A Pilot Multi-Omics Approach Unveils Strong Immune Activation in the First Ten Days of Life in Extremely Preterm Infants
Abstract
1. Introduction
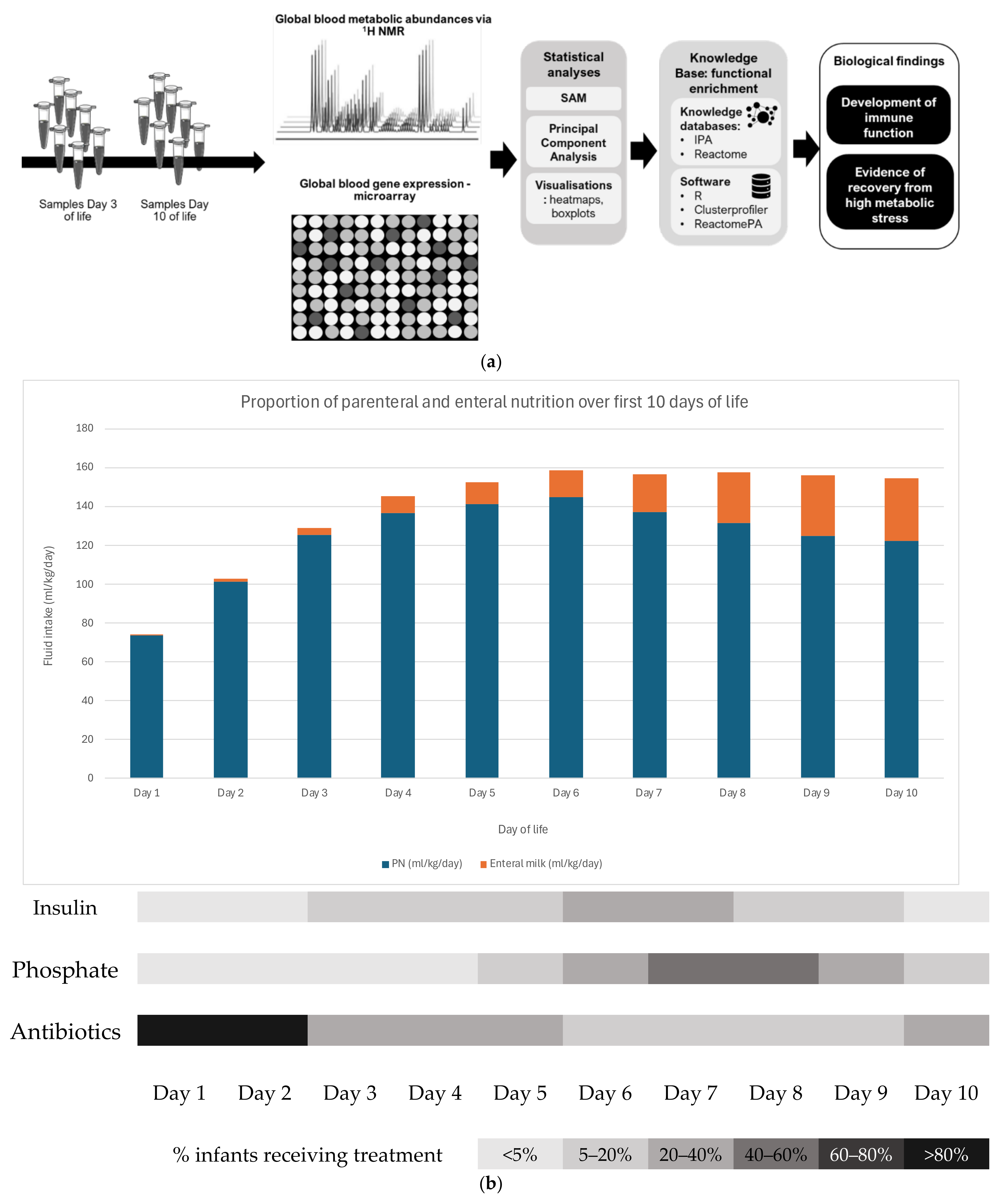
2. Methods
2.1. Patient Recruitment and Sample Collection
2.2. RNA Extraction and Microarray Data Acquisition and Analysis
2.3. H NMR Metabolomics Data Acquisition and Analysis
2.4. Real-Time qPCR
3. Results
3.1. Patient Recruitment and Characteristics
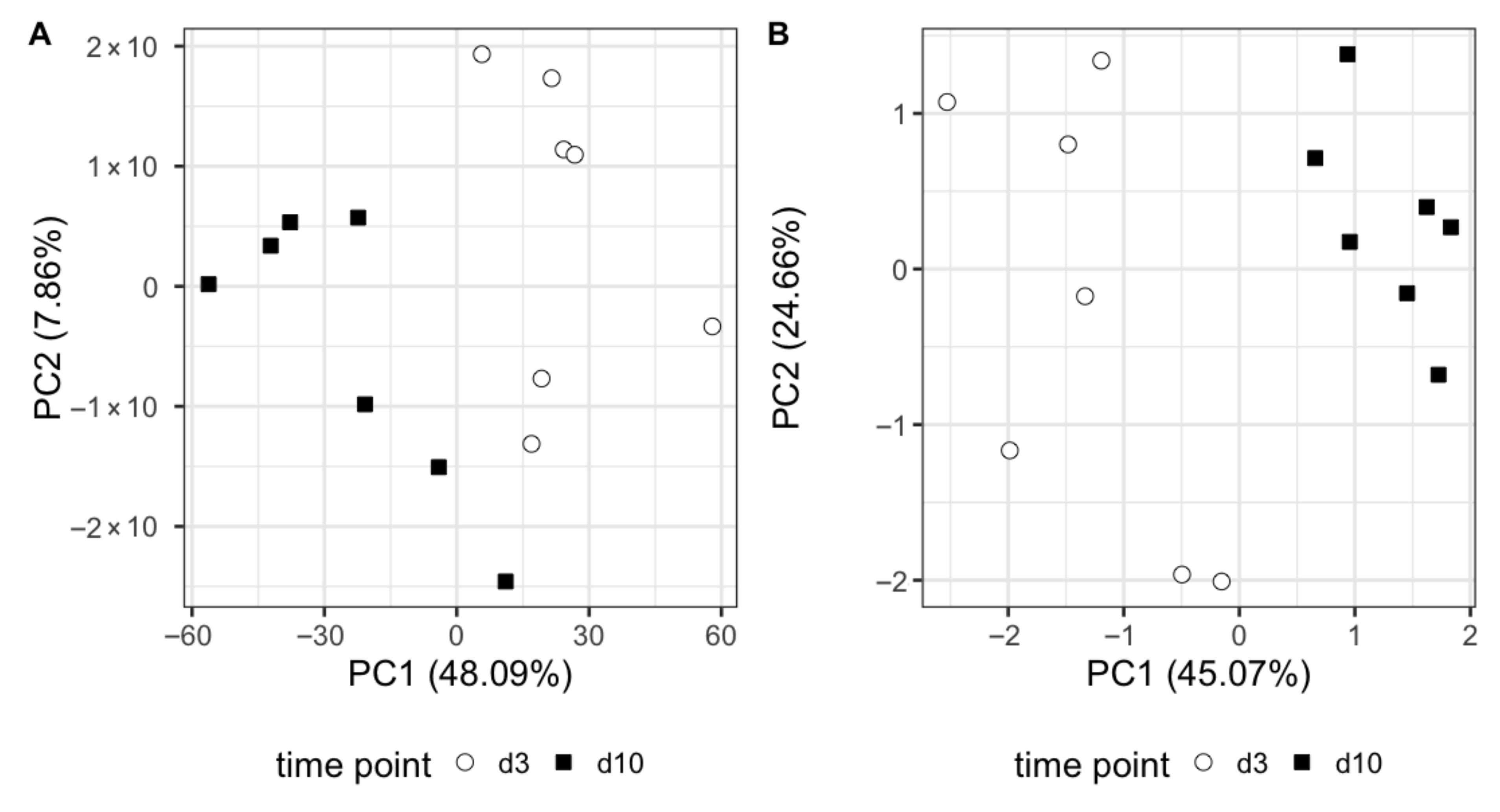
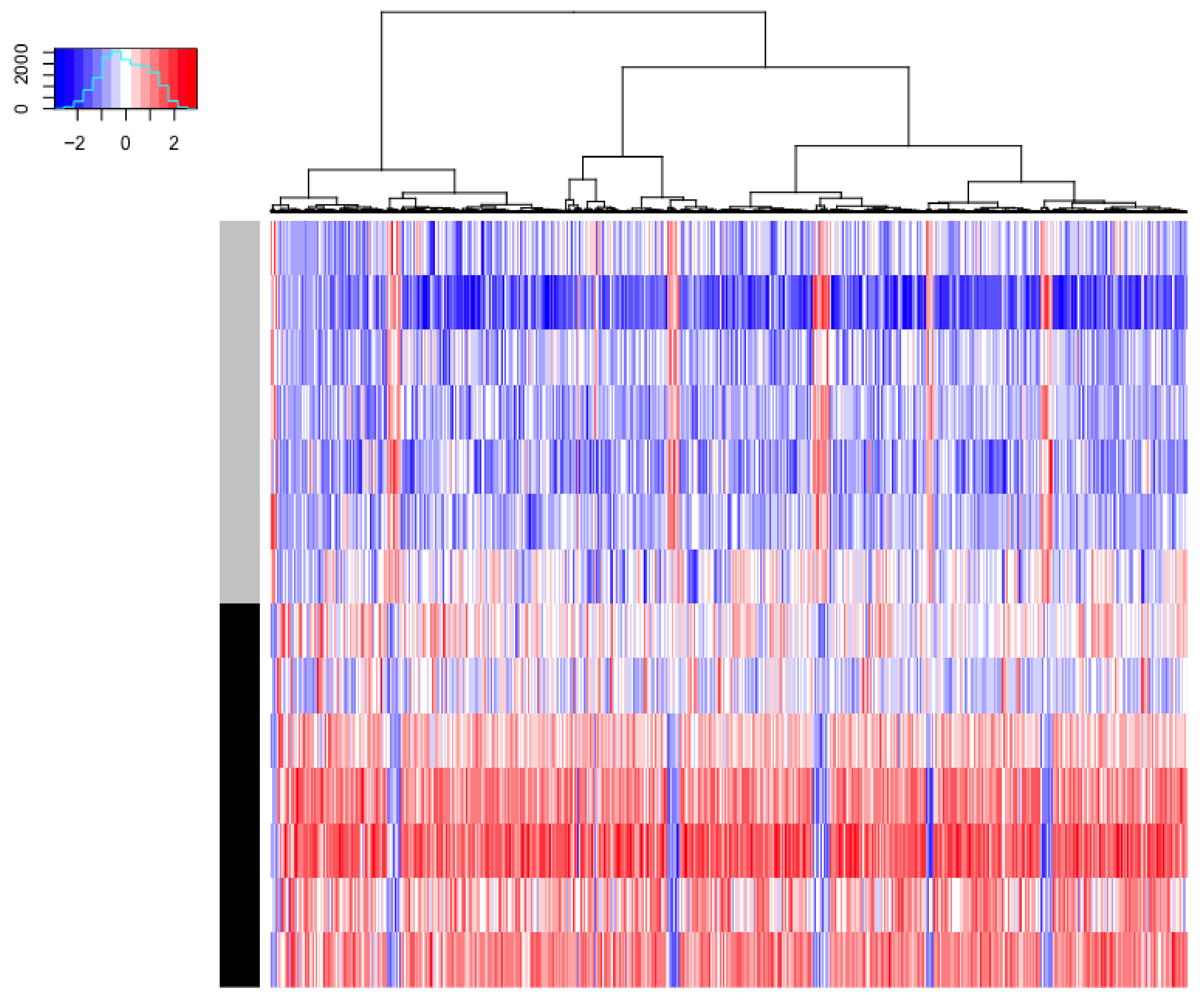
3.2. Omics Analysis
3.3. Real-Time qPCR Validation
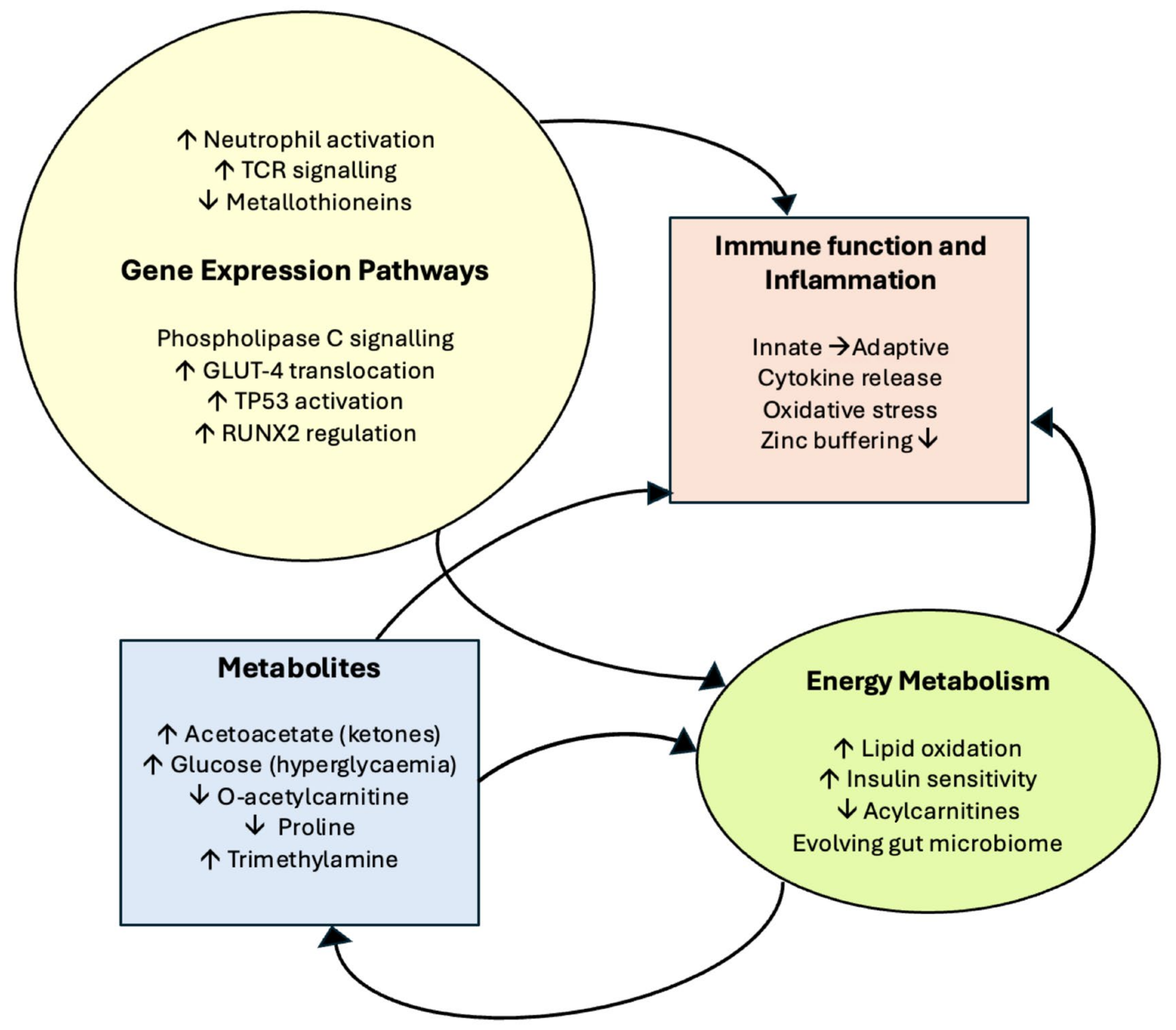
3.4. Results Summary
4. Discussion
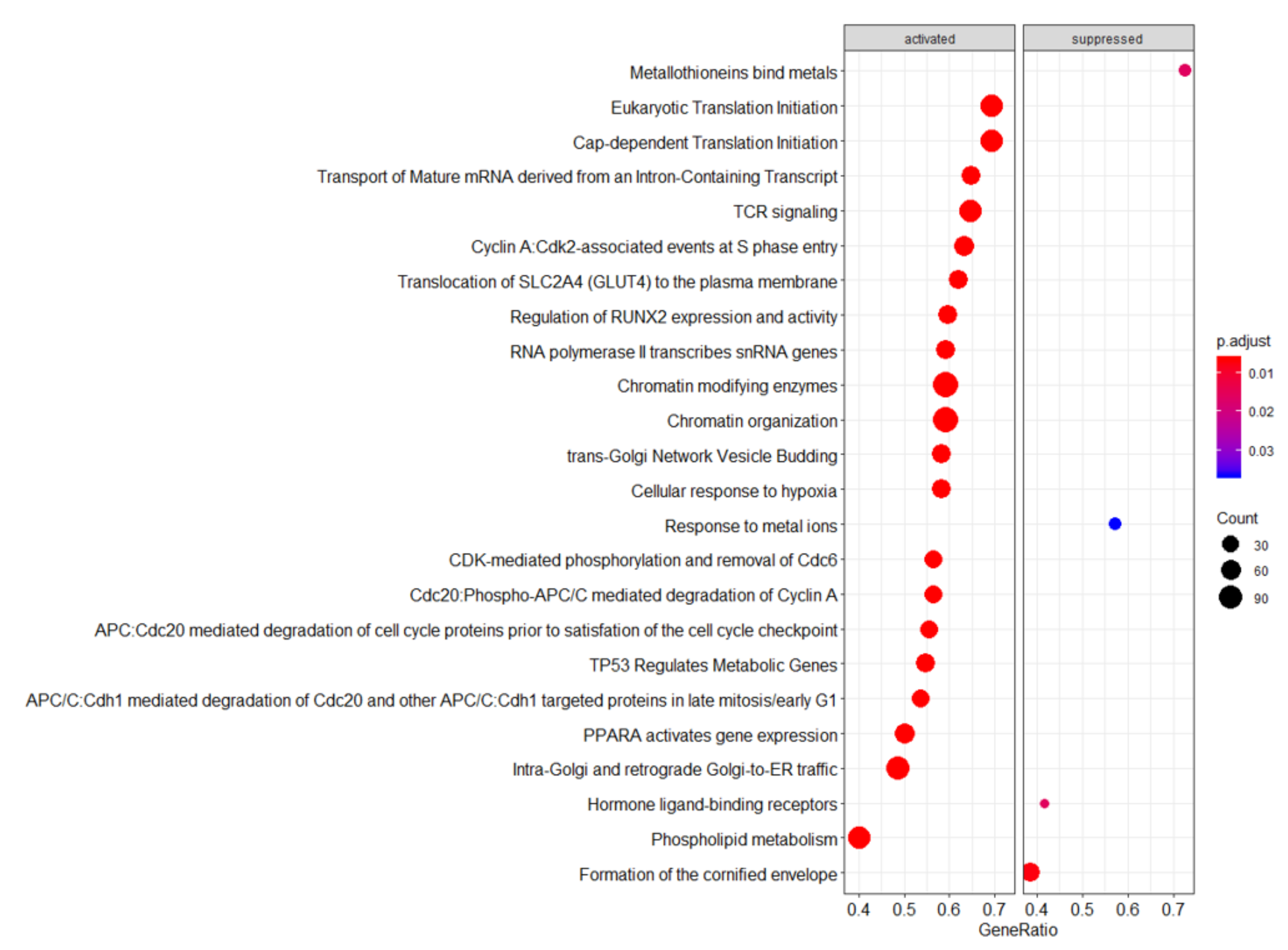
4.1. Ingenuity Pathway Analysis (IPA)—Significantly Upregulated Pathways
4.1.1. fMLP Signalling in Neutrophils
4.1.2. Fcγ Receptor-Mediated Phagocytosis by Macrophages and Monocytes
4.1.3. Phospholipase C Signalling
4.2. Reactome—Highlighted Significant Pathways
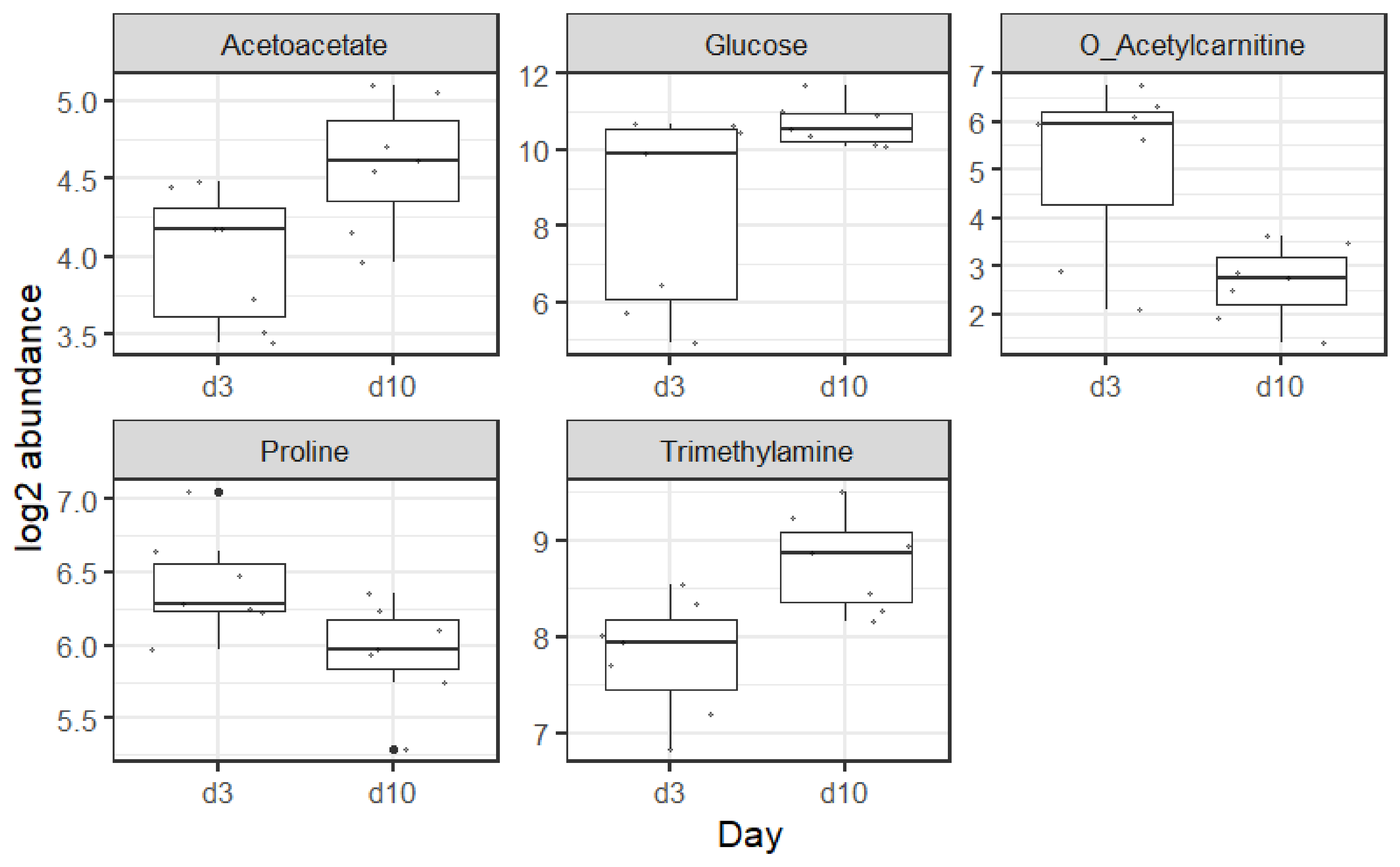
4.3. Metabolites
4.3.1. Acetoacetate
4.3.2. Glucose
4.3.3. O-Acetylcarnitine
4.3.4. Proline
4.3.5. Trimethylamine
4.4. Limitations of the Study
4.5. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PN | Parenteral nutrition |
| GALT | Gut-associated lymphoid tissue |
| VPI | Very preterm infant |
| µl | Microlitre |
| RNA | Ribonucleic acid |
| NICU | Neonatal intensive care unit |
| CRP | C-reactive protein |
| DNAse | Deoxyribonuclease |
| DNA | Deoxyribonucleic acid |
| ng | Nanogram |
| rpm | Revolutions per minute |
| µm | Micrometre |
| RIN | RNA integrity number |
| SAM | Significance Analysis of Microarrays |
| FDR | False discovery rate |
| PCA | Principal Component Analysis |
| IPA | Ingenuity®Pathway Analysis™ |
| B–H | Benjamini–Hochberg |
| NMR | Nuclear magnetic resonance |
| Hz | Hertz |
| DSS | Sodium 3-(trimethysilyl)propane-1-1sulfonate |
| ppm | Parts per million |
| PCR | Polymerase chain reaction |
| cDNA | Complementary deoxyribonucleic acid |
| Ct | Cycle threshold |
| SD | Standard deviation |
| g | Grams |
| TCR | T-cell receptor |
| fMLP | N-Formylmethionyl-leucyl-phenylalanine |
| PC | Principal component |
| d3 | Day 3 |
| d10 | Day 10 |
| IgG | Immunoglobulin G |
| PLC | Phospholipase C |
| GA | Gestational age |
| GLUT 4 | Glucose transporter 4 |
References
- Van Goudoever, J.B.; Carnielli, V.; Darmaun, D.; de Pipaon, M.S. The ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Amino acids. Clin. Nutr. 2018, 37, 2315–2323. [Google Scholar] [CrossRef]
- Morgan, C.; Tan, M. Attainment targets for protein intake using standardised, concentrated and individualised neonatal parenteral nutrition regimens. Nutrients 2019, 11, 2167. [Google Scholar] [CrossRef]
- Cormack, B.E.; Jiang, Y.; Harding, J.E.; Crowther, C.A.; Bloomfield, F.H. Relationships between Neonatal Nutrition and Growth to 36 Weeks’ Corrected Age in ELBW Babies–Secondary Cohort Analysis from the Provide Trial. Nutrients 2020, 12, 760. [Google Scholar] [CrossRef]
- De Cloet, J.; Simal, I.; Benoot, K.; Goossens, L. Assessment of the Adherence to ESPGHAN 2018 Guidelines in the Neonatal Intensive Care Unit of the Ghent University Hospital: A Retrospective Study. Nutrients 2023, 15, 2324. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Strømmen, K.; Blakstad, E.W.; Almaas, A.N.; Westerberg, A.C.; Brække, K.; Rønnestad, A.; Nakstad, B.; Berg, J.P.; Veierød, M.B.; et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia—A randomized, controlled trial. Clin. Nutr. 2013, 32, 207–212. [Google Scholar] [CrossRef]
- van Puffelen, E.; Vanhorebeek, I.; Joosten, K.F.M.; Wouters, P.J.; Van den Berghe, G.; Verbruggen, S.C. Early versus late parenteral nutrition in critically ill, term neonates: A preplanned secondary subgroup analysis of the PEPaNIC multicentre, randomised controlled trial. Lancet Child. Adolesc. Health 2018, 2, 505–515. [Google Scholar] [CrossRef]
- Vanhorebeek, I.; Verbruggen, S.; Casaer, M.P.; Gunst, J.; Wouters, P.J.; Hanot, J.; Guerra, G.G.; Vlasselaers, D.; Joosten, K.; Van den Berghe, G. Effect of early supplemental parenteral nutrition in the paediatric ICU: A preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir. Med. 2017, 5, 475–483. [Google Scholar] [CrossRef]
- Angelika, D.; Etika, R.; Utomo, M.T.; Mirha, S.; Handayani, K.D.; Ugrasena, I.D.G. The glucose infusion rate of parenteral nutrition in the first week of life in preterm infants: An observational study. Ital. J. Pediatr. 2021, 47, 219. [Google Scholar] [CrossRef]
- Muk, T.; Brunse, A.; Henriksen, N.L.; Aasmul-Olsen, K.; Nguyen, D.N. Glucose supply and glycolysis inhibition shape the clinical fate of Staphylococcus epidermidis-infected preterm newborns. JCI Insight 2022, 7, e157234. [Google Scholar] [CrossRef]
- Shouman, B.; Abdel-Hady, H.; Badr, R.I.; Hammad, E.; Salama, M.F. Dose of intravenous lipids and rate of bacterial clearance in preterm infants with blood stream infections. Eur. J. Pediatr. 2012, 171, 811–816. [Google Scholar] [CrossRef]
- Kapoor, V.; Malviya, M.N.; Soll, R. Lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst. Rev. 2019, 6, CD013163. [Google Scholar] [CrossRef] [PubMed]
- Walsh, V.; McGuire, W. Immunonutrition for Preterm Infants. Neonatology 2019, 115, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sadeghirad, B.; Morgan, R.L.; Zeratkaar, D.; Chang, Y.; Crandon, H.N.; Couban, R.; Foroutan, F.; Florez, I.D. Amino acids for the prevention of mortality and morbidity in preterm infants: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 18333. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.P.; Rostas, S.; Rogers, S.; Martin, C.R.; Calkins, K.L. Parenteral Nutrition in the Neonatal Intensive Care Unit: Intravenous Lipid Emulsions. Clin. Perinatol. 2023, 50, 575–589. [Google Scholar] [CrossRef]
- Patel, J.J.; Miller, K.R.; Rosenthal, C.; Rosenthal, M.D. When Is It Appropriate to Use Arginine in Critical Illness? Nutr. Clin. Pract. 2016, 31, 438–444. [Google Scholar] [CrossRef]
- Tsafaras, G.P.; Ntontsi, P.; Xanthou, G. Advantages and Limitations of the Neonatal Immune System. Front. Pediatr. 2020, 8, 5. [Google Scholar] [CrossRef]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; Salles, É.D.S.L.; Bhatia, J.; Hale, V.L.; Baban, B. Innate Immunity of Neonates and Infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef]
- Atyeo, C.; Alter, G. The multifaceted roles of breast milk antibodies. Cell 2021, 184, 148–1499. [Google Scholar] [CrossRef]
- Holm, S.R.; Jenkins, B.J.; Cronin, J.G.; Jones, N.; Thornton, C.A. A role for metabolism in determining neonatal immune function. Pediatr. Allergy Immunol. 2021, 32, 1616–1628. [Google Scholar] [CrossRef]
- MAQC Consortium; Shi, L.; Reid, L.H.; Jones, W.D.; Shippy, R.; Warrington, J.A.; Baker, S.C.; Collins, P.J.; de Longueville, F.; Kawasaki, E.S.; et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat. Biotechnol. 2006, 24, 1151–1161. [Google Scholar] [CrossRef]
- Tibshirani, A.R.; Chu, G.; Narasimhan, B.; Li, J. SAM: Significance Analysis of Microarrays. Available online: http://cran.r-project.org/web/packages/samr/index.html (accessed on 22 June 2023).
- R Core Team, R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 21 June 2023).
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Yu, G.; He, Q.Y. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 2016, 12, 477–479. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. Ser. A 2011, 174, 245–246. [Google Scholar] [CrossRef]
- He, X.; Parenti, M.; Grip, T.; Domellöf, M.; Lönnerdal, B.; Hernell, O.; Timby, N.; Slupsky, C.M. Metabolic phenotype of breast-fed infants, and infants fed standard formula or bovine MFGM supplemented formula: A randomized controlled trial. Sci. Rep. 2019, 9, 339. [Google Scholar] [CrossRef]
- Wu, Z.; Tien, N.T.N.; Bæk, O.; Zhong, J.; Klabunde, B.; Nguyen, T.T.; Yen, N.T.H.; Long, N.P.; Nguyen, D.N. Regulation of host metabolism and defense strategies to survive neonatal infection. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167482. [Google Scholar] [CrossRef]
- Schiffmann, E.; Corcoran, B.A.; Wahl, S.M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl. Acad. Sci. USA 1975, 72, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Selvatici, R.; Falzarano, S.; Mollica ASpisani, S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur. J. Pharmacol. 2006, 534, 1–11. [Google Scholar] [CrossRef]
- Junker, F.; Gordon, J.; Qureshi, O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front. Immunol. 2020, 11, 1393. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, F.; Phospholipase, C. Encyclopedia of Biological Chemistry: Second Edition; Academic Press: Cambridge, MA, USA, 2013; pp. 467–471. [Google Scholar] [CrossRef]
- Rahman, M.T.; Haque, N.; Abu Kasim, N.H.; De Ley, M. Origin, Function, and Fate of Metallothionein in Human Blood. Rev. Physiol. Biochem. Pharmacol. 2017, 173, 41–62. [Google Scholar] [CrossRef]
- Adamo, A.M.; Oteiza, P.I. Zinc deficiency and neurodevelopment: The case of neurons. Biofactors 2010, 36, 117–124. [Google Scholar] [CrossRef]
- Kumasaka, S.; Negishi, Y.; Morita, R.; Migita, M.; Shima, Y. Immunological role of zinc in preterm neonates. Immunol. Med. 2025, 48, 78–88. [Google Scholar] [CrossRef]
- Polberger, S.; Fletcher, M.P.; Graham, T.W.; Vruwink, K.; Gershwin, M.E.; Lönnerdal, B. Effect of infant formula zinc and iron level on zinc absorption, zinc status, and immune function in infant rhesus monkeys. J. Pediatr. Gastroenterol. Nutr. 1996, 22, 134–143. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Malavolta, M.; Muti, E.; Costarelli, L.; Cipriano, C.; Piacenza, F.; Tesei, S.; Giacconi, R.; Lattanzio, F. Zinc, metallothioneins and longevity: Interrelationships with niacin and selenium. Curr. Pharm. Des. 2008, 14, 2719–2732. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Wu, M.; Huang, J. Identification of Inflammatory Genes, Pathways, and Immune Cells in Necrotizing Enterocolitis of Preterm Infant by Bioinformatics Approaches. Biomed. Res. Int. 2021, 2021, 5568724. [Google Scholar] [CrossRef]
- Hawdon, J.M.; Ward Platt, M.P.; Aynsley-Green, A. Patterns of metabolic adaptation for preterm and term infants in the first neonatal week. Arch. Dis. Child. 1992, 67, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Bloom, S.R.; Aynsley Green, A. Metabolic and endocrine events at the time of the first feed of human milk in preterm and term infants. Arch. Dis. Child. 1978, 53, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.K.; Tebani, A.; Malmodin, D.; Pedersen, A.; Hellgren, G.; Löfqvist, C.; Hansen-Pupp, I.; Uhlén, M.; Hellström, A. Longitudinal Serum Metabolomics in Extremely Premature Infants: Relationships With Gestational Age, Nutrition, and Morbidities. Front. Neurosci. 2022, 16, 830884. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C. What is the evidence base for using insulin treatment to prevent or treat neonatal hyperglycaemia? Early Hum. Dev. 2015, 91, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Beardsall, K. Hyperglycaemia in the Newborn Infant. Physiology Verses Pathology. Front. Pediatr. 2021, 9, 641306. [Google Scholar] [CrossRef]
- Vargas, E.; Podder, V.; Carrillo Sepulveda, M.A. Physiology, Glucose Transporter Type 4. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Thiele, I.G.; Niezen-Koning, K.E.; van Gennip, A.H.; Aarnoudse, J.G. Increased plasma carnitine concentrations in preeclampsia. Obstet. Gynecol. 2004, 103, 876–880. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Oleg, A.; Shchelochkov, O.A.; Cook, D.E.; Berberich, S.L.; Copeland, S.; Dagle, J.M.; Murray, J.C. The influence of maternal disease on metabolites measured as part of newborn screening. J. Matern. Fetal Neonatal Med. 2013, 26, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Younge, N.E.; Newgard, C.B.; Cotton, C.M.; Goldberg, R.N.; Muehlbauer, M.J.; Bain, J.R.; Stevens, R.D.; O’Connell, T.M.; Rawls, J.F.; Seed, P.C.; et al. Disrupted Maturation of the Microbiota and Metabolome among Extremely Preterm Infants with Postnatal Growth Failure. Sci. Rep. 2019, 9, 8167. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Faes, C.; Debevec, T.; Rytz, C.; Millet, G.; Pialoux, V. Preterm birth and oxidative stress: Effects of acute physical exercise and hypoxia physiological responses. Redox Biol. 2018, 17, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Stella, A.M.G.; Calvani, M.; Butterfield, D.A. Acetylcarnitine and cellular stress response: Roles in nutritional redox homeostasis and regulation of longevity genes. J. Nutr. Biochem. 2006, 17, 73–88. [Google Scholar] [CrossRef]
- Tomlinson, C.; Rafii, M.; Sgro, M.; Ball, R.O.; Pencharz, P. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates. Pediatr. Res. 2011, 69, 46–50. [Google Scholar] [CrossRef]
- Morgan, C.; Burgess, L. High Protein Intake Does Not Prevent Low Plasma Levels of Conditionally Essential Amino Acids in Very Preterm Infants Receiving Parenteral Nutrition. J. Parenter. Enter. Nutr. 2017, 41, 455–462. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline deficiency. J. Nutr. Biochem. 1990, 1, 332–349. [Google Scholar] [CrossRef]
- Bavineni, M.; Wassenaar, T.M.; Agnihotri, K.; Ussery, D.W.; Lüscher, T.F.; Mehta, J.L. Mechanisms linking preterm birth to onset of cardiovascular disease later in adulthood. Eur. Heart J. 2019, 40, 1107–1112. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and trimethylamine N-oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef]
- Saugstad, O.D.; Kwinta, P.; Wollen, E.J.; Bik-Multanowski, M.; Madetko-Talowska, A.; Jagła, M.; Tomasik, T.; Pietrzyk, J.J. Impact of antenatal glucocorticosteroids on whole-genome expression in preterm babies. Acta Paediatr. Int. J. Paediatr. 2013, 102, 349–355. [Google Scholar] [CrossRef]
- Stevenson, D.K.; Chang, A.L.; Wong, R.J.; Reiss, J.D.; Gaudillière, B.; Sylvester, K.G.; Ling, X.B.; Angst, M.S.; Shaw, G.M.; Katz, M.; et al. Towards a new taxonomy of preterm birth. J. Perinatol. 2025, 45, 1158–1162. [Google Scholar] [CrossRef]
- Leiva, T.; Lueschow, S.; Burge, K.; Devette, C.; McElroy, S.; Chaaban, H. Biomarkers of necrotizing enterocolitis in the era of machine learning and omics. Semin. Perinatol. 2023, 47, 151693. [Google Scholar] [CrossRef]
- Ng, S.; Strunk, T.; Jiang, P.; Muk, T.; Sangild, P.T.; Currie, A. Precision medicine for neonatal sepsis. Front. Mol. Biosci. 2018, 5, 70. [Google Scholar] [CrossRef]
- Toldi, G.; Hummler, H.; Pillay, T. T Lymphocytes, Multi-Omic Interactions and Bronchopulmonary Dysplasia. Front. Pediatr. 2021, 9, 694034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgess, L.; Caamaño Gutiérrez, E.; Flanagan, B.F.; Sylvestre, D.A.; Slupsky, C.M.; Turner, M.A.; Morgan, C. A Pilot Multi-Omics Approach Unveils Strong Immune Activation in the First Ten Days of Life in Extremely Preterm Infants. Metabolites 2025, 15, 659. https://doi.org/10.3390/metabo15100659
Burgess L, Caamaño Gutiérrez E, Flanagan BF, Sylvestre DA, Slupsky CM, Turner MA, Morgan C. A Pilot Multi-Omics Approach Unveils Strong Immune Activation in the First Ten Days of Life in Extremely Preterm Infants. Metabolites. 2025; 15(10):659. https://doi.org/10.3390/metabo15100659
Chicago/Turabian StyleBurgess, Laura, Eva Caamaño Gutiérrez, Brian F. Flanagan, Duncan Alexander Sylvestre, Carolyn M. Slupsky, Mark A. Turner, and Colin Morgan. 2025. "A Pilot Multi-Omics Approach Unveils Strong Immune Activation in the First Ten Days of Life in Extremely Preterm Infants" Metabolites 15, no. 10: 659. https://doi.org/10.3390/metabo15100659
APA StyleBurgess, L., Caamaño Gutiérrez, E., Flanagan, B. F., Sylvestre, D. A., Slupsky, C. M., Turner, M. A., & Morgan, C. (2025). A Pilot Multi-Omics Approach Unveils Strong Immune Activation in the First Ten Days of Life in Extremely Preterm Infants. Metabolites, 15(10), 659. https://doi.org/10.3390/metabo15100659





