Linking Elastin in Skeletal Muscle Extracellular Matrix to Metabolic and Aerobic Function in Type 2 Diabetes: A Secondary Analysis of a Lower Leg Training Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
2.3. Single-Leg Exercise Training (SLET)
2.4. Graded Exercise Test
2.5. Insulin Sensitivity
2.6. Magnetic Resonance Spectroscopy
2.7. Near-Infrared Spectroscopy
2.8. Second Harmonic Generation Imaging
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Baseline Collagen Abundance and Organization
3.3. Skeletal Muscle Extracellular Matrix Components Before and After Exercise Training
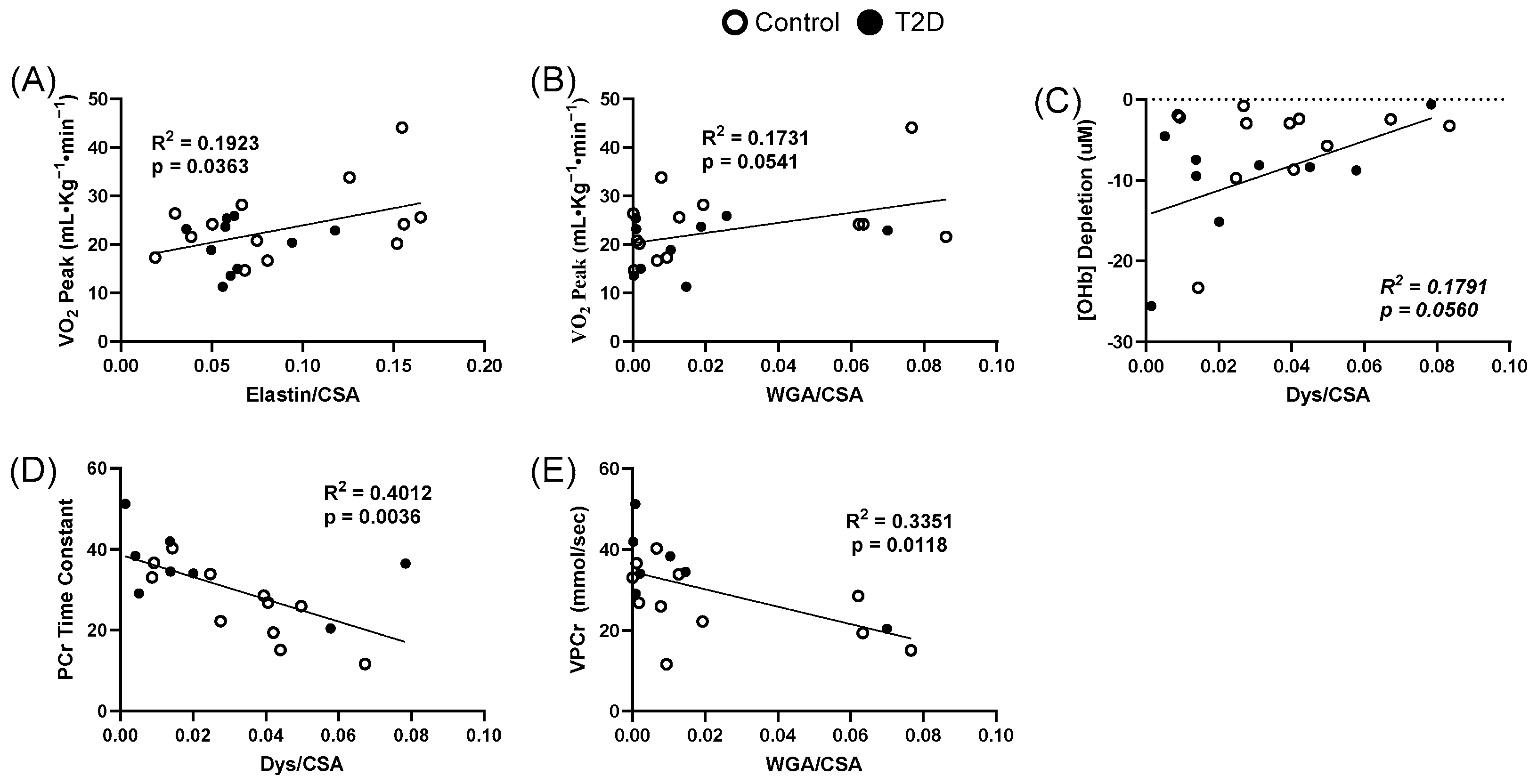
3.4. Extracellular Matrix Relationships to Clinical Measures
3.5. Extracellular Matrix Relationships to Oxidative Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| BMI | Body mass index |
| BP | Blood pressure |
| CRF | Cardiorespiratory fitness |
| CSA | Cross-sectional area |
| Dys | Dystrophin |
| ECM | Extracellular matrix |
| EDPs | Elastin degradation products |
| GIR | Glucose infusion rate |
| GLP-1 | Glucagon-like peptide-1 |
| Hb | Hemoglobin |
| HHb | Deoxyhemoglobin |
| MRI | Magnetic resonance imaging |
| MRS | Magnetic resonance spectroscopy |
| NIRS | Near-infrared spectroscopy |
| OHb | Oxyhemoglobin |
| OWC | Overweight/Obese Controls |
| Pi | Inorganic Phosphate |
| SHG | Second harmonic generation |
| SLET | Single Leg Exercise Training |
| T2D | Type 2 diabetes |
| tHb | Total hemoglobin |
| VPCr | Rate of phosphocreatine synthesis |
| WGA | Wheat germ agglutinin |
Appendix A
| p Value/R2 | Collagen Area | Collagen Density | Hyaluronic Acid | Elastin | Proteoglycans | Dystrophin |
|---|---|---|---|---|---|---|
| Glucose Infusion Rate | 0.848/0.001 | 0.098/0.119 | 0.197/0.081 | 0.001/0.426 | 0.769/0.004 | 0.134/0.108 |
| VO2Peak | 0.172/0.079 | 0.099/0.119 | 0.237/0.065 | 0.036/0.192 | 0.054/0.173 | 0.388/0.035 |
| Increase in Total Hemoglobin | 0.965/0.000 | 0.41/0.032 | 0.872/0.001 | 0.579/0.019 | 0.930/0.000 | 0.496/0.024 |
| Oxyhemoglobin Accumulation | 0.879/0.001 | 0.800/0.003 | 0.457/0.029 | 0.629/0.014 | 0.336/0.048 | 0.056/0.179 |
| Deoxyhemoglobin Accumulation | 0.887/0.000 | 0.827/0.002 | 0.358/0.044 | 0.846/0.002 | 0.316/0.052 | 0.102/0.134 |
| Velocity of PCr Turnover | 0.461/0.028 | 0.214/0.079 | 0.578/0.018 | 0.317/0.058 | 0.01/0.335 | 0.004/0.401 |
| Maximal Respiration | 0.250/0.068 | 0.568/0.017 | 0.987/0.000 | 0.576/0.018 | 0.894/0.001 | 0.136/0.125 |
| ADP Time Constant | 0.183/0.091 | 0.678/0.009 | 0.786/0.004 | 0.559/0.020 | 0.556/0.020 | 0.172/0.106 |
| PCr Time Constant | 0.425/0.033 | 0.855/0.001 | 0.919/0.000 | 0.507/0.026 | 0.744/0.006 | 0.003/0.401 |
| Muscle Strength | 0.429/0.014 | 0.273/0.056 | 0.0504/0.090 | 0.364/0.020 | 0.478/0.012 | 0.789/0.001 |
References
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025. [Google Scholar]
- Baena-Diez, J.M.; Penafiel, J.; Subirana, I.; Ramos, R.; Elosua, R.; Marin-Ibanez, A.; Guembe, M.J.; Rigo, F.; Tormo-Diaz, M.J.; Moreno-Iribas, C.; et al. Risk of Cause-Specific Death in Individuals With Diabetes: A Competing Risks Analysis. Diabetes Care 2016, 39, 1987–1995. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Kurl, S.; Salonen, R.; Rauramaa, R.; Salonen, J.T. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: A prospective population-based cohort study. Eur. Heart J. 2004, 25, 1428–1437. [Google Scholar] [CrossRef]
- Nadeau, K.J.; Zeitler, P.S.; Bauer, T.A.; Brown, M.S.; Dorosz, J.L.; Draznin, B.; Reusch, J.E.; Regensteiner, J.G. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J. Clin. Endocrinol. Metab. 2009, 94, 3687–3695. [Google Scholar] [CrossRef]
- Lav Madsen, P.; Sejersen, C.; Nyberg, M.; Sorensen, M.H.; Hellsten, Y.; Gaede, P.; Bojer, A.S. The cardiovascular changes underlying a low cardiac output with exercise in patients with type 2 diabetes mellitus. Front. Physiol. 2024, 15, 1294369. [Google Scholar] [CrossRef]
- Mac Ananey, O.; Malone, J.; Warmington, S.; O’Shea, D.; Green, S.; Egana, M. Cardiac output is not related to the slowed O2 uptake kinetics in type 2 diabetes. Med. Sci. Sports Exerc. 2011, 43, 935–942. [Google Scholar] [CrossRef]
- Regensteiner, J.G.; Bauer, T.A.; Reusch, J.E.; Quaife, R.A.; Chen, M.Y.; Smith, S.C.; Miller, T.M.; Groves, B.M.; Wolfel, E.E. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med. Sci. Sports Exerc. 2009, 41, 977–984. [Google Scholar] [CrossRef]
- Cree-Green, M.; Scalzo, R.L.; Harrall, K.; Newcomer, B.R.; Schauer, I.E.; Huebschmann, A.G.; McMillin, S.; Brown, M.S.; Orlicky, D.; Knaub, L.; et al. Supplemental Oxygen Improves In Vivo Mitochondrial Oxidative Phosphorylation Flux in Sedentary Obese Adults With Type 2 Diabetes. Diabetes 2018, 67, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Cree-Green, M.; Newcomer, B.R.; Brown, M.S.; Baumgartner, A.D.; Bergman, B.; Drew, B.; Regensteiner, J.G.; Pyle, L.; Reusch, J.E.; Nadeau, K.J. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes 2015, 64, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Le Page, A.; Khalil, A.; Vermette, P.; Frost, E.H.; Larbi, A.; Witkowski, J.M.; Fulop, T. The role of elastin-derived peptides in human physiology and diseases. Matrix Biol. 2019, 84, 81–96. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.L.; Pattamaprapanont, P.; Cooney, E.M.; Nava, R.C.; Mitri, J.; Hafida, S.; Lessard, S.J. Canagliflozin Prevents Hyperglycemia-Associated Muscle Extracellular Matrix Accumulation and Improves the Adaptive Response to Aerobic Exercise. Diabetes 2022, 71, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.D.; Davis, A.; Sherrill, C.; Westwood, B.; Hawkins, G.A.; Palmer, N.D.; Chou, J.W.; Reeves, T.; Cox, L.A.; Kavanagh, K. Skeletal muscle extracellular matrix remodeling with worsening glycemic control in nonhuman primates. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R226–R235. [Google Scholar] [CrossRef]
- Ugwoke, C.K.; Cvetko, E.; Umek, N. Skeletal Muscle Microvascular Dysfunction in Obesity-Related Insulin Resistance: Pathophysiological Mechanisms and Therapeutic Perspectives. Int. J. Mol. Sci. 2022, 23, 847. [Google Scholar] [CrossRef]
- Wasserman, D.H.; Wang, T.J.; Brown, N.J. The Vasculature in Prediabetes. Circ. Res. 2018, 122, 1135–1150. [Google Scholar] [CrossRef]
- Kang, L.; Ayala, J.E.; Lee-Young, R.S.; Zhang, Z.; James, F.D.; Neufer, P.D.; Pozzi, A.; Zutter, M.M.; Wasserman, D.H. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes 2011, 60, 416–426. [Google Scholar] [CrossRef]
- Hulett, N.A.; Scalzo, R.L.; Reusch, J.E.B. Glucose Uptake by Skeletal Muscle within the Contexts of Type 2 Diabetes and Exercise: An Integrated Approach. Nutrients 2022, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, R.L.; Schauer, I.E.; Rafferty, D.; Knaub, L.A.; Kvaratskhelia, N.; Johnson, T.K.; Pott, G.B.; Abushamat, L.A.; Whipple, M.O.; Huebschmann, A.G.; et al. Single-leg exercise training augments in vivo skeletal muscle oxidative flux and vascular content and function in adults with type 2 diabetes. J. Physiol. 2022, 600, 963–978. [Google Scholar] [CrossRef]
- Kriska, A. Ethnic and cultural issues in assessing physical activity. Res. Q. Exerc. Sport. 2000, 71 (Suppl. 2), 47–53. [Google Scholar] [CrossRef]
- Wolff, C.A.; Konopka, A.R.; Suer, M.K.; Trappe, T.A.; Kaminsky, L.A.; Harber, M.P. Increased cardiorespiratory fitness and skeletal muscle size following single-leg knee extension exercise training. J. Sports Med. Phys. Fit. 2019, 59, 934–940. [Google Scholar] [CrossRef]
- Regensteiner, J.G.; Bauer, T.A.; Reusch, J.E.; Brandenburg, S.L.; Sippel, J.M.; Vogelsong, A.M.; Smith, S.; Wolfel, E.E.; Eckel, R.H.; Hiatt, W.R. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J. Appl. Physiol. 1998, 85, 310–317. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, G.L.; Scalzo, R.L.; Schweder, M.M.; Larson, D.G.; Luckasen, G.J.; Irwin, D.; Hamilton, K.L.; Schroeder, T.; Bell, C. Sympathetic inhibition attenuates hypoxia induced insulin resistance in healthy adult humans. J. Physiol. 2012, 590, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Cree-Green, M.; Newcomer, B.R.; Brown, M.; Hull, A.; West, A.D.; Singel, D.; Reusch, J.E.; McFann, K.; Regensteiner, J.G.; Nadeau, K.J. Method for controlled mitochondrial perturbation during phosphorus MRS in children. Med. Sci. Sports Exerc. 2014, 46, 2030–2036. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Newcomer, B.R.; Hunter, G.R.; Hetherington, H.P.; Weinsier, R.L. 31P MRS measurement of mitochondrial function in skeletal muscle: Reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed. 2000, 13, 14–27. [Google Scholar] [CrossRef]
- Bamman, M.M.; Caruso, J.F. Resistance exercise countermeasures for space flight: Implications of training specificity. J. Strength. Cond. Res. 2000, 14, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Huang, S.Y.; Regensteiner, J.G.; Micco, A.J.; Ishimoto, G.; Manco-Johnson, M.; Drose, J.; Reeves, J.T. Venous occlusion plethysmography reduces arterial diameter and flow velocity. J. Appl. Physiol. 1989, 66, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, B.R.; Boska, M.D. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve 1997, 20, 336–346. [Google Scholar] [CrossRef]
- van Boogaart, A. Magnetic Resonance User Interface Software Package, 96.3 ed.; Delft Technical University Press: Delft, The Netherlands, 1997. [Google Scholar]
- Rico-Sanz, J.; Thomas, E.L.; Jenkinson, G.; Mierisova, S.; Iles, R.; Bell, J.D. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J. Appl. Physiol. 1999, 87, 2068–2072. [Google Scholar] [CrossRef]
- Mason McClatchey, P.; Bauer, T.A.; Regensteiner, J.G.; Schauer, I.E.; Huebschmann, A.G.; Reusch, J.E.B. Dissociation of local and global skeletal muscle oxygen transport metrics in type 2 diabetes. J. Diabetes Its Complicat. 2017, 31, 1311–1317. [Google Scholar] [CrossRef]
- Vuillemin, N.; Mahou, P.; Debarre, D.; Gacoin, T.; Tharaux, P.L.; Schanne-Klein, M.C.; Supatto, W.; Beaurepaire, E. Efficient second-harmonic imaging of collagen in histological slides using Bessel beam excitation. Sci. Rep. 2016, 6, 29863. [Google Scholar] [CrossRef]
- Liu, Y.; Keikhosravi, A.; Mehta, G.S.; Drifka, C.R.; Eliceiri, K.W. Methods for Quantifying Fibrillar Collagen Alignment. Methods Mol. Biol. 2017, 1627, 429–451. [Google Scholar] [CrossRef] [PubMed]
- Danckaert, A.; Trignol, A.; Le Loher, G.; Loubens, S.; Staels, B.; Duez, H.; Shorte, S.L.; Mayeuf-Louchart, A. MuscleJ2: A rebuilding of MuscleJ with new features for high-content analysis of skeletal muscle immunofluorescence slides. Skelet. Muscle 2023, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Coletta, D.K. Obesity and type 2 diabetes mellitus: Insights from skeletal muscle extracellular matrix remodeling. Am. J. Physiol. Cell Physiol. 2025, 328, C1752–C1763. [Google Scholar] [CrossRef]
- Attrill, E.; Ramsay, C.; Ross, R.; Richards, S.; Sutherland, B.A.; Keske, M.A.; Eringa, E.; Premilovac, D. Metabolic-vascular coupling in skeletal muscle: A potential role for capillary pericytes? Clin. Exp. Pharmacol. Physiol. 2020, 47, 520–528. [Google Scholar] [CrossRef]
- Caballero, A.E. Metabolic and vascular abnormalities in subjects at risk for type 2 diabetes: The early start of a dangerous situation. Arch. Med. Res. 2005, 36, 241–249. [Google Scholar] [CrossRef]
- Lehti, T.M.; Silvennoinen, M.; Kivela, R.; Kainulainen, H.; Komulainen, J. Effects of streptozotocin-induced diabetes and physical training on gene expression of extracellular matrix proteins in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E900–E907. [Google Scholar] [CrossRef]
- Ibanez-Fonseca, A.; Santiago Maniega, S.; Gorbenko Del Blanco, D.; Catalan Bernardos, B.; Vega Castrillo, A.; Alvarez Barcia, A.J.; Alonso, M.; Aguado, H.J.; Rodriguez-Cabello, J.C. Elastin-Like Recombinamer Hydrogels for Improved Skeletal Muscle Healing Through Modulation of Macrophage Polarization. Front. Bioeng. Biotechnol. 2020, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Ishikawa, M.; Kamei, N.; Miyaki, S.; Adachi, N.; Inoue, K.; Kawabata, S. Skeletal muscle injury treatment using the Silk Elastin(R) injection in a rat model. Regen. Ther. 2024, 26, 180–187. [Google Scholar] [CrossRef]
- Blaise, S.; Romier, B.; Kawecki, C.; Ghirardi, M.; Rabenoelina, F.; Baud, S.; Duca, L.; Maurice, P.; Heinz, A.; Schmelzer, C.E.; et al. Elastin-derived peptides are new regulators of insulin resistance development in mice. Diabetes 2013, 62, 3807–3816. [Google Scholar] [CrossRef]
- Wolfe, B.L.; Rich, C.B.; Goud, H.D.; Terpstra, A.J.; Bashir, M.; Rosenbloom, J.; Sonenshein, G.E.; Foster, J.A. Insulin-like growth factor-I regulates transcription of the elastin gene. J. Biol. Chem. 1993, 268, 12418–12426. [Google Scholar] [CrossRef]
- Kang, L.; Mokshagundam, S.; Reuter, B.; Lark, D.S.; Sneddon, C.C.; Hennayake, C.; Williams, A.S.; Bracy, D.P.; James, F.D.; Pozzi, A.; et al. Integrin-Linked Kinase in Muscle Is Necessary for the Development of Insulin Resistance in Diet-Induced Obese Mice. Diabetes 2016, 65, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Vandromme, S.E.; Reid, M.E.; Taite, L.J. Synergistic activity of alphavbeta3 integrins and the elastin binding protein enhance cell-matrix interactions on bioactive hydrogel surfaces. Biomacromolecules 2012, 13, 1420–1428. [Google Scholar] [CrossRef]
- Scandolera, A.; Odoul, L.; Salesse, S.; Guillot, A.; Blaise, S.; Kawecki, C.; Maurice, P.; El Btaouri, H.; Romier-Crouzet, B.; Martiny, L.; et al. The Elastin Receptor Complex: A Unique Matricellular Receptor with High Anti-tumoral Potential. Front. Pharmacol. 2016, 7, 32. [Google Scholar] [CrossRef]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef]
- Kasprzycka, P.; Ciemerych, M.A.; Zimowska, M. Differential Regulation of MMP Activity by TGFβ1 in Fast-and Slow-Twitch Muscle Repair: Insights from EDL and Soleus Muscle-Derived Myoblasts. Front. Cell Dev. Biol. 2025, 13, 1592512. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef]
- Richardson, D.K.; Kashyap, S.; Bajaj, M.; Cusi, K.; Mandarino, S.J.; Finlayson, J.; DeFronzo, R.A.; Jenkinson, C.P.; Mandarino, L.J. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J. Biol. Chem. 2005, 280, 10290–10297. [Google Scholar] [CrossRef]
- Kang, L.; Lantier, L.; Kennedy, A.; Bonner, J.S.; Mayes, W.H.; Bracy, D.P.; Bookbinder, L.H.; Hasty, A.H.; Thompson, C.B.; Wasserman, D.H. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes 2013, 62, 1888–1896. [Google Scholar] [CrossRef]
- Dantas, W.S.; Roschel, H.; Murai, I.H.; Gil, S.; Davuluri, G.; Axelrod, C.L.; Ghosh, S.; Newman, S.S.; Zhang, H.; Shinjo, S.K.; et al. Exercise-Induced Increases in Insulin Sensitivity After Bariatric Surgery Are Mediated By Muscle Extracellular Matrix Remodeling. Diabetes 2020, 69, 1675–1691. [Google Scholar] [CrossRef] [PubMed]
- Ananthakumar, A.; Liu, Y.; Fernandez, C.E.; Truskey, G.A.; Voora, D. Modeling statin myopathy in a human skeletal muscle microphysiological system. PLoS ONE 2020, 15, e0242422. [Google Scholar] [CrossRef] [PubMed]
- Rebalka, I.A.; Cao, A.W.; May, L.L.; Tarnopolsky, M.A.; Hawke, T.J. Statin administration activates system xC(-) in skeletal muscle: A potential mechanism explaining statin-induced muscle pain. Am. J. Physiol. Cell Physiol. 2019, 317, C894–C899. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- LaBelle, S.A.; Poulson, A.M., IV; Maas, S.A.; Rauff, A.; Ateshian, G.A.; Weiss, J.A. Spatial Configurations of 3D Extracellular Matrix Collagen Density and Anisotropy Simultaneously Guide Angiogenesis. PLoS Comput. Biol. 2023, 19, e1011553. [Google Scholar] [CrossRef] [PubMed]
- Trebacz, H.; Barzycka, A. Mechanical Properties and Functions of Elastin: An Overview. Biomolecules 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Draicchio, F.; Behrends, V.; Tillin, N.A.; Hurren, N.M.; Sylow, L.; Mackenzie, R. Involvement of the extracellular matrix and integrin signalling proteins in skeletal muscle glucose uptake. J. Physiol. 2022, 600, 4393–4408. [Google Scholar] [CrossRef]
- Rodriguez-Cruz, M.; Sanchez, R.; Escobar, R.E.; Cruz-Guzman, O.R.; Lopez-Alarcon, M.; Bernabe Garcia, M.; Coral-Vazquez, R.; Matute, G.; Velazquez Wong, A.C. Evidence of Insulin Resistance and Other Metabolic Alterations in Boys with Duchenne or Becker Muscular Dystrophy. Int. J. Endocrinol. 2015, 2015, 867273. [Google Scholar] [CrossRef]
- Beroud, C.; Tuffery-Giraud, S.; Matsuo, M.; Hamroun, D.; Humbertclaude, V.; Monnier, N.; Moizard, M.P.; Voelckel, M.A.; Calemard, L.M.; Boisseau, P.; et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum. Mutat. 2007, 28, 196–202. [Google Scholar] [CrossRef]
- Kellogg, D.L., 3rd; McCammon, K.M.; Hinchee-Rodriguez, K.S.; Adamo, M.L.; Roman, L.J. Neuronal nitric oxide synthase mediates insulin- and oxidative stress-induced glucose uptake in skeletal muscle myotubes. Free Radic. Biol. Med. 2017, 110, 261–269. [Google Scholar] [CrossRef]
- Tammineni, E.R.; Manno, C.; Oza, G.; Figueroa, L. Skeletal muscle disorders as risk factors for type 2 diabetes. Mol. Cell Endocrinol. 2025, 599, 112466. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.C.; Rayavarapu, S.; Hogarth, M.W.; Van der Meulen, J.H.; Horn, A.; Defour, A.; Takeda, S.; Brown, K.J.; Hathout, Y.; Nagaraju, K.; et al. Mitochondria mediate cell membrane repair and contribute to Duchenne muscular dystrophy. Cell Death Differ. 2017, 24, 330–342. [Google Scholar] [CrossRef]



| Control (n = 24) | T2D (n = 21) | |
|---|---|---|
| Age (y) | 52 ± 13 | 61 ± 9 |
| Sex (% Female) | 45.8 | 47.4 |
| BMI (Kg/m2) | 30.0 ± 4.6 | 31.9 ± 8.0 |
| Body Fat (%) | 34.2 ± 8.2 | 37.2 ± 8.0 |
| SBP (mmHG) * | 118.8 ± 11.7 | 125.0 ± 11.0 |
| DBP (mmHG) | 80.3 ± 8.0 | 79.5 ± 7.1 |
| Duration of DM (y) * | 0 ± 0 | 8.8 ± 7.0 |
| HbA1c (%) * | 5.3 ± 0.3 | 6.3 ± 0.7 |
| Current Metformin Use (%) * | 0 | 88.9 |
| Total Cholesterol (mmol/L) * | 4.544 ± 0.854 | 4.042 ± 0.885 |
| HDL (mmol/L) | 1.311 ± 0.287 | 1.161 ± 0.331 |
| LDL (mmol/L) | 2.848 ± 0.799 | 2.359 ± 0.786 |
| Current Statin Use (%) * | 8.3 | 42.9 |
| GIR (mL/Kg·min) * | 7.1 ± 2.3 | 4.8 ± 1.9 |
| VO2Peak (mL/Kg·min) * | 24.5 ± 7.8 | 18.7 ± 4.8 |
| VPCr (mmol/s) * | 0.223 ± 0.088 | 0.155 ± 0.077 |
| Oxyhemoglobin Depletion (µM) | −3.4 ± 5.3 | −6.2 ± 7.0 |
| Maximal Voluntary Contraction (Kg) | 43.2 ± 15.2 | 39.0 ± 8.0 |
| Fiber Size (µm) | 184.1 ± 33.6 | 206.1 ± 35.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulett, N.A.; Knaub, L.A.; Schauer, I.E.; Regensteiner, J.G.; Scalzo, R.L.; Reusch, J.E.B. Linking Elastin in Skeletal Muscle Extracellular Matrix to Metabolic and Aerobic Function in Type 2 Diabetes: A Secondary Analysis of a Lower Leg Training Intervention. Metabolites 2025, 15, 655. https://doi.org/10.3390/metabo15100655
Hulett NA, Knaub LA, Schauer IE, Regensteiner JG, Scalzo RL, Reusch JEB. Linking Elastin in Skeletal Muscle Extracellular Matrix to Metabolic and Aerobic Function in Type 2 Diabetes: A Secondary Analysis of a Lower Leg Training Intervention. Metabolites. 2025; 15(10):655. https://doi.org/10.3390/metabo15100655
Chicago/Turabian StyleHulett, Nicholas A., Leslie A. Knaub, Irene E. Schauer, Judith G. Regensteiner, Rebecca L. Scalzo, and Jane E. B. Reusch. 2025. "Linking Elastin in Skeletal Muscle Extracellular Matrix to Metabolic and Aerobic Function in Type 2 Diabetes: A Secondary Analysis of a Lower Leg Training Intervention" Metabolites 15, no. 10: 655. https://doi.org/10.3390/metabo15100655
APA StyleHulett, N. A., Knaub, L. A., Schauer, I. E., Regensteiner, J. G., Scalzo, R. L., & Reusch, J. E. B. (2025). Linking Elastin in Skeletal Muscle Extracellular Matrix to Metabolic and Aerobic Function in Type 2 Diabetes: A Secondary Analysis of a Lower Leg Training Intervention. Metabolites, 15(10), 655. https://doi.org/10.3390/metabo15100655





