Chemometric Discrimination of Korean and Chinese Kimchi Using Untargeted Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Kimchi Sample
2.2. pH, Salinity, and Titratable Acidity
2.3. GC-MS Analysis
2.4. UPLC-Q-TOF MS Analysis
2.5. Data Processing
2.6. Predictive Modeling of Kimchi Biomarkers
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Kimchi Samples
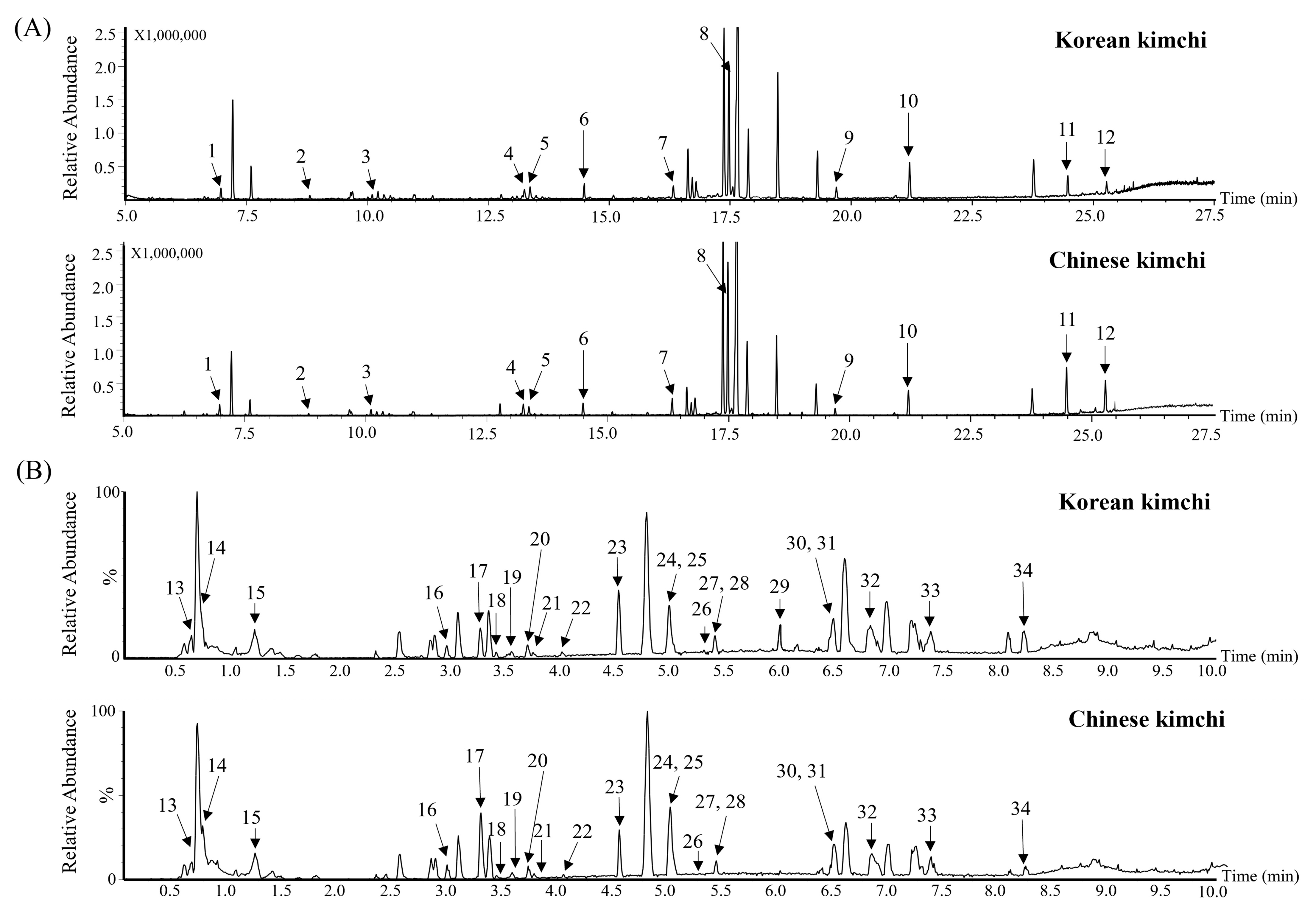
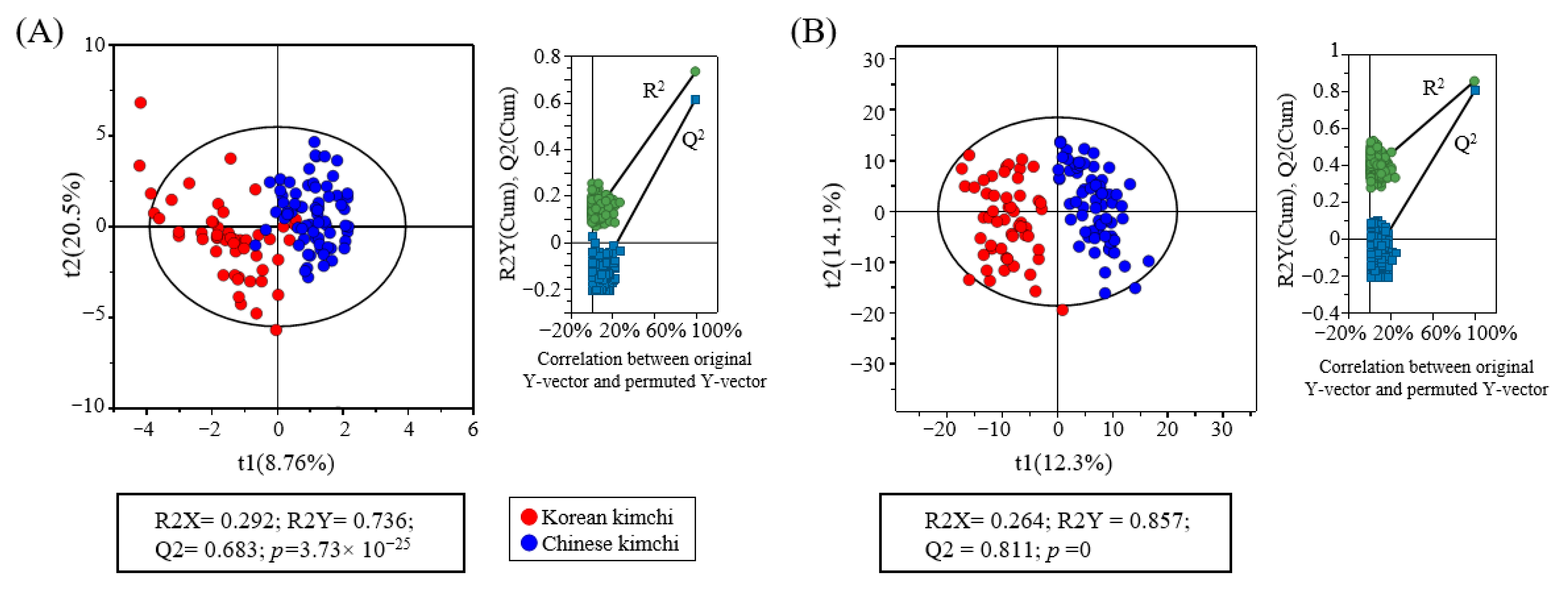
3.2. Metabolomic Analysis
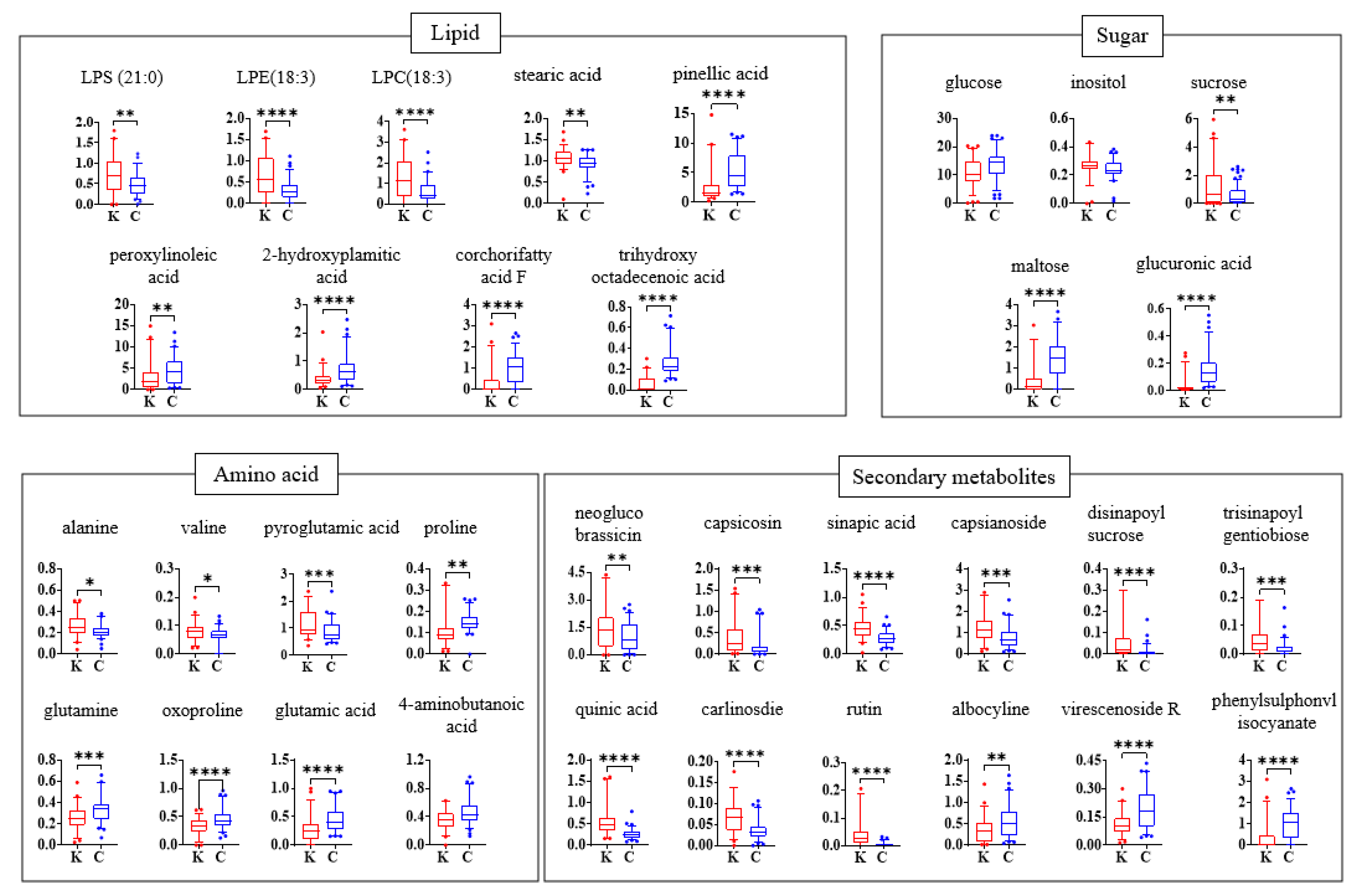
3.3. Relative Abundance of Identified Metabolites
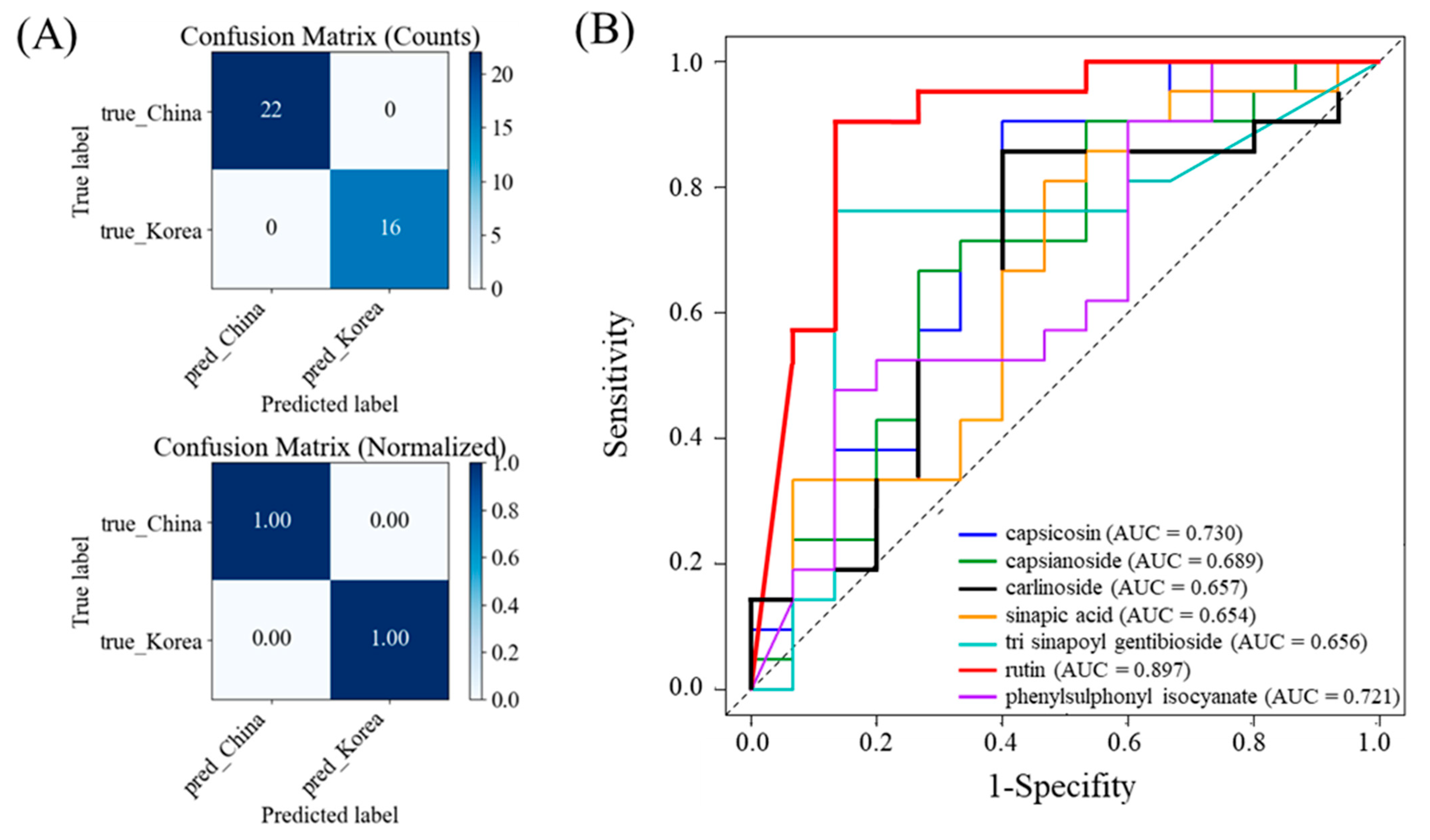
3.4. Discriminative Performance of Key Metabolites for Kimchi Origin Discrimination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chien, H.J.; Zheng, Y.F.; Wang, W.C.; Kuo, C.Y.; Hsu, Y.M.; Lai, C.C. Determination of adulteration, geographical origins, and species of food by mass spectrometry. Mass Spectrom. Rev. 2023, 42, 2273–2323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Gao, F.; Su, W.; Li, T.; Wang, Y. Foodomics as a Tool for Evaluating Food Authenticity and Safety from Field to Table: A Review. Foods 2025, 14, 15. [Google Scholar] [CrossRef]
- Gökmen, V. Importance of Food Authentication and Origin Testing. Food Chem. X 2023, 18, 100708. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic Nose as a Tool for Monitoring the Authenticity of Food. A Review. Food Anal. Meth. 2017, 10, 1800–1816. [Google Scholar] [CrossRef]
- Salihah, N.T.; Hossain, M.M.; Lubis, H.; Ahmed, M.U. Trends and advances in food analysis by real-time polymerase chain reaction. J. Food Sci. Technol. 2016, 53, 2196–2209. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Zotos, A.; Thomatou, A.-A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), a Useful Tool in Authenticity of Agricultural Products’ and Foods’ Origin. Foods 2022, 11, 3705. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469. [Google Scholar] [CrossRef]
- Selamat, J.; Rozani, N.A.A.; Murugesu, S. Application of the Metabolomics Approach in Food Authentication. Molecules 2021, 26, 7565. [Google Scholar] [CrossRef]
- Donarski, J.A.; Jones, S.A.; Charlton, A.J. Application of Cryoprobe 1H Nuclear Magnetic Resonance Spectroscopy and Multivariate Analysis for the Verification of Corsican Honey. J. Agric. Food Chem. 2008, 14, 5451–5456. [Google Scholar] [CrossRef]
- Solovyev, P.A.; Fauhl-Hassek, C.; Riedl, J.; Esslinger, S.; Bontempo, L.; Camin, F. NMR spectroscopy in wine authentication: An official control perspective. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2040–2062. [Google Scholar] [CrossRef]
- Woodcock, T.; Downey, G.; O’Donnell, C.P. Confirmation of Declared Provenance of European Extra Virgin Olive Oil Samples by NIR Spectroscopy. J. Agric. Food Chem. 2008, 56, 11520–11525. [Google Scholar] [CrossRef]
- Vaclavik, L.; Lacina, O.; Hajslova, J.; Zweigenbaum, J. The use of high performance liquid chromatography–quadrupole time-of-flight mass spectrometry coupled to advanced data mining and chemometric tools for discrimination and classification of red wines according to their variety. Anal. Chim. Acta 2011, 685, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Choi, W.; Park, J.H.; Lim, J.; Kwon, S.W. Determination of coffee origins by integrated metabolomic approach of combining multiple analytical data. Food Chem. 2010, 121, 1260–1268. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.A.; Capitani, D.; Iaffaldano, N.; Rosato, M.P.; Ragni, P.; Reale, A.; Sorrentino, E.; D’Amico, I.; Coppola, R. NMR metabolic profiling of organic and aqueous sea bass extracts: Implications in the discrimination of wild and cultured sea bass. Talanta 2008, 77, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Yang, H.J.; Kim, M.J.; Han, E.S.; Kim, H.J.; Kwon, D.Y. Metabolomic analysis of meju during fermentation by ultra performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS). Food Chem. 2011, 127, 1056–1064. [Google Scholar] [CrossRef]
- Namgung, H.J.; Park, H.J.; Cho, I.H.; Choi, H.K.; Kwon, D.Y.; Shim, S.M.; Kim, Y.S. Metabolite profiling of doenjang, fermented soybean paste, during fermentation. J. Sci. Food Agric. 2010, 90, 1926–1935. [Google Scholar] [CrossRef]
- Yang, S.O.; Kim, S.H.; Cho, S.; Lee, J.H.; Kim, Y.S.; Yun, S.S.; Choi, H.K. Classification of Fermented Soymilk during Fermentation by 1H NMR Coupled with Principal Component Analysis and Elucidation of Free-Radical Scavenging Activities. Biosci. Biotechnol. Biochem. 2009, 73, 1184–1188. [Google Scholar] [CrossRef]
- Trimigno, A.; Bøge Lyndgaard, C.; Atladóttir, G.A.; Aru, V.; Balling Engelsen, S.; Harder Clemmensen, L.K. An NMR Metabolomics Approach to Investigate Factors Affecting the Yoghurt Fermentation Process and Quality. Metabolites 2020, 10, 293. [Google Scholar] [CrossRef]
- Cha, J.; Kim, Y.B.; Park, S.E.; Lee, S.H.; Roh, S.W.; Son, H.S. Does kimchi deserve the status of a probiotic food? Crit. Rev. Food Sci. Nutr. 2024, 64, 6512–6525. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, E.J.; Park, S.E.; Cho, K.M.; Kwon, S.J.; Roh, S.W.; Kwak, S.; Whon, T.W.; Son, H.S. Impact of essential and optional ingredients on microbial and metabolic profiles of kimchi. Food Chem. X 2024, 22, 101348. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Bae, M.; Kim, M.J.; Jang, H.Y.; Jung, S.; Lee, J.H.; Hwang, I.M. Rapid Quantitative Analysis of Metabolites in Kimchi Using LC-Q-Orbitrap MS. ACS Omega 2023, 8, 3896–3904. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.; Bong, Y.S.; Lee, K.S.; Hwang, G.W. Determination of the Geographical Origin of Kimchi by 1H NMR-Based Metabolite Profiling. Biosci. Biotechnol. Biochem. 2012, 76, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Bong, Y.S.; Gautam, M.K.; La, M.R.; Lee, K.S. Geographic Origins of Korean and Chinese Kimchi Determined by Multiple Elements. Biosci. Biotechnol. Biochem. 2012, 76, 2096–2100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hur, S.H.; Kim, H.; Kim, Y.K.; An, J.M.; Lee, J.H.; Kim, H.J. Geographical origin discrimination of kimchi using ICP-OES and ICP-MS combined with multivariate analysis. Food Chem. 2023, 423, 136235. [Google Scholar][Green Version]
- Kim, D.W.; Kim, B.M.; Lee, H.J.; Jang, G.J.; Song, S.H.; Lee, J.I.; Lee, S.B.; Shim, J.M.; Lee, K.W.; Kim, J.H.; et al. Effects of Different Salt Treatments on the Fermentation Metabolites and Bacterial Profiles of Kimchi. J. Food Sci. 2017, 82, 1124–1131. [Google Scholar] [CrossRef]
- Ghasemo, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Lee, H.Y.; Haque, A.; Cho, K.M. Changes in physicochemical property and lactic acid bacterial community during kimchi fermentation at different temperatures. J. Appl. Biol. Chem. 2020, 63, 429–437. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kwon, H.; Kim, J.K.; Park, C.H.; Sathasivam, R.; Park, S.U. Comparative Analysis of Glucosinolate and Phenolic Compounds in Green and Red Kimchi Cabbage (Brassica rapa L. ssp. pekinensis) Hairy Roots after Exposure to Light and Dark Conditions. Horticulturae 2023, 9, 466. [Google Scholar]
- Kim, S.Y.; Ha, J.H. Rapid determination of the geographical origin of kimchi by Fourier transform near-infrared spectroscopy coupled with chemometric techniques. Sci. Rep. 2024, 14, 24581. [Google Scholar] [CrossRef]
- In, J.M.; Kim, H.M.; Jin, H.J.; Kim, D.C.; Oh, N.S.; Chae, H.J. Production of a Fermented Korean Pear Puree using a New Strain Leuconostoc mesenteroides KACC 91495P Isolated from Kimchi. J. Appl. Boil. Chem. 2010, 53, 51–55. [Google Scholar] [CrossRef][Green Version]
- Rentería-Ortega, M.; Colín-Alvarez, M.d.L.; Gaona-Sánchez, V.A.; Chalapud, M.C.; García-Hernández, A.B.; León-Espinosa, E.B.; Valdespino-León, M.; Serrano-Villa, F.S.; Calderón-Domínguez, G. Characterization and Applications of the Pectin Extracted from the Peel of Passiflora tripartita var. mollissima. Membranes 2023, 13, 797. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Paramithiotis, S.; Shin, H.S. Kimchi and Other Widely Consumed Traditional Fermented Foods of Korea: A Review. Front. Microbiol. 2016, 7, 1493. [Google Scholar] [CrossRef]
- Liu, L.; She, X.; Chen, X.; Qian, Y.; Tao, Y.; Li, Y.; Guo, S.; Xiang, W.; Liu, G.; Rao, Y. Microbiota Succession and Chemical Composition Involved in the Radish Fermentation Process in Different Containers. Front. Microbiol. 2020, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, M.J.; Choi, Y.-J.; Park, S.J.; Lee, M.-A.; Min, S.G.; Park, S.-H.; Seo, H.-Y.; Yun, Y.-R. Free Amino Acid and Volatile Compound Profiles of Jeotgal Alternatives and Its Application to Kimchi. Foods 2021, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Jeon, H.-S.; Yoo, J.-Y.; Kim, J.-H. Some Important Metabolites Produced by Lactic Acid Bacteria Originated from Kimchi. Foods 2021, 10, 2148. [Google Scholar] [CrossRef] [PubMed]
- Langa, S.; Santos, S.; Flores, J.A.; Peirotén, Á.; Rodríguez, S.; Curiel, J.A.; Landete, J.M. Selection of GABA-Producing Lactic Acid Bacteria Strains by Polymerase Chain Reaction Using Novel gadB and gadC Multispecies Primers for the Development of New Functional Foods. Int. J. Mol. Sci. 2024, 25, 13696. [Google Scholar]
- Lee, K.W.; Shim, J.M.; Yao, Z.; Kim, J.A.; Kim, J.H. Properties of Kimchi Fermented with GABA-Producing Lactic Acid Bacteria as a Starter. J. Microbiol. Biotechnol. 2018, 28, 534–541. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lim, J.Y.; Kang, M.J.; Yang, J.H.; Chung, Y.B.; Park, S.H.; Min, S.G.; Lee, M.A. Changes in bacterial composition and metabolite profiles during kimchi fermentation with different garlic varieties. Heliyon 2024, 10, e24283. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, C.; Ye, Q.; Liu, C.; Wan, H.; Ruan, M.; Zhou, G.; Wang, R.; Li, Z.; Diao, M.; et al. The Influence of Different Factors on the Metabolism of Capsaicinoids in Pepper (Capsicum annuum L.). Plants 2024, 13, 2887. [Google Scholar] [CrossRef]
- Arrizabalaga-Larrañaga, A.; Campmajó, G.; Saurina, J.; Núñez, O.; Santos, F.J.; Moyano, E. Determination of capsaicinoids and carotenoids for the characterization and geographical origin authentication of paprika by UHPLC–APCI–HRMS. LWT-Food Sci. Technol. 2021, 139, 110533. [Google Scholar]
- Park, B.; Yang, J.S.; Moon, E.W.; Seo, H.Y.; Ha, J.H. Influence of Capsaicinoids Content on the Microbial Community during Kimchi Fermentation. J. Microbiol. Biotechnol. 2019, 29, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.B.; Park, S.J.; Choi, Y.J.; Yun, Y.R.; Lee, M.A.; Park, S.H.; Min, S.G.; Seo, H.Y. Metabolic shift during fermentation in kimchi according to capsaicinoid concentration. Heliyon 2024, 10, e24441. [Google Scholar] [CrossRef] [PubMed]
- Baroni, M.V.; Fabani, M.P.; Adan, F.; Podio, N.S.; Wunderlin, D.A. Effect of geographical location, processing and simulated digestion on antioxidant characteristics of quince (Cydonia oblonga). Heliyon 2022, 8, e11435. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Beta, T. Discrimination of geographical origin of Napirira bean (Phaseolus vulgaris L.) based on phenolic profiles and antioxidant activity. J. Food Compos. Anal. 2017, 62, 217–222. [Google Scholar] [CrossRef]
- Ishaq, M.; Zhao, L.; Soliman, M.M.; Althobaiti, S.; Al-Harthi, H.F.; Albattal, S.B.; Chengtao, W. Ameliorative impacts of Sinapic acid against monosodium urate crystal-induced gouty arthritis and inflammation through different signaling pathways. Toxicol. Res. 2024, 13, tfae130. [Google Scholar]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. Ferulic, Sinapic, 3,4-Dimethoxycinnamic Acid and Indomethacin Derivatives with Antioxidant, Anti-Inflammatory and Hypolipidemic Functionality. Antioxidants 2023, 12, 1436. [Google Scholar] [CrossRef]
- Su, G.; Yu, C.; Liang, S.; Wang, W.; Wang, H. Multi-omics in food safety and authenticity in terms of food components. Food chem. 2024, 437, 137943. [Google Scholar] [CrossRef]
- Liang, C.; Xu, Z.; Liu, P.; Guo, S.; Xiao, P.; Duan, J.-a. Integrating different detection techniques and data analysis methods for comprehensive food authenticity verification. Food Chem. 2025, 463, 141471. [Google Scholar] [CrossRef]
| Korean Kimchi | Chinese Kimchi | |
|---|---|---|
| pH | 4.905 ± 0.533 | 4.886 ± 0.524 |
| salinity (%) | 3.454 ± 0.763 | 3.153 ± 0.665 * |
| TA (% lactic acid) | 0.219 ± 0.059 | 0.218 ± 0.055 |
| RT (min) | Compound | RI | VIP | p-Value | Fold Change (vs. K) |
|---|---|---|---|---|---|
| 6.98 | alanine | 1097 | 1.06 | 5.43 × 10−3 | −1.23 |
| 8.82 | valine | 1210 | 0.97 | 1.14 × 10−2 | −1.21 |
| 10.10 | proline | 1292 | 1.17 | 7.24 × 10−3 | 1.25 |
| 13.25 | oxoproline | 1516 | 0.88 | 1.20 × 10−2 | −1.19 |
| 13.37 | 4-aminobutanoic acid | 1525 | 1.65 | 6.34 × 10−5 | 1.33 |
| 14.49 | glutamic acid | 1612 | 1.86 | 5.82 × 10−6 | 1.74 |
| 16.34 | glutamine | 1766 | 1.10 | 9.18 × 10−3 | 1.26 |
| 17.67 | glucose | 1843 | 1.45 | 3.73 × 10−2 | 1.29 |
| 19.70 | inositol | 1981 | 0.88 | 6.45 × 10−2 | −1.14 |
| 21.22 | stearic acid | 2132 | 1.14 | 2.30 × 10−4 | −1.12 |
| 24.48 | sucrose | 2609 | 1.24 | 4.27 × 10−3 | −2.20 |
| 25.28 | maltose | 2723 | 2.23 | 4.35 × 10−9 | 3.20 |
| RT (min) | Compound | Exact Mass (M-H) | MS Fragments | VIP | p-Value | Fold Change (vs. K) |
|---|---|---|---|---|---|---|
| 0.69 | glucuronic acid | 193.035 | 113 | 1.68 | 1.19 × 10−10 | 4.56 |
| 0.74 | quinic acid | 191.055 | 179 | 1.68 | 1.25 × 10−10 | −2.18 |
| 1.20 | pyroglutamic acid | 128.033 | 116 | 1.00 | 5.04 × 10−5 | −1.38 |
| 2.93 | phenylsulphonyl isocyanate | 181.990 | 119 | 1.32 | 1.78 × 10−6 | 2.68 |
| 3.27 | neoglucobrassicin | 477.063 | 422, 609 | 0.85 | 3.00 × 10−3 | −1.56 |
| 3.40 | rutin | 609.147 | 284 | 1.31 | 1.29 × 10−6 | −7.45 |
| 3.52 | carlinoside | 579.137 | 399 | 1.39 | 3.36 × 10−7 | −1.83 |
| 3.71 | sinapic acid | 223.060 | 149, 164,193 | 1.59 | 1.73 × 10−9 | −1.64 |
| 3.85 | disinapoyl sucrose | 753.225 | 529, 205 | 1.08 | 9.54 × 10−5 | −6.27 |
| 4.09 | trisinapoyl gentiobiose | 959.287 | 735, 529, 511, 205 | 1.03 | 1.92 × 10−4 | −2.58 |
| 4.53 | capsianoside | 1129.536 | 937, 497, 343 | 1.06 | 2.70 × 10−5 | −1.55 |
| 5.00 | pinellic acid | 329.231 | 211, 229, 171, 139 | 1.33 | 1.42 × 10−6 | 2.00 |
| 5.00 | corchorifatty acid F | 327.218 | 211, 171, 229, 291 | 1.65 | 4.12 × 10−10 | 3.20 |
| 5.24 | virescenoside R | 643.336 | 293, 481 | 1.17 | 2.07 × 10−6 | 1.63 |
| 5.33 | trihydroxy octadecenoic acid | 329.232 | 171, 201 | 2.16 | 8.03 × 10−19 | 4.70 |
| 5.41 | albocyline | 307.191 | 116, 329, 805 | 0.90 | 1.00 × 10−3 | 1.55 |
| 5.95 | capsicosin | 1287.595 | 915 | 1.05 | 3.32 × 10−4 | −2.23 |
| 6.43 | LPE (18:3) | 474.262 | 277, 196 | 1.33 | 1.06 × 10−6 | −2.04 |
| 6.45 | LPC (18:3) | 562.316 | 502, 277 | 1.23 | 8.31 × 10−6 | −2.03 |
| 6.88 | peroxy linoleic acid | 295.226 | 277, 233 | 0.99 | 1.72 × 10−10 | 1.54 |
| 7.38 | LPS (21:0) | 566.348 | 281, 152, 506 | 0.88 | 2.00 × 10−3 | −1.45 |
| 8.22 | 2-hydroxypalmitic acid | 271.227 | 225, 255 | 1.13 | 5.81 × 10−6 | 1.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.-A.; Kim, D.-S.; Kim, H.-D.; Kim, K.-B.; Ham, K.-S.; Lee, Y.; Kim, H.-J. Chemometric Discrimination of Korean and Chinese Kimchi Using Untargeted Metabolomics. Metabolites 2025, 15, 640. https://doi.org/10.3390/metabo15100640
Nguyen Q-A, Kim D-S, Kim H-D, Kim K-B, Ham K-S, Lee Y, Kim H-J. Chemometric Discrimination of Korean and Chinese Kimchi Using Untargeted Metabolomics. Metabolites. 2025; 15(10):640. https://doi.org/10.3390/metabo15100640
Chicago/Turabian StyleNguyen, Quynh-An, Dong-Shin Kim, Hyo-Dong Kim, Kyu-Bin Kim, Kyung-Sik Ham, Yonghoon Lee, and Hyun-Jin Kim. 2025. "Chemometric Discrimination of Korean and Chinese Kimchi Using Untargeted Metabolomics" Metabolites 15, no. 10: 640. https://doi.org/10.3390/metabo15100640
APA StyleNguyen, Q.-A., Kim, D.-S., Kim, H.-D., Kim, K.-B., Ham, K.-S., Lee, Y., & Kim, H.-J. (2025). Chemometric Discrimination of Korean and Chinese Kimchi Using Untargeted Metabolomics. Metabolites, 15(10), 640. https://doi.org/10.3390/metabo15100640







