Research on the Changing Characteristics of Milk Composition and Serum Metabolites Across Various Lactation Periods in Xinggao Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animals
2.2. Sample Collection and Index Determination
2.2.1. Milk Composition Analysis
2.2.2. Blood Sample Collection and Serum Metabolomics Determination
2.3. Statistical Analysis
3. Results
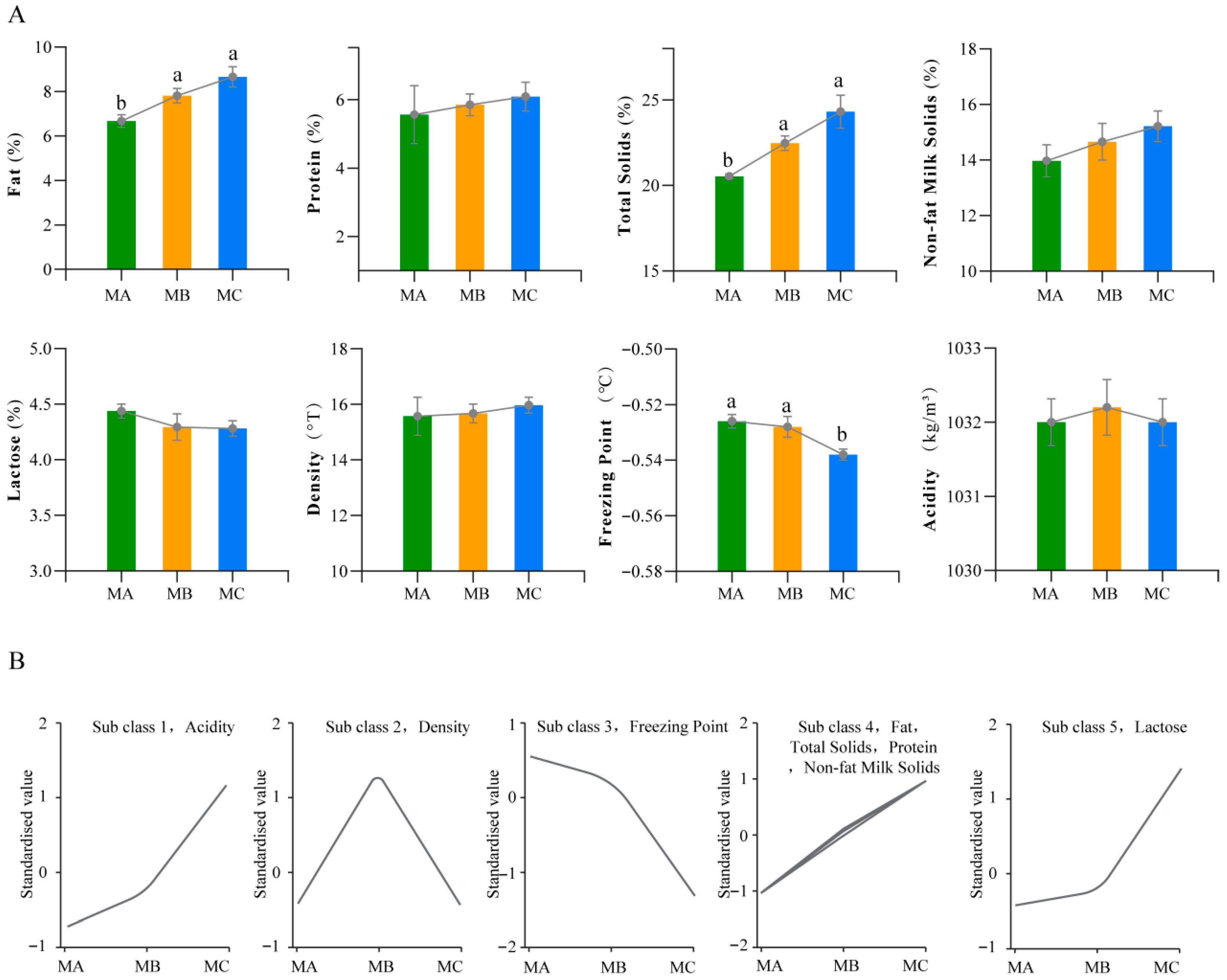
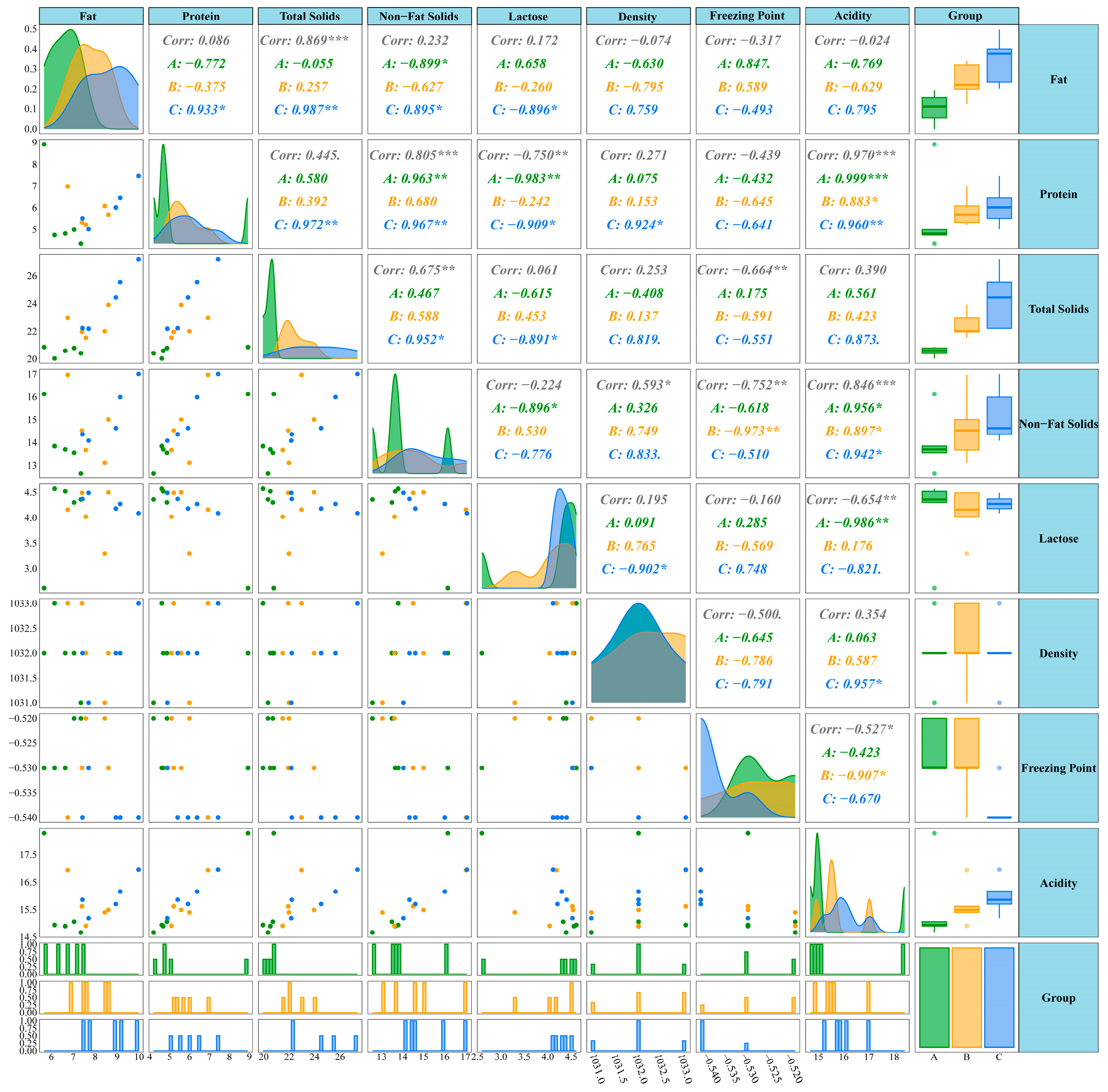
3.1. Changes in Milk Composition at Different Lactation Stages
3.2. PCA
3.3. PLS-DA
3.4. Differential Metabolite Screening
3.5. Classification of Differential Metabolites
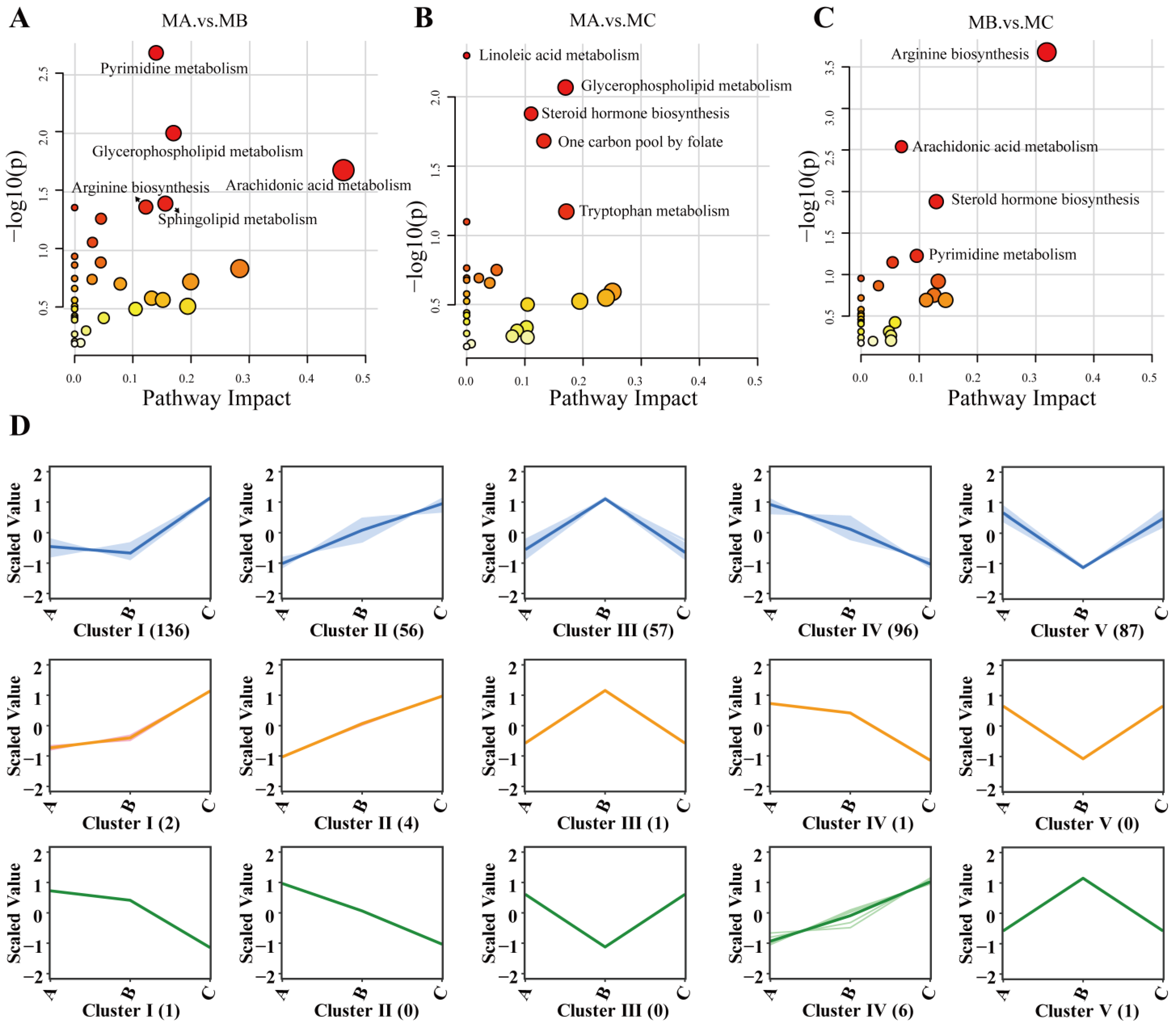
3.6. KEGG Pathway Analysis of Differential Metabolites
3.7. K-Means Clustering Analysis of Differential Metabolites and Milk Components
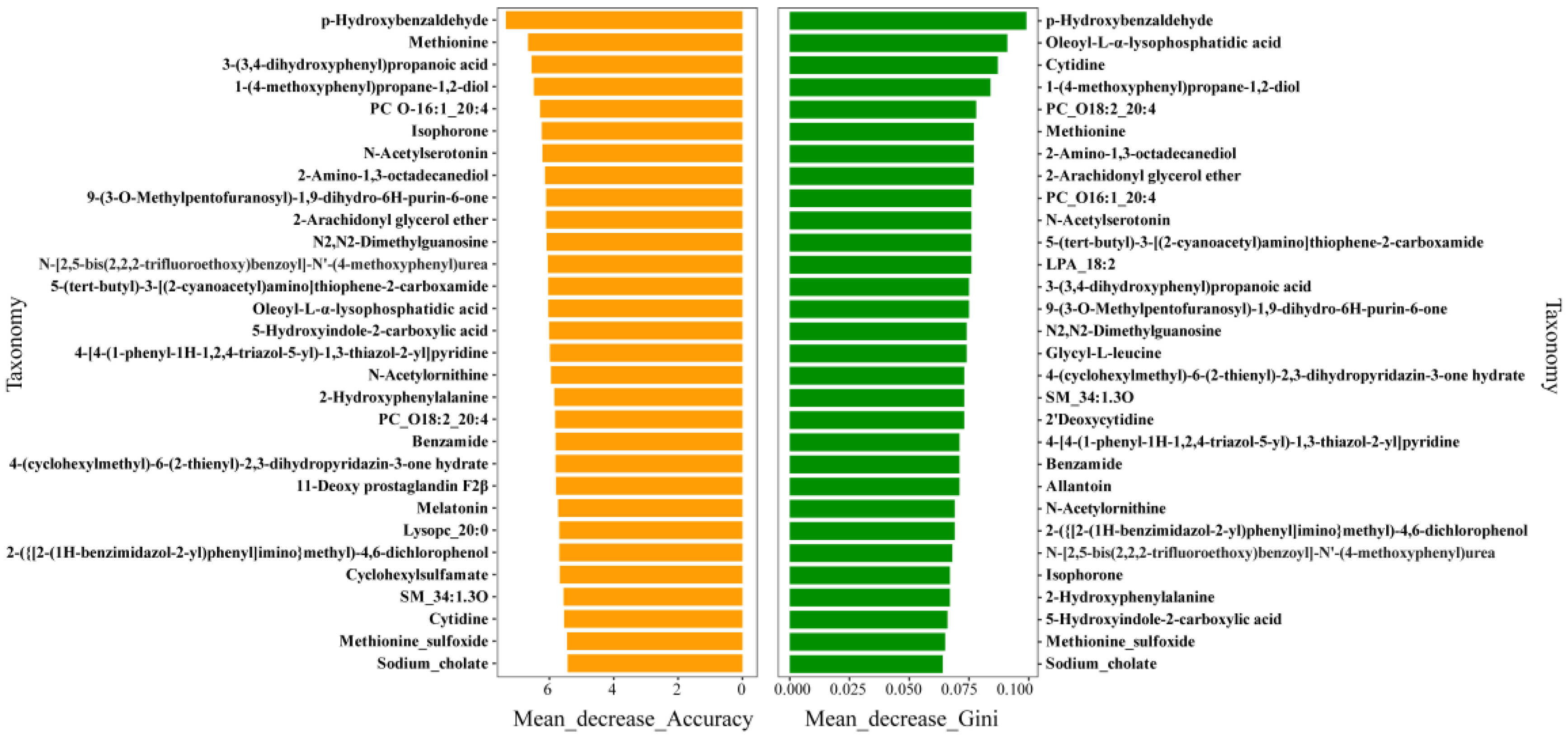
3.8. Random Forest Analysis of Differential Metabolites
4. Discussion
4.1. Changes in Milk Composition During Different Lactation Periods
4.2. Metabolic Changes in Lactating Ewes
4.2.1. Metabolic Differences Between MA and MB
4.2.2. Metabolic Differences Between MA and MC
4.2.3. Metabolic Differences Between MB and MC
4.3. Correlation Between Milk Composition and Serum Metabolome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MA | Early lactation period |
| MB | Mid-lactation period |
| MC | Late lactation period |
| DHEA | Dehydroepiandrosterone |
References
- Agenbag, B.; Swinbourne, A.M.; Petrovski, K.; van Wettere, W.H.E.J. Lambs need colostrum: A review. Livest. Sci. 2021, 251, 104624. [Google Scholar] [CrossRef]
- Rako, A.; Kalit, M.T.; Kalit, S. Effect of sheep’s milk composition on strength and syneresis of rennet-induced milk gel during lactation. Food Technol. Biotechnol. 2019, 57, 426. [Google Scholar] [CrossRef]
- Mestawet, T.A.; Girma, A.; Ådnøy, T.; Devold, T.G.; Narvhus, J.A.; Vegarud, G.E. Milk production, composition and variation at different lactation stages of four goat breeds in Ethiopia. Small Ruminant Res. 2012, 105, 176–181. [Google Scholar] [CrossRef]
- Westhoff, T.A.; Borchardt, S.; Mann, S. Invited review: Nutritional and management factors that influence colostrum production and composition in dairy cows. J. Dairy Sci. 2024, 107, 4109–4128. [Google Scholar] [CrossRef] [PubMed]
- Freitas-de-Melo, A.; Orihuela, A.; Hötzel, M.J.; Ungerfeld, R. What Do We Know and Need to Know About Weaning in Sheep? An Overview of Weaning Practises, Stress and Welfare. Front. Anim. Sci. 2022, 3, 823188. [Google Scholar] [CrossRef]
- Kenéz, Á.; Dänicke, S.; Rolle-Kampczyk, U.; von Bergen, M.; Huber, K. A metabolomics approach to characterize phenotypes of metabolic transition from late pregnancy to early lactation in dairy cows. Metabolomics 2016, 12, 165. [Google Scholar] [CrossRef]
- Li, Q.; Chao, T.; Wang, Y.; Xuan, R.; Guo, Y.; He, P.; Zhang, L.; Wang, J. Comparative metabolomics reveals serum metabolites changes in goats during different developmental stages. Sci. Rep. 2024, 14, 7291. [Google Scholar] [CrossRef]
- Xiang, X.; Chang, H.; Khan, S.; Khan, I.M.; Zhu, F.; Yang, L.; Duan, G.; Li, J.; Nassar, N.; Dai, F. Comparative analysis of milk production, composition, and plasma metabolomics across various dairy goat breeds in Yunnan Province, China. Front. Vet. Sci. 2025, 12, 1552786. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, A.; Lv, X.; Fan, D.; Chen, Q.; Wu, Y.; Zhou, C.; Tan, Z. Combined metabolomics and biochemical analyses of serum and milk revealed parity-related metabolic differences in Sanhe dairy cattle. Metabolites 2024, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Rempel, L.A.; Miles, J.R.; Oliver, W.T.; Broeckling, C.D. Non-targeted plasma metabolome of early and late lactation gilts. Front. Mol. Biosci. 2016, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shen, N.; Wang, S.; Liu, Y.; Peng, P.; Shi, L.; Han, B.; Wang, K.; Sun, D. Integrating transcriptomic and metabolomic profiles of primiparous Holstein cows across multiple lactation periods reveals the regulatory mechanism underlying milk component traits. J. Dairy Sci. 2025, 108, 10377–10390. [Google Scholar] [CrossRef] [PubMed]
- NY/T 2659-2014; Rapid Determination of Fat, Protein, Lactose, and Total Solids in Bovine Milk—Infrared Spectroscopy Method. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2014.
- Zipp, K.A.; Knierim, U. Effects of whole-day versus half-day cow-calf contact on cows’ and calves’ performance. Animal 2024, 18, 101318. [Google Scholar] [CrossRef]
- Barth, K. Effects of suckling on milk yield and milk composition of dairy cows in cow–calf contact systems. J. Dairy Res. 2020, 87, 133–137. [Google Scholar] [CrossRef]
- Ontsouka, C.E.; Bruckmaier, R.M.; Blum, J.W. Fractionized Milk Composition During Removal of Colostrum and Mature Milk. J. Dairy Sci. 2003, 86, 2005–2011. [Google Scholar] [CrossRef]
- Dhaoui, A.; Chniter, M.; Atigui, M.; Dbara, M.; Seddik, M.; Hammadi, M. Factors affecting the milk yield and composition over lactation of prolific D’man ewes in Tunisian oases. Trop. Anim. Health Prod. 2019, 51, 507–518. [Google Scholar] [CrossRef]
- Yilmaz, O.; Çak, B.; Bolacali, M. Effects of lactation stage, age, birth type and body weight on chemical composotion of Red Karaman sheep milk. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 2011, 17, 383–386. [Google Scholar] [CrossRef]
- Zagorska, J.; Ciprovica, I. Evaluation of factors affecting freezing point of milk. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2013, 7, 106–111. [Google Scholar] [CrossRef]
- Erduran, H. Effect of parturition time and photoperiod on milk production, quality, and somatic cell count traits of pure and crossbred goats in a different production system. Trop. Anim. Health Prod. 2023, 55, 145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Tan, J.; Li, L.X.; Wang, Y.; Liu, M.; Jiang, L.S.; Zhao, Y.C. Longitudinal characterization of serum metabolome and lipidome reveals that the ceramide profile is associated with metabolic health in early postpartum cows experiencing different lipolysis. J. Dairy Sci. 2024, 107, 7446–7468. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Tang, G.; Yu, J.; Chen, J.; Li, Z.; Cao, Y.; Lei, X.; Deng, L.; Wu, S.; et al. Multi-omics revealed the long-term effect of ruminal keystone bacteria and the microbial metabolome on lactation performance in adult dairy goats. Microbiome 2023, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Liu, X.; Li, X.; Wang, Z.; Shao, P.; Jiao, T.; He, Y.; Zhao, S. Succession of rumen microbiota and metabolites across different reproductive periods in different sheep breeds and their impact on the growth and development of offspring lambs. Microbiome 2024, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Liu, X.; Pu, X.; He, Y.; Wang, J.; Zhao, S.; Shao, P.; Wang, F.; Xie, Z.; Chen, X.; et al. Characterizing the dynamics of the rumen microbiota, its metabolites, and blood metabolites across reproductive stages in Small-tailed Han sheep. Microbiol. Spectr. 2023, 11, e286723. [Google Scholar] [CrossRef]
- Mohammadi, V.; Anassori, E.; Jafari, S. Measure of energy related biochemical metabolites changes during peri-partum period in Makouei breed sheep. Vet. Res. Forum 2016, 7, 35–39. [Google Scholar]
- El-Sayed, A.A.; Sallam, A.M.; Abou-Soliman, I. Metabolic profile and gene expression pattern of cytokines and antioxidants markers during different physiological stages in Barki ewes. BMC Vet. Res. 2024, 20, 206. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Muscio, A.; Sevi, A. Relationship between welfare and udder health indicators in dairy ewes. Vet. Res. Commun. 2006, 30, 83–94. [Google Scholar] [CrossRef]
- Finucane, K.A.; McFadden, T.B.; Bond, J.P.; Kennelly, J.J.; Zhao, F. Onset of lactation in the bovine mammary gland: Gene expression profiling indicates a strong inhibition of gene expression in cell proliferation. Funct. Integr. Genom. 2008, 8, 251–264. [Google Scholar] [CrossRef]
- Jena, M.K.; Khan, F.B.; Ali, S.A.; Abdullah, A.; Sharma, A.K.; Yadav, V.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Sahoo, A.K.; et al. Molecular complexity of mammary glands development: A review of lactogenic differentiation in epithelial cells. Artif. Cell. Nanomed. Biotechnol. 2023, 51, 491–508. [Google Scholar] [CrossRef]
- Garavito, M.F.; Narvaez-Ortiz, H.Y.; Zimmermann, B.H. Pyrimidine Metabolism: Dynamic and Versatile Pathways in Pathogens and Cellular Development. J. Genet. Genom. 2015, 42, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, M. Metabolomic profiles in yak mammary gland tissue during the lactation cycle. PLoS ONE 2019, 14, e219220. [Google Scholar] [CrossRef]
- RILLEMA, J.A. Characteristics of the prolactin stimulation of uridine metabolism in mammary gland explants. Endocrinology 1975, 96, 1307–1311. [Google Scholar] [CrossRef]
- Wilms, J.N.; Hare, K.S.; Fischer-Tlustos, A.J.; Vahmani, P.; Dugan, M.; Leal, L.N.; Steele, M.A. Fatty acid profile characterization in colostrum, transition milk, and mature milk of primi-and multiparous cows during the first week of lactation. J. Dairy Sci. 2022, 105, 2612–2630. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, F.; Fleith, M.; Goyer, A.; Samuel, T.M.; Elmelegy-Masserey, I.; Fontannaz, P.; Cruz-Hernandez, C.; Thakkar, S.K.; Monnard, C.; De Castro, C.A. Human milk fatty acid composition and its association with maternal blood and adipose tissue fatty acid content in a cohort of women from Europe. Eur. J. Nutr. 2022, 61, 2167–2182. [Google Scholar] [CrossRef] [PubMed]
- Häussler, S.; Sadri, H.; Ghaffari, M.H.; Sauerwein, H. Symposium review: Adipose tissue endocrinology in the periparturient period of dairy cows. J. Dairy Sci. 2022, 105, 3648–3669. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.; Gross, J.J.; van Dorland, A.; Tetens, J.; Thaller, G.; Schlather, M.; Bruckmaier, R.; Simianer, H. Gene-based mapping and pathway analysis of metabolic traits in dairy cows. PLoS ONE 2015, 10, e122325. [Google Scholar] [CrossRef]
- Kumar, P.; Ahmed, M.A.; Abubakar, A.A.; Hayat, M.N.; Kaka, U.; Ajat, M.; Goh, Y.M.; Sazili, A.Q. Improving animal welfare status and meat quality through assessment of stress biomarkers: A critical review. Meat Sci. 2023, 197, 109048. [Google Scholar] [CrossRef]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef]
- Izawa, S.; Sugaya, N.; Shirotsuki, K.; Yamada, K.C.; Ogawa, N.; Ouchi, Y.; Nagano, Y.; Suzuki, K.; Nomura, S. Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biol. Psychol. 2008, 79, 294–298. [Google Scholar] [CrossRef]
- Fustini, M.; Galeati, G.; Gabai, G.; Mammi, L.E.; Bucci, D.; Baratta, M.; Accorsi, P.A.; Formigoni, A. Overstocking dairy cows during the dry period affects dehydroepiandrosterone and cortisol secretion. J. Dairy Sci. 2017, 100, 620–628. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Gaber, S.A.; Mokhemer, S.A.; Kandeel, M.; Sedik, W.F.; Nair, A.B.; Venugopala, K.N.; Khalil, H.E.; Al-Dhubiab, B.E.; Mohamed, M.Z. Pregnenolone inhibits doxorubicin-induced cardiac oxidative stress, inflammation, and apoptosis—Role of matrix metalloproteinase 2 and NADPH oxidase 1. Pharmaceuticals 2023, 16, 665. [Google Scholar] [CrossRef]
- Milivojevic, V.; Charron, L.; Fogelman, N.; Hermes, G.; Sinha, R. Pregnenolone reduces stress-induced craving, anxiety, and autonomic arousal in individuals with cocaine use disorder. Biomolecules 2022, 12, 1593. [Google Scholar] [CrossRef]
- Milivojevic, V.; Sullivan, L.; Tiber, J.; Fogelman, N.; Simpson, C.; Hermes, G.; Sinha, R. Pregnenolone effects on provoked alcohol craving, anxiety, HPA axis, and autonomic arousal in individuals with alcohol use disorder. Psychopharmacologia 2023, 240, 101–114. [Google Scholar] [CrossRef]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Athie, F.; Bachman, K.C.; Head, H.H.; Hayen, M.J.; Wilcox, C.J. Estrogen Administered at Final Milk Removal Accelerates Involution of Bovine Mammary Gland. J. Dairy Sci. 1996, 79, 220–226. [Google Scholar] [CrossRef]
- Agenäs, S.; Lundström, I.; Holtenius, K. The effect of 17β-estradiol on lactose in plasma and urine in dairy cows in late lactation. J. Dairy Res. 2019, 86, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Yart, L.; Finot, L.; Lollivier, V.; Dessauge, F. Oestradiol enhances apoptosis in bovine mammary epithelial cells in vitro. J. Dairy Res. 2013, 80, 113–121. [Google Scholar] [CrossRef]
- Ingram, J.C.; Woolridge, M.W.; Greenwood, R.J.; McGrath, L. Maternal predictors of early breast milk output. Acta. Paediatr. 1999, 88, 493–499. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Gilbreath, K.R.; Posey, E.A.; Sun, Y. L-Arginine nutrition and metabolism in ruminants. In Recent Advances in Animal Nutrition and Metabolism; Springer: New York, NY, USA, 2021; pp. 177–206. [Google Scholar]
- Roets, E.; Verbeke, R.; Massart-Leën, A.; Peeters, G. Metabolism of [14C] citrulline in the perfused sheep and goat udder. Biochem. J. 1974, 144, 435–446. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Sevrin, T.; Sirvins, C.; David, A.; Aguesse, A.; Gandon, A.; Castellano, B.; Darmaun, D.; Boquien, C.; Alexandre-Gouabau, M. Dietary Arginine Supplementation during Gestation and Lactation Increases Milk Yield and Mammary Lipogenesis in Rats. J. Nutr. 2021, 151, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shen, Y.; Jawad, M.; Wu, T.; Maloney, S.K.; Wang, M.; Chen, N.; Blache, D. Effect of arginine supplementation on the production of milk fat in dairy cows. J. Dairy Sci. 2022, 105, 8115–8129. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Wu, Z.; Wang, B.; Fu, S.; Te, R.; Da, L.; Wang, L.; Qin, Q.; He, X. Research on the Changing Characteristics of Milk Composition and Serum Metabolites Across Various Lactation Periods in Xinggao Sheep. Metabolites 2025, 15, 678. https://doi.org/10.3390/metabo15100678
Yuan J, Wu Z, Wang B, Fu S, Te R, Da L, Wang L, Qin Q, He X. Research on the Changing Characteristics of Milk Composition and Serum Metabolites Across Various Lactation Periods in Xinggao Sheep. Metabolites. 2025; 15(10):678. https://doi.org/10.3390/metabo15100678
Chicago/Turabian StyleYuan, Jingda, Zhenbo Wu, Biao Wang, Shaoyin Fu, Rigele Te, Lai Da, Liwei Wang, Qing Qin, and Xiaolong He. 2025. "Research on the Changing Characteristics of Milk Composition and Serum Metabolites Across Various Lactation Periods in Xinggao Sheep" Metabolites 15, no. 10: 678. https://doi.org/10.3390/metabo15100678
APA StyleYuan, J., Wu, Z., Wang, B., Fu, S., Te, R., Da, L., Wang, L., Qin, Q., & He, X. (2025). Research on the Changing Characteristics of Milk Composition and Serum Metabolites Across Various Lactation Periods in Xinggao Sheep. Metabolites, 15(10), 678. https://doi.org/10.3390/metabo15100678




