Differential Effects of Probiotic Strains on Chronic Stress-Exacerbated Colonic Motility in Rats: A Comparative Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Experiment Design
2.3. Physiological Measurements
2.4. Microbiome Analysis
2.5. Tissue Collection and Protein Extraction
2.6. Western Blot Analysis
2.7. Tissue Collection and Fixation
2.8. Immunohistochemistry (IHC) Analysis
2.9. Physiological Measurements
3. Results
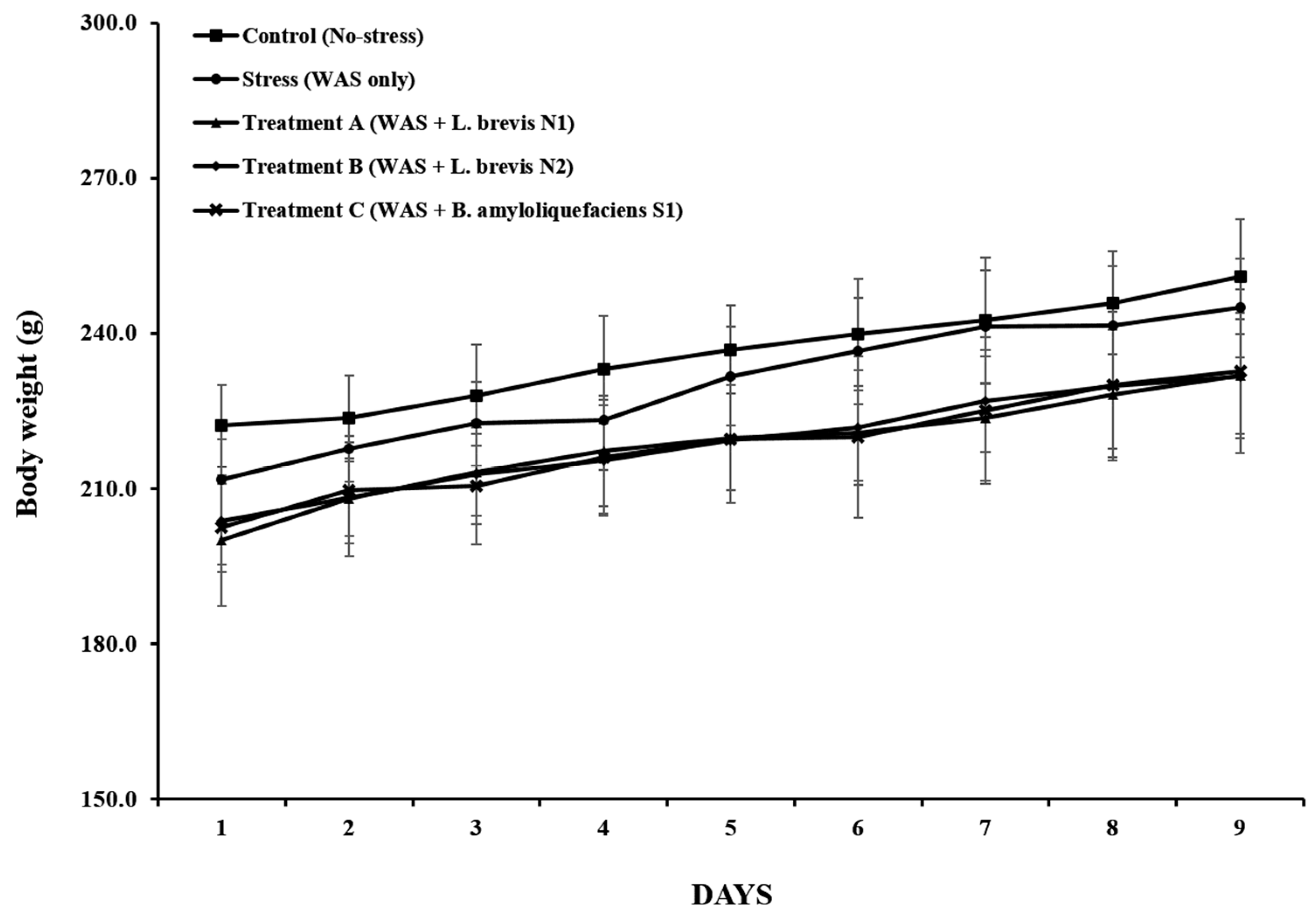
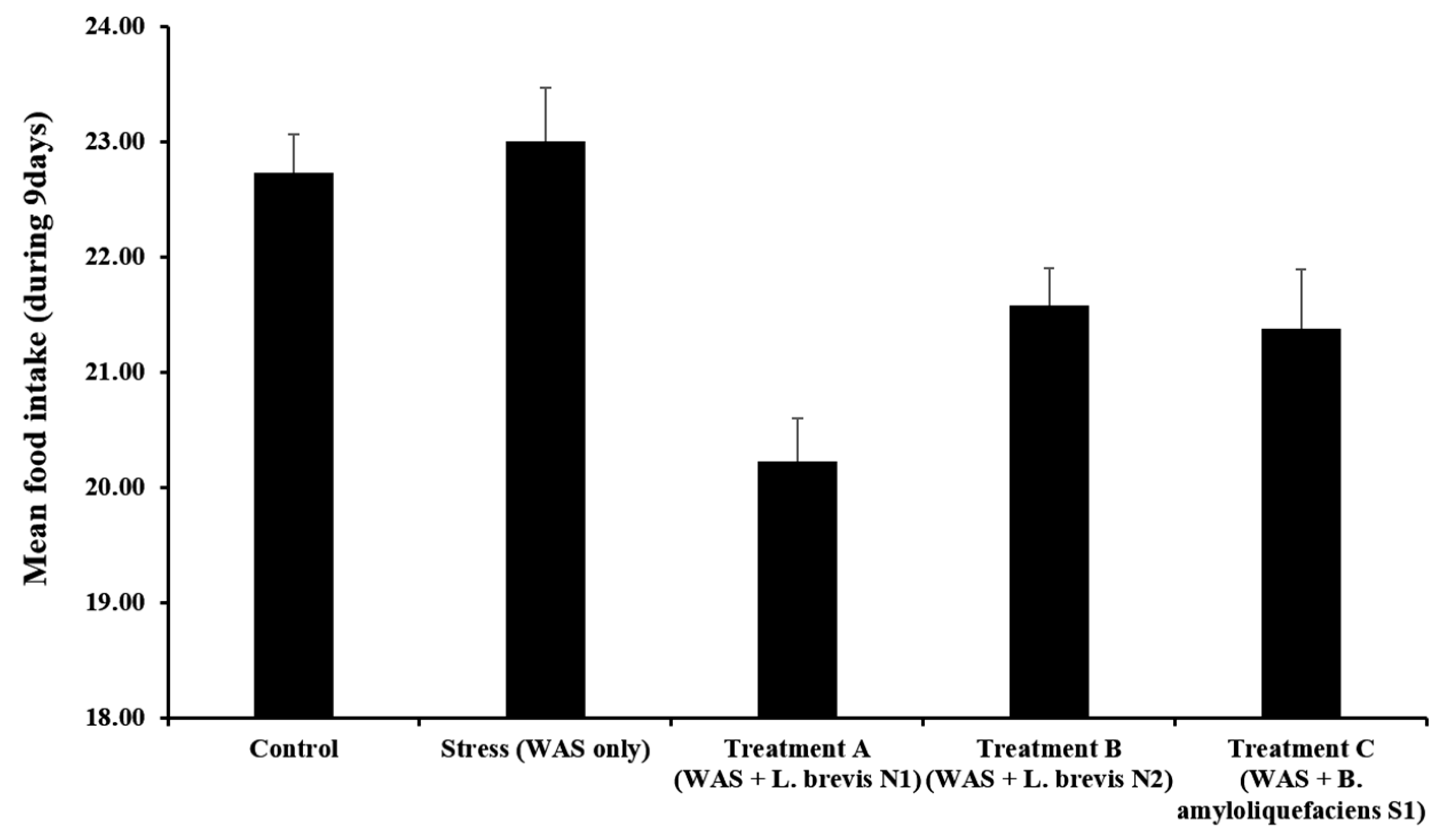
3.1. Body Weight and Food Intake
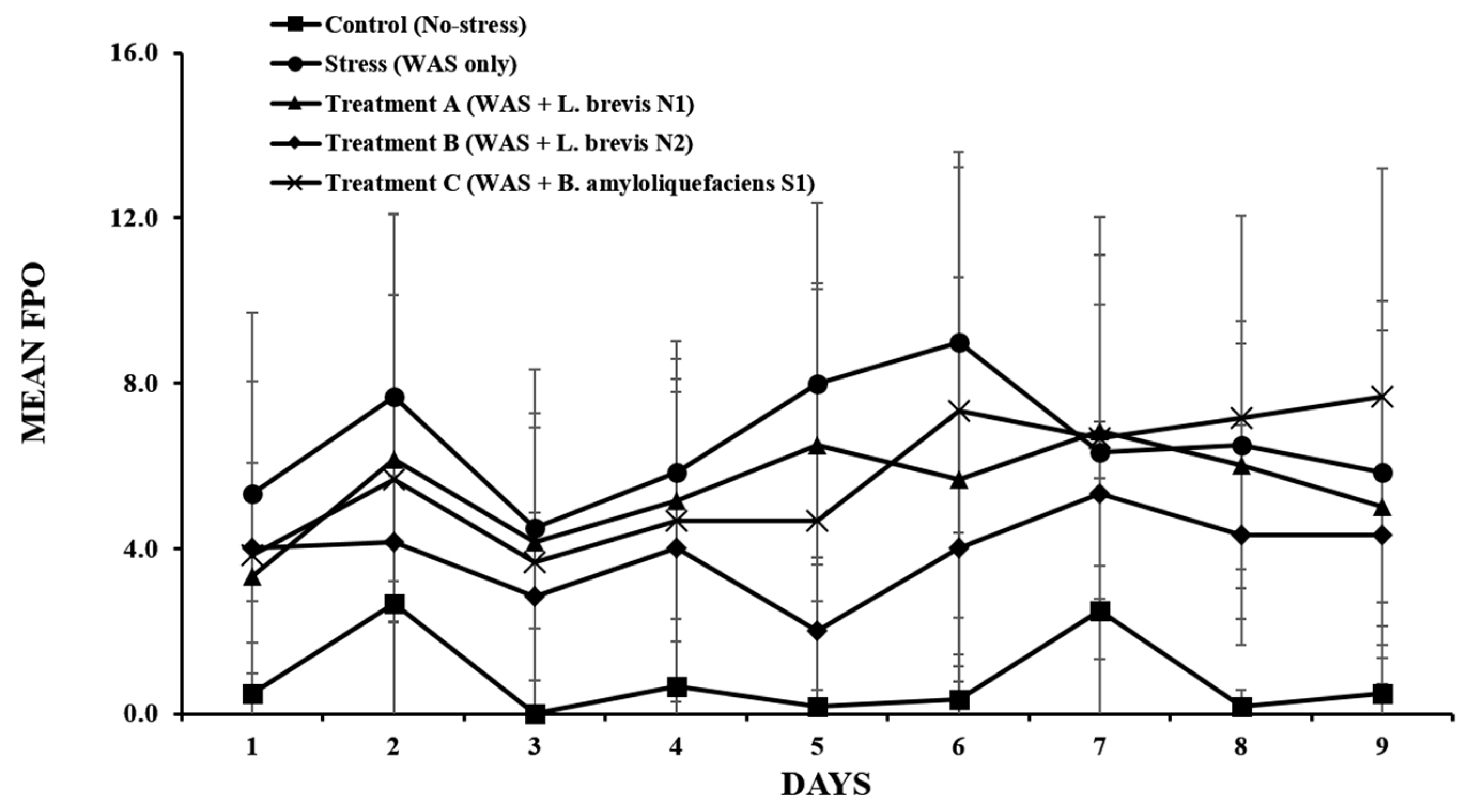
3.2. Daily FPO
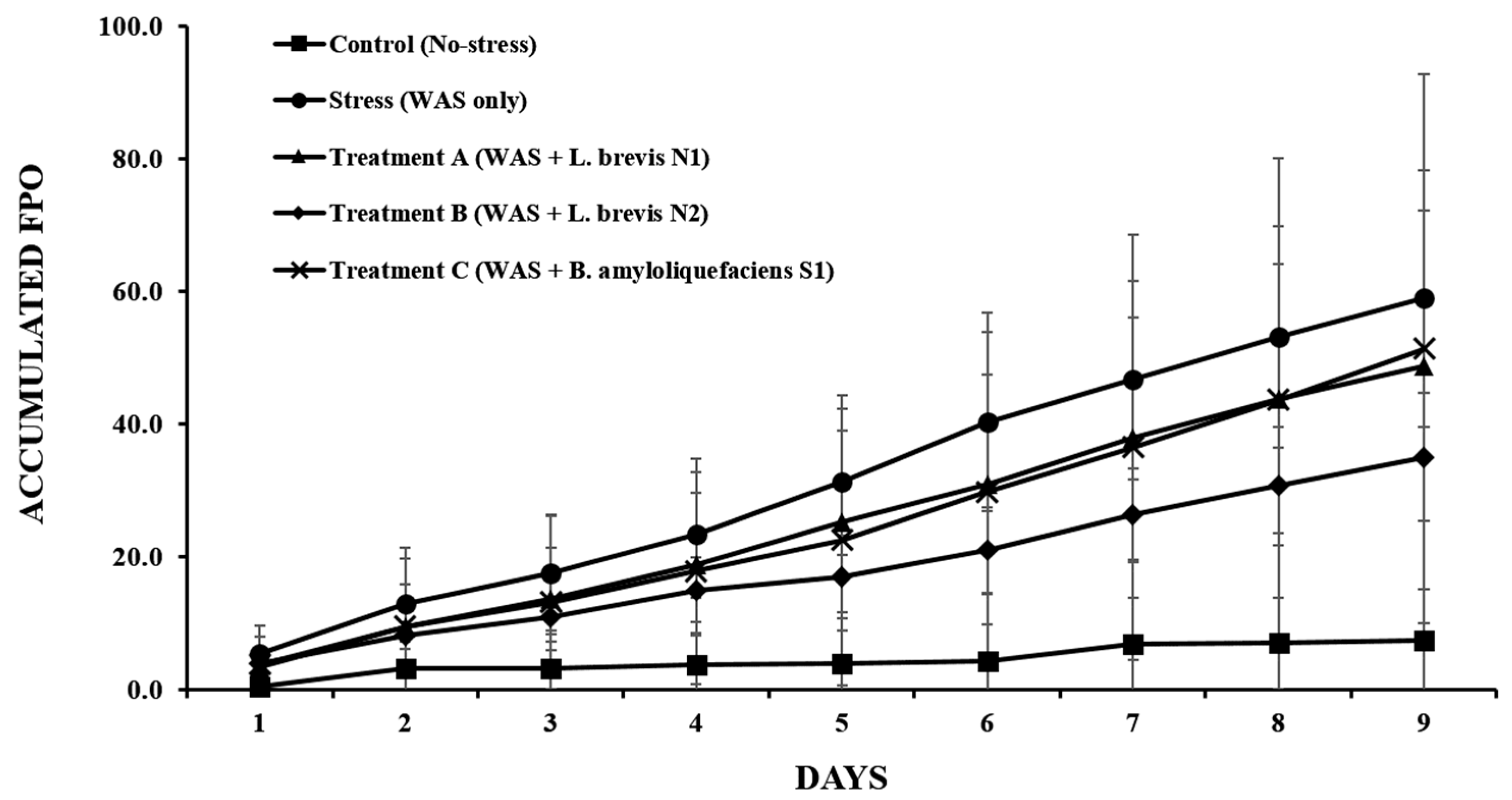
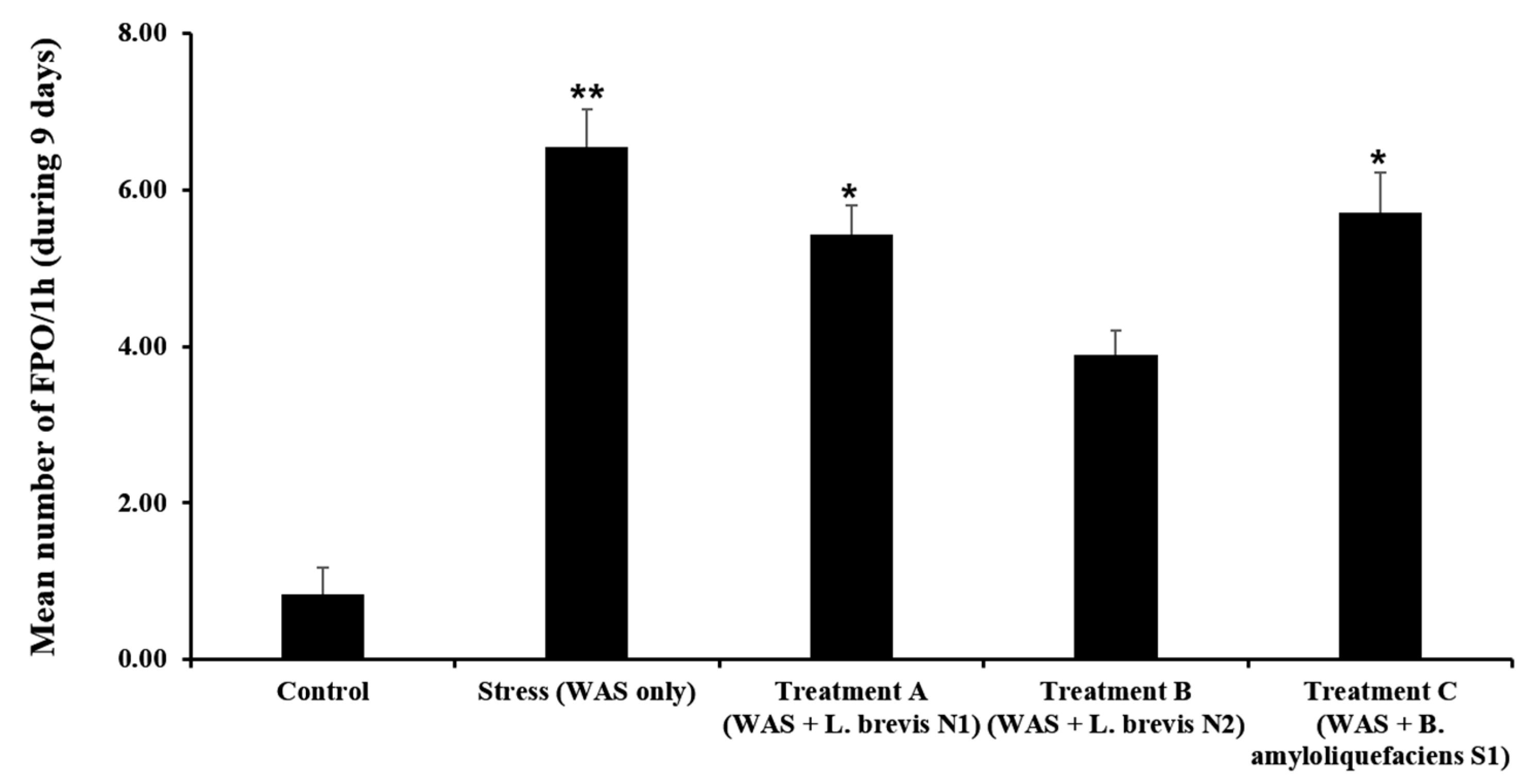
3.3. Accumulated FPO
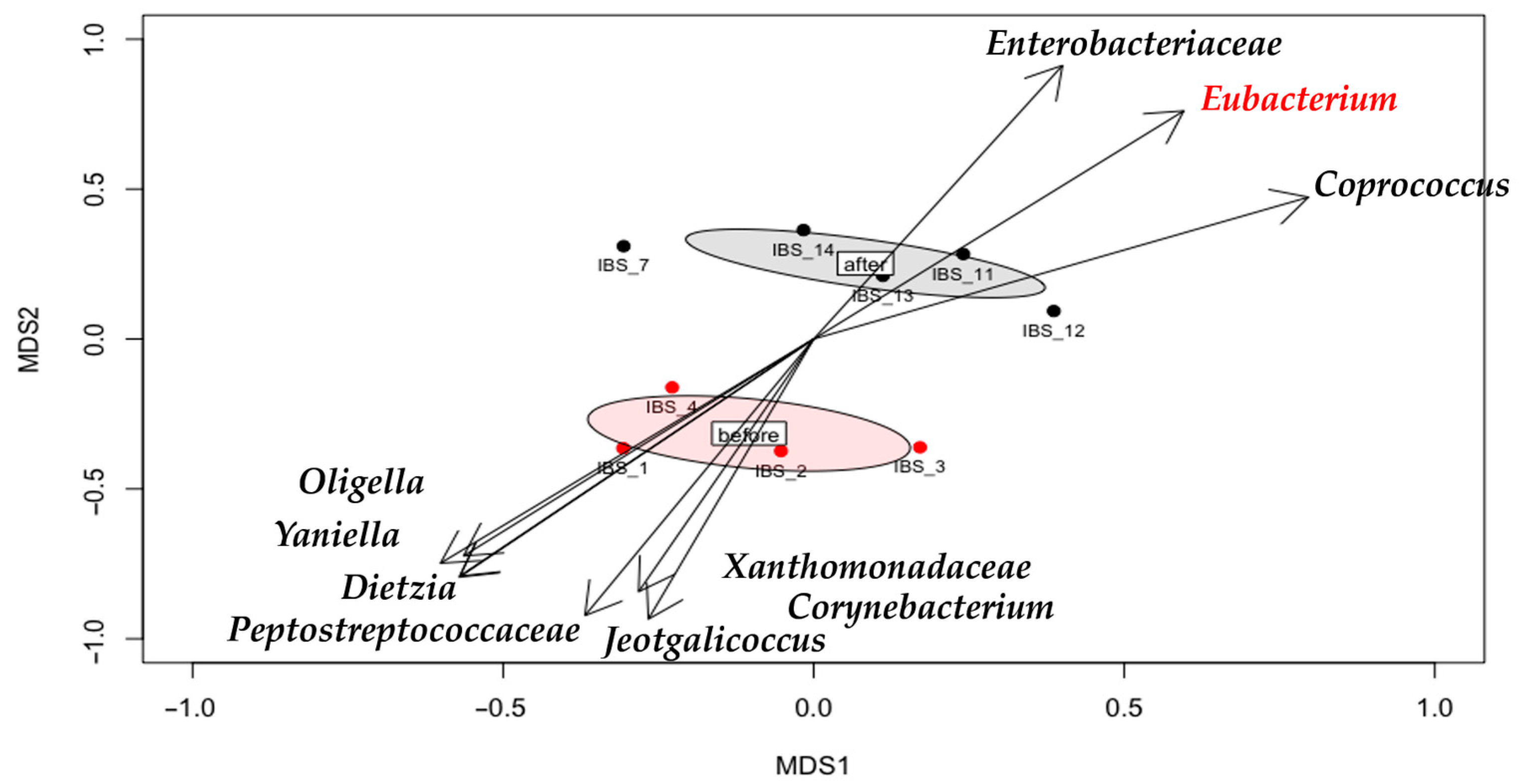
3.4. Gut Microbiota Shifts
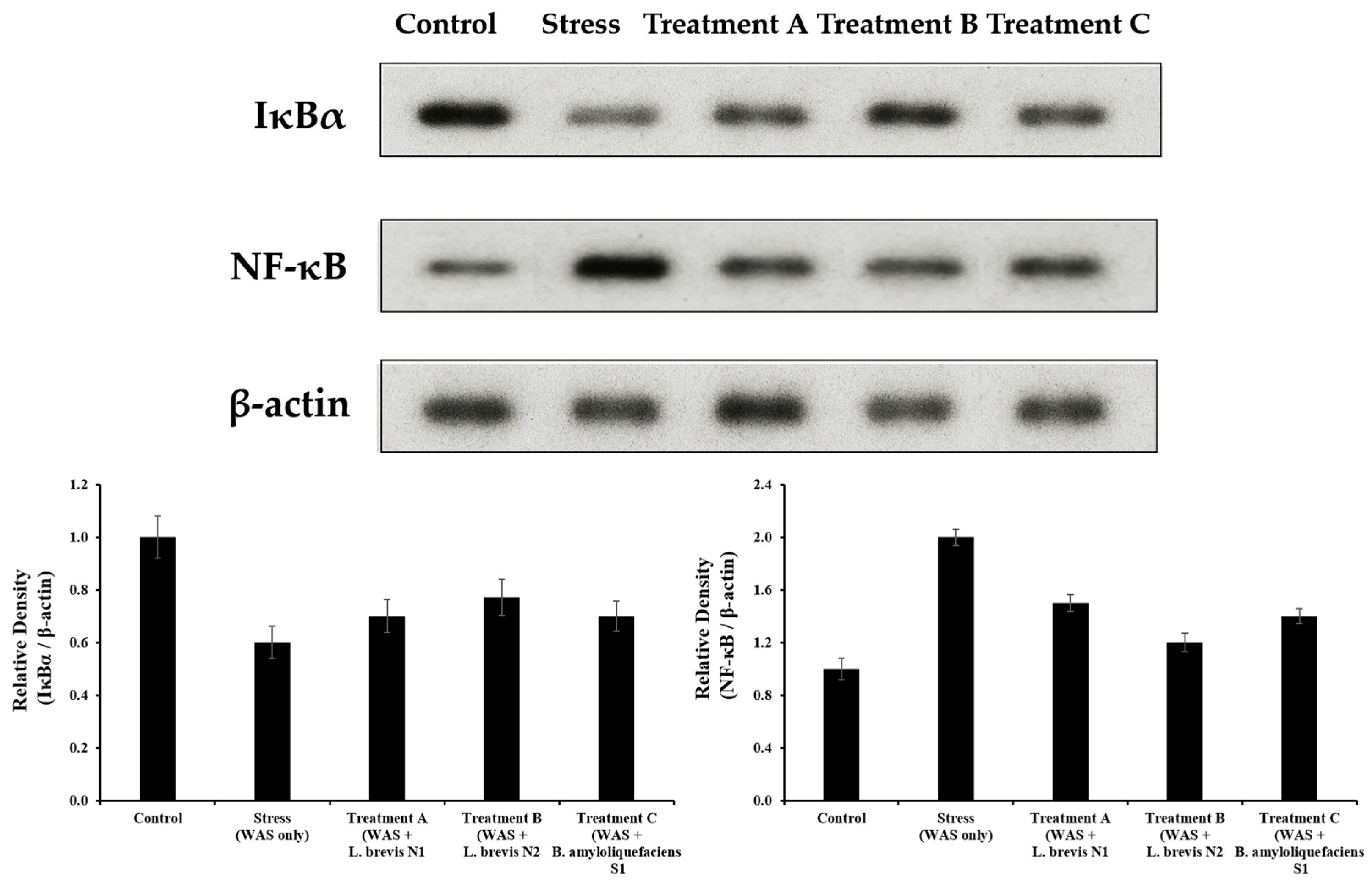
3.5. NF-κB and IκBα Protein Expression
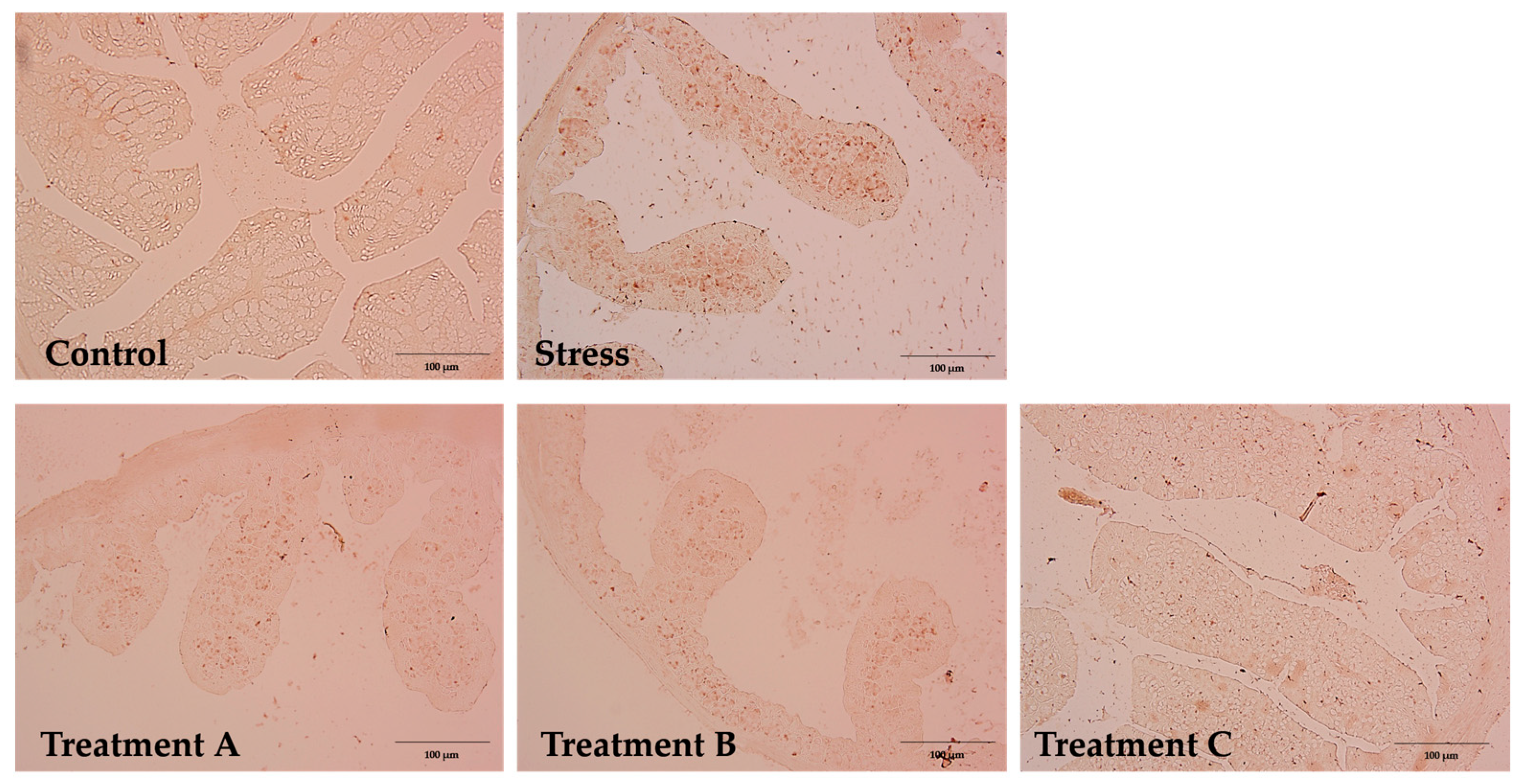
3.6. IHC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drm Drossman, D.A. Functional Gastrointestinal Disorders: Rome IV Update. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Triantos, C. Microbiome Shifts and Their Impact on Gut Physiology in Irritable Bowel Syndrome. Int. J. Mol. Sci. 2024, 25, 12395. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Tache, Y.; Bonaz, B. Corticotropin-Releasing Factor Receptors and Stress-Related Alterations of Gut Motor Function. J. Clin. Investig. 2007, 117, 33–40. [Google Scholar] [CrossRef]
- Bradesi, S.; Schwetz, I.; Ennes, H.S.; Lamy, C.M.; Ohning, G.; Fanselow, M.; Mayer, E.A. Repeated Exposure to Water Avoidance Stress in Rats: A New Model for Sustained Visceral Hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G42–G53. [Google Scholar] [CrossRef]
- Larauche, M.; Mulak, A.; Taché, Y. Stress-Related Alterations of Visceral Sensation: Animal Models for Irritable Bowel Syndrome Study. J. Neurogastroenterol. Motil. 2011, 17, 213–234. [Google Scholar] [CrossRef]
- Hussain, Z.; Kim, H.W.; Huh, C.W.; Lee, Y.J.; Park, H. The Effect of Peripheral CRF Peptide and Water Avoidance Stress on Colonic and Gastric Transit in Guinea Pigs. Yonsei Med. J. 2017, 58, 872–877. [Google Scholar] [CrossRef]
- Ohland, C.L.; Macnaughton, W.K. Probiotic Bacteria and Intestinal Epithelial Barrier Function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of Probiotics in Irritable Bowel Syndrome: Updated Systematic Review with Meta-Analysis. World J. Gastroenterol. 2015, 21, 3072–3084. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Sanders, M.E.; Guarner, F.; Guerrant, R.; Holt, P.R.; Quigley, E.M.M.; Sartor, R.B.; Sherman, P.M.; Mayer, E.A. An Update on the Use and Investigation of Probiotics in Health and Disease. Gut 2013, 62, 787–796. [Google Scholar] [CrossRef]
- Kim, H.J.; Camilleri, M.; McKinzie, S.; Lempke, M.B.; Burton, D.D.; Thomforde, G.M.; Zinsmeister, A.R. A Randomized Controlled Trial of a Probiotic, VSL#3, on Gut Transit and Symptoms in Diarrhoea-Predominant Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2003, 17, 895–904. [Google Scholar] [CrossRef]
- De Palma, G.; Collins, S.M.; Bercik, P. The Microbiota-Gut-Brain Axis in Functional Gastrointestinal Disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef]
- Kwon, J.G.; Park, K.S.; Park, J.H.; Park, J.M.; Park, C.H.; Lee, K.J.; Park, H.J.; Rhee, J.C.; The Korean Society of Neurogastroenterology and Motility. Guidelines for the Treatment of Irritable Bowel Syndrome. Korean J. Gastroenterol. 2011, 57, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterization of Bacillus Probiotics Available for Human Use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Quigley, E.M.M. Probiotics in the Management of Irritable Bowel Syndrome and Inflammatory Bowel Disease. Curr. Opin. Gastroenterol. 2013, 29, 184–189. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-κB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Dorrington, M.G.; Pham, N.-A.; Hyatt, A.; Bruce, E.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a Social Stressor Alters the Structure of the Intestinal Microbiota. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Probiotics and Immune Health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Shirvani-Rad, S.; Tabatabaei-Malazy, O.; Mohseni, S.; Hasani-Ranjbar, S.; Soroush, A.-R.; Hoseini-Tavassol, Z.; Ejtahed, H.-S.; Larijani, B. Probiotics as a Complementary Therapy for Management of Obesity: A Systematic Review. Evid. Based Complement. Alternat. Med. 2021, 2021, 6688450. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A. Technical Aspects of Immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef]
- Khodabakhsh, P.; Khoie, N.; Dehpour, A.-R.; Abdollahi, A.; Ghazi-Khansari, M.; Shafaroodi, H. Montelukast suppresses the development of irritable bowel syndrome phenotype possibly through modulating NF-κB signaling in an experimental model. Inflammopharmacology 2022, 30, 313–325. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kanazawa, M.; Fukudo, S.; Drossman, D.A. Biopsychosocial Model of Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2011, 17, 131–139. [Google Scholar] [CrossRef]
- Qin, H.-Y.; Cheng, C.-W.; Tang, X.-D.; Bian, Z.-X. Impact of Psychological Stress on Irritable Bowel Syndrome. World J. Gastroenterol. 2014, 20, 14126–14131. [Google Scholar] [CrossRef]
- Suda, K.; Setoyama, H.; Nanno, M.; Matsumoto, S.; Kawai, M. Involvement of parasympathetic pelvic efferent pathway in psychological stress-induced defecation. World J. Gastroenterol. 2013, 19, 1200–1209. [Google Scholar] [CrossRef]
- Bagchi, M.; Mimes, M.; Williams, C.; Balmoori, J.; Ye, X.; Stohs, S.; Bagchi, D. Acute and Chronic Stress-Induced Oxidative Gastrointestinal Injury in Rats, and the Protective Ability of a Novel Grape Seed Proanthocyanidin Extract. Nutr. Res. 1999, 19, 1189–1199. [Google Scholar] [CrossRef]
- Campana, R.; van Hemert, S.; Baffone, W. Strain-Specific Probiotic Properties of Lactic Acid Bacteria and Their Interference with Human Intestinal Pathogens Invasion. Gut Pathog. 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, J.H.; Park, S.Y.; Kim, S.C.; Kim, J.Y.; Lee, J.H.; Park, J.S.; Lee, Y.H.; Kang, M.W.; Rhee, M.H.; et al. Stress-Induced Alterations in Mast Cell Numbers and PAR-2 Expression in the Colon. J. Korean Med. Sci. 2010, 25, 1330–1335. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota–Gut–Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the Gut Microbiota in Intestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, P.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The Anxiolytic Effect of Bifidobacterium longum NCC3001 Involves Vagal Pathways. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, Y.B.; Kim, J.H.; Kwon, H.C.; Kim, D.K.; Cho, S.W. The Alteration of Enterochromaffin Cell, Mast Cell, and Lamina Propria T Lymphocyte Numbers in Irritable Bowel Syndrome and Its Relationship with Psychological Factors. J. Gastroenterol. Hepatol. 2008, 23, 1689–1694. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain–Gut–Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Evrensel, A.; Ceylan, M.E. The Gut–Brain Axis: The Missing Link in Depression. Clin. Psychopharmacol. Neurosci. 2015, 13, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Mahony, S.M. The Microbiome-Gut-Brain Axis: From Bowel to Behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Liebregts, T.; Adam, B.; Bredack, C.; Röth, A.; Heinzel, S.; Lester, S.; Talley, N.J. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef]
- Schreiber, S.; Nikolaus, S.; Hampe, J. Activation of nuclear factor kappa B in inflammatory bowel disease. Gut 1998, 42, 477–484. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cui, L.-H.; Wang, X.-H.; Yan, Z.-H.; Li, C.; Gong, S.-D.; Zheng, Y.; Luo, Z.; Wang, Y. Modulation of Inflammation by Toll-Like Receptor 4/Nuclear Factor-Kappa B in Diarrhea-Predominant Irritable Bowel Syndrome. Oncotarget 2017, 8, 113957–113965. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lv, Y.; Liu, H.; Lin, Z.; Zhu, N.; Huang, B. Baicalein Alleviates Chronic Acute Stress-Induced Irritable Bowel Syndrome-Like Symptoms in Rats via Modulating the ODC1/NF-κB Pathway and Oxidative Stress. Biochem. Biophys. Res. Commun. 2025, 771, 152058. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Jiang, Y.; Yin, Q.; Li, X.; Xiong, Y.; Li, B.; Xu, X.; Hu, H.; Qian, G. Sinisan Alleviates Stress-Induced Intestinal Dysfunction and Depressive-Like Behaviors in Mice with Irritable Bowel Syndrome by Enhancing the Intestinal Barrier and Modulating Central 5-Hydroxytryptamine. Int. J. Mol. Sci. 2024, 25, 10262. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]

| Group | Material | Dose | n |
|---|---|---|---|
| Control (No-stress) | negative control (10% skim milk) | - | 6 |
| Stress (WAS only) | negative control (10% skim milk) | - | 6 |
| Treatment A | WAS + Lactobacillus brevis N1 | 1 × 109 CFU | 6 |
| Treatment B | WAS + Lactobacillus brevis N2 | 1 × 109 CFU | 6 |
| Treatment C | WAS + Bacillus amyloliquefaciens S1 | 1 × 109 CFU | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-S.; Lee, S.; Kim, S. Differential Effects of Probiotic Strains on Chronic Stress-Exacerbated Colonic Motility in Rats: A Comparative Evaluation. Metabolites 2025, 15, 677. https://doi.org/10.3390/metabo15100677
Lee Y-S, Lee S, Kim S. Differential Effects of Probiotic Strains on Chronic Stress-Exacerbated Colonic Motility in Rats: A Comparative Evaluation. Metabolites. 2025; 15(10):677. https://doi.org/10.3390/metabo15100677
Chicago/Turabian StyleLee, Yun-Seong, Soyu Lee, and Sooah Kim. 2025. "Differential Effects of Probiotic Strains on Chronic Stress-Exacerbated Colonic Motility in Rats: A Comparative Evaluation" Metabolites 15, no. 10: 677. https://doi.org/10.3390/metabo15100677
APA StyleLee, Y.-S., Lee, S., & Kim, S. (2025). Differential Effects of Probiotic Strains on Chronic Stress-Exacerbated Colonic Motility in Rats: A Comparative Evaluation. Metabolites, 15(10), 677. https://doi.org/10.3390/metabo15100677





