Insights from Metabolomics Profiling of MSUD in Pediatrics Toward Disease Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Ethical Approval
2.3. Study Participants and Sample Collection
2.4. Metabolite Extraction
2.5. LC-HRMS Metabolomics Analysis
2.6. Metabolomics Data Processing, Annotation, and Statistical Analysis
3. Results
3.1. Demographic and Clinical Features of Pediatric Participants
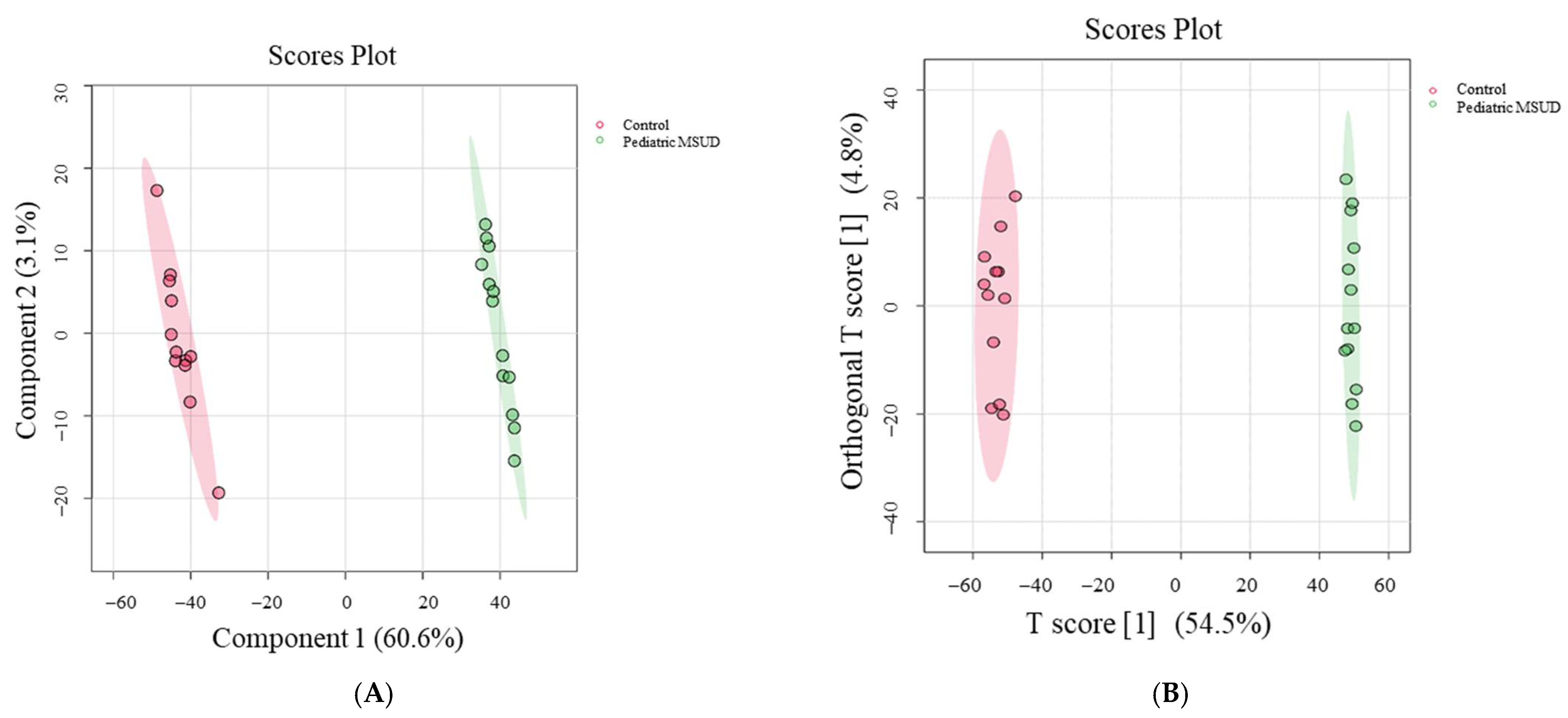
3.2. Untargeted Metabolomics Profile of MSUD Patients
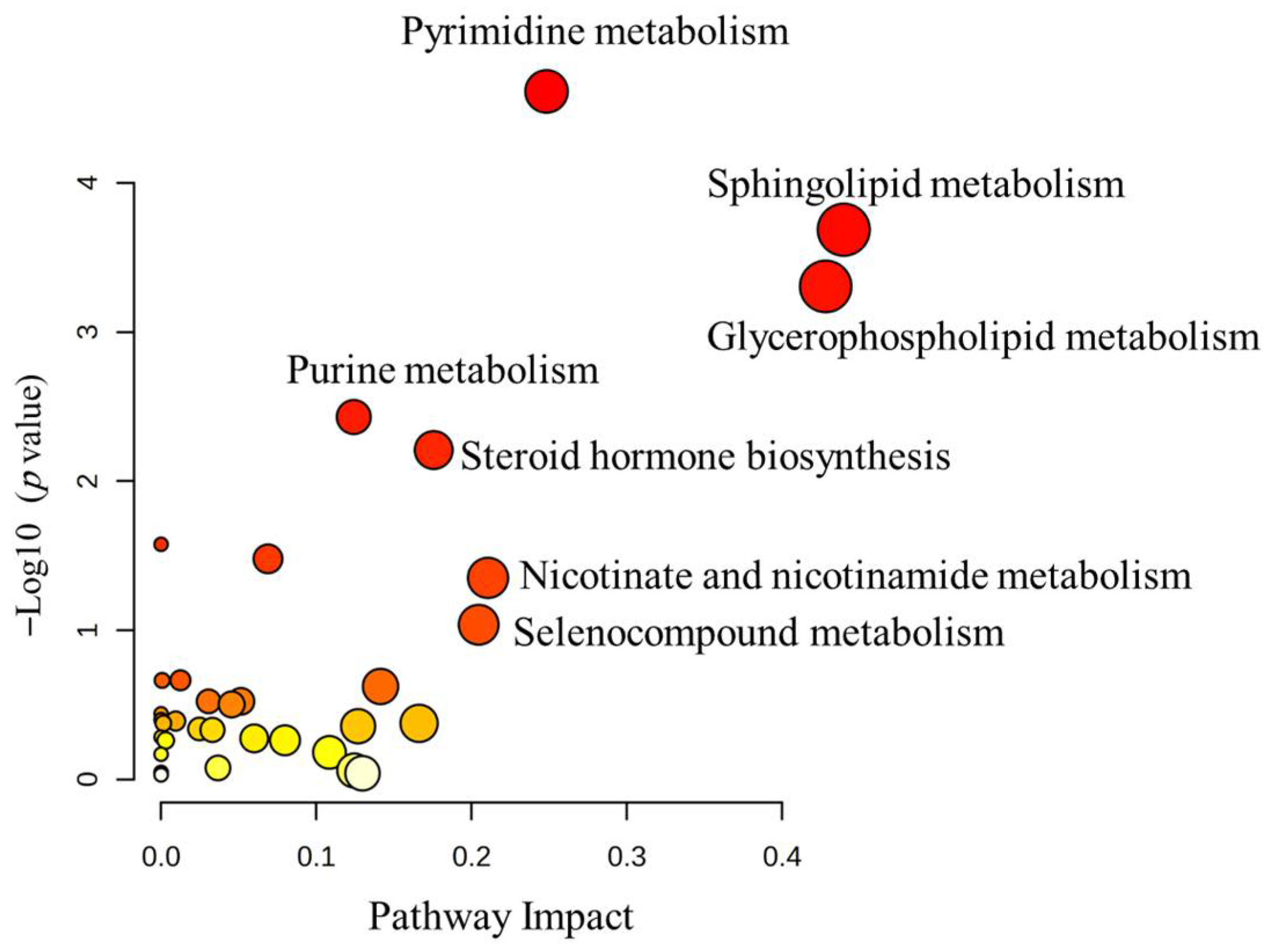
3.3. Analysis of Metabolomic Pathway
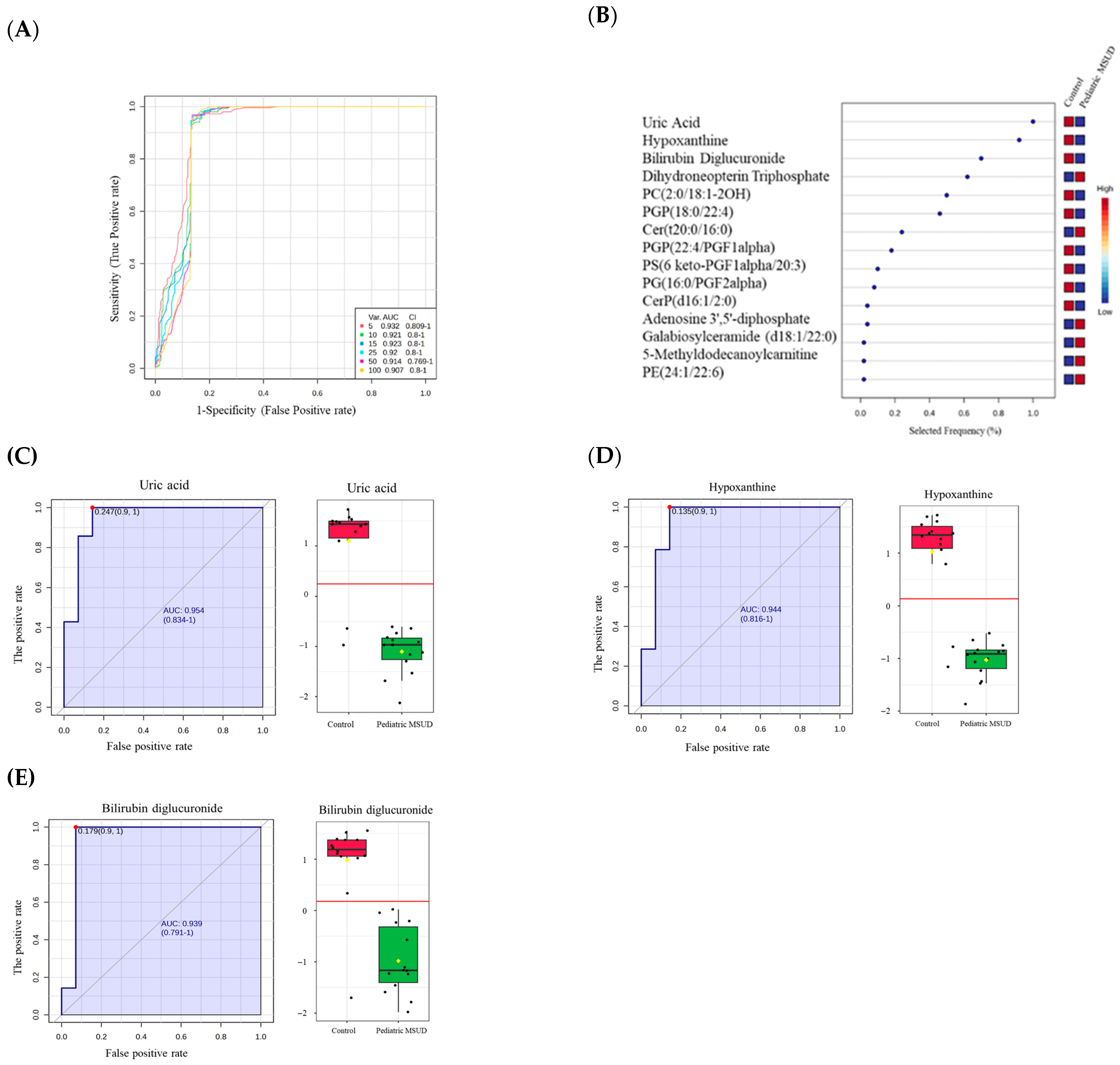
3.4. Biomarkers Analysis of Pediatric MSUD
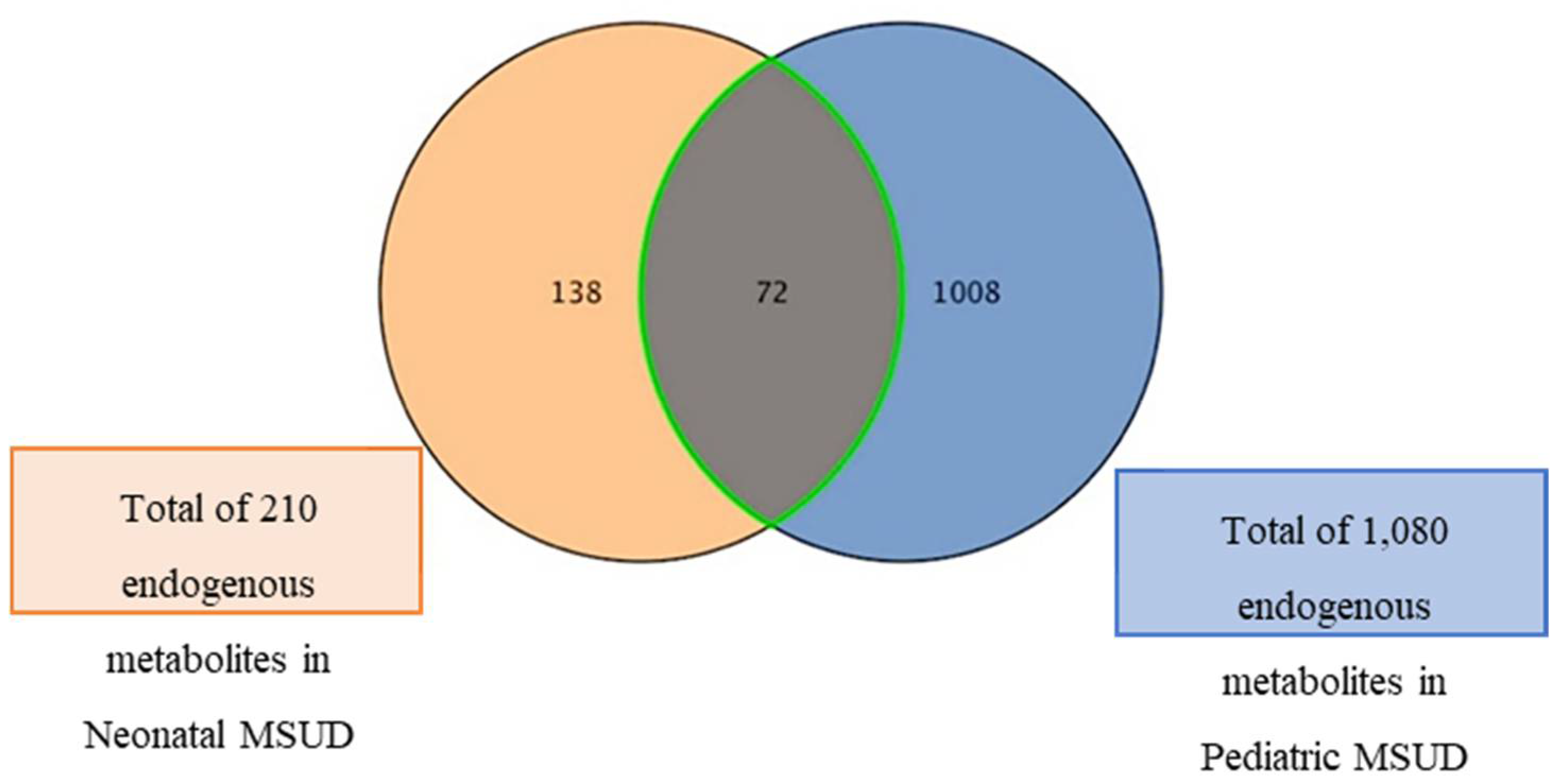
3.5. Dysregulated Metabolites Shared Between Neonatal and Pediatric MSUD Patients
4. Discussion
4.1. Untargeted Metabolomics Offers a Framework for Characterizing Metabolic Trajectories Across Developmental Stages
4.2. Different Metabolomics Patterns Between Pediatric MSUD Patients and Healthy Controls
4.3. Distinct Biomarkers of Pediatric MSUD Compared to Neonatal MSUD
4.4. Inverse Expression of Dysregulated Metabolites Shared Between Neonatal and Pediatric MSUD Patients
4.5. Limitations and Future Directions of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, P.R.; Gass, J.M.; Vairo, F.P.E.; Farnham, K.M.; Atwal, H.K.; Macklin, S.; Klee, E.W.; Atwal, P.S. Maple syrup urine disease: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Frazier, D.M.; Allgeier, C.; Homer, C.; Marriage, B.J.; Ogata, B.; Rohr, F.; Splett, P.L.; Stembridge, A.; Singh, R.H. Nutrition management guideline for maple syrup urine disease: An evidence- and consensus-based approach. Mol. Genet. Metab. 2014, 112, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Mazariegos, G.V.; Morton, D.H.; Sindhi, R.; Soltys, K.; Nayyar, N.; Bond, G.; Shellmer, D.; Shneider, B.; Vockley, J.; Strauss, K.A. Liver transplantation for classical maple syrup urine disease: Long-term follow-up in 37 patients and comparative United Network for Organ Sharing experience. J. Pediatr. 2012, 160, 116–121.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lu, D.; Xu, F.; Ji, W.; Zhan, X.; Gao, X.; Qiu, W.; Zhang, H.; Liang, L.; Gu, X.; et al. Newborn screening of maple syrup urine disease and the effect of early diagnosis. Clin. Chim. Acta 2023, 548, 117483. [Google Scholar] [CrossRef]
- Younes, S.; Elkahlout, R.; Kilani, H.; Okashah, S.; Sharshani, H.A.; Rezoug, Z.; Zayed, H.; Al-Dewik, N. Spectrum of genetic variants associated with maple syrup urine disease in the Middle East, North Africa, and Türkiye (MENAT): A systematic review. BMC Med. Genom. 2025, 18, 1. [Google Scholar] [CrossRef]
- Schmidt, J.L.; Castellanos-Brown, K.; Childress, S.; Bonhomme, N.; Oktay, J.S.; Terry, S.F.; Kyler, P.; Davidoff, A.; Greene, C. The impact of false-positive newborn screening results on families: A qualitative study. Genet. Med. 2012, 14, 76–80. [Google Scholar] [CrossRef]
- Sajeev, M.; Chin, S.; Ho, G.; Bennetts, B.; Sankaran, B.P.; Gutierrez, B.; Devanapalli, B.; Tolun, A.A.; Wiley, V.; Fletcher, J.; et al. Challenges in Diagnosing Intermediate Maple Syrup Urine Disease by Newborn Screening and Functional Validation of Genomic Results Imperative for Reproductive Family Planning. Int. J. Neonatal Screen. 2021, 7, 25. [Google Scholar] [CrossRef]
- Yang, X.; Yang, R.; Zhang, T.; Tan, D.J.; Pan, R.; Chen, Z.; Wu, D.; Chen, C.; Xu, Y.; Zhang, L.; et al. Genotypic and phenotypic spectrum of maple syrup urine disease in Zhejiang of China. QJM Int. J. Med. 2024, 117, 717–727. [Google Scholar] [CrossRef]
- Tebani, A.; Abily-Donval, L.; Afonso, C.; Marret, S.; Bekri, S. Clinical Metabolomics: The New Metabolic Window for Inborn Errors of Metabolism Investigations in the Post-Genomic Era. Int. J. Mol. Sci. 2016, 17, 1167. [Google Scholar] [CrossRef]
- Wurth, R.; Turgeon, C.; Stander, Z.; Oglesbee, D. An evaluation of untargeted metabolomics methods to characterize inborn errors of metabolism. Mol. Genet. Metab. 2024, 141, 108115. [Google Scholar] [CrossRef]
- Becker, S.; Kortz, L.; Helmschrodt, C.; Thiery, J.; Ceglarek, U. LC–MS-based metabolomics in the clinical laboratory. J. Chromatogr. B 2012, 883–884, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Khan, A.; Christensen, M.; Zhou, X.; Lund, A.; Grønborg, S.W.; Wibrand, F.; Østergaard, E.; Moritz, T. A diagnostic algorithm for inherited metabolic disorders using untargeted metabolomics. Metabolomics 2025, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.Z.; AlMalki, R.H.; Mogren, M.A.; Sebaa, R.; Alanazi, M.; Jacob, M.; Alodaib, A.; Alfares, A.; Rahman, A.M.A. Exploratory Untargeted Metabolomics of Dried Blood Spot Samples from Newborns with Maple Syrup Urine Disease. Int. J. Mol. Sci. 2024, 25, 5720. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.D.; Newby, L.K.; Eckstrand, J.; Wixted, D.; Singh, R.H. Lipid changes in the metabolome of a single case study with maple syrup urine disease (MSUD) after five days of improved diet adherence of controlled branched-chain amino acids (BCAA). Mol. Genet. Metab. Rep. 2020, 25, 100651. [Google Scholar] [CrossRef]
- Fortis, S.P.; Grier, A.L.; Reisz, J.A.; Dzieciatkowska, M.; Cendali, F.I.; Kauffman, V.; Morton, D.H.; D’aLessandro, A. Advancing the Biochemical Understanding of Maple Syrup Urine Disease and the Impact of Liver Transplantation: A Pilot Study. J. Proteome Res. 2025, 24, 3088–3104. [Google Scholar] [CrossRef]
- Tejedor, J.R.; Soriano-Sexto, A.; Beccari, L.; Castejón-Fernández, N.; Correcher, P.; Sainz-Ledo, L.; Alba-Linares, J.J.; Urdinguio, R.G.; Ugarte, M.; Fernández, A.F. Integration of multi-omics layers empowers precision diagnosis through unveiling pathogenic mechanisms on maple syrup urine disease. J. Inherit. Metab. Dis. 2025, 48, e12829. [Google Scholar] [CrossRef]
- Thistlethwaite, L.R.; Li, X.; Burrage, L.C.; Riehle, K.; Hacia, J.G.; Braverman, N.; Wangler, M.F.; Miller, M.J.; Elsea, S.H.; Milosavljevic, A. Clinical diagnosis of metabolic disorders using untargeted metabolomic profiling and disease-specific networks learned from profiling data. Sci. Rep. 2022, 12, 1. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Werner, E.; Heilier, J.-F.; Ducruix, C.; Ezan, E.; Junot, C.; Tabet, J.-C. Mass spectrometry for the identification of the discriminating signals from metabolomics: Current status and future trends. J. Chromatogr. B 2008, 871, 143–163. [Google Scholar] [CrossRef]
- Alodaib, A.; Carpenter, K.; Wiley, V.; Sim, K.; Christodoulou, J.; Wilcken, B. An improved ultra performance liquid chromatography-tandem mass spectrometry method for the determination of alloisoleucine and branched chain amino acids in dried blood samples. Ann. Clin. Biochem. 2011, 48, 468–470. [Google Scholar] [CrossRef]
- Dunn, W.B.; Wilson, I.D.; Nicholls, A.W.; Broadhurst, D. The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. Bioanalysis 2012, 4, 2249–2264. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2021, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar] [PubMed]
- Pontzer, H.; Yamada, Y.; Sagayama, H.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; Blaak, E.E. Daily energy expenditure through the human life course. Science 2021, 373, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Ku, L.C.; Smith, P.B.; Ku, L.C.; Smith, P.B. Dosing in neonates: Special considerations in physiology and trial design. Pediatr. Res. 2015, 77, 1. [Google Scholar] [CrossRef]
- Longo, L.D. Fetal–Neonatal Growth and Metabolism. In The Rise of Fetal and Neonatal Physiology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Tucker, M.H.; Tiwari, P.; Carter, B.S. The physiology, assessment, and treatment of neonatal pain. Semin. Fetal Neonatal Med. 2023, 28, 101465. [Google Scholar] [CrossRef]
- Jia, X.; Fan, J.; Wu, X.; Cao, X.; Ma, L.; Abdelrahman, Z.; Zhao, F.; Zhu, H.; Bizzarri, D.; Akker, E.B.v.D.; et al. A Novel Metabolomic Aging Clock Predicting Health Outcomes and Its Genetic and Modifiable Factors. Adv. Sci. 2024, 11, e2406670. [Google Scholar] [CrossRef]
- Moco, S.; Collino, S.; Rezzi, S.; Martin, F.-P.J. Metabolomics perspectives in pediatric research. Pediatr. Res. 2013, 73, 2. [Google Scholar] [CrossRef]
- Mutz, J.; Iniesta, R.; Lewis, C.M. Metabolomic age (MileAge) predicts health and life span: A comparison of multiple machine learning algorithms. Sci. Adv. 2024, 10, eadp3743. [Google Scholar] [CrossRef]
- Panyard, D.J.; Yu, B.; Snyder, M.P. The metabolomics of human aging: Advances, challenges, and opportunities. Sci. Adv. 2022, 8, eadd6155. [Google Scholar] [CrossRef]
- Reiss, J.D.; Mataraso, S.J.; Holzapfel, L.F.; Marić, I.; Kasowski, M.M.; Martin, C.R.; Long, J.Z.; Stevenson, D.K.; Shaw, G.M.; on behalf of the Stanford Metabolic Health Center. Applications of Metabolomics and Lipidomics in the Neonatal Intensive Care Unit. NeoReviews 2025, 26, e100–e114. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yong, V.W. Oxidized phospholipids as novel mediators of neurodegeneration. Trends Neurosci. 2022, 45, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134 Pt 7, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Funchal, C.; Latini, A.; Jacques-Silva, M.C.; Santos AQd Buzin, L.; Gottfried, C.; Wajner, M.; Pessoa-Pureur, R. Morphological alterations and induction of oxidative stress in glial cells caused by the branched-chain α-keto acids accumulating in maple syrup urine disease. Neurochem. Int. 2006, 49, 640–650. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies Keir, J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Kulikova, V.A.; Gromyko, D.V.; Nikiforov, A.A. The Regulatory Role of NAD in Human and Animal Cells. Biochem. Biokhimiia 2018, 83, 800–812. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Mileti, L.N.; Baleja, J.D.; Mileti, L.N.; Baleja, J.D. The Role of Purine Metabolism and Uric Acid in Postnatal Neurologic Development. Molecules 2025, 30, 839. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Yıldız, Y.; Akcan Yıldız, L.; Dursun, A.; Tokatlı, A.; Coşkun, T.; Tekşam, Ö.; Sivri, H.S. Predictors of acute metabolic decompensation in children with maple syrup urine disease at the emergency department. Eur. J. Pediatr. 2020, 179, 7. [Google Scholar] [CrossRef]
- Kumbhar, S.; Musale, M.; Jamsa, A.; Kumbhar, S.; Musale, M.; Jamsa, A. Bilirubin metabolism: Delving into the cellular and molecular mechanisms to predict complications. Egypt. J. Intern. Med. 2024, 36, 1. [Google Scholar] [CrossRef]
- Grijalva, J.; Vakili, K. Neonatal liver physiology. Semin. Pediatr. Surg. 2013, 22, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Neal-Kluever, A.; Fisher, J.; Grylack, L.; Kakiuchi-Kiyota, S.; Halpern, W. Physiology of the Neonatal Gastrointestinal System Relevant to the Disposition of Orally Administered Medications. Drug Metab. Dispos. 2019, 47, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Anthony, R.; McKinlay, C.J. Adaptation for life after birth: A review of neonatal physiology. Anaesth. Intensive Care Med. 2023, 24, 1–9. [Google Scholar] [CrossRef]
- Chakkarapani, A.A.; Roehr, C.C.; Hooper, S.B.; te Pas, A.B.; Gupta, S. Transitional circulation and hemodynamic monitoring in newborn infants. Pediatr. Res. 2023, 96, 3. [Google Scholar] [CrossRef]
- Jones, C.T.; Harding, J.E.; Robinson, J.S.; Lafeber, H.N.; Rolph, T.P. Aspects of the Regulation of Fetal Growth. In Advances in Animal and Comparative Physiology; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Ruggiero, A.; Ariano, A.; Triarico, S.; Capozza, M.A.; Ferrara, P.; Attinà, G. Neonatal pharmacology and clinical implications. Drugs Context 2019, 8, 212608. [Google Scholar] [CrossRef]
- Oyarzábal, A.; Musokhranova, U.; Barros, L.F.; García-Cazorla, A. Energy metabolism in childhood neurodevelopmental disorders. eBioMedicine 2021, 69, 103474. [Google Scholar]
- Bougneres, P.F.; Lemmel, C.; Ferre, P.; Bier, D.M. Ketone body transport in the human neonate and infant. J. Clin. Investig. 1986, 77, 42–48. [Google Scholar] [CrossRef]

| Demographic and Clinical Features | Pediatric MSUD (n = 14) | Control (n = 14) | p-Value | |||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Age (years) | 11 | ±0.277 | 10.85 | ±0.345 | 0.72 | |
| Gender | Female (%) | 50 | NA | 50 | NA | NA |
| Male (%) | 50 | NA | 50 | NA | NA | |
| Biomarker | Xleucine (Cutoff: <245 µM) | 1003.36 | ±65.14 | 218.7 | ±4.96 | 1.59 × 10−11 ** |
| Valine (Cutoff: <290 µM) | 622.5 | ±44.63 | 260.7 | 4.01 × 10−8 ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, A.Z.; AlMalki, R.H.; Sebaa, R.; Al Mogren, M.; Alanazi, M.; Sumaily, K.M.; Alodaib, A.; Mujamammi, A.H.; Jacob, M.; Sabi, E.M.; et al. Insights from Metabolomics Profiling of MSUD in Pediatrics Toward Disease Progression. Metabolites 2025, 15, 658. https://doi.org/10.3390/metabo15100658
Alotaibi AZ, AlMalki RH, Sebaa R, Al Mogren M, Alanazi M, Sumaily KM, Alodaib A, Mujamammi AH, Jacob M, Sabi EM, et al. Insights from Metabolomics Profiling of MSUD in Pediatrics Toward Disease Progression. Metabolites. 2025; 15(10):658. https://doi.org/10.3390/metabo15100658
Chicago/Turabian StyleAlotaibi, Abeer Z., Reem H. AlMalki, Rajaa Sebaa, Maha Al Mogren, Mohammad Alanazi, Khalid M. Sumaily, Ahmad Alodaib, Ahmed H. Mujamammi, Minnie Jacob, Essa M. Sabi, and et al. 2025. "Insights from Metabolomics Profiling of MSUD in Pediatrics Toward Disease Progression" Metabolites 15, no. 10: 658. https://doi.org/10.3390/metabo15100658
APA StyleAlotaibi, A. Z., AlMalki, R. H., Sebaa, R., Al Mogren, M., Alanazi, M., Sumaily, K. M., Alodaib, A., Mujamammi, A. H., Jacob, M., Sabi, E. M., Alfares, A., & Abdel Rahman, A. M. (2025). Insights from Metabolomics Profiling of MSUD in Pediatrics Toward Disease Progression. Metabolites, 15(10), 658. https://doi.org/10.3390/metabo15100658







