Effect of β-Caryophyllene on PPAR-γ, NF-κB, and CNR2: Implications for Gut–Brain Axis Communication in a Murine Model of Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Western Blot
2.2. Reverse Transcription—(RTq)—PCR
2.3. Statistical Analysis
3. Results
3.1. Differential Regulation of PPAR-γ and NF-κB/p65 by BCP in the Small Intestine and Colon
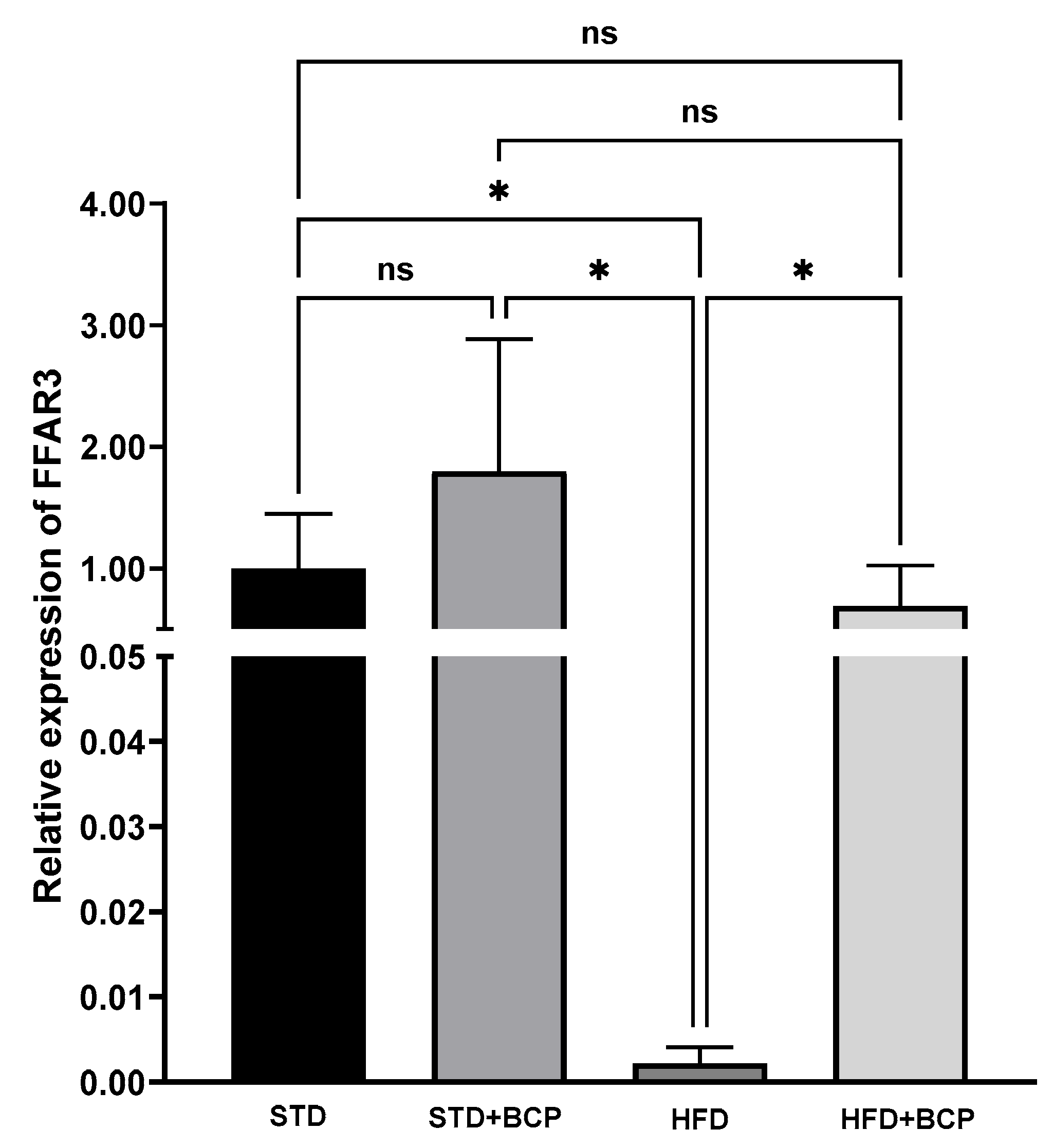
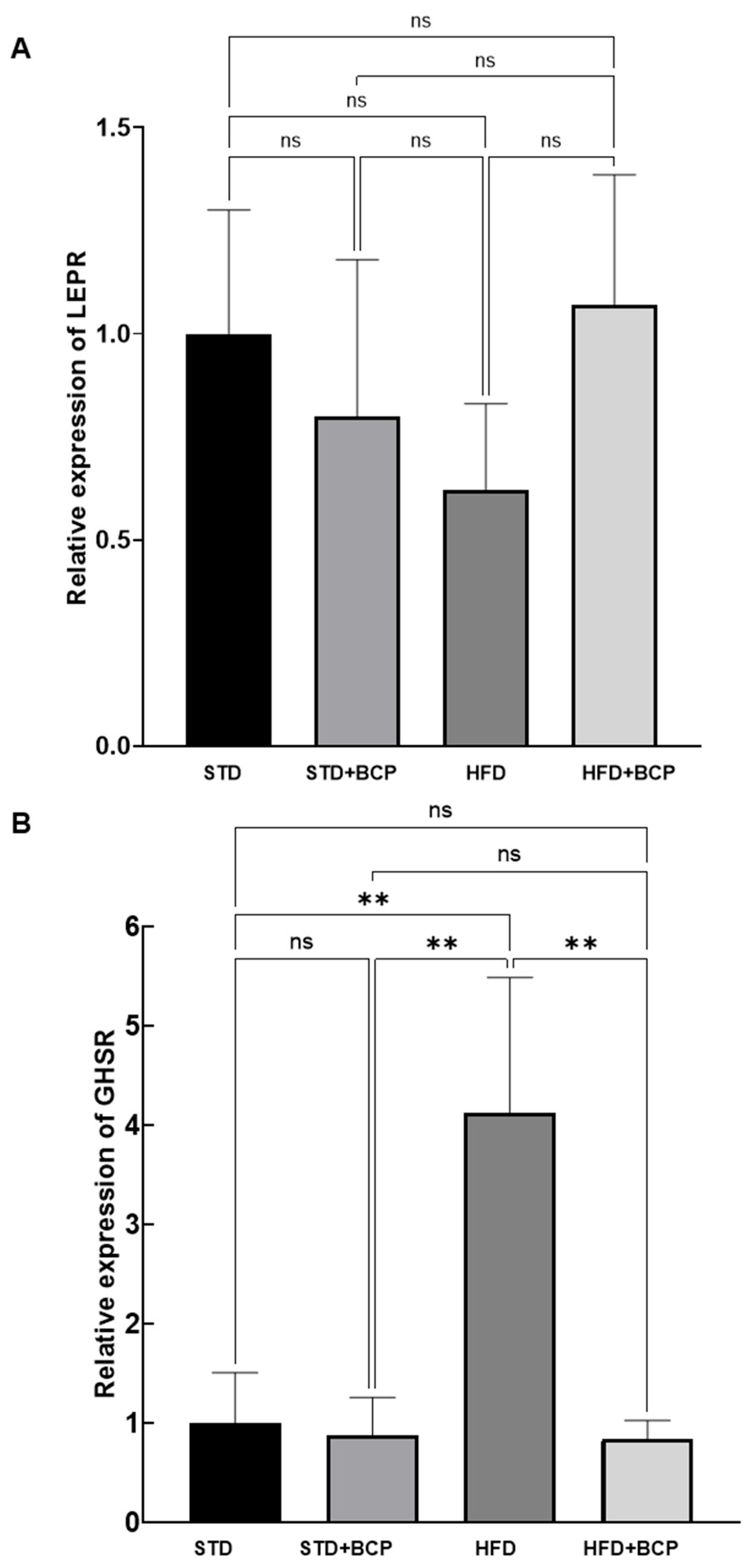
3.2. BCP Modulates the Gene Expression of Hypothalamic Receptors for SCFAs, Leptin, and Ghrelin
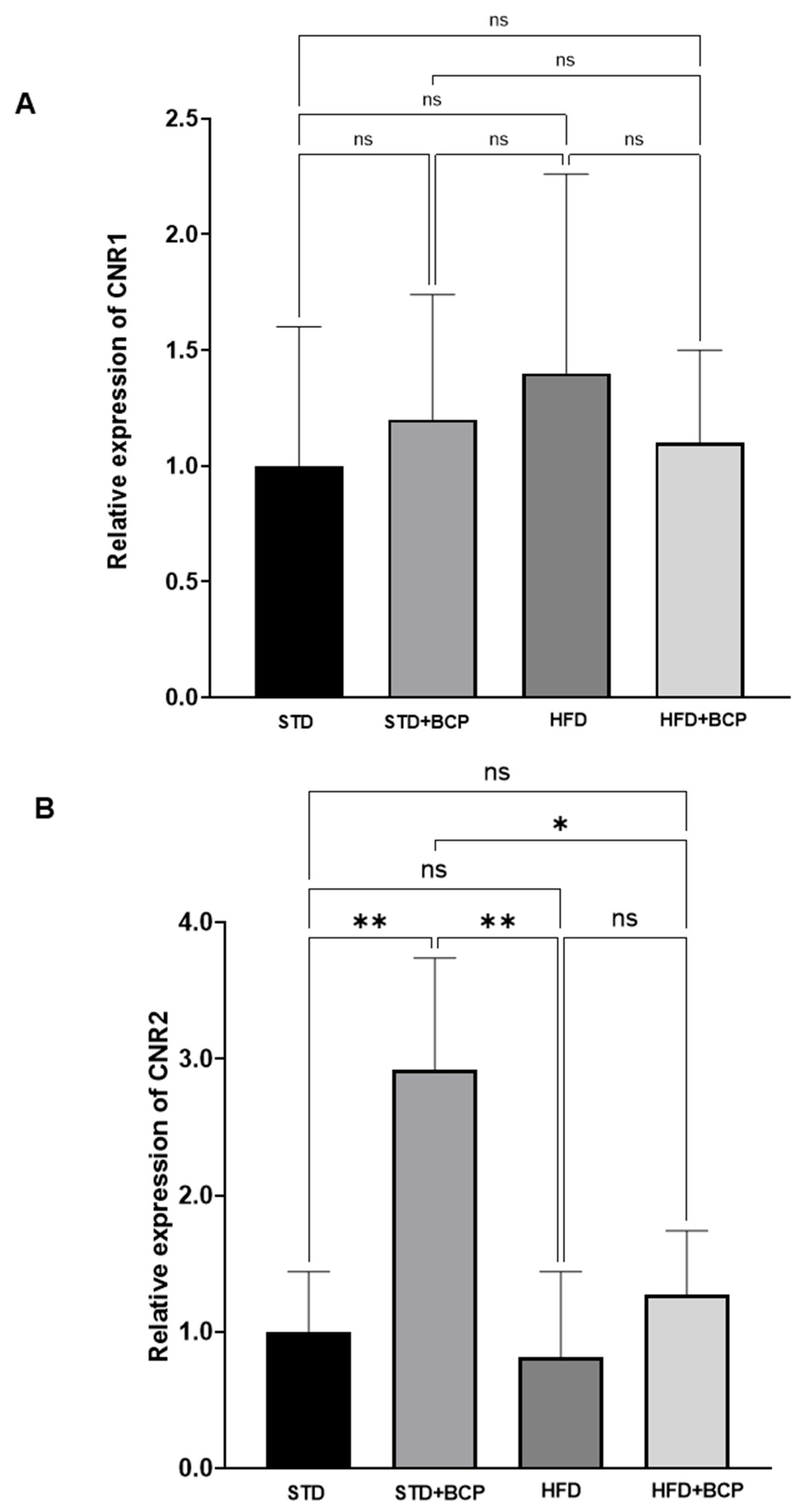
3.3. Effect of BCP on Hypothalamic Endocannabinoid System Receptor Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | Bicinchoninic Acid |
| BCP | β-Caryophyllene |
| CNR1 | Cannabinoid Receptor 1 |
| CNR2 | Cannabinoid Receptor 2 |
| CNS | Central Nervous System |
| FFAR3 | Free Fatty Acid Receptor 3 |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GHSR | Growth Hormone Secretagogue Receptor (Ghrelin Receptor) |
| GLP-1 | Glucagon-Like Peptide-1 |
| HFD | High-Fat Diet |
| HFD + BCP | High-Fat Diet + β-Caryophyllene |
| IgG | Immunoglobulin G |
| IL-8 | Interleukin 8 |
| LEPR | Leptin Receptor |
| NF-κB/p65 | Nuclear Factor Kappa B, p65 subunit |
| OD | Optical Density |
| PPARγ/PPAR-γ | Peroxisome Proliferator-Activated Receptor Gamma |
| PBS | Phosphate-Buffered Saline |
| PCR | Polymerase Chain Reaction |
| PVDF | Polyvinylidene Fluoride |
| qPCR | Quantitative Polymerase Chain Reaction |
| RIPA | Radioimmunoprecipitation Assay (lysis buffer) |
| RNA | Ribonucleic Acid |
| SCFA | Short-Chain Fatty Acids |
| SD | Standard Deviation |
| STD | Standard Diet |
| STD + BCP | Standard Diet + β-Caryophyllene |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRIzol | Tri-Reagent for RNA isolation |
| WHO | World Health Organization |
Appendix A
Appendix A.1. Calculation of the Sample Size
- n = sample size
- Z = confidence level considering the p to be used = 1.96
- σ = variation obtained in the measured parameter = 5.6
- δ = desired variation in the parameter = 3
Appendix A.2. Primers Used for the Amplification of the Genes of Interest
| Forward Oligo | Sequence | Reverse Oligo | Sequence |
|---|---|---|---|
| FW1 -CNR1 | CCTTGCAGATACCACCTTCC | REV1-CNR1 | CTGAAGGAAGTTAGAGGGAATTTCTG |
| FW1-CNR2 | CCTCGTACCTGTTCATCAGCAG | REV1-CNR2 | GTGAAGGTCATGGTCACACTGC |
| FW1- FFAR3 | GCTTCTTTCTTGGCAATTACTGG | REV1- FFAR3 | GTTTAGCAAAAGTAAGTCCACAGC |
| FW1- GHSR | CTAACGTCACGCTGGACCTG | REV1- GHSR | GTTGCCCGAGATGCCCAC |
| FW1- LEPR | GCACTTAACCTGGCATATCCAATCTC | REV1- LEPR | GGATAACTCAGGAACGTAGATACCAC |
| FW1-GAPDH | GAAGGTCGGTGTGAACGGATTTGG | REV1-GAPDH | CGTGAGTGGAGTCATACTGGAACATG |
References
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- WHO. Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 23 March 2023).
- Srivastava, G.; Apovian, C.M. Current Pharmacotherapy for Obesity. Nat. Rev. Endocrinol. 2018, 14, 12–24. [Google Scholar] [CrossRef]
- Gadde, K.M.; Apolzan, J.W.; Berthoud, H.-R. Pharmacotherapy for Patients with Obesity. Clin. Chem. 2018, 64, 118–129. [Google Scholar] [CrossRef]
- Kan, H.; Bae, J.P.; Dunn, J.P.; Buysman, E.K.; Gronroos, N.N.; Swindle, J.P.; Bengtson, L.G.S.; Ahmad, N. Real-World Primary Nonadherence to Antiobesity Medications. J. Manag. Care Spec. Pharm. 2023, 29, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting Obesity with Plant-Derived Pancreatic Lipase Inhibitors: A Comprehensive Review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Natural Dietary and Medicinal Plants with Anti-Obesity Therapeutics Activities for Treatment and Prevention of Obesity during Lock Down and in Post-COVID-19 Era. Appl. Sci. 2021, 11, 7889. [Google Scholar] [CrossRef]
- Zamani, B.; Daneshzad, E.; Siassi, F.; Guilani, B.; Bellissimo, N.; Azadbakht, L. Association of Plant-Based Dietary Patterns with Psychological Profile and Obesity in Iranian Women. Clin. Nutr. 2020, 39, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Jupp, P.W. Selected Environmental Factors in a Complex Systems Approach to Managing Obesity. Obes. Med. 2020, 19, 100275. [Google Scholar] [CrossRef]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural Anti-Obesity Agents and Their Therapeutic Role in Management of Obesity: A Future Trend Perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Lu, M.; Cao, Y.; Xiao, J.; Song, M.; Ho, C.-T. Molecular Mechanisms of the Anti-Obesity Effect of Bioactive Ingredients in Common Spices: A Review. Food Funct. 2018, 9, 4569–4581. [Google Scholar] [CrossRef]
- Giuseppe, D.; Angela, D.; Davide, R.; Pamela, M. Effects of a Combination of Berberis Aristata, Silybum Marianum and Monacolin on Lipid Profile in Subjects at Low Cardiovascular Risk; A Double-Blind, Randomized, Placebo-Controlled Trial. IJMS 2017, 18, 343. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L. Berberine, a Plant Alkaloid with Lipid- and Glucose-Lowering Properties: From in Vitro Evidence to Clinical Studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Bennett, B.T.; Wilson, J.C.; Edens, N.K.; Pereira, S.L. Epigallocatechin-3-Gallate Improves Plantaris Muscle Recovery after Disuse in Aged Rats. Exp. Gerontol. 2014, 50, 82–94. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.H.; Park, J.I.; Oh, H.T.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. (-)-Epigallocatechin-3-Gallate Stimulates Myogenic Differentiation through TAZ Activation. Biochem. Biophys. Res. Commun. 2017, 486, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Mirza, K.A.; Pereira, S.L.; Edens, N.K.; Tisdale, M.J. Attenuation of Muscle Wasting in Murine C2C12 Myotubes by Epigallocatechin-3-Gallate. J. Cachexia Sarcopenia Muscle 2014, 5, 339–345. [Google Scholar] [CrossRef]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The Effects of Curcumin Supplementation on High-sensitivity C-reactive Protein, Serum Adiponectin, and Lipid Profile in Patients with Type 2 Diabetes: A Randomized, Double-blind, Placebo-controlled Trial. Phytother. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef]
- Chong, P.-W.; Beah, Z.-M.; Grube, B.; Riede, L. IQP-GC-101 Reduces Body Weight and Body Fat Mass: A Randomized, Double-Blind, Placebo-Controlled Study. Phytother. Res. 2014, 28, 1520–1526. [Google Scholar] [CrossRef]
- Gonnelli, S.; Caffarelli, C.; Stolakis, K.; Cuda, C.; Giordano, N.; Nuti, R. Efficacy and Tolerability of a Nutraceutical Combination (Red Yeast Rice, Policosanols, and Berberine) in Patients with Low-Moderate Risk Hypercholesterolemia: A Double-Blind, Placebo-Controlled Study. Curr. Ther. Res. 2015, 77, 1–6. [Google Scholar] [CrossRef]
- Widjajakusuma, E.C.; Jonosewojo, A.; Hendriati, L.; Wijaya, S.; Ferawati; Surjadhana, A.; Sastrowardoyo, W.; Monita, N.; Muna, N.M.; Fajarwati, R.P.; et al. Phytochemical Screening and Preliminary Clinical Trials of the Aqueous Extract Mixture of Andrographis Paniculata (Burm. f.) Wall. Ex Nees and Syzygium Polyanthum (Wight.) Walp Leaves in Metformin Treated Patients with Type 2 Diabetes. Phytomedicine 2019, 55, 137–147. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Azimullah, S.; Meeran, M.F.N.; Saraswathiamma, D.; Jha, N.K.; Sadek, B.; Adeghate, E.; Tariq, S.; Marzooqi, S.A.; Ojha, S. β-Caryophyllene, a Dietary Phytocannabinoid, Alleviates Diabetic Cardiomyopathy in Mice by Inhibiting Oxidative Stress and Inflammation Activating Cannabinoid Type-2 Receptors. ACS Pharmacol. Transl. Sci. 2023, 6, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Djafarian, K.; Mofidi Nejad, M.; Yekaninejad, M.S.; Javanbakht, M.H. The Effect of β-Caryophyllene on Food Addiction and Its Related Behaviors: A Randomized, Double-Blind, Placebo-Controlled Trial. Appetite 2022, 178, 106160. [Google Scholar] [CrossRef]
- Rodríguez-Mejía, U.U.; Viveros-Paredes, J.M.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Zepeda-Nuño, J.S.; Velázquez-Juárez, G.; Delgado-Rizo, V.; García-Iglesias, T.; Camacho-Padilla, L.G.; Varela-Navarro, E.; et al. β-Caryophyllene: A Therapeutic Alternative for Intestinal Barrier Dysfunction Caused by Obesity. Molecules 2022, 27, 6156. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Protects against Diet-Induced Dyslipidemia and Vascular Inflammation in Rats: Involvement of CB2 and PPAR-γ Receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef]
- Dammann, K.; Khare, V.; Lang, M.; Claudel, T.; Harpain, F.; Granofszky, N.; Evstatiev, R.; Williams, J.M.; Pritchard, D.M.; Watson, A.; et al. PAK1 Modulates a PPARγ/NF-κB Cascade in Intestinal Inflammation. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015, 1853, 2349–2360. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The Gut Microbiota-Immune-Brain Axis: Therapeutic Implications. CR Med. 2025, 6, 101982. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Shaker, O.G.; Ismail, M.F.; Sayed, N.H. Peroxisome Proliferator-Activated Receptor Gamma in Obesity and Colorectal Cancer: The Role of Epigenetics. Sci. Rep. 2017, 7, 10714. [Google Scholar] [CrossRef]
- Krentz, A.J.; Bailey, C.J. Oral Antidiabetic Agents: Current Role in Type 2 Diabetes Mellitus. Drugs 2005, 65, 385–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Tan, B.; Qu, Y.; Liu, H.; Deng, H.; Chen, Y.; Wang, R.; Tian, J.; Zhu, J. Gut Microbiota and Its Derived SCFAs Regulate the HPGA to Reverse Obesity-Induced Precocious Puberty in Female Rats. Front. Endocrinol. 2022, 13, 1051797. [Google Scholar] [CrossRef] [PubMed]
- Franco-Arroyo, N.N.; Viveros-Paredes, J.M.; Zepeda-Morales, A.S.M.; Roldán, E.; Márquez-Aguirre, A.L.; Zepeda-Nuño, J.S.; Velázquez-Juárez, G.; Fafutis-Morris, M.; López-Roa, R.I. β-Caryophyllene, a Dietary Cannabinoid, Protects Against Metabolic and Immune Dysregulation in a Diet-Induced Obesity Mouse Model. J. Med. Food 2022, 25, 993–1002. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Y.; Gao, L.; Yang, Z.; Lin, J.; Ren, S.; Li, F.; Chen, J.; Wang, Z.; Dong, Z.; et al. PPAR-γ Integrates Obesity and Adipocyte Clock through Epigenetic Regulation of Bmal1. Theranostics 2022, 12, 1589–1606. [Google Scholar] [CrossRef]
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARgamma as a New Therapeutic Target in Inflammatory Bowel Diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef]
- Su, W.; Bush, C.R.; Necela, B.M.; Calcagno, S.R.; Murray, N.R.; Fields, A.P.; Thompson, E.A. Differential Expression, Distribution, and Function of PPAR-Gamma in the Proximal and Distal Colon. Physiol. Genom. 2007, 30, 342–353. [Google Scholar] [CrossRef]
- Engin, A. Lipid Storage, Lipolysis, and Lipotoxicity in Obesity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 97–129. ISBN 978-3-031-63657-8. [Google Scholar]
- Roberts, L.D.; Murray, A.J.; Menassa, D.; Ashmore, T.; Nicholls, A.W.; Griffin, J.L. The Contrasting Roles of PPARδ and PPARγ in Regulating the Metabolic Switch between Oxidation and Storage of Fats in White Adipose Tissue. Genome Biol. 2011, 12, R75. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.P.; Serra, M.P.; Boi, M.; Boi, M.; Carta, A.; Carta, A.; Murru, E.; Murru, E.; Carta, G.; Carta, G.; et al. Anti-Inflammatory Effect of Beta-Caryophyllene Mediated by the Involvement of TRPV1, BDNF and trkB in the Rat Cerebral Cortex after Hypoperfusion/Reperfusion. Int. J. Mol. Sci. 2022, 23, 3633. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, R.; Cheng, L.; Yang, J.; Zhu, L. Peroxisome Proliferator-Activated Receptor Gamma Activation Promotes Intestinal Barrier Function by Improving Mucus and Tight Junctions in a Mouse Colitis Model. Dig. Liver Dis. 2018, 50, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Caioni, G.; Viscido, A.; d’Angelo, M.; Panella, G.; Castelli, V.; Merola, C.; Frieri, G.; Latella, G.; Cimini, A.; Benedetti, E. Inflammatory Bowel Disease: New Insights into the Interplay between Environmental Factors and PPARγ. Int. J. Mol. Sci. 2021, 22, 985. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.-Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids—At the Crossroads between the Gut Microbiota and Host Metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The Endocannabinoid System Links Gut Microbiota to Adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Jwa, S.-K. Efficacy of β-Caryophyllene for Periodontal Disease Related Factors. Arch. Oral Biol. 2019, 100, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- O’Rahilly, S.; Farooqi, I.S. Biopatología de La Obesidad. In Harrison. Principios de Medicina Interna, 21st ed.; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J.L., Eds.; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M.; Gavini, C.K.; Jesse, J.; Aubert, G.; Gornick, E.; Bonomo, R.; Gautron, L.; Layden, B.T.; Mansuy-Aubert, V. Vagal Neuron Expression of the Microbiota-Derived Metabolite Receptor, Free Fatty Acid Receptor (FFAR3), Is Necessary for Normal Feeding Behavior. Mol. Metab. 2021, 54, 101350. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Meeran, M.F.N.; Sharma, C.; Sadek, B.; Kaabi, J.A.; Ojha, S.K. Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications. Nutrients 2020, 12, 2963. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-Chain Fatty Acids and Ketones Directly Regulate Sympathetic Nervous System via G Protein-Coupled Receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pech-Jiménez, C.; Carrera-Quintanar, L.; Viveros-Paredes, J.M.; Marquez-Sandoval, Y.F.; Jave-Suárez, L.F.; Zepeda-Morales, A.S.M.; Velázquez-Juárez, G.; López-Roa, R.I. Effect of β-Caryophyllene on PPAR-γ, NF-κB, and CNR2: Implications for Gut–Brain Axis Communication in a Murine Model of Diet-Induced Obesity. Metabolites 2025, 15, 638. https://doi.org/10.3390/metabo15100638
Pech-Jiménez C, Carrera-Quintanar L, Viveros-Paredes JM, Marquez-Sandoval YF, Jave-Suárez LF, Zepeda-Morales ASM, Velázquez-Juárez G, López-Roa RI. Effect of β-Caryophyllene on PPAR-γ, NF-κB, and CNR2: Implications for Gut–Brain Axis Communication in a Murine Model of Diet-Induced Obesity. Metabolites. 2025; 15(10):638. https://doi.org/10.3390/metabo15100638
Chicago/Turabian StylePech-Jiménez, Cristina, Lucrecia Carrera-Quintanar, Juan Manuel Viveros-Paredes, Yolanda Fabiola Marquez-Sandoval, Luis Felipe Jave-Suárez, Adelaida Sara Minia Zepeda-Morales, Gilberto Velázquez-Juárez, and Rocio Ivette López-Roa. 2025. "Effect of β-Caryophyllene on PPAR-γ, NF-κB, and CNR2: Implications for Gut–Brain Axis Communication in a Murine Model of Diet-Induced Obesity" Metabolites 15, no. 10: 638. https://doi.org/10.3390/metabo15100638
APA StylePech-Jiménez, C., Carrera-Quintanar, L., Viveros-Paredes, J. M., Marquez-Sandoval, Y. F., Jave-Suárez, L. F., Zepeda-Morales, A. S. M., Velázquez-Juárez, G., & López-Roa, R. I. (2025). Effect of β-Caryophyllene on PPAR-γ, NF-κB, and CNR2: Implications for Gut–Brain Axis Communication in a Murine Model of Diet-Induced Obesity. Metabolites, 15(10), 638. https://doi.org/10.3390/metabo15100638








