Cardiometabolic Therapies Shape Non-Coding RNA Landscapes in Cardiovascular Fibrosis
Abstract
1. Introduction
2. Metabolic Modulators and Their Influence on ncRNAs
2.1. Metformin
2.2. SGLT2 Inhibitors
2.3. PPARγ Agonists (Pioglitazone and Rosiglitazone)
2.4. GLP-1 Receptor Agonists (Liraglutide)
2.5. Fatty Acid Oxidation Inhibitors (Trimetazidine)
2.6. Cardiometabolic Therapies and CircularRNAs: A Current Knowledge Gap
3. Therapeutic Implications: The Interplay Between Metabolic Modulators and ncRNAs Offers Novel Therapeutic Strategies and Markers of Treatment Efficacy for Cardiovascular Fibrosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caporali, A.; Anwar, M.; Devaux, Y.; Katare, R.; Martelli, F.; Srivastava, P.K.; Pedrazzini, T.; Emanueli, C. Non-Coding RNAs as Therapeutic Targets and Biomarkers in Ischaemic Heart Disease. Nat. Rev. Cardiol. 2024, 21, 556–573. [Google Scholar] [CrossRef]
- Mckinsey, T.A.; Foo, R.; Anene-Nzelu, C.G.; Travers, J.G.; Vagnozzi, R.J.; Weber, N.; Thum, T. Emerging Epigenetic Therapies of Cardiac Fibrosis and Remodelling in Heart Failure: From Basic Mechanisms to Early Clinical Development. Cardiovasc. Res. 2022, 118, 3482–3498. [Google Scholar] [CrossRef]
- Juni, R.P.; t’ Hart, K.C.; Houtkooper, R.H.; Boon, R.A. Long Noncoding RNAs in Cardiometabolic Disorders. FEBS Lett. 2022, 596, 1367–1387. [Google Scholar] [CrossRef]
- Pagano, F.; Picchio, V.; Bordin, A.; Cavarretta, E.; Nocella, C.; Cozzolino, C.; Floris, E.; Angelini, F.; Sordano, A.; Peruzzi, M.; et al. Progressive Stages of Dysmetabolism Are Associated with Impaired Biological Features of Human Cardiac Stromal Cells Mediated by the Oxidative State and Autophagy. J. Pathol. 2022, 258, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-García, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the Risk of Cardiometabolic Diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Farkouh, M.E.; Newman, J.D.; Garvey, W.T. Cardiometabolic-Based Chronic Disease, Adiposity and Dysglycemia Drivers: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Jakubik, D.; Fitas, A.; Eyileten, C.; Jarosz-Popek, J.; Nowak, A.; Czajka, P.; Wicik, Z.; Sourij, H.; Siller-Matula, J.M.; De Rosa, S.; et al. MicroRNAs and Long Non-Coding RNAs in the Pathophysiological Processes of Diabetic Cardiomyopathy: Emerging Biomarkers and Potential Therapeutics. Cardiovasc. Diabetol. 2021, 20, 55. [Google Scholar] [CrossRef]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic Potential of RNA-Enriched Extracellular Vesicles: The next Generation in RNA Delivery via Biogenic Nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef]
- Wu, H.; Hu, Y.; Jiang, C.; Chen, C. Global Scientific Trends in Research of Epigenetic Response to Exercise: A Bibliometric Analysis. Heliyon 2024, 10, e25644. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, F.; Cai, Y.; Zhang, W.; Dun, Y. Effect of Long-Term Exercise Training on LncRNAs Expression in the Vascular Injury of Insulin Resistance. J. Cardiovasc. Transl. Res. 2018, 11, 459–469. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Zeng, W.; Chandy, M.; Wu, J.C. Cardiac Fibrosis: From Mechanisms and Models to Medicines. Trends Pharmacol. Sci. 2025. [Google Scholar] [CrossRef]
- Cabrera-Fuentes, H.A.; Barreto, G.; Perez-Campos, E.; Nivon-Torres, G.F.; Garcia González, A.A.; Al-Suhaim, E.A.; Liehn, E.A. Targeting Inflammation and Fibrosis in Cardiovascular Disease: Emerging Mechanisms and Therapies. FASEB J. 2025, 39, e71008. [Google Scholar] [CrossRef]
- Chimenti, I.; Pagano, F.; Cozzolino, C.; Icolaro, F.; Floris, E.; Picchio, V. The Role of Cardiac Fibroblast Heterogeneity in Myocardial Fibrosis and Its Novel Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 5882. [Google Scholar] [CrossRef] [PubMed]
- Żarek-Starzewska, A.; Klimczak-Tomaniak, D.; Mądrecka, A.; Sygitowicz, G.; Janiszewski, M.; Kuch, M. From Type 2 Diabetes Mellitus To Diabetic Cardiomyopathy—A Systematic Review On The Role Of MicroRNA. Curr. Diabetes Rep. 2025, 25, 42. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Martin-Montalvo, A.; Dluzen, D.F.; Zhang, Y.; Bernier, M.; Zonderman, A.B.; Becker, K.G.; Gorospe, M.; de Cabo, R.; Evans, M.K. Metformin-Mediated Increase in DICER1 Regulates MicroRNA Expression and Cellular Senescence. Aging Cell 2016, 15, 572–581. [Google Scholar] [CrossRef]
- Luo, M.; Tan, X.; Mu, L.; Luo, Y.; Li, R.; Deng, X.; Chen, N.; Ren, M.; Li, Y.; Wang, L.; et al. MiRNA-21 Mediates the Antiangiogenic Activity of Metformin through Targeting PTEN and SMAD7 Expression and PI3K/AKT Pathway. Sci. Rep. 2017, 7, 43427. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Duan, L.; Wei, S.; Liu, J.; Tian, L.; Quan, J.; Zhang, Q.; Liu, J.; Yang, J. Metformin Ameliorates Skeletal Muscle Insulin Resistance by Inhibiting MiR-21 Expression in a High-Fat Dietary Rat Model. Oncotarget 2017, 8, 98029. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wan, L.; Han, B.; Ma, S.; Pan, H.; Wei, J.; Cui, X. Metformin Suppresses Cardiac Fibroblast Proliferation under High-Glucose Conditions via Regulating the Mitochondrial Complex I Protein Grim-19 Involved in the Sirt1/Stat3 Signaling Pathway. Free Radic. Biol. Med. 2023, 206, 1–12. [Google Scholar] [CrossRef]
- Xu, M.; Li, L.P.; He, X.; Lu, X.Z.; Bi, X.Y.; Li, Q.; Xue, X.R. Metformin Induction of Heat Shock Factor 1 Activation and the Mitochondrial Unfolded Protein Response Alleviate Cardiac Remodeling in Spontaneously Hypertensive Rats. FASEB J. 2024, 38, e23654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, T.; Cai, X.; Li, G.; Li, N.; Zhou, H. LncRNA and MRNA Expression Characteristic and Bioinformatic Analysis in Myocardium of Diabetic Cardiomyopathy Mice. BMC Genom. 2024, 25, 312. [Google Scholar] [CrossRef]
- Safavi, K.; Abedpoor, N.; Hajibabaie, F.; Kaviani, E. Mitigating Diabetic Cardiomyopathy: The Synergistic Potential of Sea Buckthorn and Metformin Explored via Bioinformatics and Chemoinformatics. Biology 2025, 14, 361. [Google Scholar] [CrossRef]
- Ran, L.; Pan, W.; Feng, J.; Tang, L. Long Non-Coding RNA MALAT1: A Crucial Factor in Fibrotic Diseases. Mol. Ther. Nucleic Acids 2025, 36, 102630. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, L.; Song, J.; Wang, Z.; Huang, X.; Guo, Z.; Chen, F.; Zhao, X. Long Noncoding RNA MALAT1 Mediates Cardiac Fibrosis in Experimental Postinfarct Myocardium Mice Model. J. Cell Physiol. 2019, 234, 2997–3006. [Google Scholar] [CrossRef]
- Sathishkumar, C.; Prabu, P.; Mohan, V.; Balasubramanyam, M. Linking a Role of LncRNAs (Long Non-Coding RNAs) with Insulin Resistance, Accelerated Senescence, and Inflammation in Patients with Type 2 Diabetes. Hum. Genom. 2018, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Boozarpour, S.; Sabouri, H.; Ghalandarayeshi, S.; Babaee, N.; Lashkarboloki, M.; Banikarimi, S.A. Expression of Circulating Long Non-Coding MALAT1 and GAS5 under Metformin Treatment in Type 2 Diabetic Patients. Gene Rep. 2024, 35, 101905. [Google Scholar] [CrossRef]
- Giordo, R.; Posadino, A.M.; Mangoni, A.A.; Pintus, G. Metformin-Mediated Epigenetic Modifications in Diabetes and Associated Conditions: Biological and Clinical Relevance. Biochem. Pharmacol. 2023, 215, 115732. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, Y.; Zhang, M.; Lin, Y.; Wong, T.S.; Guan, B.; Meng, Y.; Hu, B.; Liu, F.N.; Yin, L.; et al. Mechanism of the Cardioprotective Effect of Empagliflozin on Diabetic Nephropathy Mice Based on the Basis of Proteomics. Proteome Sci. 2024, 22, 9. [Google Scholar] [CrossRef]
- Ridwan, M.; Dimiati, H.; Syukri, M.; Lesmana, R.; Zaini, L.M. Effects of SGLT2-Inhibitor on The Expression of MicroRNA-21, Transforming Growth Factor-Β1, and Matrix Metalloproteinase-2 in The Process of Cardiac Fibrosis in Hyperglycemic Model Rats. Indones. Biomed. J. 2024, 16, 48–55. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 Contributes to Myocardial Disease by Stimulating MAP Kinase Signalling in Fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Mehrtabar, S. Inhibitory Effect of MicroRNA-21 on Pathways and Mechanisms Involved in Cardiac Fibrosis Development. Ther. Adv. Cardiovasc. Dis. 2024, 18, 7539447241253134. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 Inhibition with Empagliflozin Attenuates Myocardial Oxidative Stress and Fibrosis in Diabetic Mice Heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Rolski, F.; Mączewski, M. Cardiac Fibrosis: Mechanistic Discoveries Linked to SGLT2 Inhibitors. Pharmaceuticals 2025, 18, 313. [Google Scholar] [CrossRef]
- Kiyak-Kirmaci, H.; Hazar-Yavuz, A.N.; Polat, E.B.; Alsaadoni, H.; Cilingir-Kaya, O.T.; Aktas, H.S.; Elcioglu, H.K. Effects of Empagliflozin and Dapagliflozin, SGLT2 Inhibitors, on MiRNA Expressions in Diabetes-Related Cardiovascular Damage in Rats. J. Diabetes Complicat. 2025, 39, 109063. [Google Scholar] [CrossRef]
- Mone, P.; Lombardi, A.; Kansakar, U.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Marzocco, S.; De Gennaro, S.; Famiglietti, M.; Macina, G.; et al. Empagliflozin Improves the MicroRNA Signature of Endothelial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Diabetes. J. Pharmacol. Exp. Ther. 2023, 384, 116–122. [Google Scholar] [CrossRef]
- Dooley, J.; Garcia-Perez, J.E.; Sreenivasan, J.; Schlenner, S.M.; Vangoitsenhoven, R.; Papadopoulou, A.S.; Tian, L.; Schonefeldt, S.; Serneels, L.; Deroose, C.; et al. The MicroRNA-29 Family Dictates the Balance between Homeostatic and Pathological Glucose Handling in Diabetes and Obesity. Diabetes 2016, 65, 53–61. [Google Scholar] [CrossRef]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The MiR-29 Family: Genomics, Cell Biology, and Relevance to Renal and Cardiovascular Injury. Physiol. Genom. 2012, 44, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Jensen, D.M.; Wang, J.; Liu, Y.; Geurts, A.M.; Kriegel, A.J.; Liu, P.; Ying, R.; Zhang, G.; Casati, M.; et al. MiR-29 Contributes to Normal Endothelial Function and Can Restore It in Cardiometabolic Disorders. EMBO Mol. Med. 2018, 1, e8046. [Google Scholar] [CrossRef]
- Li, G.; Zhao, C.; Fang, S. SGLT2 Promotes Cardiac Fibrosis Following Myocardial Infarction and Is Regulated by MiR-141. Exp. Ther. Med. 2021, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-L.; Wang, Y.-Y.; Yu, Z.-F.; Tian, M.-M.; Li, H. Peroxisome Proliferator-Activated Receptor-Gamma Activation Attenuates Diabetic Cardiomyopathy via Regulation of the TGF-β/ERK Pathway and Epithelial-to-Mesenchymal Transition. Life Sci. 2018, 213, 269–278. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, H.Y.; Yu, H.T.; Sun, M.; Zhang, Y.Y.; Xu, M.; Gao, W. MiRNA-711-SP1-Collagen-I Pathway Is Involved in the Anti-Fibrotic Effect of Pioglitazone in Myocardial Infarction. Sci. China Life Sci. 2013, 56, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, V.; De Pascale, M.R.; Zullo, A.; Soricelli, A.; Infante, T.; Mancini, F.P.; Napoli, C. Evidence of Epigenetic Tags in Cardiac Fibrosis. J. Cardiol. 2017, 69, 401–408. [Google Scholar] [CrossRef]
- Chen, S.; Puthanveetil, P.; Feng, B.; Matkovich, S.J.; Dorn, G.W.; Chakrabarti, S. Cardiac MiR-133a Overexpression Prevents Early Cardiac Fibrosis in Diabetes. J. Cell Mol. Med. 2014, 18, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Hu, Z.; Lin, Y.; Zhang, C.; Perez-Polo, J.R. Downregulation of MicroRNA-29 by Antisense Inhibitors and a PPAR-Γagonist Protects against Myocardial Ischaemia-Reperfusion Injury. Cardiovasc. Res. 2010, 87, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Li, T.; Zhuang, Y.; Li, Z.; Yang, W.; Huang, Q.; Li, D.; Wu, H.; Zhang, G.; Yang, T.; et al. Involvement of LncR-30245 in Myocardial Infarction-Induced Cardiac Fibrosis Through Peroxisome Proliferator-Activated Receptor-γ-Mediated Connective Tissue Growth Factor Signalling Pathway. Can. J. Cardiol. 2019, 35, 480–489. [Google Scholar] [CrossRef]
- Miao, X.; Davoudi, M.; Alitotonchi, Z.; Ahmadi, E.S.; Amraee, F.; Alemi, A.; Afrisham, R. Managing Cardiovascular Events, Hyperglycemia, and Obesity in Type 2 Diabetes through MicroRNA Regulation Linked to Glucagon-like Peptide-1 Receptor Agonists. Diabetol. Metab. Syndr. 2025, 17, 13. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.; Ma, X.; Wang, Y.; Zhang, Q.; Zheng, N.; Zhang, J.; Li, C. Liraglutide Inhibits AngII-Induced Cardiac Fibroblast Proliferation and ECM Deposition through Regulating MiR-21/PTEN/PI3K Pathway. Cell Tissue Bank. 2023, 24, 125–137. [Google Scholar] [CrossRef]
- Capuani, B.; Pacifici, F.; Della-Morte, D.; Lauro, D. Glucagon Like Peptide 1 and MicroRNA in Metabolic Diseases: Focusing on GLP1 Action on MiRNAs. Front. Endocrinol. 2018, 9, 719. [Google Scholar] [CrossRef]
- Magenta, A.; Ciarapica, R.; Capogrossi, M.C. The Emerging Role of MiR-200 Family in Cardiovascular Diseases. Circ. Res. 2017, 120, 1399–1402. [Google Scholar] [CrossRef]
- Guo, J.; Fang, W.; Sun, L.; Lu, Y.; Dou, L.; Huang, X.; Sun, M.; Pang, C.; Qu, J.; Liu, G.; et al. Reduced MiR-200b and MiR-200c Expression Contributes to Abnormal Hepatic Lipid Accumulation by Stimulating JUN Expression and Activating the Transcription of Srebp1. Oncotarget 2016, 7, 36207–36219. [Google Scholar] [CrossRef]
- Zhao, X.D.; Qin, R.H.; Yang, J.J.; Xu, S.S.; Tao, H.; Ding, X.S.; Shi, K.H. DNMT3A Controls MiR-200b in Cardiac Fibroblast Autophagy and Cardiac Fibrosis. Inflamm. Res. 2018, 67, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Chacko, L.; Bhattacharya, H.; Vallamkondu, J.; Nag, S.; Dey, A.; Karmakar, T.; Reddy, P.H.; Kandimalla, R.; Dewanjee, S. Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies. Biomedicines 2023, 11, 1126. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, J.; Tong, Q.; Lin, Q.; Qian, L.; Park, Y.; Zheng, Y. The Role of ERK1/2 in the Development of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2016, 17, 2001. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, X.; Wei, P.; Wang, Y.; Li, P.; Shao, K. Regulation of Pyroptosis in Cardiovascular Pathologies: Role of Noncoding RNAs. Mol. Ther. Nucleic Acids 2021, 25, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-F.; Wang, R.; Chen, W.; Cao, Y.-D.; Li, L.-P.; Chen, X. MiR-133a-3p Attenuates Cardiomyocyte Hypertrophy through Inhibiting Pyroptosis Activation by Targeting IKKε. Acta Histochem. 2021, 123, 151653. [Google Scholar] [CrossRef]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; Van Der Made, I.; Herias, V.; Van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. MiR-133 and MiR-30 Regulate Connective Tissue Growth Factor: Implications for a Role of MicroRNAs in Myocardial Matrix Remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, W.; Yang, T.; Meng, X.; Jiang, Z.; Tao, L.; Wang, L. Upregulation of Circular RNA CircNFIB Attenuates Cardiac Fibrosis by Sponging MiR-433. Front. Genet. 2019, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Schmidt, K.; Groß, S.; Lu, D.; Xiao, K.; Neufeldt, D.; Cushman, S.; Lehmann, N.; Thum, S.; Pfanne, A.; et al. Circular RNA CircIGF1R Controls Cardiac Fibroblast Proliferation through Regulation of Carbohydrate Metabolism. Sci. Rep. 2025, 15, 20331. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.D.; Fang, X.H.; Zhu, J.N.; Yang, J.; Pan, R.; Yuan, S.J.; Zeng, N.; Yang, Z.Z.; Yang, H.; et al. Circular RNA CircRNA_000203 Aggravates Cardiac Hypertrophy via Suppressing MiR-26b-5p and MiR-140-3p Binding to Gata4. Cardiovasc. Res. 2020, 116, 1323–1334. [Google Scholar] [CrossRef]
- Jeong, A.; Lim, Y.; Kook, T.; Kwon, D.H.; Cho, Y.K.; Ryu, J.; Lee, Y.G.; Shin, S.; Choe, N.; Kim, Y.S.; et al. Circular RNA CircSMAD4 Regulates Cardiac Fibrosis by Targeting MiR-671-5p and FGFR2 in Cardiac Fibroblasts. Mol. Ther. Nucleic Acids 2023, 34, 102071. [Google Scholar] [CrossRef]
- Bibi, A.; Madè, A.; Greco, S.; Garcia-Manteiga, J.M.; Tascini, A.S.; Tastsoglou, S.; Zaccagnini, G.; Leszek, P.; Gaetano, C.; Martelli, F. Circular PVT1 Promotes Cardiac Fibroblast Activation Interacting with MiR-30a-5p and MiR-125b-5p. Cell Death Dis. 2025, 16, 325. [Google Scholar] [CrossRef]
- Song, Y.; Guo, F.; Liu, Y.; Huang, F.; Fan, X.; Zhao, L.; Qin, G. Identification of Circular RNAs and Functional Competing Endogenous RNA Networks in Human Proximal Tubular Epithelial Cells Treated with Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin in Diabetic Kidney Disease. Bioengineered 2022, 13, 3911–3929. [Google Scholar] [CrossRef]
- Bai, Z.; Xie, T.; Liu, T.; Chen, Z.; Yu, L.; Zhang, C.; Luo, J.; Chen, L.; Zhao, X.; Xiao, Y. An Integrated RNA Sequencing and Network Pharmacology Approach Reveals the Molecular Mechanism of Dapagliflozin in the Treatment of Diabetic Nephropathy. Front. Endocrinol. 2022, 13, 967822. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Wu, S.; Zhou, Z.; Ding, X.; Shi, R.; Thorne, R.F.; Zhang, X.D.; Hu, W.; Wu, M. CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell Metab. 2019, 30, 157–173.e7. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qin, M.; Hong, H.; Xue, X.; Fang, J.; Jiang, L.; Kuang, Y.; Gao, L. Circular RNA Hsa_circ_0072309 Inhibits the Proliferation, Invasion and Migration of Gastric Cancer Cells via Inhibition of PI3K/AKT Signaling by Activating PPARγ/PTEN Signaling. Mol. Med. Rep. 2021, 23, 349. [Google Scholar] [CrossRef]
- Li, S.S.; Pan, L.; Zhang, Z.Y.; Zhou, M.D.; Chen, X.F.; Qian, L.L.; Dai, M.; Lu, J.; Yu, Z.M.; Dang, S.; et al. Diabetes Promotes Myocardial Fibrosis via AMPK/EZH2/PPAR-γ Signaling Pathway. Diabetes Metab. J. 2024, 48, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, Y.; Li, S.; Chen, Y.; Li, L.; Cao, Y.; Mingyao, M.; Shi, P.; Song, C.; Li, B.; et al. Activation of AMPK Attenuated Cardiac Fibrosis by Inhibiting CDK2 via P21/P27 and MiR-29 Family Pathways in Rats. Mol. Ther. Nucleic Acids 2017, 8, 277–290. [Google Scholar] [CrossRef]
- Lee, J.; Moon, J.H. Targeting Cardiac Fibrosis in Diabetic Heart Failure: The Role of the EZH2, AMPK, and PPAR-γ Pathways (Diabetes Metab J 2024;48:716-29). Diabetes Metab. J. 2024, 48, 1176–1178. [Google Scholar] [CrossRef]
- Chen, Z.T.; Gao, Q.Y.; Wu, M.X.; Wang, M.; Sun, R.L.; Jiang, Y.; Guo, Q.; Guo, D.C.; Liu, C.Y.; Chen, S.X.; et al. Glycolysis Inhibition Alleviates Cardiac Fibrosis After Myocardial Infarction by Suppressing Cardiac Fibroblast Activation. Front. Cardiovasc. Med. 2021, 8, 701745. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Casu, A.; Kovacova, Z.; Petrilli, A.M.; Sideleva, O.; Tharp, W.G.; Pratley, R.E. Coordinated Regulation of Gene Expression and MicroRNA Changes in Adipose Tissue and Circulating Extracellular Vesicles in Response to Pioglitazone Treatment in Humans with Type 2 Diabetes. Front. Endocrinol. 2022, 13, 955593. [Google Scholar] [CrossRef]
- Formichi, C.; Fignani, D.; Nigi, L.; Grieco, G.E.; Brusco, N.; Licata, G.; Sabato, C.; Ferretti, E.; Sebastiani, G.; Dotta, F. Circulating MicroRNAs Signature for Predicting Response to GLP1-RA Therapy in Type 2 Diabetic Patients: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 9454. [Google Scholar] [CrossRef]
- Yu, H.; Davoudi, M.; Sadegh-Nejadi, S.; Miao, X.; Bagherieh, M.; Afrisham, R. Impact of Monotherapy and Combination Therapy with Glucagon-like Peptide-1 Receptor Agonists on Exosomal and Non-Exosomal MicroRNA Signatures in Type 2 Diabetes Mellitus: A Systematic Review. J. Transl. Med. 2025, 23, 477. [Google Scholar] [CrossRef]
- Mansouri, F.; Seyed Mohammadzad, M.H. Effects of Metformin on Changes of MiR-19a and MiR-221 Expression Associated with Myocardial Infarction in Patients with Type 2 Diabetes. Diabetes Metab. Syndr. 2022, 16, 102602. [Google Scholar] [CrossRef]
- Wander, P.L.; Bammler, T.K.; Macdonald, J.W.; Srinouanprachanh, S.; Boyko, E.J.; Enquobahrie, D.A. Plasma MiRNAs and Treatment Failure in Participants in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care 2024, 47, 471–475. [Google Scholar] [CrossRef]
- Kupec, T.; Bleilevens, A.; Iborra, S.; Najjari, L.; Wittenborn, J.; Maurer, J.; Stickeler, E. Stability of Circulating MicroRNAs in Serum. PLoS ONE 2022, 17, e0268958. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular MiRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Srinivasan, S.; Yeri, A.; Cheah, P.S.; Chung, A.; Danielson, K.; De Hoff, P.; Filant, J.; Laurent, C.D.; Laurent, L.D.; Magee, R.; et al. Small RNA Sequencing across Diverse Biofluids Identifies Optimal Methods for ExRNA Isolation. Cell 2019, 177, 446–462.e416. [Google Scholar] [CrossRef]
- Quinn, J.F.; Patel, T.; Wong, D.; Das, S.; Freedman, J.E.; Laurent, L.C.; Carter, B.S.; Hochberg, F.; Van Keuren-Jensen, K.; Huentelman, M.; et al. Extracellular RNAs: Development as Biomarkers of Human Disease. J. Extracell. Vesicles 2015, 4, 27495. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M.B.; Edelman, J.J.B.; Kao, S.C.H.; Vallely, M.P.; Van Zandwijk, N.; Reid, G. The Impact of Hemolysis on Cell-Free MicroRNA Biomarkers. Front. Genet. 2013, 4, 94. [Google Scholar] [CrossRef]
- Sandau, U.S.; Wiedrick, J.T.; McFarland, T.J.; Galasko, D.R.; Fanning, Z.; Quinn, J.F.; Saugstad, J.A. Analysis of the Longitudinal Stability of Human Plasma MiRNAs and Implications for Disease Biomarkers. Sci. Rep. 2024, 14, 2148. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Navarro, B.; Ramírez-Calvo, M.; Gil Aldea, I.; Cortell Granero, I.; López Guerrero, J.A. The Impact of Tube Type, Centrifugation Conditions, and Hemolysis on Plasma Circulating MicroRNAs. Diagnostics 2024, 14, 2369. [Google Scholar] [CrossRef]
- Marabita, F.; de Candia, P.; Torri, A.; Tegnér, J.; Abrignani, S.; Rossi, R.L. Normalization of Circulating MicroRNA Expression Data Obtained by Quantitative Real-Time RT-PCR. Brief. Bioinform. 2015, 17, 204–212. [Google Scholar] [CrossRef]
- Pawlina-Tyszko, K.; Szmatoła, T. Benchmarking of Bioinformatics Tools for NGS-Based MicroRNA Profiling with RT-QPCR Method. Funct. Integr. Genom. 2023, 23, 347. [Google Scholar] [CrossRef]
- Ammerlaan, W.; Betsou, F. Intraindividual TEMPORAL MiRNA Variability in Serum, Plasma, and White Blood Cell Subpopulations. Biopreserv. Biobank. 2016, 14, 390–397. [Google Scholar] [CrossRef]
- Keller, A.; Rounge, T.; Backes, C.; Ludwig, N.; Gislefoss, R.; Leidinger, P.; Langseth, H.; Meese, E. Sources to Variability in Circulating Human MiRNA Signatures. RNA Biol. 2017, 14, 1791–1798. [Google Scholar] [CrossRef]
- Picchio, V.; Ferrero, G.; Cozzolino, C.; Pardini, B.; Floris, E.; Tarallo, S.; Dhori, X.; Nocella, C.; Loffredo, L.; Biondi-Zoccai, G.; et al. Effect of Traditional or Heat-Not-Burn Cigarette Smoking on Circulating MiRNAs in Healthy Subjects. Eur. J. Clin. Investig. 2024, 54, e14140. [Google Scholar] [CrossRef]
- Frati, G.; Forte, M.; Di Nonno, F.; Bordin, A.; Chimenti, I.; Picchio, V.; Cavarretta, E.; Stanzione, R.; Bianchi, F.; Carnevale, R.; et al. Inhibition of MiR-155 Attenuates Detrimental Vascular Effects of Tobacco Cigarette Smoking. J. Am. Heart Assoc. 2020, 9, 17000. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vijayan, M.; Bhatti, J.S.; Reddy, P.H. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog. Mol. Biol. Transl. Sci. 2017, 146, 47–94. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, D.; Katare, R. Exosomal MicroRNAs in Diabetic Heart Disease. Cardiovasc. Diabetol. 2022, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Dai, X.; Li, Y.; Li, C.; Liu, L. Exosomes from MiR-29a-Modified Adipose-Derived Mesenchymal Stem Cells Reduce Excessive Scar Formation by Inhibiting TGF-Β2/Smad3 Signaling. Mol. Med. Rep. 2021, 24, 758. [Google Scholar] [CrossRef]
- Tang, M.; Guo, C.; Sun, M.; Zhou, H.; Peng, X.; Dai, J.; Ding, Q.; Wang, Y.; Yang, C. Effective Delivery of Osteopontin Small Interference RNA Using Exosomes Suppresses Liver Fibrosis via TGF-Β1 Signaling. Front. Pharmacol. 2022, 13, 82243. [Google Scholar] [CrossRef] [PubMed]
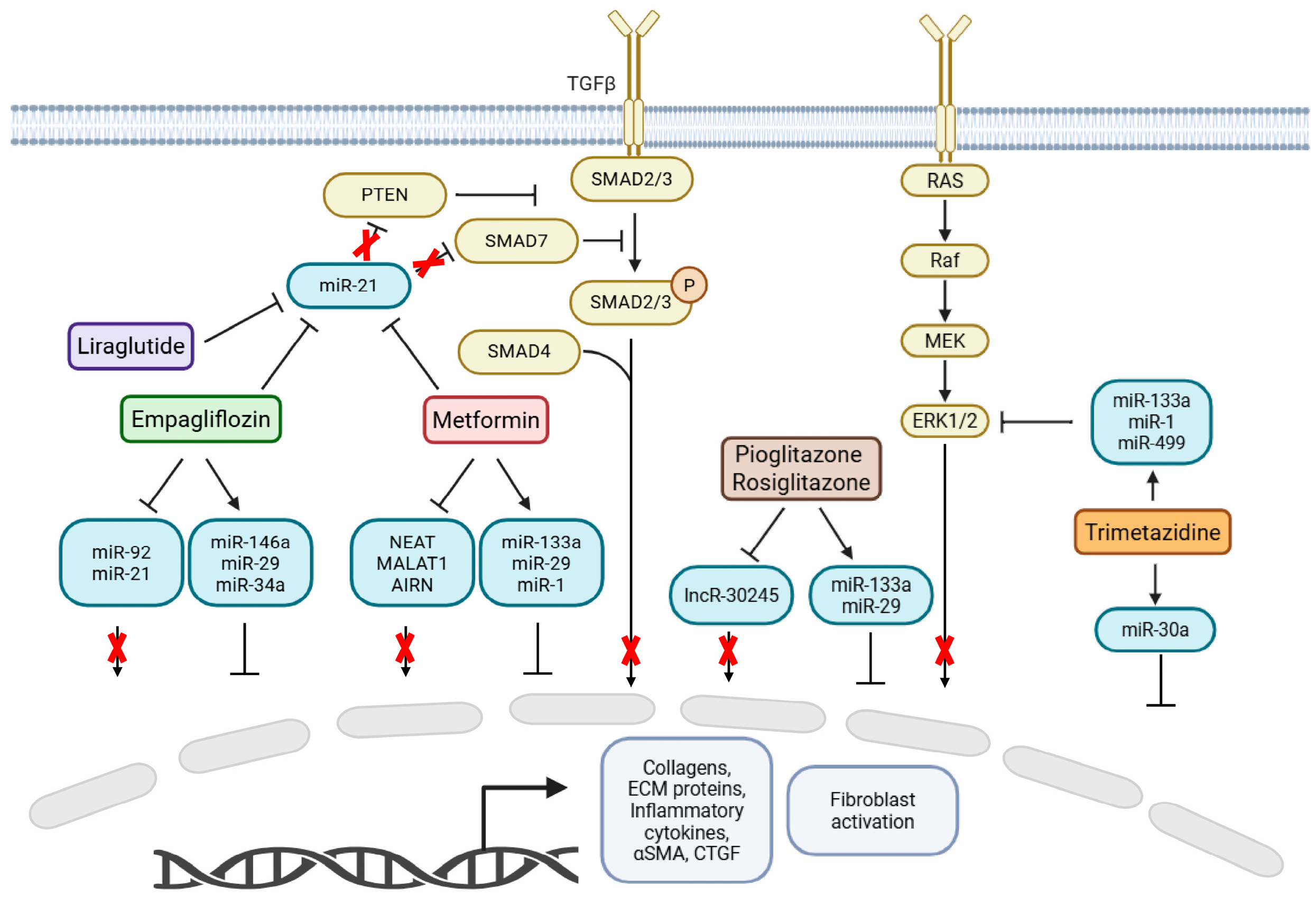
| Metformin | Details | References | Study Context |
|---|---|---|---|
| Primary Action | Activates AMPK, improves insulin sensitivity, reduces inflammation | [15] | In vivo and in vitro |
| Epigenetic Mechanism | Modulates non-coding RNAs (miRNAs and lncRNAs), affects post-transcriptional gene regulation of fibrotic genes | [15,26] | In vivo and in vitro |
| Key miRNAs Affected | miR-21 (downregulated), miR-29, miR-133, miR-1 | [16,17] | In vivo and in vitro |
| Key lncRNAs Affected | H19, NEAT1, MALAT1, AIRN | [21,26] | In vivo and in silico |
| Fibrotic Pathways Modulated | TGF-β signaling via downregulation of miR-21 and upregulation of Smad7 (inhibitory Smad) | [16] | In vitro |
| Effects on Fibroblasts | Decreased proliferation and migration; linked to Grim-19 and SIRT1 upregulation; STAT3 phosphorylation inhibited | [18] | In vivo and in vitro |
| Mitochondrial Effects | Induces mitochondrial unfolded protein response (UPR_mt) via HSF1, improving mitochondrial quality and reducing oxidative stress | [19] | In vivo and in vitro |
| Animal Models | Diabetic rat hearts, db/db mouse cardiac fibroblasts | [16,18,20] | In vivo and in vitro |
| High-throughput Studies | Identified dysregulated lncRNAs and mRNAs in diabetic cardiomyopathy (93 lncRNAs, 881 mRNAs); linked to fibrosis and apoptosis | [20] | In vivo |
| Combination Therapies | Synergistic effect with Hippophae rhamnoides L. (sea buckthorn) reducing fibrosis, inflammation via downregulating NEAT1 and MALAT1 | [21] | In vivo and in silico |
| Clinical Potential | Repositioning as epigenetic modulator in cardiac therapeutics targeting ncRNA networks to reduce fibrosis and inflammation | [15,26] | In vivo and in vitro |
| SGL2 Inhibitors | Details | References | Study Context |
|---|---|---|---|
| Primary Action | Inhibit renal glucose reabsorption, improve glycemic control | [21,22] | In vivo and in silico |
| Cardiovascular Benefits | Beyond metabolism: reduce myocardial remodeling and cardiac fibrosis via ncRNA regulation | [21,22] | In vivo and in silico |
| Key miRNAs Downregulated | miR-21 (pro-fibrotic, promotes fibroblast activation via TGF-β pathway by targeting Smad7 and PTEN), miR-92 | [22,23,24,28] | In vivo, in vitro and in silico |
| Key miRNAs Upregulated | miR-29 (antifibrotic, targets ECM genes such as collagens, elastin), miR-146a, miR-34a | [27,29,30] | In vivo and in vitro |
| Mechanistic Insights | Downregulation of miR-21 linked to decreased TGF- β/Smad pathway activity and reduced fibrosis markers (fibronectin, α-SMA) | [22,25,26] | In vivo |
| Animal Models | Diabetic mice, nicotinamide/streptozotocin-induced T2DM rats | [22,27] | In vivo and in vitro |
| Clinical Observations | Reduced circulating miR-21 and miR-92 in HFpEF patients treated with empagliflozin | [28] | In vivo and in vitro |
| miRNA-Drug Feedback Loop | miR-141 downregulated after MI; overexpression suppresses SGLT2 in cardiac fibroblasts, reducing fibrosis via TGF-β pathway | [31] | In vivo and in vitro |
| Overall Mechanism | Cardioprotective effects via dual modulation of miRNAs: downregulating pro-fibrotic and upregulating anti-fibrotic miRNAs | [21,22,29] | In vivo, in vitro and in silico |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floris, E.; Nutile, F.; Cozzolino, C.; Pontecorvi, V.; Bordin, A.; De Falco, E.; Picchio, V.; Chimenti, I.; Pagano, F. Cardiometabolic Therapies Shape Non-Coding RNA Landscapes in Cardiovascular Fibrosis. Metabolites 2025, 15, 664. https://doi.org/10.3390/metabo15100664
Floris E, Nutile F, Cozzolino C, Pontecorvi V, Bordin A, De Falco E, Picchio V, Chimenti I, Pagano F. Cardiometabolic Therapies Shape Non-Coding RNA Landscapes in Cardiovascular Fibrosis. Metabolites. 2025; 15(10):664. https://doi.org/10.3390/metabo15100664
Chicago/Turabian StyleFloris, Erica, Francesco Nutile, Claudia Cozzolino, Virginia Pontecorvi, Antonella Bordin, Elena De Falco, Vittorio Picchio, Isotta Chimenti, and Francesca Pagano. 2025. "Cardiometabolic Therapies Shape Non-Coding RNA Landscapes in Cardiovascular Fibrosis" Metabolites 15, no. 10: 664. https://doi.org/10.3390/metabo15100664
APA StyleFloris, E., Nutile, F., Cozzolino, C., Pontecorvi, V., Bordin, A., De Falco, E., Picchio, V., Chimenti, I., & Pagano, F. (2025). Cardiometabolic Therapies Shape Non-Coding RNA Landscapes in Cardiovascular Fibrosis. Metabolites, 15(10), 664. https://doi.org/10.3390/metabo15100664








