Untargeted Metabolomics Reveals the Metabolic Characteristics and Biomarkers of Antioxidant Properties of Gardeniae Fructus from Different Geographical Origins in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Metabolite Extraction
2.3. Determination of Antioxidant Activity
2.4. Untargeted LC–MS-Based Metabolomics Analysis
2.5. Data Analysis and Statistics
3. Results and Discussion
3.1. Tracing the Geographical Origins of Gardeniae Fructus by Metabolomics
3.2. Chemical Composition of Gardeniae Fructus
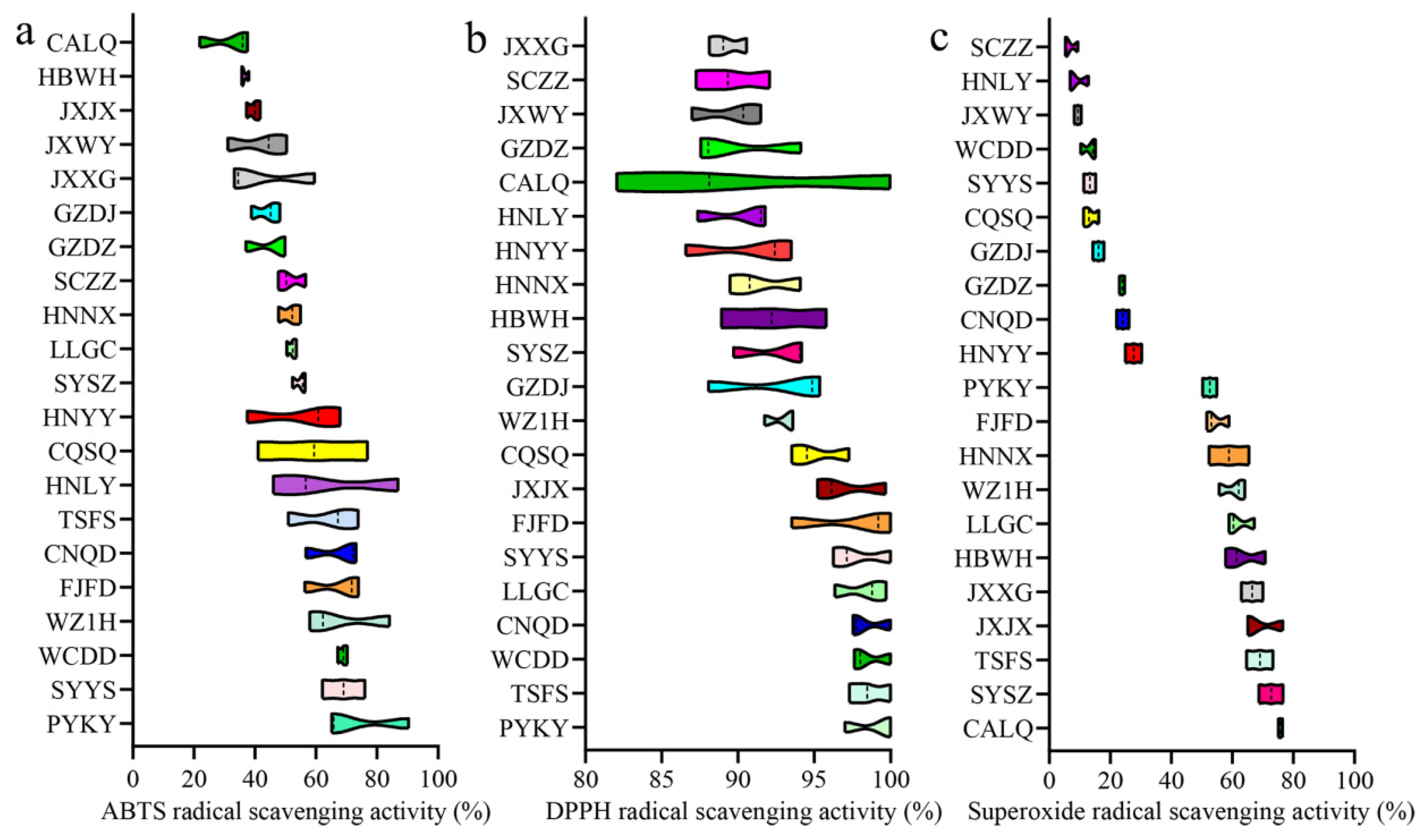
3.3. Antioxidant Activity of Gardeniae Fructus
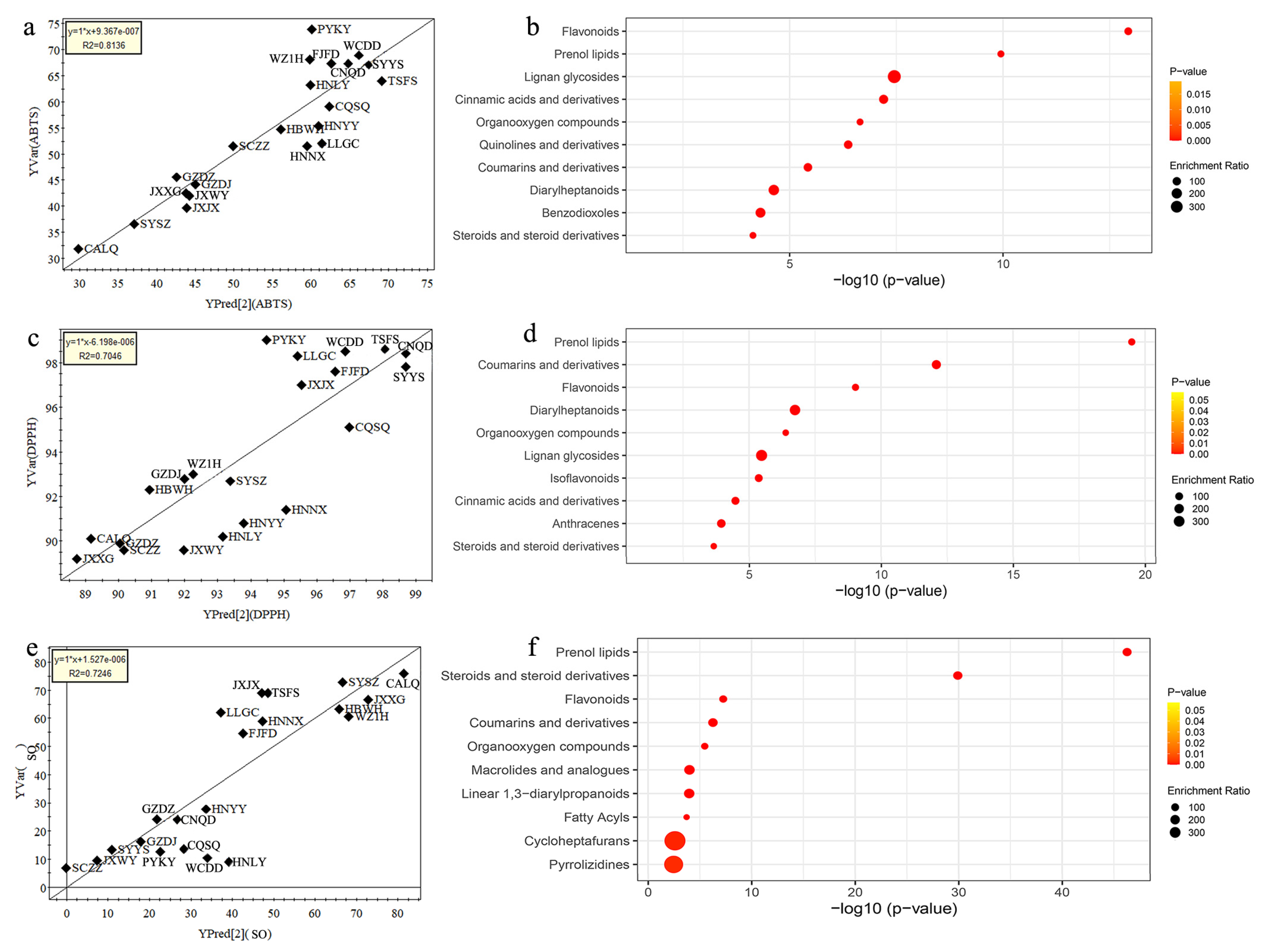
3.4. Analysis of Main Chemical Compositions Contributable to Antioxidant Activity
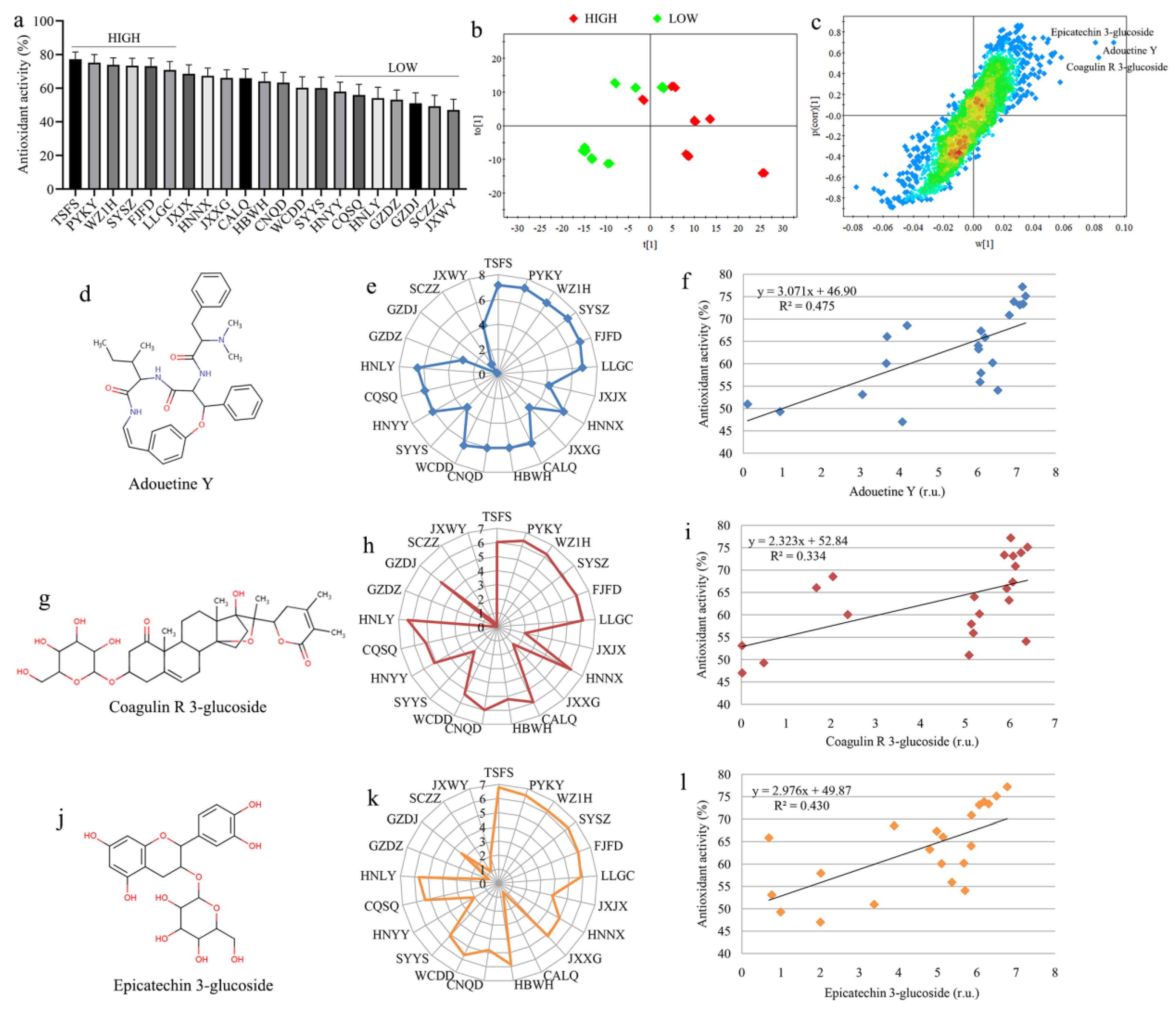
3.5. Biomarkers of High Antioxidant Property in Gardeniae Fructus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.P.; Li, M.X.; Yang, Z.Q.; Tao, W.; Wang, P.; Tian, X.Y.; Li, X.L.; Wang, W.G. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Q.; Gao, L.Q.; Yang, X.L.; Wang, M.X.; Feng, X.T.; Deng, S.Y. Genome-wide resequencing reveals genetic relationships between main Gardenia jasminoides Ellis cultivars. Horticulturae 2023, 9, 754. [Google Scholar] [CrossRef]
- Liu, N.; Chen, S.Y. Research Progress in Medicinal Value and Application of Gardenia jasminoides. Med. Plant Res. 2023, 13, 1–5. [Google Scholar] [CrossRef]
- Wu, X.Y.; Zhou, Y.; Yin, F.Z.; Mao, C.Q.; Li, L.; Cai, B.C.; Lu, T.L. Quality control and producing areas differentiation of Gardeniae Fructus for eight bioactive constituents by HPLC–DAD–ESI/MS. Phytomedicine 2014, 21, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Z.; Qin, S.S.; Han, J.Y.; Meng, J.; Liang, A.H. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J. Ethnopharmacol. 2022, 289, 114984. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Fan, Y.L.; Li, X.Y.; Ding, L.; Zhou, W.Y.; Xu, G.Z.; Wang, Y.; Zhang, Y.Z.; Ni, Q.X. Accelerated solvent extraction of antioxidant compounds from gardeniae fructus and its acetylcholinesterase inhibitory and PC12 cell protective activities. Foods 2021, 10, 2805. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Ahn, S.H.; Nguyen, U.T.; Yang, I.J.; Shin, H.M. Geniposide, a principal component of Gardeniae Fructus, protects skin from diesel exhaust particulate matter-induced oxidative damage. Evid-Based Compl. Alt. Med. 2021, 2021, 8847358. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, H.L.; Hao, S.X.; Pan, D.M.; Wang, G.J.; Yu, W.Q. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the antioxidant activity of propolis samples from different geographical regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Meenu, M.; Xu, B.J. Nutritional value and antioxidant activity of Chinese black truffle (Tuber indicum) grown in different geographical regions in China. LWT 2021, 135, 110226. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Zhang, J.; Sun, C.H.; Wang, R.N.; Wei, H.Y.; He, H.H.; Zhou, D.H.; Zhang, H.C.; Zhu, J.Y. Metabolomics revealed metabolite biomarkers of antioxidant properties and flavonoid metabolite accumulation in purple rice after grain filling. Food Chem. X 2023, 18, 100720. [Google Scholar] [CrossRef]
- Darwish, A.G.; Moniruzzaman, M.; Tsolova, V.; El-Sharkawy, I. Integrating metabolomics and gene expression underlying potential biomarkers compounds associated with antioxidant activity in southern grape seeds. Metabolites 2023, 13, 210. [Google Scholar] [CrossRef]
- Kaur, P.; Darwish, A.G.; El-Sharkawy, I.; Singh, A.; Subramanian, J. Comparative antioxidant activity and untargeted metabolomic analyses of sour cherry cultivars based on ultra-performance–time of flight–mass spectrometry. Plants 2024, 13, 1511. [Google Scholar] [CrossRef]
- Qamar, H.; Li, Y.F.; He, R.; Waqas, M.; Song, M.; Deng, D.; Cui, Y.Y.; Yang, P.; Liu, Z.C.; Qammar, B.; et al. Integrated metabolomics and metagenomics unveiled biomarkers of antioxidant potential in fermented brewer’s grains. Antioxidants 2024, 13, 872. [Google Scholar] [CrossRef]
- Gong, K.Y.; Yin, X.L.; Ying, N.; Wu, M.J.; Lyu, Y.X.; Zheng, H.; Jiang, L.L. Rapid and accurate detection of Dendrobium officinale adulterated with lower-price species using NMR characteristic markers integrated with artificial neural network. J. Food Meas. Charact. 2024, 18, 4845–4852. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Solorzano, E.R.; Roverso, M.; Bogialli, S.; Bortoli, M.; Orian, L.; Badocco, D.; Pettenuzzo, S.; Favaro, G.; Pastore, P. Antioxidant activity of Zuccagnia-type propolis: A combined approach based on LC-HRMS analysis of bioanalytical-guided fractions and computational investigation. Food Chem. 2024, 461, 140827. [Google Scholar] [CrossRef]
- Pang, Z.Q.; Chong, J.; Zhou, G.Y.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.Z.; Xia, J.G. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Li, A.R.; Li, A.Q.; Luo, C.; Liu, B. Geographical specificity of metabolomic traits among Amomum villosum Lour. Fruits from different areas in China. LWT 2024, 191, 115619. [Google Scholar] [CrossRef]
- Sun, Q.F.; Wu, F.R.; Wu, W.; Yu, W.J.; Zhang, G.W.; Huang, X.Y.; Hao, Y.B.; Luo, L.P. Identification and quality evaluation of Lushan Yunwu tea from different geographical origins based on metabolomics. Food Res. Int. 2024, 186, 114379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Wang, X.Y.; Sa, Y.P.; Li, L.Y.; Wang, W.B.; Yang, L.L.; Ding, S.Q.; Wilson, G.D.; Yang, Y.Y.; Zhang, Y.; et al. A comparative UHPLC-QTOF-MS/MS-based metabolomics approach reveals the metabolite profiling of wolfberry sourced from different geographical origins. Food Chem. X 2024, 21, 101221. [Google Scholar] [CrossRef]
- Li, Y.H.; Liang, L.; Xu, C.H.; Yang, T.M.; Wang, Y.X. UPLC-Q-TOF/MS-based untargeted metabolomics for discrimination of navel oranges from different geographical origins of China. LWT 2021, 137, 110382. [Google Scholar] [CrossRef]
- Li, Z.M.; Tan, M.M.; Deng, H.X.; Yang, X.; Yu, Y.; Zhou, D.R.; Dong, H. Geographical origin differentiation of rice by LC–MS-based non-targeted metabolomics. Foods 2022, 11, 3318. [Google Scholar] [CrossRef]
- Zhang, S.B.; Li, C.; Gu, W.; Qiu, R.L.; Chao, J.G.; Pei, L.F.; Ma, L.J.; Guo, Y.F.; Tian, R. Metabolomics analysis of dandelions from different geographical regions in China. Phytochem. Anal. 2021, 32, 899–906. [Google Scholar] [CrossRef]
- Man, K.Y.; Chan, C.O.; Tang, H.H.; Dong, N.P.; Capozzi, F.; Wong, K.H.; Kwok, K.W.H.; Chan, H.M.; Mok, D.K.W. Mass spectrometry-based untargeted metabolomics approach for differentiation of beef of different geographic origins. Food Chem. 2021, 338, 127847. [Google Scholar] [CrossRef]
- Shin, J.; Yang, J.; Kim, H.; Sim, Y.; Cha, E.; Yang, J.Y. Development of metabolomic biomarkers to discriminate the geographical origin of Korean and Russian snow crabs using CE-TOF/MS. Food Chem. 2024, 451, 139286. [Google Scholar] [CrossRef]
- Zhao, G.H.; Zhao, W.F.; Han, L.S.; Ding, J.; Chang, Y.Q. Metabolomics analysis of sea cucumber (Apostichopus japonicus) in different geographical origins using UPLC–Q-TOF/MS. Food Chem. 2020, 333, 127453. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, X.Y.; Cai, M.; Gu, H.T.; E, H.C.; Li, X.B.; Zhang, Y.M.; Lu, H.; Zhou, C.Y. Metabolomics analysis of Morchella sp. from different geographical origins of China using UPLC-Q-TOF-MS. Front. Nutr. 2022, 9, 865531. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the antioxidant and anti-inflammatory activities of selected plant compounds and their metal ions complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Almoraie, N.M.; Shatwan, I.M. The potential effects of dietary antioxidants in obesity: A comprehensive review of the literature. Healthcare 2024, 12, 416. [Google Scholar] [CrossRef]
- Fatima, M.T.; Bhat, A.A.; Nisar, S.; Fakhro, K.A.; Al-Shabeeb Akil, A.S. The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders: An assessment of the benefit profile. Heliyon 2022, 9, e12698. [Google Scholar] [CrossRef]
- Luo, M.C.; Zhou, L.; Huang, Z.; Li, B.W.; Nice, E.C.; Xu, J.; Huang, C.H. Antioxidant therapy in cancer: Rationale and progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids1. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Baczewska-Dąbrowska, A.H.; Dmuchowski, W.; Gozdowski, D.; Gworek, B.; Jozwiak, A.; Swiezewska, E.; Dąbrowski, P.; Suwara, I. The importance of prenol lipids in mitigating salt stress in the leaves of Tilia× euchlora trees. Trees 2022, 36, 393–404. [Google Scholar] [CrossRef]
- Rabeh, K.; Sbabou, L.; Rachidi, F.; Ferradouss, A.; Laghmari, G.; Aasfar, A.; Arroussi, H.E.; Ouajdi, M.; Antry, S.E.; Belkadi, B.; et al. Lipidomic profiling of Argania spinosa L. (Skeels) following drought stress. Applied Biochem. Biotech. 2023, 195, 1781–1799. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radical Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.F.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Filipský, T.; Říha, M.; Macáková, K.; Anzenbacherová, E.; Karlíčková, J.; Mladěnka, P. Antioxidant effects of coumarins include direct radical scavenging, metal chelation and inhibition of ROS-producing enzymes. Curr. Top. Med. Chem. 2015, 15, 415–431. [Google Scholar] [CrossRef]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Cur. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Gunaherath, G.M.K.B.; Gunatilaka, A.L. Plant steroids: Occurrence, biological significance, and their analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 1–31. [Google Scholar]
- Mooradian, A.D. Antioxidant properties of steroids. J. Steroid Biochem. Mol. Biol. 1993, 45, 509–511. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, T.; Yang, R.X.; Tang, H.X.; Qiang, L.; Liu, Y.P. Anti-inflammatory steroids from the fruits of Artocarpus heterophyllus. Nat. Prod. Res. 2021, 35, 3071–3077. [Google Scholar] [CrossRef]
- Patel, S.S.; Savjani, J.K. Systematic review of plant steroids as potential antiinflammatory agents: Current status and future perspectives. J. Phytopharmacol. 2015, 4, 121–125. [Google Scholar] [CrossRef]
- Ahmad, A.; Gupta, G.; Afzal, M.; Kazmi, I.; Anwar, F. Antiulcer and antioxidant activities of a new steroid from Morus alba. Life Sci. 2013, 92, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Miya, G.M.; Oriola, A.O.; Payne, B.; Cuyler, M.; Lall, N.; Oyedeji, A.O. Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties. Molecules 2023, 28, 3434. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Khan, H. Advances in antioxidant potential of natural alkaloids. Curr. Bioact. Compd. 2017, 13, 101–108. [Google Scholar] [CrossRef]
- Yamin, R.; Ahmad, I.; Khalid, H.; Perveen, A.; Abbasi, S.W.; Nishan, U.; Sheheryar, S.; Moura, A.A.; Ahmed, S.; Ullah, R.; et al. Identifying plant-derived antiviral alkaloids as dual inhibitors of SARS-CoV-2 main protease and spike glycoprotein through computational screening. Front. Pharmacol. 2024, 15, 1369659. [Google Scholar] [CrossRef]
- Mustafa, S.; Nazir, M.; Riaz, N.; Saleem, M.; Tauseef, S.; Tousif, M.I.; Abbas, Z.; Kamran, A.B.; Alaerjani, W.M.A.; Alarfaji, S.S.; et al. In vitro and in silico bioactivities and chemical profiling of Nepeta leucolaena to validate its use in nutraceutical or biopharmaceutical applications. Process Biochem. 2023, 125, 61–74. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]



| Sample No. | Origins | Longitude (E) | Latitude (N) | Altitude (m) |
|---|---|---|---|---|
| JXXG | Xingan, Jiangxi | 115°18′47.47″ | 27°44′45.01″ | 122 |
| JXWY | Wuyuan, Jiangxi | 117°46′51.87″ | 29°25′52.55″ | 126 |
| JXJX | Jinxi, Jiangxi | 116°42′39.07″ | 28°05′49.09″ | 159 |
| HNYY | Yueyang, Hunan | 113°22′48.02″ | 29°30′01.02″ | 660 |
| HNNX | Ningxiang, Hunan | 112°36′36.04″ | 28°10′47.99″ | 920 |
| HNLY | Liuyang, Hunan | 113°15′30.24″ | 28°02′07.42″ | 76 |
| HBWH | Wuhan, Hubei | 114°26′48.83″ | 31°06′46.89″ | 110 |
| GZDJ | Dejiang, Guizhou | 108°10′08.65″ | 28°15′41.22″ | 710 |
| GZDZ | Danzhai, Guizhou | 107°48′42.63″ | 26°19′57.55″ | 828 |
| FJFD | Fuding, Fujian | 120°15′11.88″ | 27°25′39.31″ | 295 |
| CQSQ | Sanquan, Chongqing | 107°15′36.03″ | 29°05′23.98″ | 1050 |
| SCZZ | Zizhong, Sichuan | 104°43′36.89″ | 29°45′09.91″ | 437 |
| CALQ | Chunan, Zhejiang | 119°14′02.77″ | 29°56′12.87″ | 445 |
| CNQD | Cangnan, Zhejiang | 120°17′41.43″ | 27°24′32.66″ | 312 |
| PYKY | Pingyang, Zhejiang | 120°29′46.33″ | 27°41′01936″ | 404 |
| TSFS | Taishun, Zhejiang | 120°14′28.01″ | 27°26′24.97″ | 430 |
| WCDD | Wencheng, Zhejiang | 120°09′37.86″ | 27°50′28.60″ | 508 |
| SYYS | Yongjia, Zhejiang | 120°33′59.70″ | 28°10′14.08″ | 101 |
| LLGC | Yongjia, Zhejiang | 120°32′24.04″ | 28°13′12.04″ | 280 |
| SYSZ | Yongjia, Zhejiang | 120°35′24.06″ | 28°17′24.34″ | 460 |
| WZ1H | Yongjia, Zhejiang | 120°32′19.18″ | 28°17′19.18″ | 408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Jiang, L.; Yin, X.; Zhang, S.; Duan, X.; Chen, J.; Liu, Y.; Zheng, H.; Tao, Z. Untargeted Metabolomics Reveals the Metabolic Characteristics and Biomarkers of Antioxidant Properties of Gardeniae Fructus from Different Geographical Origins in China. Metabolites 2025, 15, 38. https://doi.org/10.3390/metabo15010038
Jiang W, Jiang L, Yin X, Zhang S, Duan X, Chen J, Liu Y, Zheng H, Tao Z. Untargeted Metabolomics Reveals the Metabolic Characteristics and Biomarkers of Antioxidant Properties of Gardeniae Fructus from Different Geographical Origins in China. Metabolites. 2025; 15(1):38. https://doi.org/10.3390/metabo15010038
Chicago/Turabian StyleJiang, Wu, Lingling Jiang, Xiaoli Yin, Shuhui Zhang, Xiaojing Duan, Jiadong Chen, Yingying Liu, Hong Zheng, and Zhengming Tao. 2025. "Untargeted Metabolomics Reveals the Metabolic Characteristics and Biomarkers of Antioxidant Properties of Gardeniae Fructus from Different Geographical Origins in China" Metabolites 15, no. 1: 38. https://doi.org/10.3390/metabo15010038
APA StyleJiang, W., Jiang, L., Yin, X., Zhang, S., Duan, X., Chen, J., Liu, Y., Zheng, H., & Tao, Z. (2025). Untargeted Metabolomics Reveals the Metabolic Characteristics and Biomarkers of Antioxidant Properties of Gardeniae Fructus from Different Geographical Origins in China. Metabolites, 15(1), 38. https://doi.org/10.3390/metabo15010038





