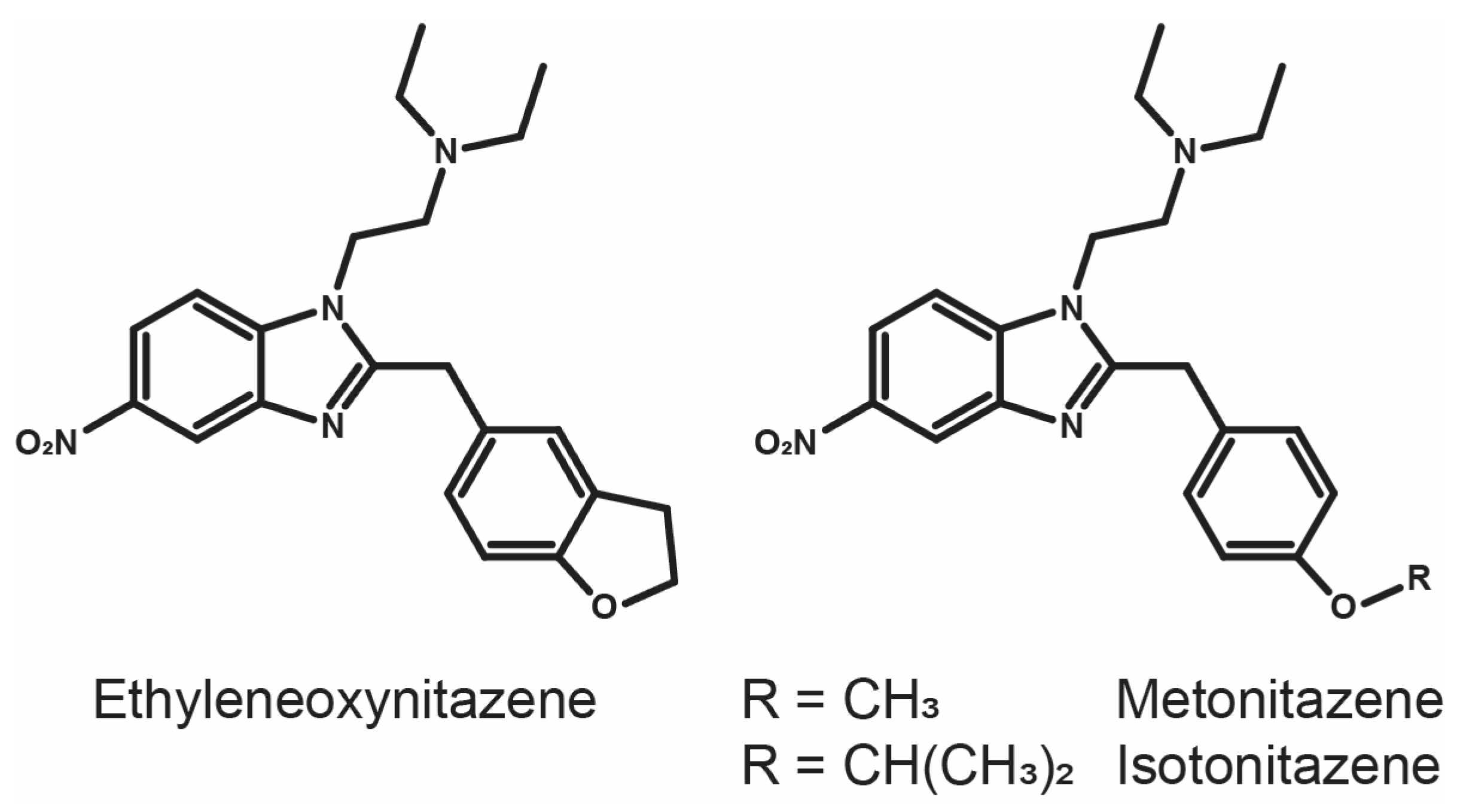
In Vitro Metabolism of a Benzofuran-Substituted Nitazene: Ethyleneoxynitazene
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. In Silico Metabolite Predictions
2.3. Hepatocyte Incubation
2.4. Sample Preparation
2.5. LC-HRMS/MS Analysis
2.5.1. LC Conditions
2.5.2. HRMS/MS Conditions
2.6. Data Mining
3. Results
3.1. In Silico Metabolite Predictions
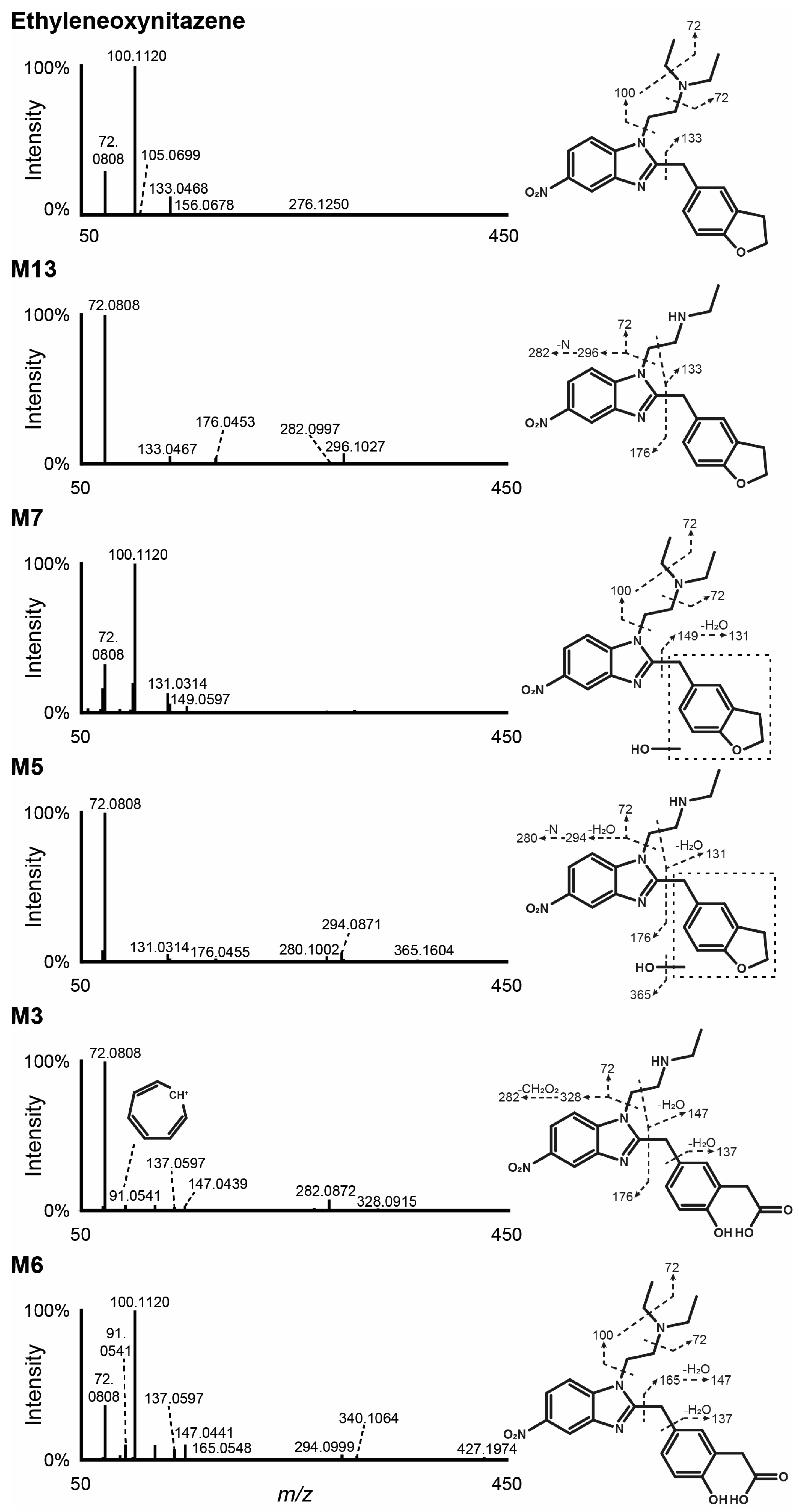
3.2. Ethyleneoxynitazene LC-HRMS/MS Fragmentation
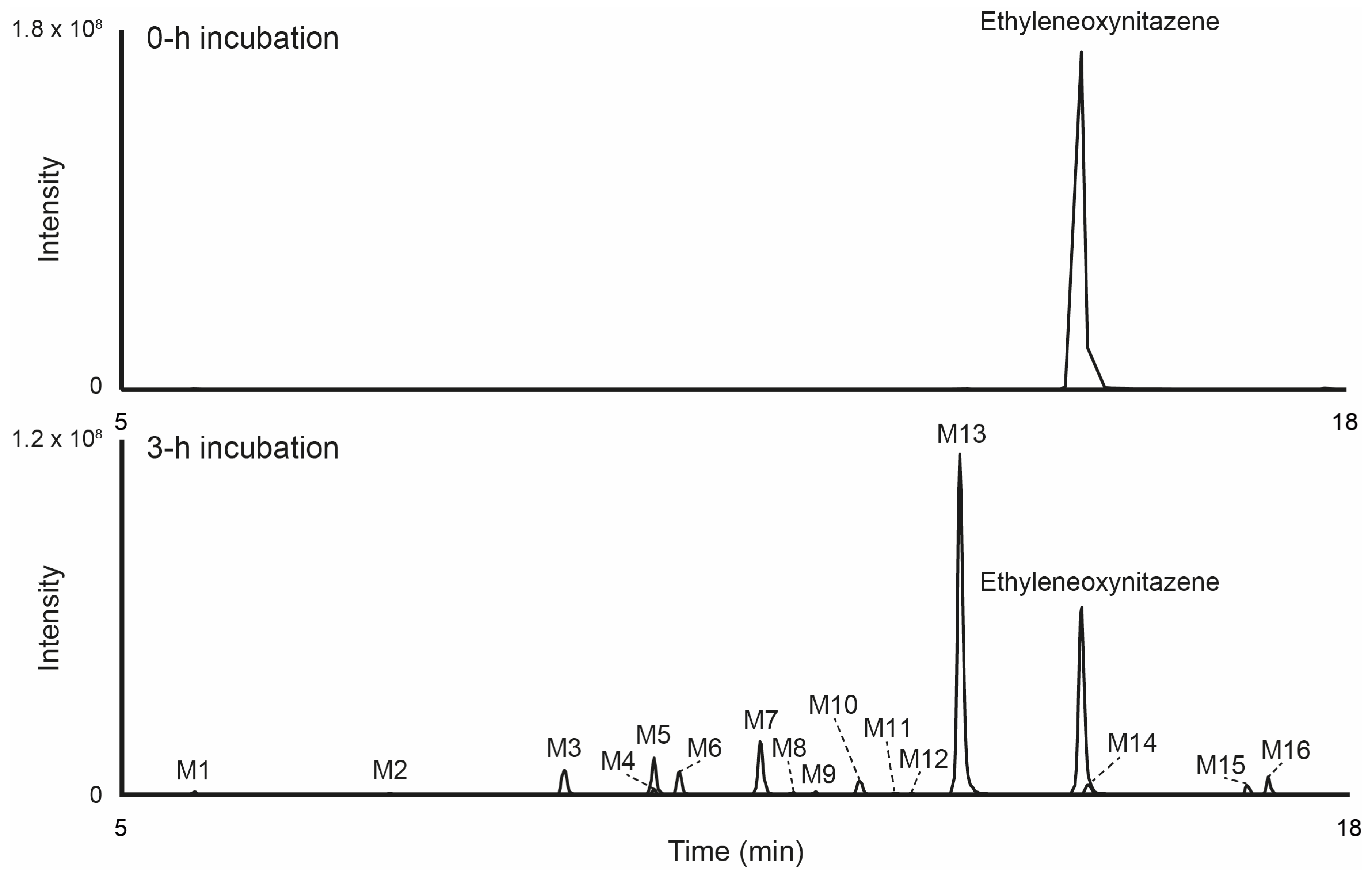
3.3. Metabolite Identification in Hepatocyte Incubations
3.3.1. N-Deethylation
3.3.2. Hydroxylation
3.3.3. Combination of N-Deethylation and Hydroxylation
3.3.4. Combination of Hydroxylation and Oxidative Cleavage
3.3.5. Combination of N-Deethylation, Hydroxylation, and Oxidative Cleavage
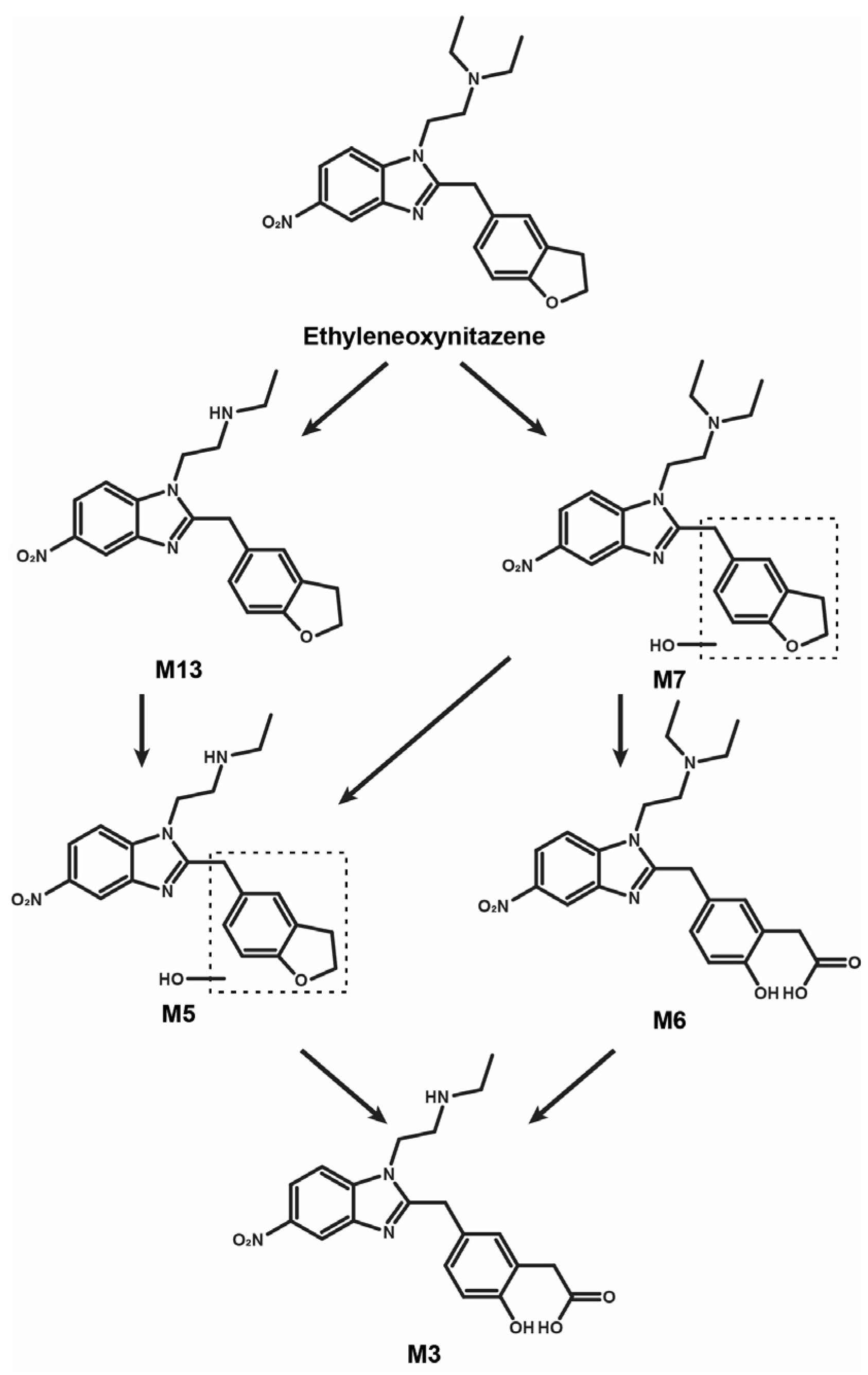
4. Discussion
4.1. Ethyleneoxynitazene Metabolic Pathway
4.2. In Silico Metabolite Predictions
4.3. Metabolite Biomarkers of Ethyleneoxynitazene Consumption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGC | Automatic gain control |
| ddMS2 | Data-dependent tandem mass spectrometry |
| FWHM | full width at half maximum |
| FullMS | Full-scan mass spectrometry |
| HESI | Heated electrospray ionization |
| HRMS/MS | High-resolution tandem mass spectrometry |
| IT | Injection time |
| LC | Liquid chromatography |
| MP | Mobile phase |
| NCE | Normalized collision energy |
| NSO | New synthetic opioid |
| RT | Retention time |
| SMILES | Simplified molecular-input line-entry system |
| sWME | Supplemented Williams’ medium E |
References
- Prekupec, M.P.; Mansky, P.A.; Baumann, M.H. Misuse of novel synthetic opioids: A deadly new trend. J. Addict. Med. 2017, 11, 156–265. [Google Scholar] [CrossRef]
- Gardner, E.A.; McGrath, S.A.; Dowling, D.; Bai, D. The opioid crisis: Prevalence and markets of opioids. Forensic Sci. Rev. 2022, 11, 43–70. [Google Scholar]
- Zawilska, J.B.; Adamowicz, P.; Kurpeta, M.; Wojcieszak, J. Non-fentanyl new synthetic opioids—An update. Forensic Sci. Int. 2023, 349, 111775. [Google Scholar] [CrossRef]
- Juli, A.; Juli, G.; Juli, R.; Juli, L. Fentanyl: New Wave, New Age, New Addiction? Psychiatr. Danub. 2024, 36, 254–256. [Google Scholar]
- Papsun, D.M.; Krotulski, A.J.; Logan, B.K. Proliferation of novel synthetic opioids in postmortem investigations after core-structure scheduling for fentanyl-related substances. Am. J. Forensic Med. Pathol. 2022, 43, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Meng, S.; Shi, J.; Lu, L. Control of fentanyl-related substances in China. Lancet Psychiatry 2019, 6, e15. [Google Scholar] [CrossRef]
- Montanari, E.; Madeo, G.; Pichini, S.; Busardò, F.P.; Carlier, J. Acute intoxications and fatalities associated with benzimidazole opioid (Nitazene Analog) use: A systematic review. Ther. Drug Monit. 2022, 44, 494–510. [Google Scholar] [CrossRef]
- Kozell, L.B.; Eshleman, A.J.; Wolfrum, K.M.; Swanson, T.L.; Schutzer, K.A.; Schutzer, W.E.; Abbas, A.I. Pharmacology of newly identified nitazene variants reveals structural determinants of affinity, potency, selectivity for mu opioid receptors. Neuropharmacology 2025, 276, 110512. [Google Scholar] [CrossRef] [PubMed]
- Glatfelter, G.C.; Vandeputte, M.M.; Chen, L.; Walther, D.; Tsai, M.-H.M.; Shi, L.; Stove, C.P.; Baumann, M.H. Alkoxy chain length governs the potency of 2-benzylbenzimidazole ‘nitazene’ opioids associated with human overdose. Psychopharmacology 2023, 240, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.M.; Tsai, M.-H.M.; Chen, L.; Glatfelter, G.C.; Walther, D.; Stove, C.P.; Shi, L.; Baumann, M.H. Comparative neuropharmacology of structurally distinct non-fentanyl opioids that are appearing on recreational drug markets worldwide. Drug Alcohol Depend. 2023, 249, 109939. [Google Scholar] [CrossRef]
- European Union Drugs Agency (EUDA). Case Report EDND-CR-2023-226. Available online: https://ednd2.emcdda.europa.eu/ednd/report/view/9737 (accessed on 1 August 2025).
- Hunger, A.; Kebrle, J.; Rossi, A.; Hoffmann, K. Synthesis of analgesically active benzimidazole derivatives with basic substitutions. Experientia 1957, 13, 400–401. [Google Scholar] [CrossRef]
- Hunger, A.; Kebrle, J.; Rossi, A.; Hoffmann, K. Benzimidazol-derivate und verwandte heterocyclen VI. Synthese von phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-essigsäure-estern und -amiden. Helv. Chim. Acta 1960, 43, 1727–1733. [Google Scholar] [CrossRef]
- Vandeputte, M.M.; Glatfelter, G.C.; Walther, D.; Ujvary, I.; Iula, D.; Baumann, M.; Stove, C.P. When a prophecy comes true: Ethyleneoxynitazene as a ‘prophetic’ member of the emerging class of 2-benzylbenzimidazole ‘nitazene’ synthetic opioids. Emerg. Trends Drugs Addict. Health 2024, 4, 100129. [Google Scholar] [CrossRef]
- Vandeputte, M.M.; Van Uytfanghe, K.; Layle, N.K.; St Germaine, D.M.; Iula, D.M.; Stove, C.P. Synthesis, chemical characterization, and-opioid receptor activity assessment of the emerging group of nitazene new synthetic opioids. ACS Chem. Neurosci. 2021, 12, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Taoussi, O.; Berardinelli, D.; Zaami, S.; Tavoletta, F.; Basile, G.; Kronstrand, R.; Auwärter, V.; Busardò, F.P.; Carlier, J. Human metabolism of four synthetic benzimidazole opioids: Isotonitazene, metonitazene, etodesnitazene, and metodesnitazene. Arch. Toxicol. 2024, 98, 2101–2116. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, G.R.; Fasinu, P.S. Metabolic characterization of the new benzimidazole synthetic opioids—Nitazenes. Front. Pharmacol. 2024, 15, 1434573. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Papsun, D.M.; Walton, S.E.; Logan, B.K. Metonitazene in the United States—Forensic toxicology assessment of a potent new synthetic opioid using liquid chromatography mass spectrometry. Drug Test. Anal. 2021, 13, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Krotulski, A.J.; Papsun, D.M.; Kacinko, S.L.; Logan, B.K. Isotonitazene Quantitation and Metabolite Discovery in Authentic Forensic Casework. J. Anal. Toxicol. 2021, 44, 521–530. [Google Scholar] [CrossRef]
- Berardinelli, D.; Taoussi, O.; Carlier, J.; Tini, A.; Zaami, S.; Sundermann, T.; Busardò, F.P.; Auwarter, V. In vitro, in vivo metabolism and quantification of the novel synthetic opioid N-piperidinyl etonitazene (etonitazepipne). Clin. Chem. Lab. Med. 2024, 62, 1580–1590. [Google Scholar] [CrossRef]
- Kanamori, T.; Okada, Y.; Segawa, H.; Yamamuro, T.; Kuwayama, K.; Tsujikawa, K.; Iwata, Y.T. Metabolism of highly potent synthetic opioid nitazene analogs: N-ethyl-N-(1-glucuronyloxyethyl) metabolite formation and degradation to N-desethyl metabolites during enzymatic hydrolysis. Drug Test. Anal. 2025, 17, 238–249. [Google Scholar] [CrossRef]
- Magny, R.; Schiestel, T.; M’Rad, A.; Lefrère, B.; Raphalen, J.-H.; Ledochowski, S.; Labat, L.; Mégarbane, B.; Houzé, P. Comparison of the metabolic profiles associated with protonitazene and protonitazepyne in two severe poisonings. Metabolites 2025, 15, 371. [Google Scholar] [CrossRef]
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A comprehensive computational tool for small molecule metabolism prediction and metabolite identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.; Berardinelli, D.; Montanari, E.; Sirignano, A.; Di Trana, A.; Busardò, F.P. 3F-α-pyrrolydinovalerophenone (3F-α-PVP) in vitro human metabolism: Multiple in silico predictions to assist in LC-HRMS/MS analysis and targeted/untargeted data mining. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1193, 123162. [Google Scholar] [CrossRef]
- Di Trana, A.; Brunetti, P.; Giorgetti, R.; Marinelli, E.; Zaami, S.; Busardò, F.P.; Carlier, J. In silico prediction, LC-HRMS/MS analysis, and targeted/untargeted data-mining workflow for the profiling of phenylfentanyl in vitro metabolites. Talanta 2021, 235, 122740. [Google Scholar] [CrossRef]
- De Vrieze, L.M.; Walton, S.E.; Pottie, E.; Papsun, D.; Logan, B.K.; Krotulski, A.J.; Stove, C.P.; Vandeputte, M.M. In vitro structure-activity relationships and forensic case series of emerging 2-benzylbenzimidazole ‘nitazene’ opioids. Arch. Toxicol. 2024, 98, 2999–3018. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime (UNODC). UNODC EWA: Increasing Availability of Nitazenes Calls for Global Response. Available online: https://www.unodc.org/LSS/Announcement/Details/b47cf39e-f557-4001-98a8-536af5673e9e (accessed on 1 August 2025).
- Rietjens, I.M.C.M.; Dussort, P.; Günther, H.; Hanlon, P.; Honda, H.; Mally, A.; O’hagan, S.; Scholz, G.; Seidel, A.; Swenberg, J.; et al. Exposure assessment of process-related contaminants in food by biomarker monitoring. Arch. Toxicol. 2018, 92, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, T.; Okada, Y.; Segawa, H.; Yamamuro, T.; Kuwayama, K.; Tsujikawa, K.; Togawa-Iwata, Y. Detection and confirmation of the ring-opened carboxylic acid metabolite of a new synthetic opioid furanylfentanyl. Forensic Toxicol. 2021, 39, 114–122. [Google Scholar] [CrossRef]
| ID | Biotransformation | Elemental Composition | RT, min | m/z [M+H]+ [M−H]− | Mass Error, (Δppm) | Peak Area in Incubates: [M+H]+ [M−H]− |
|---|---|---|---|---|---|---|
| M1 | Nitro reduction | C22H28N4O | 5.78 | 365.2343 ND | −1.95 - | 3.6 × 106 ND |
| M2 | Nitro reduction + N-Acetylation | C24H30N4O2 | 7.83 | 407.2441 ND | −0.13 - | 1.5 × 106 ND |
| M3 | N-Deethylation + Hydroxylation + Dihydrofuran opening to COOH | C20H22N4O5 | 9.68 | 399.1661 397.1530 | −0.49 3.16 | 3.6 × 107 2.2 × 107 |
| M4 | Hydration | C22H28N4O4 | 10.62 | 413.2179 411.2054 | −1.04 3.94 | 6.5 × 106 2.1 × 106 |
| M5 | N-Deethylation + Hydroxylation | C20H22N4O4 | 10.63 | 383.1712 ND | −0.47 - | 4.8 × 107 ND |
| M6 | Hydroxylation + Dihydrofuran opening to COOH | C22H26N4O5 | 10.91 | 427.1970 425.1841 | −1.40 2.49 | 3.2 × 107 1.8 × 107 |
| M7 | Hydroxylation | C22H26N4O4 | 11.76 | 411.2023 409.1889 | −0.93 1.88 | 7.7 × 107 1.3 × 106 |
| M8 | Hydroxylation + O-Glucuronidation | C28H34N4O10 | 12.11 | 587.2347 585.2219 | −0.12 2.88 | 3.4 × 106 2.9 × 106 |
| M9 | N-Deethylation + Oxidation | C20H20N4O4 | 12.33 | 381.1552 ND | −1.39 - | 3.5 × 106 ND |
| M10 | N-Deethylation + N-Deethylation | C18H18N4O3 | 12.81 | 339.1455 ND | 0.98 - | 2.2 × 107 ND |
| M11 | Hydroxylation + Oxidative deamination | C18H17N3O5 | 13.18 | 356.1245 ND | 1.13 - | 3.3 × 106 ND |
| M12 | Oxidation | C22H24N4O4 | 13.36 | 409.1876 ND | 1.39 - | 2.3 × 106 ND |
| M13 | N-Deethylation | C20H22N4O3 | 13.86 | 367.1759 ND | −1.54 - | 5.1 × 108 ND |
| Parent | Ethyleneoxynitazene | C22H26N4O3 | 15.16 | 395.2073 ND | −1.18 - | 3.1 × 108 ND |
| M14 | N-Deethylation + Desaturation | C20H20N4O3 | 15.24 | 365.1608 ND | −0.05 - | 1.6 × 107 ND |
| M15 | N-Deethylation + Oxidation | C20H20N4O4 | 16.91 | 381.1560 ND | 0.70 - | 1.2 × 107 ND |
| M16 | Oxidative deamination | C18H17N3O4 | 17.13 | 340.1298 ND | 1.81 - | 2.2 × 107 ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taoussi, O.; Ovat, D.Y.; Tavoletta, F.; Tini, A.; Bambagiotti, G.; Carlier, J.; Auwärter, V.; Busardò, F.P.; Berardinelli, D. In Vitro Metabolism of a Benzofuran-Substituted Nitazene: Ethyleneoxynitazene. Metabolites 2025, 15, 679. https://doi.org/10.3390/metabo15100679
Taoussi O, Ovat DY, Tavoletta F, Tini A, Bambagiotti G, Carlier J, Auwärter V, Busardò FP, Berardinelli D. In Vitro Metabolism of a Benzofuran-Substituted Nitazene: Ethyleneoxynitazene. Metabolites. 2025; 15(10):679. https://doi.org/10.3390/metabo15100679
Chicago/Turabian StyleTaoussi, Omayema, Duygu Yeşim Ovat, Francesco Tavoletta, Anastasio Tini, Giulia Bambagiotti, Jeremy Carlier, Volker Auwärter, Francesco Paolo Busardò, and Diletta Berardinelli. 2025. "In Vitro Metabolism of a Benzofuran-Substituted Nitazene: Ethyleneoxynitazene" Metabolites 15, no. 10: 679. https://doi.org/10.3390/metabo15100679
APA StyleTaoussi, O., Ovat, D. Y., Tavoletta, F., Tini, A., Bambagiotti, G., Carlier, J., Auwärter, V., Busardò, F. P., & Berardinelli, D. (2025). In Vitro Metabolism of a Benzofuran-Substituted Nitazene: Ethyleneoxynitazene. Metabolites, 15(10), 679. https://doi.org/10.3390/metabo15100679










