Metabolic Characteristics of PGPR-Induced Growth Promotion in Alfalfa (Medicago sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Preparation of Bacterial Suspension
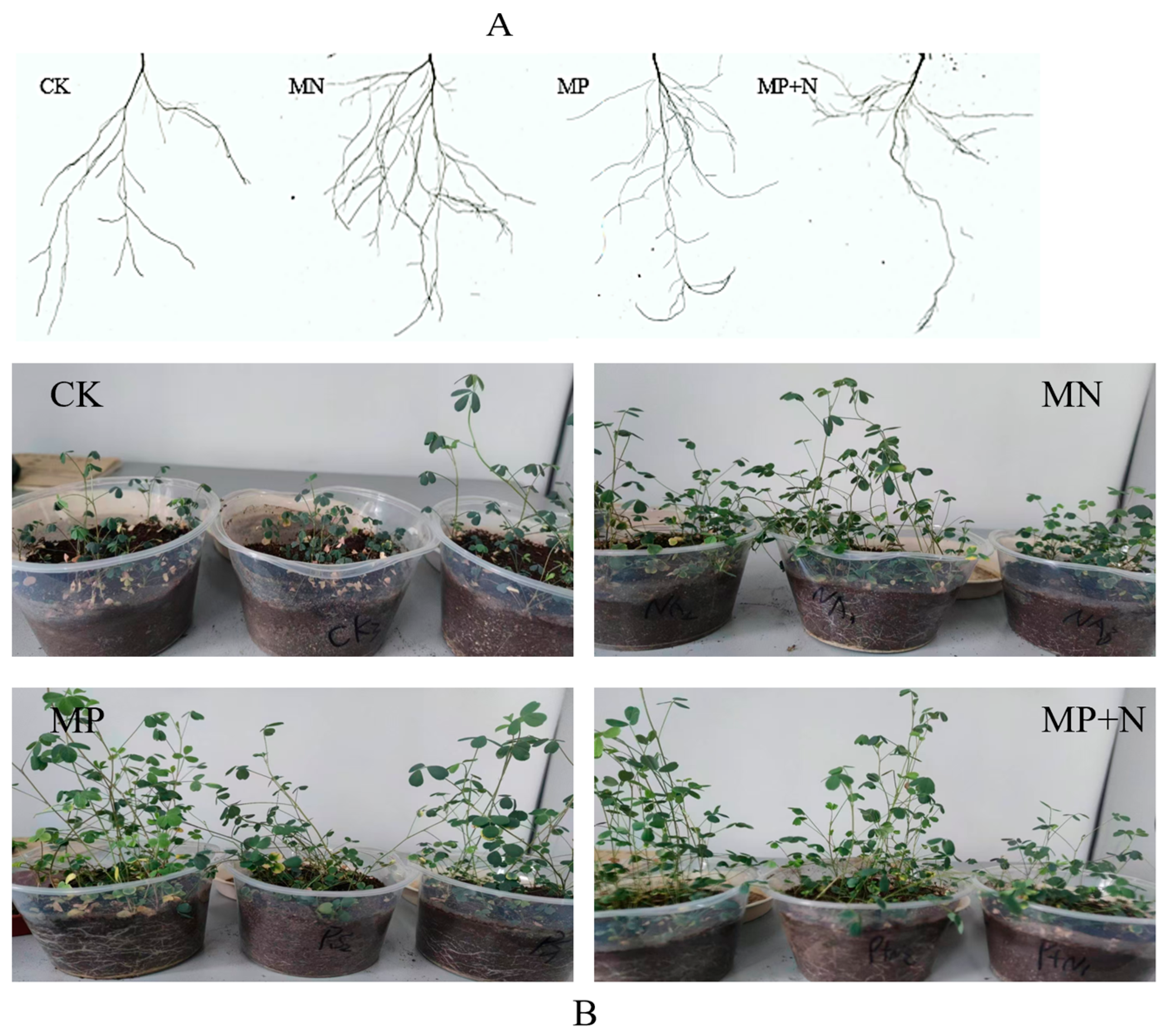
2.3. Preparation of Plant Pot Experiments
2.4. Metabolomics Assay Methods
2.5. Data Analysis
3. Results
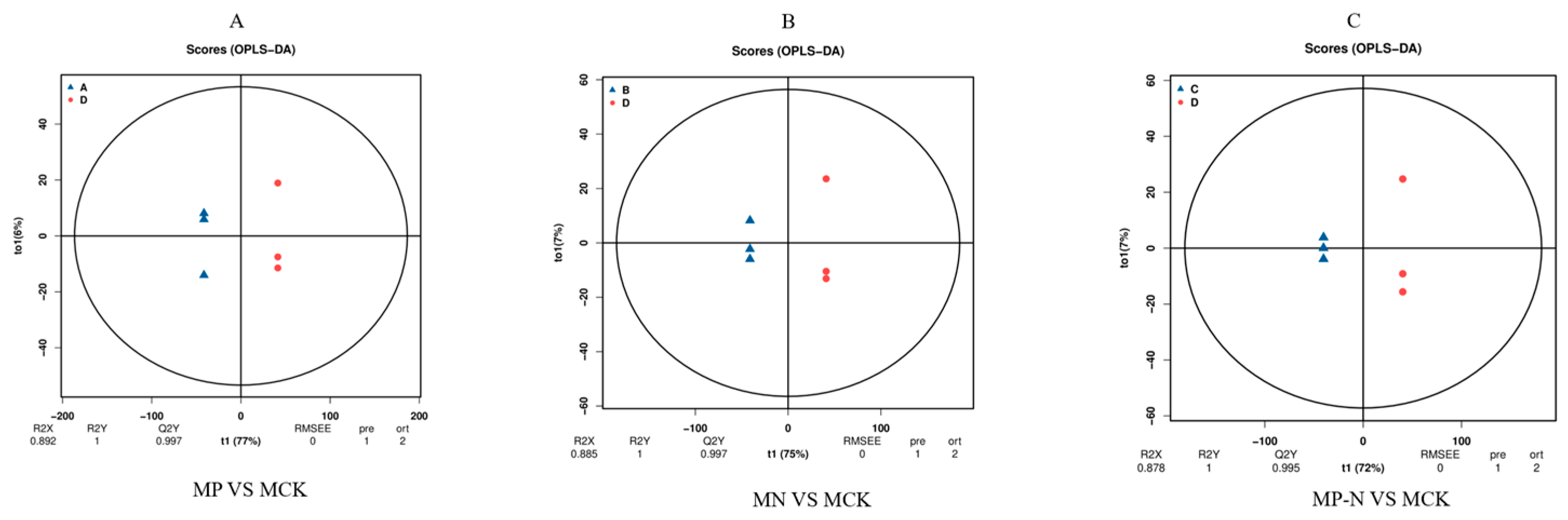
3.1. Orthogonal Partial Least Squares Discriminant Analysis
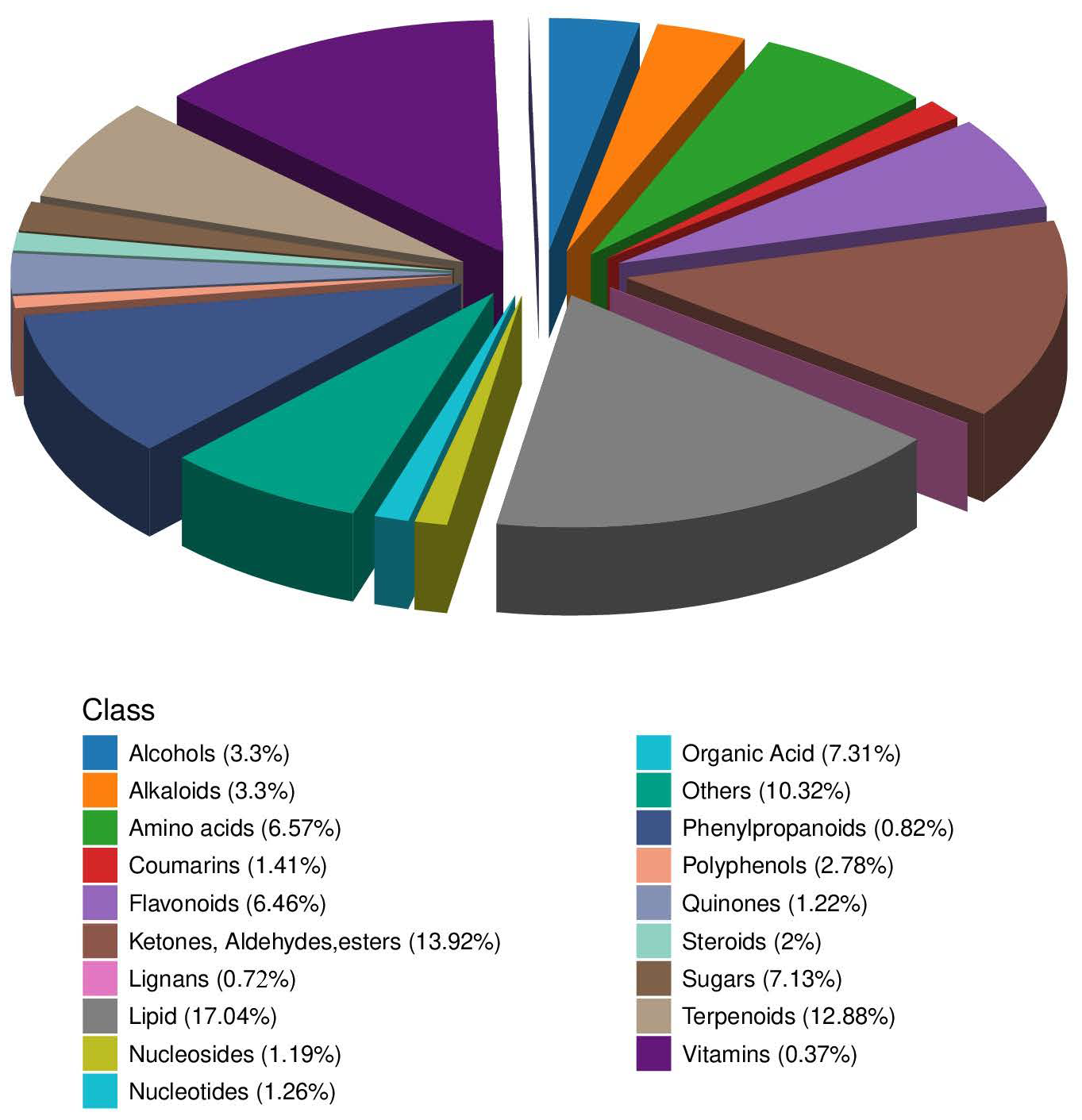
3.2. Description of Material Detection Results
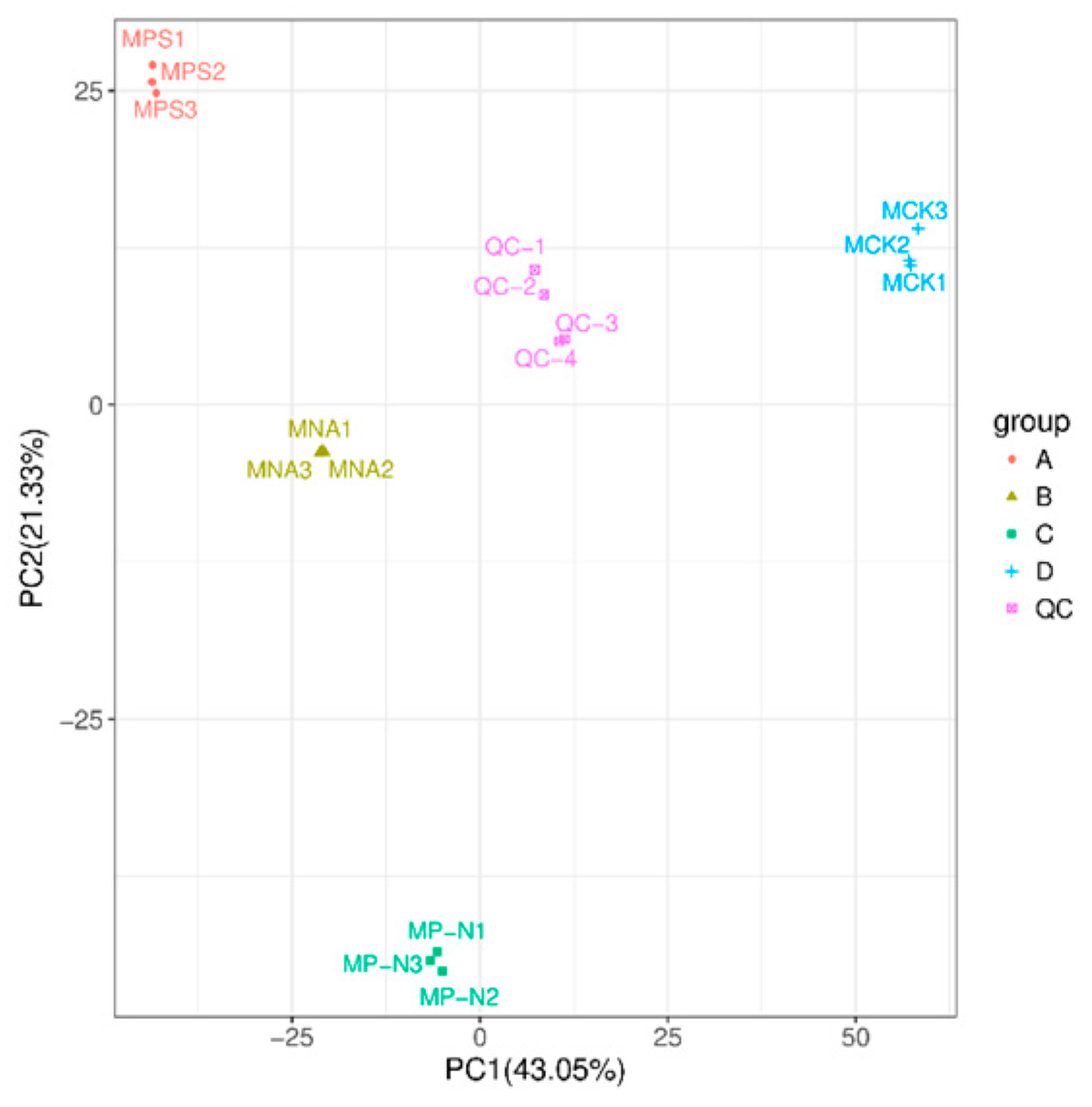
3.3. Principal Component Analysis (PCA)
3.4. Screening and Identification of Differential Metabolites
3.5. Differential Metabolite Analysis
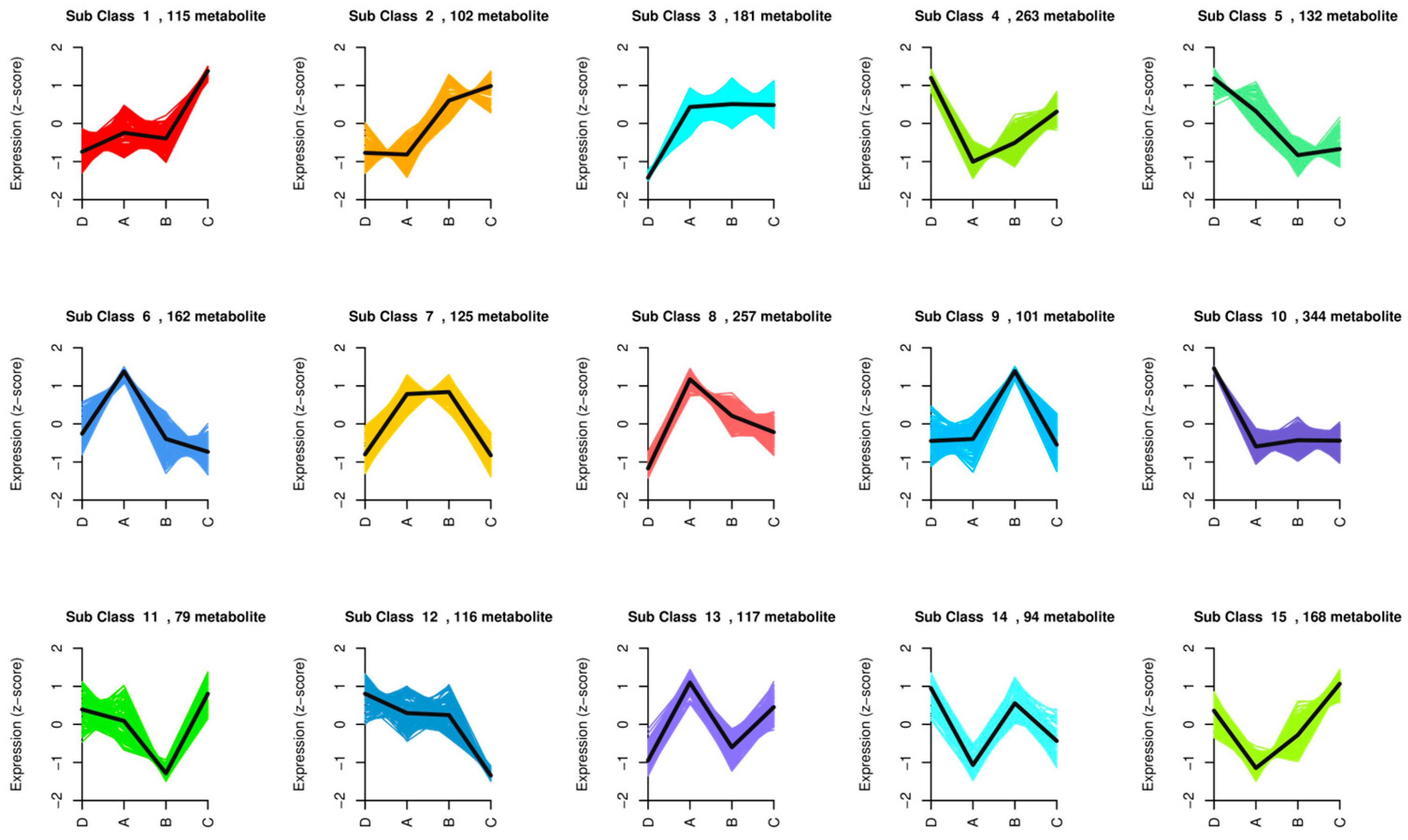
3.5.1. K-Means Analysis of Differential Metabolites
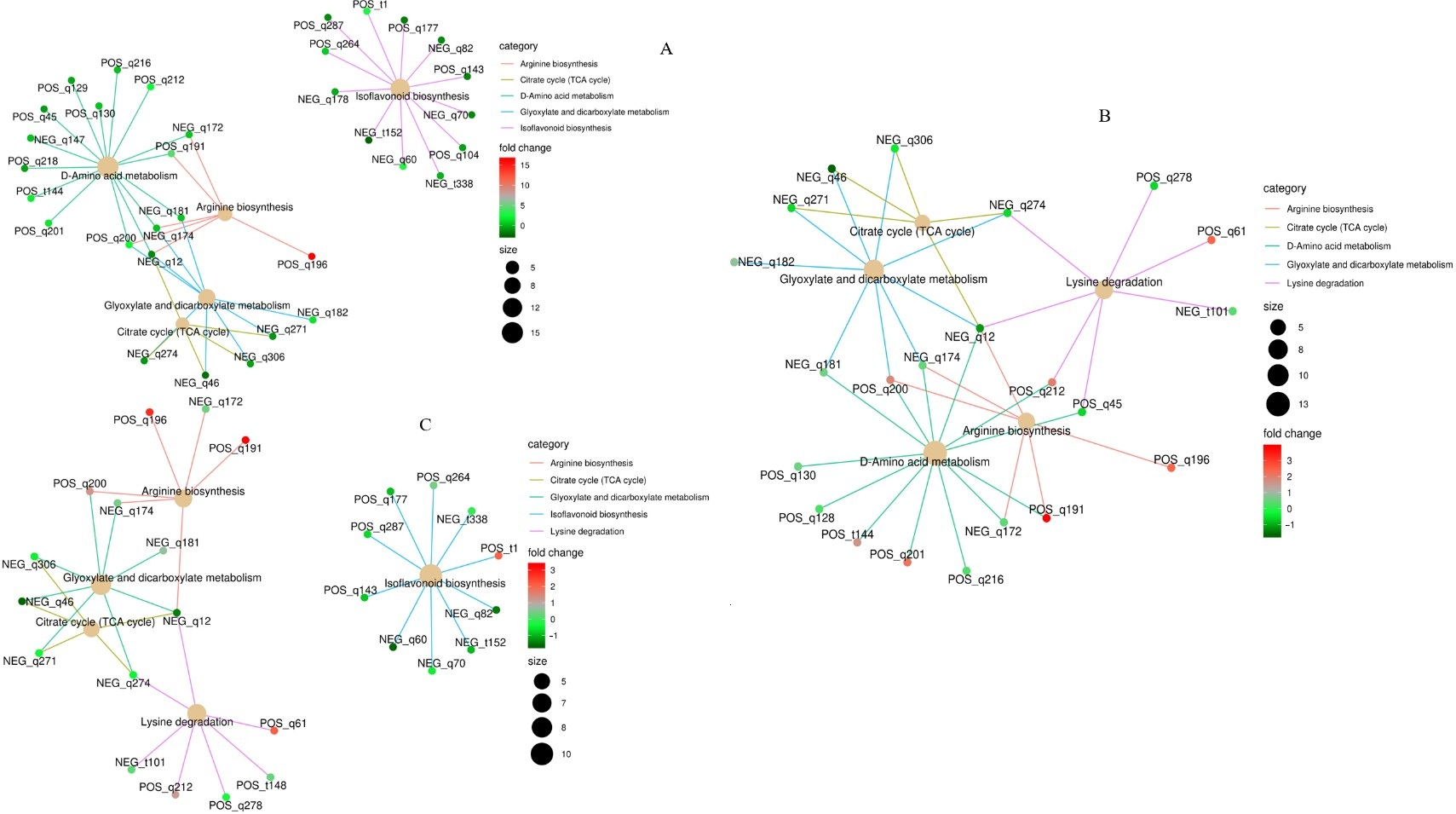
3.5.2. KEGG Functional Annotation and Enrichment Analysis of Differential Metabolites
3.6. Functional Analysis of Differential Metabolites in Each Treatment Group
4. Discussion
4.1. Key Nutritional Components in the Growth of Alfalfa
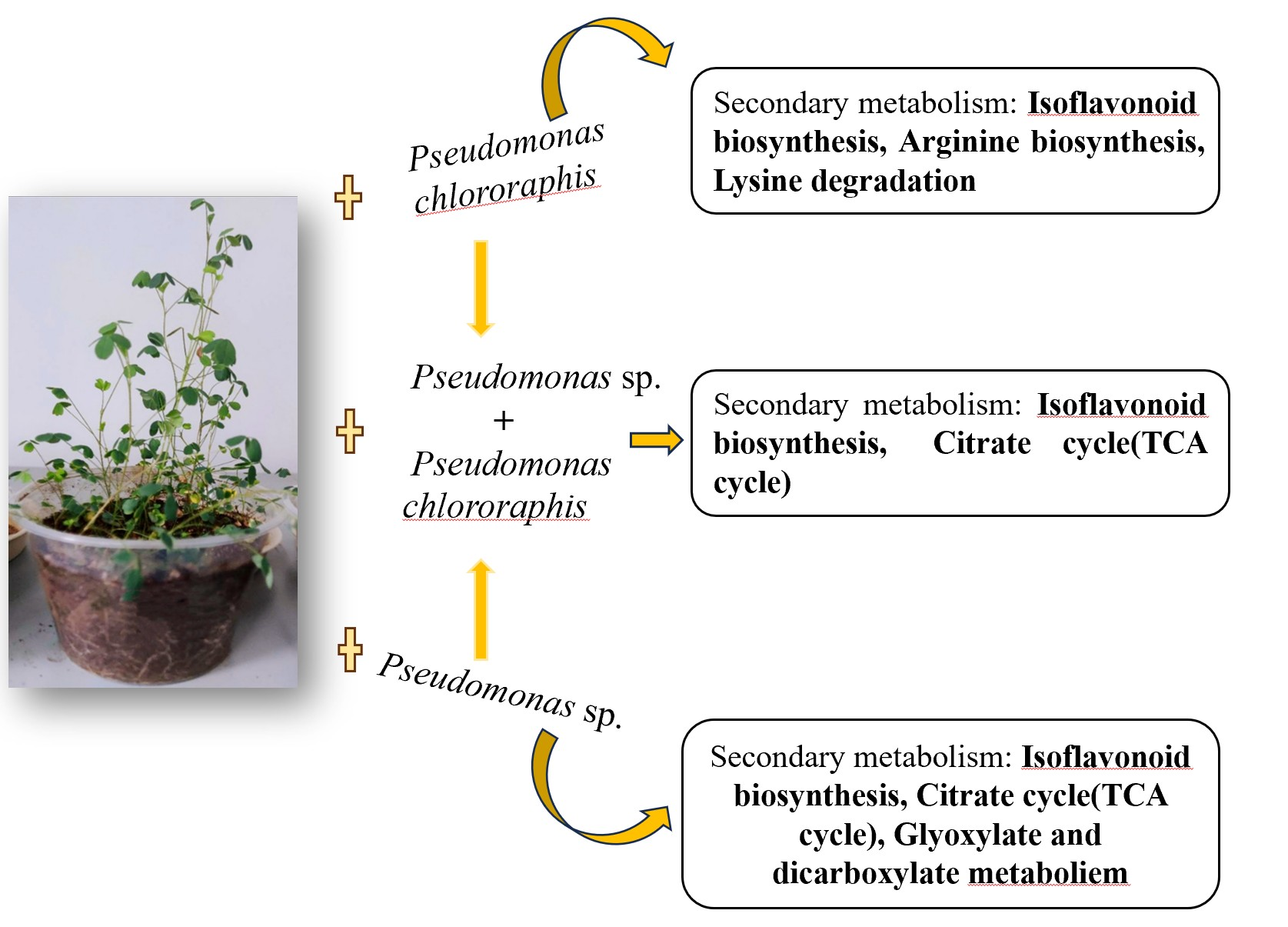
4.2. Differential Metabolites in Response to Different Bacterial Treatments
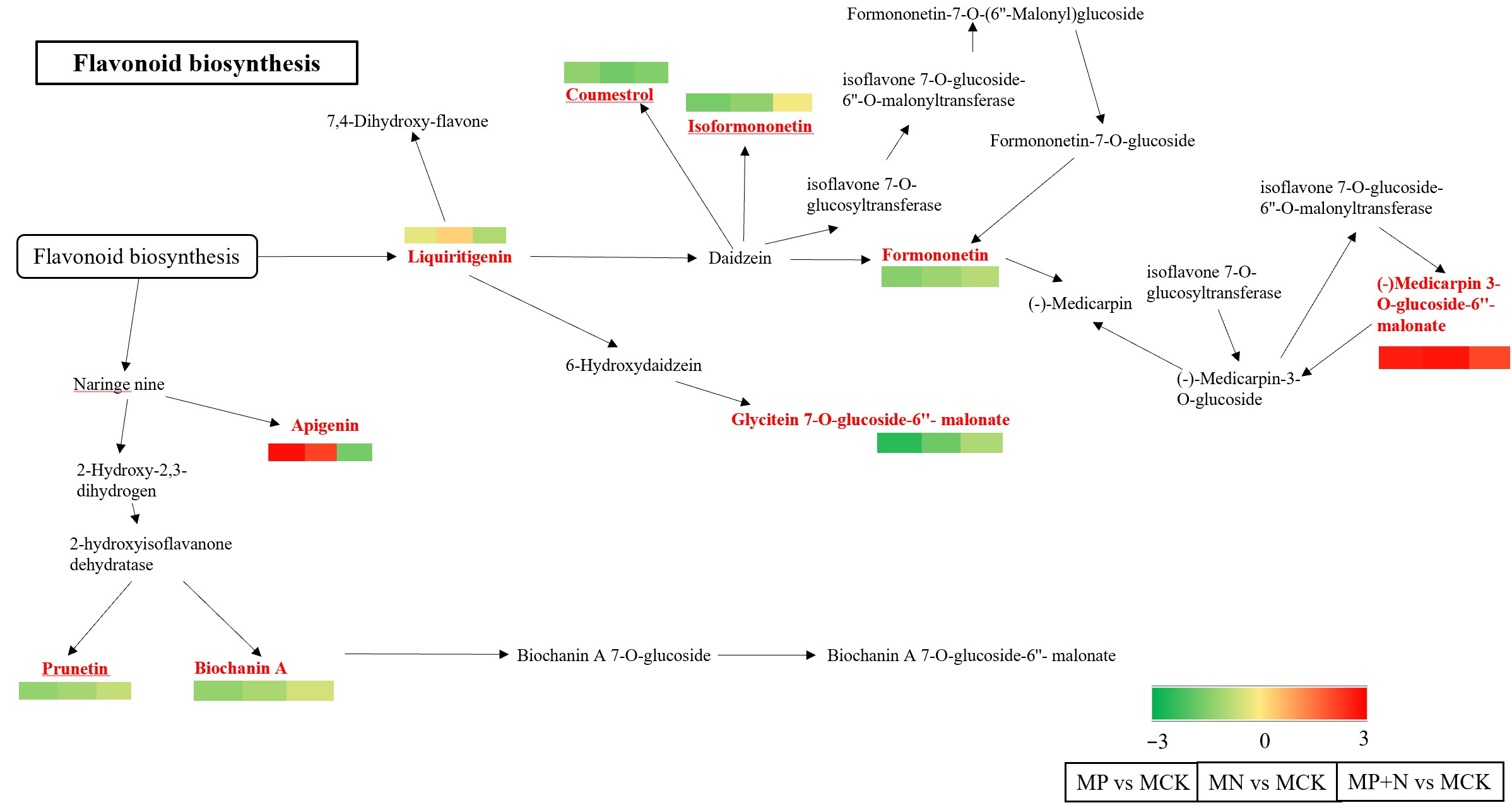
4.3. The Primary Metabolic Pathway Through Which PGPR Strains Induce Alfalfa Growth Is Isoflavonoid Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.; Piceno, Y.M.; Desantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the Rhizosphere Microbiome for Diseas-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne, L.Y. The Rhizosphere: A Playground and Battlefield for Soilborne Pathogens and Beneficial Microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Singh, R.K.; Ali, S.A.; Nath, P.; Sane, V.A. Activation of Ethylene-Responsive Phydroxyphenyl Pyruvate Dioxygenase Leads to Increased Tocopherol Levels During Ripening in Mango. Exp. Bot. 2011, 62, 3375–3385. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Glick, B.R. The Role of Plant Growth-Promoting Bacteria in Metal Phytoremediation. Adv. Microb. Physiol. 2017, 71, 97–132. [Google Scholar] [CrossRef] [PubMed]
- Antoun, H.; Prevost, D. Ecology of Plant Growth Promoting Rhizobacteria. In PGPR: Biocontrol and Biofertilization; Springer: Dordrecht, The Netherlands, 2005; pp. 1–38. [Google Scholar] [CrossRef]
- Davide, B.; Klaus, S.; Stijn, S.; Emiel, V.L.V.T.; Paul, S.L. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of Plant Growth-Promoting Rhizobacteria for Sustainable Development in Agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial Interactions in the Rhizosphere: Beneficial Influences of Plant Growth-Promoting Rhizobacteria on Nutrient Acquisition Process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Ambrosini, A.; De Souza, R.; Passaglia, L.M.P. Ecological Role of Bacterial Inoculants and Their Potential Impact on Soil Microbial Diversity. Plant Soil 2016, 400, 193–207. [Google Scholar] [CrossRef]
- Zhang, C.M.; Wang, C.Z.; Hu, X.F.; Liu, J.W.; He, Y. Research Progress on Nutritional Value and Applications of Alfalfa. China Feed 2005, 2005, 15–17. [Google Scholar] [CrossRef]
- Li, Y.P.; Yang, H.M.; Yang, Z.; Xie, Y.J.; Ding, W.J.; Jin, S.L.; Xing, H. The Nutritional Value of Alfalfa and Its Application in Livestock and Poultry Production. Feed Res. 2015, 38, 14–18. [Google Scholar]
- Li, W.R.; Zhang, S.Q.; Ding, S.Y.; Shan, L. Root Morphological Variation and Water Use in Alfalfa Under Drought Stress. Acta Ecol. Sin. 2010, 30, 5140–5150. [Google Scholar]
- Castonguay, Y.; Laberge, S.; Brummer, E.C.; Volenec, J.J. Alfalfa Winter Hardiness: A Research Retrospective and Integrated Perspective. Adv. Agron. 2006, 90, 203–265. [Google Scholar] [CrossRef]
- Yin, Q. Study on Spatial and Temporal Heterogeneity of Alfalfa Hay Making and Storing Technology; IMAU: Hohhot, China, 2013. [Google Scholar]
- Hong, F.Z. Forage Production is an Important Guarantee for National Food Security and Ecological Security. Pratacultural Sci. 2009, 26, 2. [Google Scholar]
- Wang, M.L. The Development of Forage Grass Industry and Its Impact on Trade. Anim. Agric. 2019, 36–39. [Google Scholar]
- Yang, C.; Wang, M.L.; Liu, Y.Z. Alfalfa Trade of China, Historical Changes, Future Trends and Suggestions. Pratacultural Sci. 2011, 5, 1711–1717. [Google Scholar]
- Tao, S.; Wang, Y.T.; Zhang, Q. Situation of Domestic Alfalfa Market and the Impact from Sino-US Trade Frictions and Its Countermeasures. Anim. Husb. Feed Sci. 2019, 40, 46–50. [Google Scholar] [CrossRef]
- Li, Y.N.; Liu, W.Q.; Pei, Y.X. Application and Challenges of Biotechnology in Alfalfa Breeding. Emerg. Sci. Technol. 2024, 3, 323–332. [Google Scholar]
- Jiang, X.Q.; Yang, Q.C.; Kang, J.M. Research Progress on Yield Loss Under Drought Stress and Drought Resistance Genetics of Alfalfa (Medicago sativa). Acta Prataculturae Sin. 2025, 34, 219–234. [Google Scholar] [CrossRef]
- Chen, C.J.; Bao, M.F.; Wang, W.H.; Shang, J.H.; Zeng, Y.X.; Sha, X.D.; Zhu, X.Z.; Wang, X.M.; Liu, W.H. Current Situation and Prospects for Drought-Resistance Breeding in Alfalfa. Acta Prataculturae Sin. 2025, 34, 204–223. [Google Scholar] [CrossRef]
- Jeon, J.S.; Rybka, D.; Natalia, C.Q.; Vos, R.D.; Jos, M.R.; Desalegn, W.E. Metabolic Signatures of Rhizobacteria-Induced Plant Growth Promotion. Plant Cell Environ. 2022, 45, 3086–3099. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on Lysogenesis I. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological Systems Database as a Model of the Real World. Nucleic Acids Res. 2025, 6, D672–D677. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; Shearman, G.C. Do Lipids Shape the Eukaryotic Cell Cycle? BBA-Mol. Cell Biol. Lipids 2018, 1863, 9–19. [Google Scholar] [CrossRef]
- Xiong, W.; Jousset, A.; Guo, S.; Karlsson, I.; Zhao, Q.Y.; Wu, H.S.; Kowalchuk, G.A.; Shen, Q.R.; Li, R.; Geisen, S. Soil Protist Communities Form a Dynamic Hub in the Soil Microbiome. ISME J. 2018, 12, 634–638. [Google Scholar] [CrossRef]
- Sugiura, Y.; Akiyama, R.; Tanaka, S.; Yano, K.; Kameoka, H.; Kawaguchi, M.; Saito, K. Myristate Can be Used as a Carbon and Energy Source for the Asymbiotic Growth of Arbuscular Mycorrhizal Fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 25779–25788. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Feng, F.Q.; Zhang, H. Quantitative Structure-Activity Relationships of Antimicrobial Fatty Acids and Derivatives Against Bacillus. J. Chin. Inst. Food Sci. Technol. 2014, 14, 191–199. [Google Scholar]
- Cheng, H.N.; Qin, D.D.; Xu, F.C.; Xu, Q.; Peng, Y.C.; Sun, L.Q.; Xu, L.; Guo, Y.; Yang, X.Q.; Xu, D.Z.; et al. Comparative Analysis of Metabolomics of Colored Hulless Barley and Colored Wheat Grains. Acta Agron. Sin. 2025, 1–12. Available online: http://kns.cnki.net/kcms/detail/11.1809.S.20241223.1041.002.html (accessed on 9 April 2025).
- Zhang, Z. Effects of Amino Acid Nitrogen on Cucumber Growth; Chongqing Southwest University: Chongqing, China, 2005. [Google Scholar]
- Song, Q.; Cao, F.Q.; Gong, Y.Y.; Cheng, X.Y.; Bi, X.Y.; Liu, L.H. Current Research Progresses of Amino Acids Uptake, Transport and Their Biological Roles in Higher Plants. J. Plant Nutr. Fertil. 2012, 18, 1507–1517. [Google Scholar] [CrossRef]
- Wang, K.; Nan, L.L.; Xia, J.; Wu, S.W.; Yang, L.L. Metabolomics Reveal Root Differential Metabolites of Different Root-Type Alfalfa Under Drought Stress. Front. Plant Sci. 2024, 15, 1341826. [Google Scholar] [CrossRef]
- Chai, H.; Yang, Z.; Li, H.; Shen, Z.B.; Zhong, P.; Li, S.S.; Wang, X.L.; Xu, Y.X.; Zhang, Y.; Song, M.C.; et al. Drought Resistance Evaluation and Metabolomics Analysis in Alfalfa Under Drought Stress. Heilongjiang Anim. Sci. Vet. Med. 2023, 94–105, 135–137. [Google Scholar] [CrossRef]
- Verrier, P.J.; Bird, D.; Burla, B.; Dassa, E.; Forestier, C.; Geisler, M.; Klein, M.; Kolukisaoglu, U.; Lee, Y.; Martinoia, E.; et al. Plant ABC Proteins—A Unified Nomenclature and Updated Inventory. Trends Plant Sci. 2008, 13, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.L.; Wang, S.H.; Song, Y.N.; Christie, P. Effects of a Dark Septate Endophyte and Extracellular Metabolites on Alfalfa Root Exudates: A Non-Targeted Metabolomics Analysis. Physiol. Plant. 2024, 176, e14165. [Google Scholar] [CrossRef]
- Wang, R.T.; Liu, J.X.; Jiang, W.Y.; Ji, P.S.; Li, Y.G. Metabolomics and Microbiomics Reveal Impacts of Rhizosphere Metabolites on Alfalfa Continuous Cropping. Front. Microbiol. 2022, 13, 833968. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Li, L.; Li, D.; Liang, Z.; Cao, Y.; Hu, T.; Yang, P. Proteomic Analysis of Alfalfa (Medicago sativa L.) Roots in Response to Rhizobium Nodulation and Salt Stress. Genes 2022, 13, 2004. [Google Scholar] [CrossRef]
- Duan, C.J.; Mei, Y.X.; Wang, Q.; Wang, Y.H.; Li, Q.; Hong, M.J.; Hu, S.; Li, S.Q.; Fang, L.C. Rhizobium Inoculation Enhanced the resistance of Alfalfa and Soil Enzymatic Activity in Cu-polluted Soils. Prepr. (Version 1) Available Res. Sq. 2021, 6. [Google Scholar] [CrossRef]
- Zang, Q.B. Determination of Flavonoids and Feed Quality Evaluation of Afalfa; Inner Mongolia University: Hohhot, China, 2019. [Google Scholar]
- Wang, J. Study on the Effect of Isoflavones on Nitrogen Efficiency of Alfalfa; Gansu Agricultural University: Lanzhou, China, 2023. [Google Scholar] [CrossRef]
- Tong, C.H.; Liu, X.J.; Wu, Y.; Zhao, Y.J.; Wang, J. Regulation of Endogenous Isoflavones on Alfalfa Nodulation and Nitrogen Fixation and Nitrogen Use Efficiency. Acta Prataculturae Sin. 2022, 31, 124–135. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, J.; Deng, B.; Liu, Z.K.; Li, J.H.; He, Y.J. Effects of Exogenous Root Exudates on Symbiosis of Alfalfa and Rhizobium. Acta Agrestia Sin. 2020, 28, 88–94. [Google Scholar] [CrossRef]
- Spaink, H.P. Root Nodulation and Infection Factors Produced by Rhizobial Bacteria. Annu. Rev. Microbiol. 2000, 54, 257–288. [Google Scholar] [CrossRef]
- Guo, C.H.; Zhu, Z.F.; Duan, Y.X.; Wang, Y.Y.; Chen, L.J. Suppression of Different Soybean Isoflavones on Heterodera Glycines. Chin. J. Oil Crop Sci. 2017, 39, 540–545. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Cheng, Q.; Zhang, L.; Cai, T.; Shi, Q.; Wang, Z.; Chang, C.; Yin, Q.; Jiang, X.; et al. Multi-Omics Analyses Reveal New Insights Into Nutritional Quality Changes of Alfalfa Leaves During the Flowering Period. Front. Plant Sci. 2022, 30, 995031. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Ge, G.T.; Liu, Y.; Wang, W.; Liu, L.Y.; Jia, Y.S. Proteomics Integrated with Metabolomics: Analysis of the Internal Causes of Nutrient Changes in Alfalfa at Different Growth Stages. BMC Plant Biol. 2018, 18, 78. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Ma, Y.; Fang, L.; Liu, Y.; Zhu, X.; Dong, H.; Wang, S. Metabolite Analysis Reveals Flavonoids Accumulation During Flower Development in Rhododendron Pulchrum Sweet (Ericaceae). PeerJ 2024, 12, e17325. [Google Scholar] [CrossRef]
- Wang, X.; Yin, J.; Wang, J.; Li, J. Integrative Analysis of Transcriptome and Metabolome Revealed the Mechanisms By Which Flavonoids and Phytohormones Regulated The Adaptation of Alfalfa Roots to NaCl Stress. Front. Plant Sci. 2023, 3, 1117868. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Luo, K. Biosynthesis and Metabolic Engineering of Isoflavonoids in Model Plants and Crops: A Review. Front. Plant Sci. 2024, 25, 1384091. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao, R.; Zhang, Y.; Li, Q.; Lei, S. Metabolic Characteristics of PGPR-Induced Growth Promotion in Alfalfa (Medicago sativa L.). Metabolites 2025, 15, 652. https://doi.org/10.3390/metabo15100652
Dao R, Zhang Y, Li Q, Lei S. Metabolic Characteristics of PGPR-Induced Growth Promotion in Alfalfa (Medicago sativa L.). Metabolites. 2025; 15(10):652. https://doi.org/10.3390/metabo15100652
Chicago/Turabian StyleDao, Rina, Ying Zhang, Qiang Li, and Shengyan Lei. 2025. "Metabolic Characteristics of PGPR-Induced Growth Promotion in Alfalfa (Medicago sativa L.)" Metabolites 15, no. 10: 652. https://doi.org/10.3390/metabo15100652
APA StyleDao, R., Zhang, Y., Li, Q., & Lei, S. (2025). Metabolic Characteristics of PGPR-Induced Growth Promotion in Alfalfa (Medicago sativa L.). Metabolites, 15(10), 652. https://doi.org/10.3390/metabo15100652





