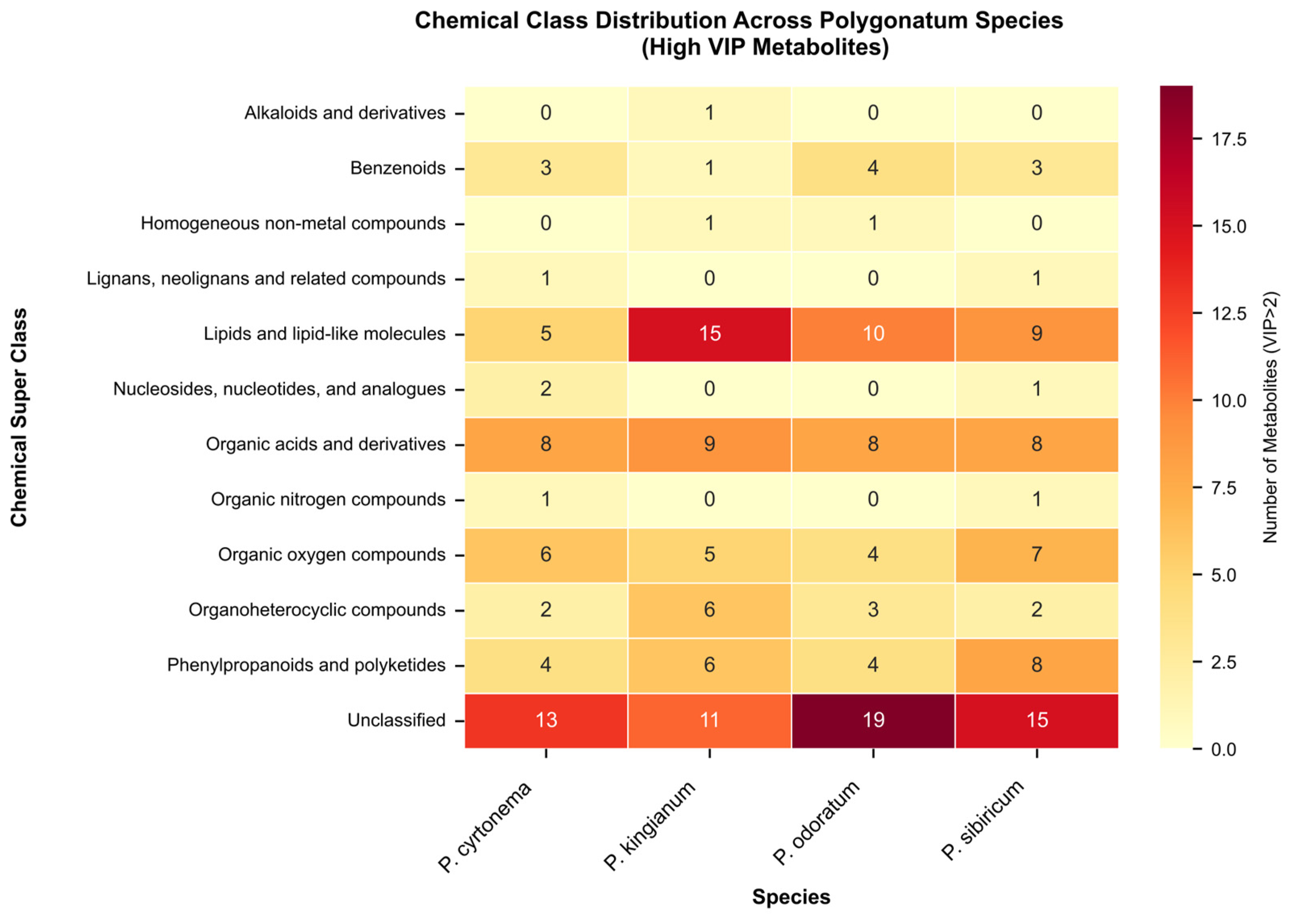
3.1. PK, PS, PC, and PO Metabolic Profiles
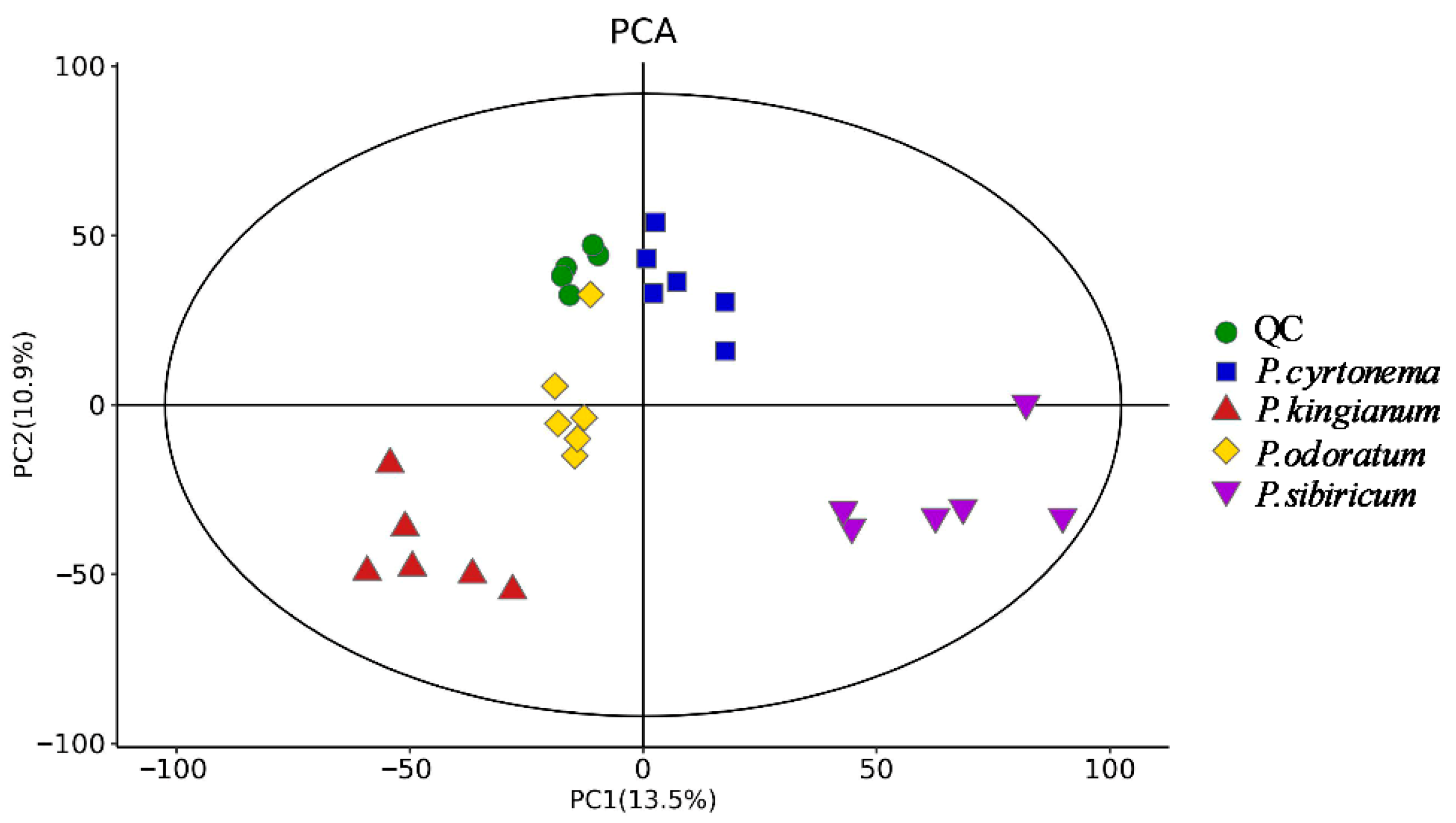
3.2. PK, PS, PC, and PO Differential Metabolites Revealed Through PCA and OPLS-DA
3.3. Characteristic Features of Carbohydrates in PK, PS, PC, and PO
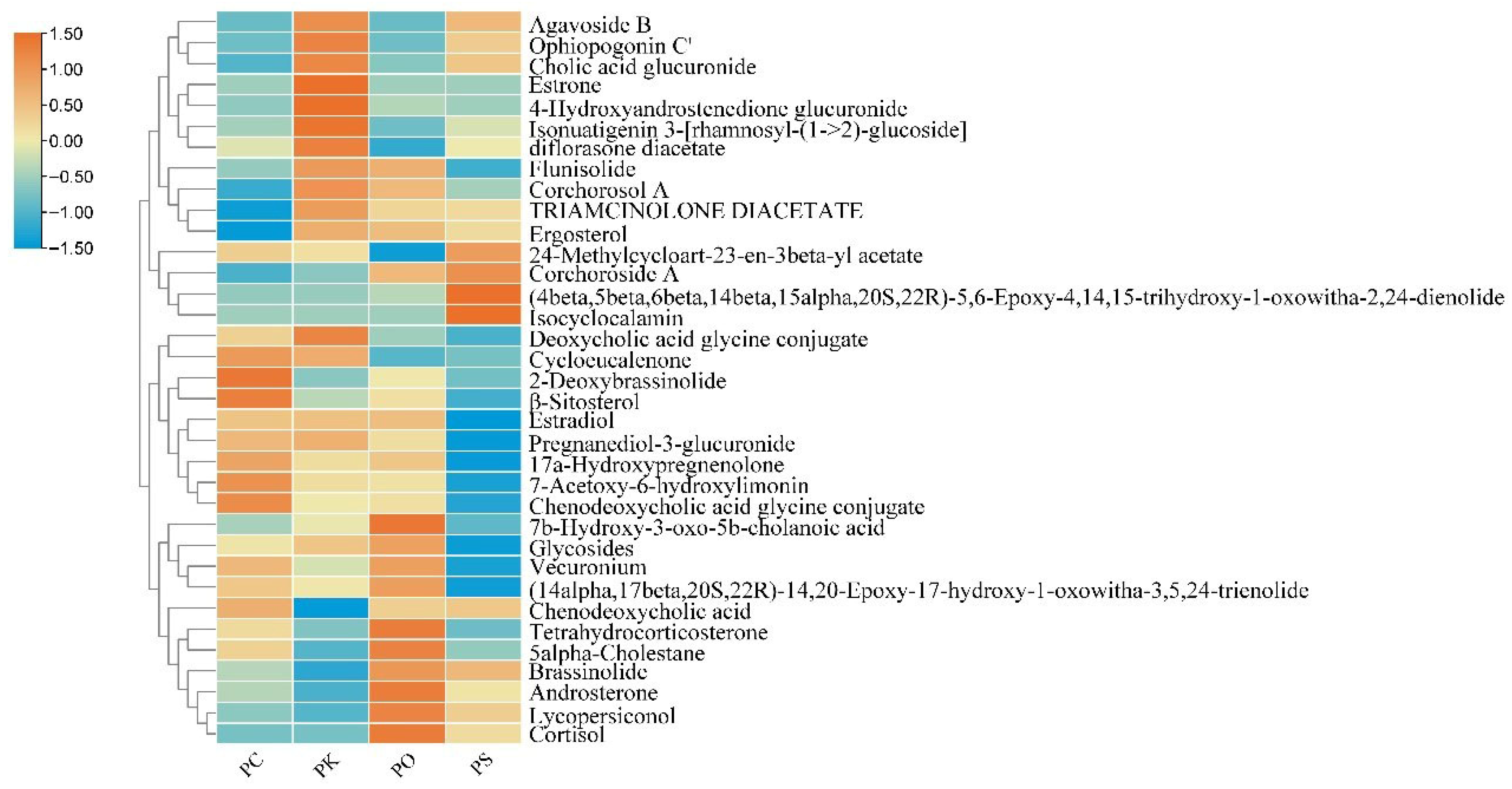
3.4. Characteristic Features of Steroids in PK, PS, PC, and PO
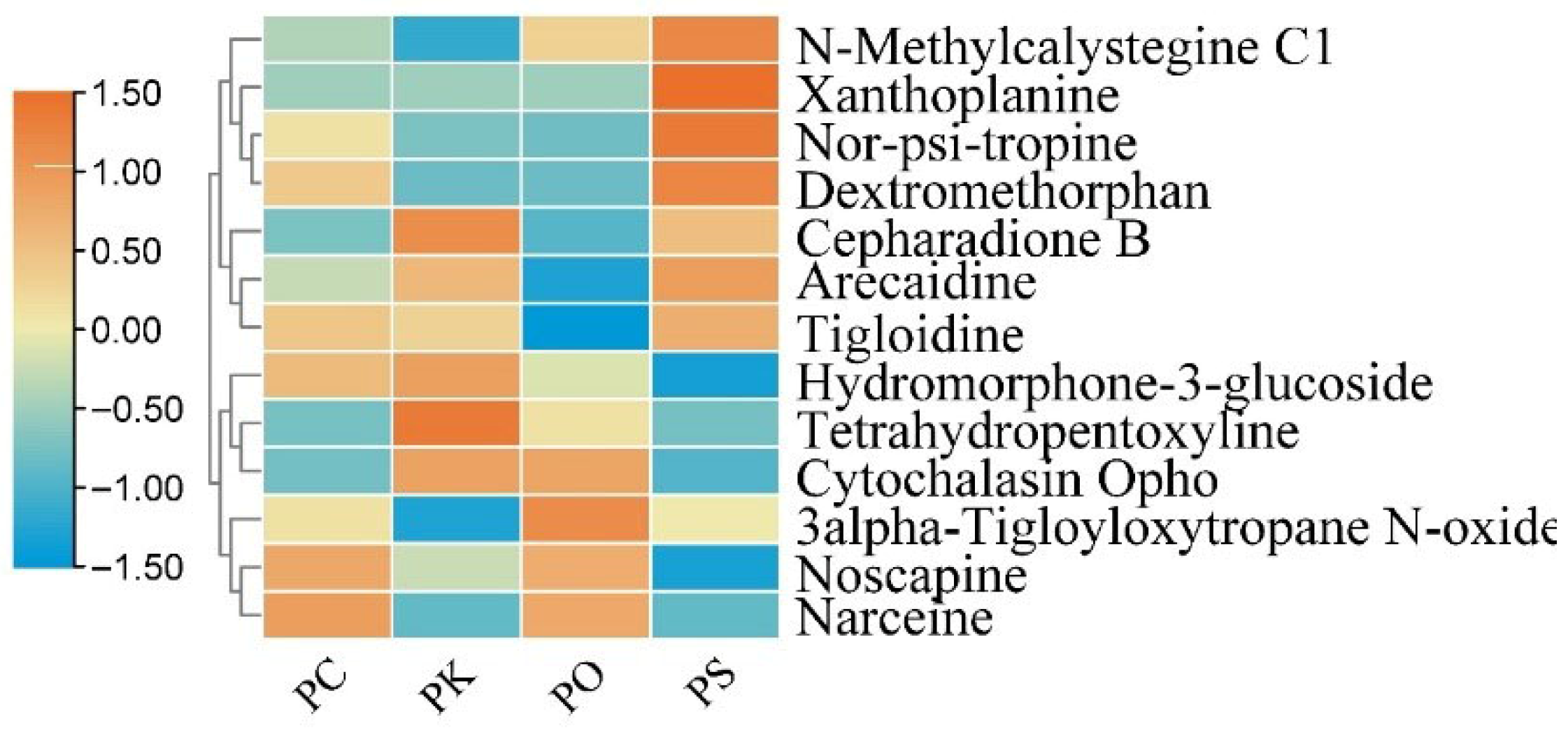
3.5. Characteristic Features of Alkaloids in PK, PS, PC, and PO
3.6. KEGG Enrichment of Metabolites That Differed in PK, PS, PC, and PO
3.7. Species-Specific Diagnostic Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MS | Mass spectrometry |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PC | Polygonatum cyrtonema |
| PCA | Principal component analysis |
| PK | Polygonatum kingianum |
| PLS-DA | Partial least squares discriminant analysis |
| PO | Polygonatum odoratum |
| PR | Polygonati rhizoma |
| PS | Polygonatum sibiricum |
References
- Ma, C.; Chang, H.; Yang, Y.; Sun, Y.; Wang, E. Textual research on genuineness of Shanxi Polygonati rhizoma. Mod. Chin. Med. 2018, 20, 887–898. [Google Scholar]
- He, Y.; Chen, Z.; Nie, X.; Wang, D.; Zhang, Q.; Peng, T.; Zhang, C.; Wu, D.; Zhang, J. Recent advances in polysaccharides from edible and medicinal Polygonati rhizoma: From bench to market. Int. J. Biol. Macromol. 2022, 195, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, C.; Zhou, W.; Li, Y.; Xie, Y.; Hu, H.; Wang, Z. Polygonati rhizoma with the homology of medicine and food: A review of ethnopharmacology, botany, phytochemistry, pharmacology and applications. J. Ethnopharmacol. 2023, 309, 116296. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jiang, J.G. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013, 4, 1727–1741. [Google Scholar] [CrossRef]
- National Pharmacopoeia Commission. National Pharmacopoeia Commission Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Ning, H.; Yuan, M.; Wu, Q.; Ping, Y.; Zhou, Z.; Xu, Y.; Yin, H. Identification of Chemical Constituents from Polygonatum cyrtonema. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 77–82. [Google Scholar]
- Chai, Y.; Luo, J.; Bao, Y. Effects of Polygonatum sibiricum saponin on hyperglycemia, gut microbiota composition and metabolic profiles in type 2 diabetes mice. Biomed. Pharmacother. 2021, 143, 112155. [Google Scholar] [CrossRef]
- Luo, J.; Chai, Y.; Zhao, M.; Guo, Q.; Bao, Y. Hypoglycemic effects and modulation of gut microbiota of diabetic mice by saponin from Polygonatum sibiricum. Food Funct. 2020, 11, 4327–4338. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.L.; Hou, S.B.; Chen, G. Chemical constituents from the rhizomes of Polygonatum sibiricum Red. and anti-inflammatory activity in RAW264.7 macrophage cells. Nat. Prod. Res. 2019, 33, 2359–2362. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J. Chemical constituents of the genus Polygonatum and their role in medicinal treatment. Nat. Prod. Commun. 2015, 10, 683–688. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.Z.; Yang, W.Z.; Yang, M.Q.; Zhang, J.Y. Research progress in chemical constituents in plants of Polygonatum and their pharmacological effects. Zhongguo Zhong Yao Za Zhi 2019, 44, 1989–2008. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Huang, X.; Kong, L. Steroidal saponins from Polygonatum cyrtonema. Chem. Nat. Compd. 2013, 49, 888–891. [Google Scholar] [CrossRef]
- Sun, L.; Li, X. Studies on Chemical Constituents of Rhizome of Polygonatum Sibiricum (II). Chin. Tradit. Herb. Drugs 2001, 32, 586–588. [Google Scholar]
- Li, X.; Lai, G.; Wang, Y.; Zhang, B.; Luo, S. Studies on chemical constituents of rhizome of Polygonatum kingianum (II). Chin. Tradit. Herb. Drugs 2008, 39, 825–828. [Google Scholar]
- Wang, Y.F.; Lu, C.H.; Lai, G.F.; Cao, J.X.; Luo, S.D. A new indolizinone from Polygonatum kingianum. Planta Medica 2003, 69, 1066–1068. [Google Scholar] [CrossRef]
- Son, K.H.; Do, J.C.; Kang, S.S. Isolation of adenosine from the rhizomes of Polygonatum sibiricum. Arch. Pharmacal Res. 1991, 14, 193–194. [Google Scholar] [CrossRef]
- Cao, M.; Zheng, X.; Chen, L. Research progress of Polygonatum sibiricum polysaccharides. China Food Addit. 2008, 52, 52–55. [Google Scholar]
- Liu, L.; Dong, Q.; Dong, X.T.; Fang, J.N.; Ding, K. Structural investigation of two neutral polysaccharides isolated from rhizome of Polygonatum sibiricum. Carbohydr. Polym. 2007, 70, 304–309. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, C.; Xu, D. Extract isolation and structural determination of polysaccharides from rhizome of Polygonatum sibiricum. Food Ferment. Ind. 2010, 036, 79–82. [Google Scholar]
- Wu, Q.; Hu, S.; Yang, G.; Mei, Z. Separation, purification and structural studies of polysaccharides from Polygonatum kingianum. Chem. Ind. For. Prod. 2005, 25, 80–82. [Google Scholar]
- Kun, W.; Yongde, Y.; Feng, T.; Hang, X.; Jia, S.; Jin, W. Sequential Extraction and Structural Analysis of polysaccharides from Polygonatum cyrtonema Hua. Nat. Prod. Res. Dev. 2014, 26, 364–369. [Google Scholar]
- Wang, W.; Zheng, Z.; Chen, J.; Duan, T.; He, H.; Tang, S. Characterization of metabolite landscape distinguishes wild from cultivated Polygonati Rhizomes by UHPLC-Q-TOF-MS untargeted metabolomics. Food Biosci. 2023, 53, 102574. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, L.; Shan, X.; Zhao, T.; Cui, M.; Hao, W.; Wei, B. Untargeted components and in vivo metabolites analyses of Polygonatum under different processing times. LWT-Food Sci. Technol. 2023, 173, 114334. [Google Scholar] [CrossRef]
- Guan, Y.; Liang, Z.; Li, R.; Guo, Y.; Dang, L.; Gong, F.; Xu, S.; Wang, T.; Bo, N.; Yang, S.; et al. Chemical composition and antioxidant activity of Polygonatum kingianum processed by the traditional method of “Nine Cycles of Steaming and Sun-Drying”. Food Chem. X 2024, 22, 101292. [Google Scholar] [CrossRef]
- Jiao, J.; Chen, L.; Sun, R.; Liu, F.; Ma, C.; Liang, Z. Comparison and analysis of the main chemical components of Polygonatum from different origins. J. Chin. Med. Mater. 2016, 39, 519–522. [Google Scholar]
- Li, L.; Liao, B.Y.; Thakur, K.; Zhang, J.G.; Wei, Z.J. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 109, 761–771. [Google Scholar] [CrossRef]
- Li, Y.; Deng, H.; Zhang, P.; Chen, L. Effect of polygonati polysaccharide on glucose metabolism in diabetic mice. Chin. J. Clin. Rehabil. 2005, 9, 90–91. [Google Scholar]
- Gong, H.; Li, W.; Yin, Y.; Li, W. Hypoglycemic activity and mechanism of Polygona-polysaccharide on diabetic rat model. Zhongguo Zhong Yao Za Zhi 2009, 34, 1149–1154. [Google Scholar] [PubMed]
- Duan, H.; Wang, B.; Zhang, Y. Antitumor effects and mechanism of rhizoma polygonati polysaccharide on H22 tumor bearing mice. Tradit. Chin. Drug Res. Clin. Pharmacol. 2014, 25, 5–7. [Google Scholar]
- Zhang, F.; Gao, Q.; Kong, L.; Zhu, Y. Study of the antitumor activity of polygonati polysaccharide. China Pract. Med. 2007, 2, 95–96. [Google Scholar]
- Zeng, G.F.; Zhang, Z.Y.; Lu, L.; Xiao, D.Q.; Xiong, C.X.; Zhao, Y.X.; Zong, S.H. Protective effects of Polygonatum sibiricum polysaccharide on ovariectomy-induced bone loss in rats. J. Ethnopharmacol. 2011, 136, 224–229. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Wagner, J.S.; Roberts, R.M.; Lubahn, D.B. Intraovarian actions of oestrogen. Reproduction 2001, 122, 215–226. [Google Scholar] [CrossRef] [PubMed]
- van den Driesche, S.; Smith, V.M.; Myers, M.; Duncan, W.C. Expression and regulation of oestrogen receptors in the human corpus luteum. Reproduction 2008, 135, 509–517. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Osol, G.; Mandala, M. Maternal uterine vascular remodeling during pregnancy. Physiology 2009, 24, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 2000, 106, 1203–1204. [Google Scholar] [CrossRef]
- Bickel, C.; Rupprecht, H.J.; Blankenberg, S.; Rippin, G.; Hafner, G.; Daunjauer, A.; Hofmann, K.-P.; Meyer, J. Serum uric acid as an independent predictor of mortality in patients with angiographically proven coronary artery disease. Am. J. Cardiol. 2002, 89, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S.; Yang, S.H.; Simpkins, J.W. Neuroprotective effects of phenolic A ring oestrogens. Novartis Found. Symp. 2000, 230, 202–213. [Google Scholar] [CrossRef]
- Gilmore, W.; Weiner, L.P.; Correale, J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol. 1997, 158, 446–451. [Google Scholar] [CrossRef]
- Jansson, L.; Holmdahl, R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm. Res. 1998, 47, 290–301. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2006, 361, 1251–1263. [Google Scholar] [CrossRef]
- Godsland, I.F.; Crook, D.; Simpson, R.; Proudler, T.; Felton, C.; Lees, B.; Anyaoku, M.; Devenport, V.; Wynn, V. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. N. Engl. J. Med. 1990, 323, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Shi, X.; Pan, S.; Li, Y.; Ma, W.; Wang, H.; Xu, C.; Li, L. Xanthoplanine attenuates macrophage polarization towards M1 and inflammation response via disruption of CrkL-STAT5 complex. Arch. Biochem. Biophys. 2020, 683, 108325. [Google Scholar] [CrossRef]
- Mueller, C.; Ness, T.J.; Younger, J.W. Low-dose dextromethorphan for the treatment of fibromyalgia pain: Results from a longitudinal, single-blind, placebo-controlled pilot trial. J. Pain Res. 2021, 14, 189–200. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Zuniga-Munoz, A.M.; Guarner-Lans, V. Beneficial effects of the amino acid glycine. Mini Rev. Med. Chem. 2017, 17, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious beneficial effect of nonessential amino acid, glycine: A review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef]
- Vieira, C.P.; Viola, M.; Carneiro, G.D.; D’Angelo, M.L.; Vicente, C.P.; Passi, A.; Pimentel, E.R. Glycine improves the remodeling process of tenocytes in vitro. Cell Biol. Int. 2018, 42, 804–814. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Dunlop, R.A.; Powell, J.T.; Banack, S.A.; Cox, P.A. L-serine: A naturally occurring amino acid with therapeutic potential. Neurotox. Res. 2018, 33, 213–221. [Google Scholar] [CrossRef] [PubMed]




| Group | Type | R2X (cum) | R2Y (cum) | Q2 (cum) | R2 | Q2 |
|---|---|---|---|---|---|---|
| PS/PO | PCA | 0.509 | ||||
| PS/PO | PLS | 0.732 | 0.996 | 0.969 | ||
| PS/PO | OPLS | 0.732 | 0.996 | 0.968 | 0.824 | −0.375 |
| PC/PO | PCA | 0.562 | ||||
| PC/PO | PLS | 0.681 | 0.996 | 0.87 | ||
| PC/PO | OPLS | 0.681 | 0.996 | 0.859 | 0.903 | −0.389 |
| PK/PO | PCA | 0.557 | ||||
| PK/PO | PLS | 0.619 | 0.996 | 0.946 | ||
| PK/PO | OPLS | 0.619 | 0.996 | 0.923 | 0.898 | −0.39 |
| PS/PK | PCA | 0.594 | ||||
| PS/PK | PLS | 0.699 | 0.997 | 0.924 | ||
| PS/PK | OPLS | 0.699 | 0.997 | 0.912 | 0.906 | −0.296 |
| PC/PK | PCA | 0.561 | ||||
| PC/PK | PLS | 0.636 | 0.998 | 0.938 | ||
| PC/PK | OPLS | 0.636 | 0.998 | 0.909 | 0.908 | −0.329 |
| PS/PC | PCA | 0.58 | ||||
| PS/PC | PLS | 0.7 | 0.995 | 0.883 | ||
| PS/PC | OPLS | 0.7 | 0.995 | 0.884 | 0.854 | −0.324 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, N.; Jiang, J.; Ye, W.; Liu, J. Metabolic Signatures of Four Polygonatum Rhizoma Species Mapped Using Untargeted Metabolomics. Metabolites 2025, 15, 682. https://doi.org/10.3390/metabo15110682
Jia N, Jiang J, Ye W, Liu J. Metabolic Signatures of Four Polygonatum Rhizoma Species Mapped Using Untargeted Metabolomics. Metabolites. 2025; 15(11):682. https://doi.org/10.3390/metabo15110682
Chicago/Turabian StyleJia, Ning, Jinlan Jiang, Wei Ye, and Jiqin Liu. 2025. "Metabolic Signatures of Four Polygonatum Rhizoma Species Mapped Using Untargeted Metabolomics" Metabolites 15, no. 11: 682. https://doi.org/10.3390/metabo15110682
APA StyleJia, N., Jiang, J., Ye, W., & Liu, J. (2025). Metabolic Signatures of Four Polygonatum Rhizoma Species Mapped Using Untargeted Metabolomics. Metabolites, 15(11), 682. https://doi.org/10.3390/metabo15110682




