Resistant Potato Starch Supplementation Increases Serum Antioxidant Levels in a Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigational Product
2.2. Study Design
2.3. Clinical Trial Conduct
2.4. Metabolomic Analysis
2.5. Statistical Analysis
3. Results
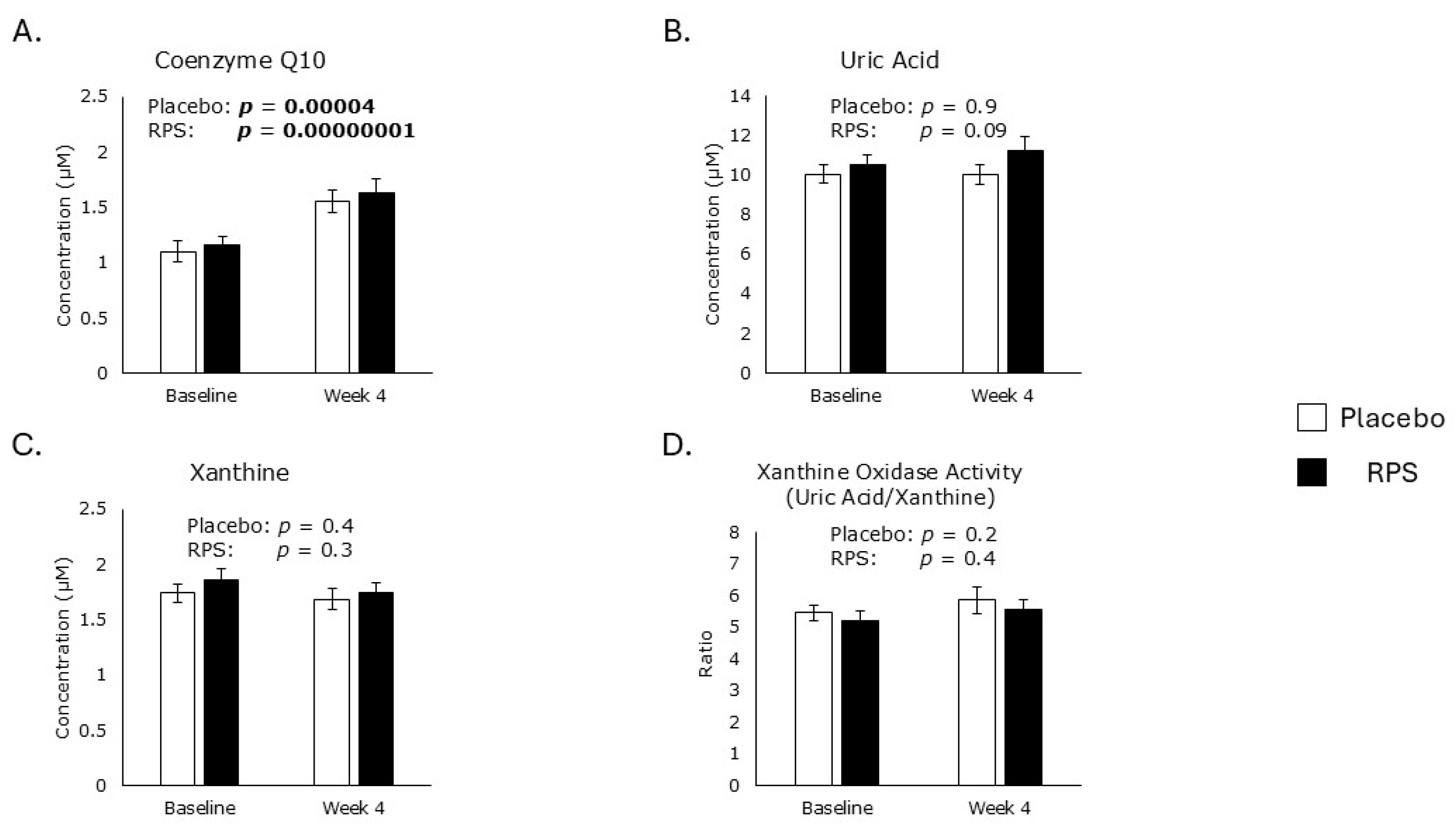
3.1. Exogenous Antioxidants
3.2. Endogenous Antioxidants
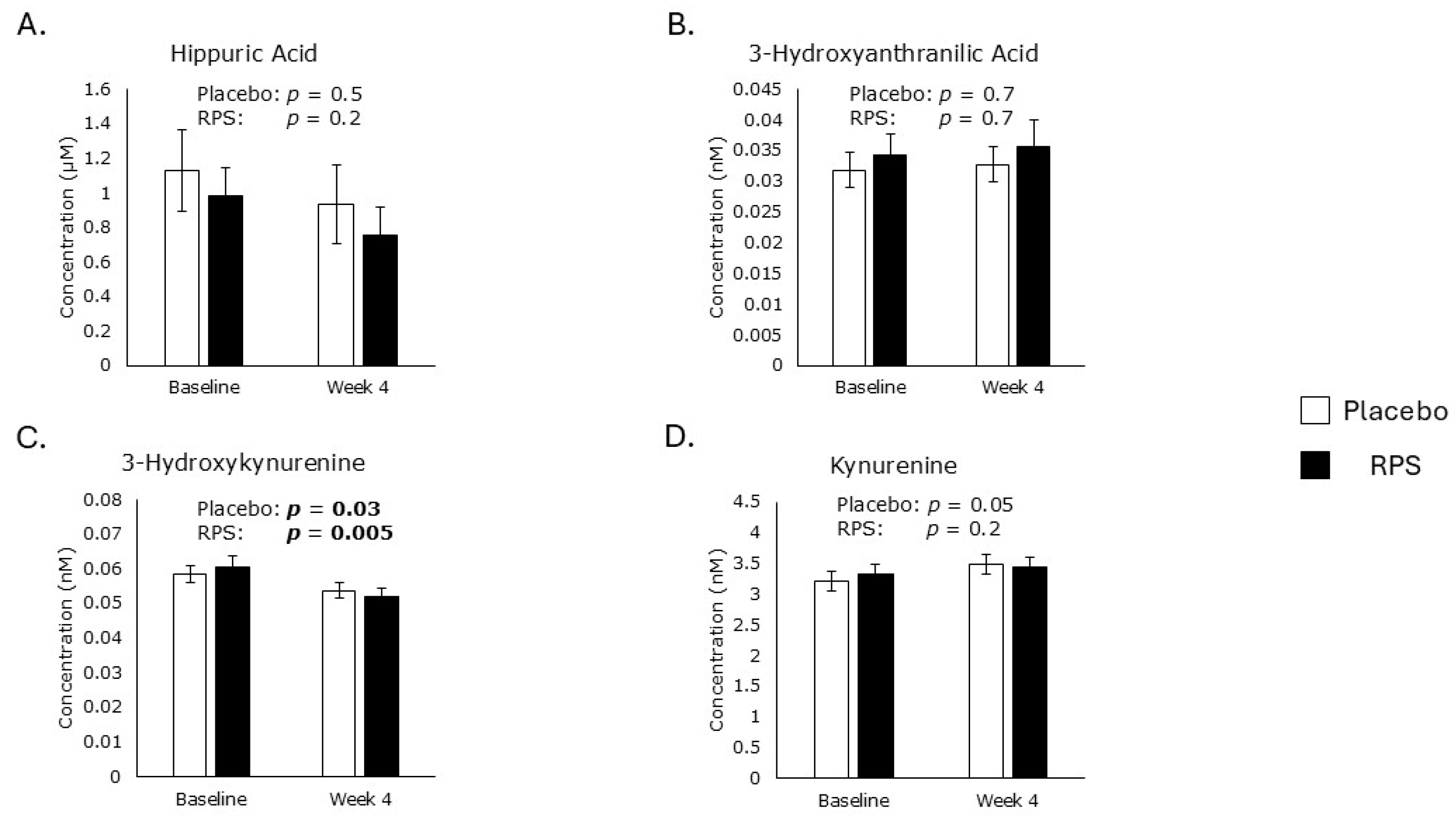
3.3. Polyphenol and Tryptophan Metabolites
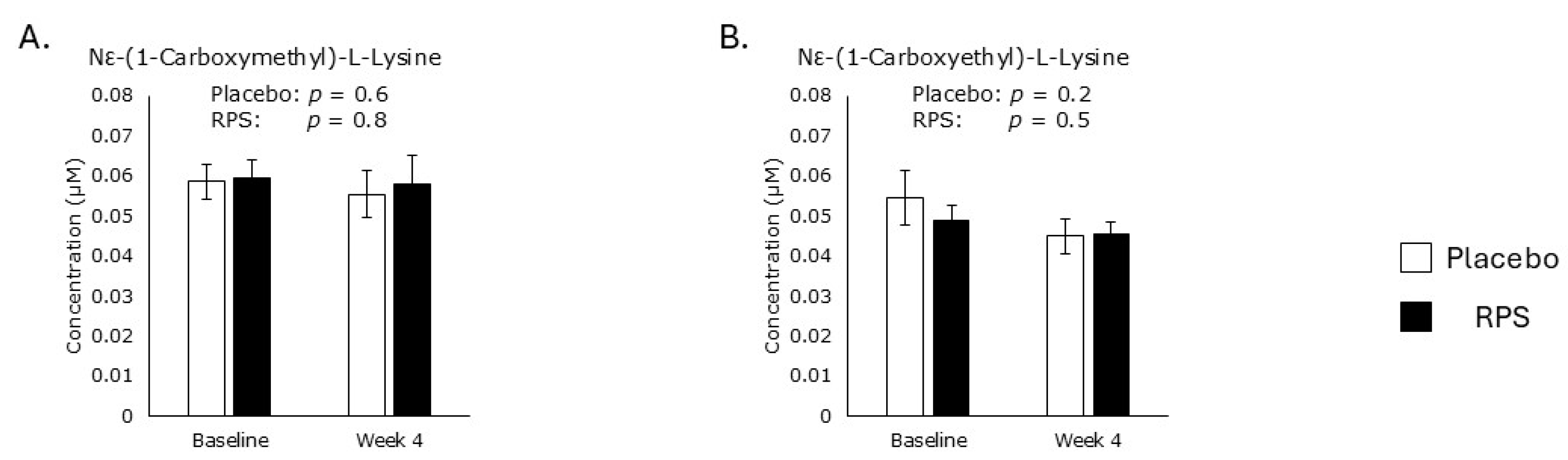
3.4. Metabolomic Markers of Oxidative Stress
3.5. Correlations Between Increases in Antioxidant Levels and Changes in Gut Microbiota
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced end products of glycated proteins |
| BMI | Body mass index |
| CoQ10 | Coenzyme Q10 |
| IS | Internal standard |
| LC-MS/MS | Liquid chromatography coupled to tandem mass spectrometry |
| QC | Quality control |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| RPS | Resistant potato starch |
| RS | Resistant starch |
| SEM | Standard error of the mean |
References
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Maciejczyk, M. The antioxidant barrier, oxidative/nitrosative stress, and protein glycation in allergy: From basic research to clinical practice. Front. Immunol. 2024, 15, 1440313. [Google Scholar] [CrossRef]
- Superti, F.; Russo, R. Alpha-Lipoic Acid: Biological Mechanisms and Health Benefits. Antioxidants 2024, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Dewsbury, M.; Hargreaves, I.P. The Ubiquinone-Ubiquinol Redox Cycle and Its Clinical Consequences: An Overview. Int. J. Mol. Sci. 2024, 25, 6765. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.V.; Rios, A.; Takeyama, R.; Santos, N.; Pires, L.; Santos, G.S.; Rodrigues, I.J.; Jeyaraman, M.; Purita, J.; Lana, J.F. Nebulized Glutathione as a Key Antioxidant for the Treatment of Oxidative Stress in Neurodegenerative Conditions. Nutrients 2024, 16, 2476. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef]
- Guerra, R.M.; Pagliarini, D.J. Coenzyme Q biochemistry and biosynthesis. Trends Biochem. Sci. 2023, 48, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I.; Zmitek, K.; Zmitek, J. Coenzyme Q10 contents in foods and fortification strategies. Crit. Rev. Food Sci. Nutr. 2010, 50, 269–280. [Google Scholar] [CrossRef]
- Intharuksa, A.; Kuljarusnont, S.; Sasaki, Y.; Tungmunnithum, D. Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and Medical Application. Molecules 2024, 29, 5760. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Dilworth, L.; Stennett, D.; Facey, A.; Omoruyi, F.; Mohansingh, S.; Omoruyi, F.O. Diabetes and the associated complications: The role of antioxidants in diabetes therapy and care. Biomed. Pharmacother. 2024, 181, 117641. [Google Scholar] [CrossRef]
- McGill, C.R.; Kurilich, A.C.; Davignon, J. The role of potatoes and potato components in cardiometabolic health: A review. Ann. Med. 2013, 45, 467–473. [Google Scholar] [CrossRef]
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.; Dong, M.; Liu, R.H. Cellular Antioxidant Activity of Common Vegetables. J. Agric. Food Chem. 2010, 58, 6621–6629. [Google Scholar] [CrossRef]
- Brown, C.R.; Culley, D.; Yang, C.-P.; Durst, R.; Wrolstad, R. Variation of Anthocyanin and Carotenoid Contents and Associated Antioxidant Values in Potato Breeding Lines. J. Am. Soc. Hort. Sci. 2005, 130, 174–180. [Google Scholar] [CrossRef]
- Health Canada. Natural Health Products Antioxidants. 2024. Available online: https://webprod.hc-sc.gc.ca/nhpid-bdipsn/dbImages/mono_antioxidants_english.pdf (accessed on 27 August 2025).
- Robertson, T.M.; Alzaabi, A.Z.; Robertson, M.D.; Fielding, B.A. Starchy carbohydrates in a healthy diet: The role of the humble potato. Nutrients 2018, 10, 1764. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Hudson, G.J.; Cummings, J.H. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 1996, 75, 749–755. [Google Scholar] [CrossRef]
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Graham, M.; Van Domselaar, G.; Forbes, J.D.; Laminman, V.; Olson, N.; DeGagne, P.; Bray, D.; et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 2018, 37, 797–807. [Google Scholar] [CrossRef]
- Bush, J.R.; Baisley, J.; Harding, S.V.; Alfa, M.J. Consumption of Solnul™ Resistant Potato Starch Produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 1582. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.R.; Han, J.; Deehan, E.C.; Harding, S.V.; Maiya, M.; Baisley, J.; Schibli, D.; Goodlett, D.R. Resistant potato starch supplementation reduces serum histamine levels in healthy adults with links to attenuated intestinal permeability. J. Funct. Foods 2023, 108, 105740. [Google Scholar] [CrossRef]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Olson, N.; DeGagne, P.; Bray, D.; Murray, B.-L.; Hiebert, B. A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic® on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults. Front. Med. 2018, 4, 260. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.R.; Iwuamadi, I.; Han, J.; Schibli, D.J.; Goodlett, D.R.; Deehan, E.C. Resistant Potato Starch Supplementation Reduces Serum Free Fatty Acid Levels and Influences Bile Acid Metabolism. Metabolites 2024, 14, 536. [Google Scholar] [CrossRef]
- Bush, J.R.; Han, J.; Goodlett, D.R. Resistant Potato Starch supplementation increases the serum levels of choline and sphingomyelins without affecting trimethylamine oxide levels. Metabolites in press.
- Paul, P.; Kaul, R.; Abdellatif, B.; Arabi, M.; Upadhyay, R.; Saliba, R.; Sebah, M.; Chaari, A. The Promising Role of Microbiome Therapy on Biomarkers of Inflammation and Oxidative Stress in Type 2 Diabetes: A Systematic and Narrative Review. Front. Nutr. 2022, 9, 906243. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, E.M.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404. [Google Scholar] [CrossRef]
- Bush, J.R.; Alfa, M.J. Consumption of resistant potato starch produces changes in gut microbiota that correlate with improvements in abnormal bowel symptoms: A secondary analysis of a clinical trial. BMC Nutr. 2024, 10, 152. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, X.-D.; Li, J.-Z.; Mo, Q.-Y.; Wang, X.; Zhao, Y.; Zhang, Y.-M.; Luo, H.-T.; Xia, D.-Y.; Ma, W.-Q.; et al. Host-derived Lactobacillus plantarum alleviates hyperuricemia by improving gut microbial community and hydrolase-mediated degradation of purine nucleosides. eLife 2024, 13, e100068. [Google Scholar] [CrossRef]
- Clarke, E.D.; Rollo, M.E.; Collins, C.E.; Wood, L.; Callister, R.; Philo, M.; Kroon, P.A.; Haslam, R.L. The Relationship between Dietary Polyphenol Intakes and Urinary Polyphenol Concentrations in Adults Prescribed a High Vegetable and Fruit Diet. Nutrients 2020, 12, 3431. [Google Scholar] [CrossRef] [PubMed]
- de Fátima Alves, L.; Moore, J.B.; Kell, D.B. The Biology and Biochemistry of Kynurenic Acid, a Potential Nutraceutical with Multiple Biological Effects. Int. J. Mol. Sci. 2024, 25, 9082. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; de Vos-Houben, J.M.J.; Niessen, P.M.G.; Miyata, T.; van Nieuwenhoven, F.; Janssen, B.J.A.; Hageman, G.; Stehouwer, C.D.A.; Schalkwijk, C. Mild oxidative damage in the diabetic rat heart is attenuated by glyoxalase-1 overexpression. Int. J. Mol. Sci. 2013, 14, 15724–15739. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Brinkmann Frye, E.; Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997, 324, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Lou, X.; Xue, J.; Shi, Z.; Zhao, Y.; Wang, F.; Chen, G. The influence of Akkermansia muciniphila on intestinal barrier function. Gut Pathog. 2024, 16, 41. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Di Matteo, G.; Felli, M.; Savatin, D.V.; Mannina, L.; Merendino, N. Evaluation of the Phenolic Components, Fiber Content, Antioxidant Activity, and Prebiotic Capacity of a Shortbread Cookie Fortified with Hazelnut Skin Waste. Foods 2024, 13, 3814. [Google Scholar] [CrossRef]
- Bachari, S.; Ghaderi-Ghahfarokhi, M.; Gavlighi, H.A.; Zarei, M. Ultrasonic depolymerization of pomegranate peel pectin: Effect of sonication time on antioxidant, α-amylase inhibitory, and prebiotic properties. Food Chem. X 2024, 24, 101901. [Google Scholar] [CrossRef]
- Yousaf, A.A.; Zeng, H.; Abbasi, K.S.; Bergholz, T.; Siddiq, M.; Dolan, K. Development and biochemical characterization of freeze-dried guava powder fortified with Lactobacillus plantarum. J. Food Sci. 2024, 89, 8644–8657. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, A.; Paikra, S.K.; Sutar, P.P.; Mishra, M. Characterization and identification of inulin from Pachyrhizus erosus and evaluation of its antioxidant and in-vitro prebiotic efficacy. J. Food Sci. Technol. 2023, 60, 328–339. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Ajuwon, K.M.; Zhong, R.; Li, T.; Chen, L.; Zhang, H.; Beckers, Y.; Everaert, N. Xylo-Oligosaccharides, Preparation and Application to Human and Animal Health: A Review. Front. Nutr. 2021, 8, 731930. [Google Scholar] [CrossRef]
- Demigné, C.; Jacobs, H.; Moundras, C.; Davicco, M.-J.; Horcajada, M.-N.; Bernalier, A.; Coxam, V. Comparison of native or reformulated chicory fructans, or non-purified chicory, on rat cecal fermentation and mineral metabolism. Eur. J. Nutr. 2008, 47, 366–374. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, T.; Wang, S.; Wang, W.; Wang, Q.; Yu, H.-X. Fructo-oligosaccharides enhance the mineral absorption and counteract the adverse effects of phytic acid in mice. Nutrition 2010, 26, 305–311. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Markiewicz, L.H.; Lamparski, G.; Juśkiewicz, J. Administration of Inulin-Supplemented Gluten-Free Diet Modified Calcium Absorption and Caecal Microbiota in Rats in a Calcium-Dependent Manner. Nutrients 2017, 9, 702. [Google Scholar] [CrossRef]
- Costa, G.; Vasconcelos, Q.; Abreu, G.; Albuquerque, A.; Vilarejo, J.; Aragão, G. Changes in nutrient absorption in children and adolescents caused by fructans, especially fructooligosaccharides and inulin. Arch. Pediatr. 2020, 27, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.U.; Kasankala, L.M.; Khan, F.A.; Al-Asmari, F.; Rahim, M.A.; Hussain, I.; Angelov, A.; Bartkiene, E.; Rocha, J.M. Impact of resistant starch: Absorption of dietary minerals, glycemic index and oxidative stress in healthy rats. Clin. Nutr. ESPEN 2024, 62, 1–9. [Google Scholar] [CrossRef]
- Drabińska, N.; Krupa-Kozak, U.; Abramowicz, P.; Jarocka-Cyrta, E. Beneficial Effect of Oligofructose-Enriched Inulin on Vitamin D and E Status in Children with Celiac Disease on a Long-Term Gluten-Free Diet: A Preliminary Randomized, Placebo-Controlled Nutritional Intervention Study. Nutrients 2018, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, Y.M.; Hall, W.L.; Ellard, S.; Lee, E.; Lodge, J.K. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br. J. Nutr. 2007, 92, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Graz, S.W.; Hazim, S.; Richardson, A.J.; Scobbie, L.; Johnstone, A.M.; Fyfe, C.; Holtrop, G.; Lobley, G.E.; Russell, W.R. Dietary carbohydrate rather than protein intake drives colonic microbial fermentation during weight loss. Eur. J. Nutr. 2019, 58, 1147–1158. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Yang, Y.; Xiang, K.; Li, H.; Sun, D.; Chen, L. Kynurenine-3-monooxygenase (KMO): From its biological functions to therapeutic effect in diseases progression. J. Cell. Physiol. 2022, 237, 4339–4355. [Google Scholar] [CrossRef]
- Aliasgharzadeh, A.; Dehghan, P.; Gargari, B.P.; Asghari-Jafarabadi, M. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: A randomised controlled clinical trial. Br. J. Nutr. 2015, 113, 321–330. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Dehghan, P.; Namazi, N. Prebiotic supplementation modulates advanced glycation end-products (AGEs), soluble receptor for AGEs (sRAGE), and cardiometabolic risk factors through improving metabolic endotoxemia: A randomized-controlled clinical trial. Eur. J. Nutr. 2020, 59, 3009–3021. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Z.; Kou, M.; Wang, X.; Yue, Z.; Zhang, M. Impact of nurse-led self-management education on type 2 diabetes: A meta-analysis. Front. Public Health 2025, 13, 1622988. [Google Scholar] [CrossRef]

| RPS | Bifidobacterium | Akkermansia | ||||
|---|---|---|---|---|---|---|
| r | R2 | p | r | R2 | p | |
| 3-Hydroxyanthranillic Acid | 0.078 | 0.006 | 0.6 | 0.188 | 0.035 | 0.2 |
| 3-Hydroxykynurenine | −0.188 | 0.035 | 0.2 | 0.142 | 0.020 | 0.3 |
| All-trans Retinol | 0.008 | 0.000 | 1.0 | 0.380 | 0.144 | 0.008 |
| α-Tocopherol | 0.030 | 0.001 | 0.8 | 0.227 | 0.052 | 0.1 |
| CoQ10 | 0.128 | 0.016 | 0.4 | 0.120 | 0.014 | 0.4 |
| Hippuric Acid | 0.128 | 0.016 | 0.4 | −0.115 | 0.013 | 0.4 |
| Kynurenine | 0.149 | 0.022 | 0.3 | 0.022 | 0.001 | 0.9 |
| NƐ-(1-carboxymethyl)-L-lysine | 0.055 | 0.003 | 0.7 | −0.116 | 0.013 | 0.4 |
| NƐ-(1-carboxyethyl)-L-lysine | −0.030 | 0.001 | 0.8 | 0.098 | 0.010 | 0.5 |
| Uric Acid | 0.038 | 0.001 | 0.8 | 0.133 | 0.018 | 0.4 |
| Xanthine | 0.046 | 0.002 | 0.8 | −0.163 | 0.026 | 0.3 |
| Placebo | Bifidobacterium | Akkermansia | ||||
|---|---|---|---|---|---|---|
| r | R2 | p | r | R2 | p | |
| 3-Hydroxyanthranillic Acid | −0.077 | 0.006 | 0.6 | −0.249 | 0.062 | 0.09 |
| 3-Hydroxykynurenine | −0.046 | 0.002 | 0.8 | −0.310 | 0.096 | 0.03 |
| All-trans Retinol | −0.083 | 0.007 | 0.6 | 0.123 | 0.015 | 0.4 |
| α-Tocopherol | −0.160 | 0.026 | 0.3 | 0.075 | 0.006 | 0.6 |
| CoQ10 | −0.325 | 0.106 | 0.02 | 0.037 | 0.001 | 0.8 |
| Hippuric Acid | −0.011 | 0.000 | 0.9 | 0.203 | 0.041 | 0.2 |
| Kynurenine | −0.134 | 0.018 | 0.4 | −0.098 | 0.010 | 0.5 |
| NƐ-(1-carboxymethyl)-L-lysine | −0.277 | 0.076 | 0.06 | 0.276 | 0.076 | 0.06 |
| NƐ-(1-carboxyethyl)-L-lysine | −0.048 | 0.002 | 0.7 | 0.048 | 0.002 | 0.7 |
| Uric Acid | −0.116 | 0.013 | 0.4 | −0.019 | 0.000 | 0.9 |
| Xanthine | 0.096 | 0.009 | 0.5 | 0.086 | 0.007 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bush, J.R.; Han, J.; Goodlett, D.R. Resistant Potato Starch Supplementation Increases Serum Antioxidant Levels in a Randomized Trial. Metabolites 2025, 15, 661. https://doi.org/10.3390/metabo15100661
Bush JR, Han J, Goodlett DR. Resistant Potato Starch Supplementation Increases Serum Antioxidant Levels in a Randomized Trial. Metabolites. 2025; 15(10):661. https://doi.org/10.3390/metabo15100661
Chicago/Turabian StyleBush, Jason R., Jun Han, and David R. Goodlett. 2025. "Resistant Potato Starch Supplementation Increases Serum Antioxidant Levels in a Randomized Trial" Metabolites 15, no. 10: 661. https://doi.org/10.3390/metabo15100661
APA StyleBush, J. R., Han, J., & Goodlett, D. R. (2025). Resistant Potato Starch Supplementation Increases Serum Antioxidant Levels in a Randomized Trial. Metabolites, 15(10), 661. https://doi.org/10.3390/metabo15100661






