Multiplexed Dilute-and-Shoot Liquid Chromatography–Multiple-Reaction Monitoring Mass Spectrometry Clinical Assay for Metanephrines and Catecholamines in Human Urine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Urine Sample Collection
2.1.2. External Quality Assurance
2.2. Methods
2.2.1. Preparation of Calibration Standards and QC Samples
2.2.2. Sample Preparation
2.2.3. LC-MRM-MS Analysis
2.2.4. HPLC-ECD Analysis
2.2.5. Method Validation
2.2.6. Data Analysis
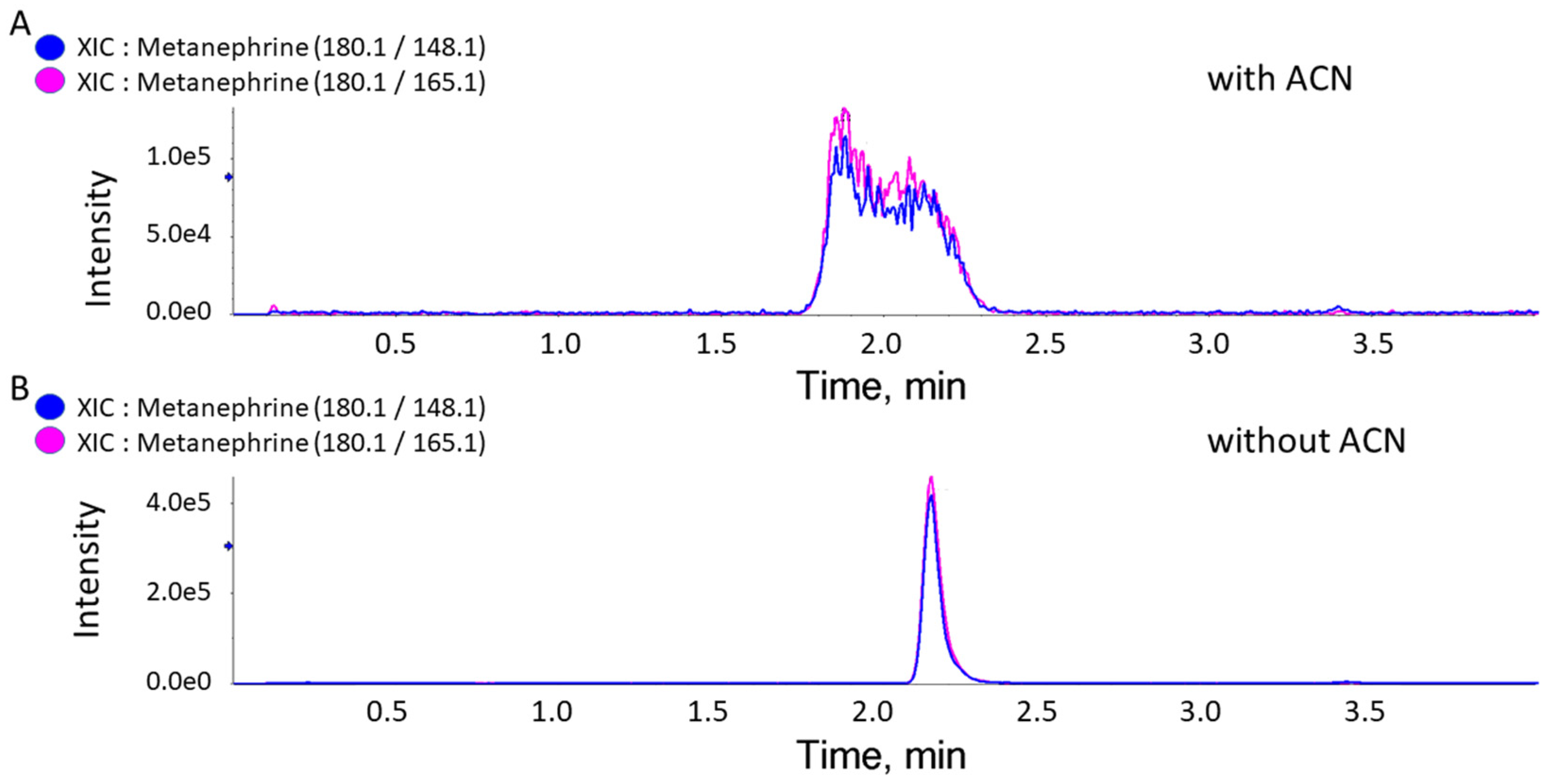
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Grouzmann, E.; Lamine, F. Determination of catecholamines in plasma and urine. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 713–723. [Google Scholar] [CrossRef] [PubMed]
- de Jong, W.H.; de Vries, E.G.; Kema, I.P. Current status and future developments of LC-MS/MS in clinical chemistry for quantification of biogenic amines. Clin. Biochem. 2011, 44, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Weinkove, C. ACP Broadsheet No 127: April 1991. Measurement of catecholamines and their metabolites in urine. J. Clin. Pathol. 1991, 44, 269–275. [Google Scholar] [CrossRef]
- Lenders, J.W.; Duh, Q.Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.P.; Grebe, S.K.; Murad, M.H.; Naruse, M.; Pacak, K.; Young, W.F., Jr.; Endocrine, S. Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef]
- Peaston, R.T.; Weinkove, C. Measurement of catecholamines and their metabolites. Ann. Clin. Biochem. 2004, 41, 17–38. [Google Scholar] [CrossRef]
- Davies, S.L.; Davison, A.S. Liquid chromatography tandem mass spectrometry for plasma metadrenalines. Clin. Chim. Acta 2019, 495, 512–521. [Google Scholar] [CrossRef]
- Cooper, R.L.; Walker, R.F. Microradioenzymic assays for the measurement of catecholamines and serotonin. Methods Enzymol. 1983, 103, 483–493. [Google Scholar] [CrossRef]
- Jones, S.R.; Mickelson, G.E.; Collins, L.B.; Kawagoe, K.T.; Wightman, R.M. Interference by pH and Ca2+ ions during measurements of catecholamine release in slices of rat amygdala with fast-scan cyclic voltammetry. J. Neurosci. Methods 1994, 52, 1–10. [Google Scholar] [CrossRef]
- Sanchis-Mallols, J.M.; Villanueva-Camañas, R.M.; Ramis-Ramos, G. Determination of unconjugated catecholamine in urine as dopamine by thermal lens spectrometry. Analyst 1992, 117, 1367–1371. [Google Scholar] [CrossRef]
- Raum, W.J. Methods of plasma catecholamine measurement including radioimmunoassay. Am. J. Physiol. 1984, 247, E4–E12. [Google Scholar] [CrossRef]
- Qasrawi, D.O.; Boyd, J.M.; Sadrzadeh, S.M.H. Measuring steroids from dried blood spots using tandem mass spectrometry to diagnose congenital adrenal hyperplasia. Clin. Chim. Acta 2021, 520, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Georges, J. Advantages and limitations of thermal lens spectrometry over conventional spectrophotometry for absorbance measurements. Talanta 1999, 48, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.; Garris, P.A.; Casto, J.M. Real-time monitoring of electrically evoked catecholamine signals in the songbird striatum using in vivo fast-scan cyclic voltammetry. J. Chem. Neuroanat. 2015, 66–67, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Bertani-Dziedzic, L.M.; Krstulovic, A.M.; Dziedzic, S.W.; Gitlow, S.E.; Cerqueira, S. Analysis of urinary metanephrines by reversed-phase high-performance liquid chromatography and electrochemical detection. Clin. Chim. Acta 1981, 110, 1–8. [Google Scholar] [CrossRef]
- Lenders, J.W.M.; Pacak, K.; Walther, M.M.; Linehan, W.M.; Mannelli, M.; Friberg, P.; Keiser, H.R.; Goldstein, D.S.; Eisenhofer, G. Biochemical Diagnosis of Pheochromocytoma: Which Test Is Best? J. Am. Med. Assoc. 2002, 287, 1427–1434. [Google Scholar] [CrossRef]
- Davidson, F.D.; Davidson, F.D. Paracetamol-associated interference in an HPLC-ECD assay for urinary free metadrenalines and catecholamines. Ann. Clin. Biochem. 2004, 41, 316–320. [Google Scholar] [CrossRef]
- Peaston, R.T.; Graham, K.S.; Chambers, E.; van der Molen, J.C.; Ball, S. Performance of plasma free metanephrines measured by liquid chromatography-tandem mass spectrometry in the diagnosis of pheochromocytoma. Clin. Chim. Acta 2010, 411, 546–552. [Google Scholar] [CrossRef]
- Shushan, B.; Roberts, W.L.; Frank, E.L.; Urry, F.M.; Kushnir, M.M. Analysis of Catecholamines in Urine by Positive-Ion Electrospray Tandem Mass Spectrometry. Clin. Chem. 2002, 48, 323–331. [Google Scholar] [CrossRef]
- Yan, J.; Kuzhiumparambil, U.; Bandodkar, S.; Solowij, N.; Fu, S. Development and validation of a simple, rapid and sensitive LC-MS/MS method for the measurement of urinary neurotransmitters and their metabolites. Anal. Bioanal. Chem. 2017, 409, 7191–7199. [Google Scholar] [CrossRef]
- Strathmann, F.G.; Hoofnagle, A.N. Current and future applications of mass spectrometry to the clinical laboratory. Am. J. Clin. Pathol. 2011, 136, 609–616. [Google Scholar] [CrossRef]
- Kline, G.A.; Boyd, J.; Leung, A.A.; Tang, A.; Sadrzadeh, H.M. Very high rate of false positive biochemical results when screening for pheochromocytoma in a large, undifferentiated population with variable indications for testing. Clin. Biochem. 2020, 77, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Mandal, R.; Wishart, D.S. A sensitive, high-throughput LC-MS/MS method for measuring catecholamines in low volume serum. Anal. Chim. Acta 2018, 1037, 159–167. [Google Scholar] [CrossRef] [PubMed]
- van Faassen, M.; Bischoff, R.; Eijkelenkamp, K.; de Jong, W.H.A.; van der Ley, C.P.; Kema, I.P. In Matrix Derivatization Combined with LC-MS/MS Results in Ultrasensitive Quantification of Plasma Free Metanephrines and Catecholamines. Anal. Chem. 2020, 92, 9072–9078. [Google Scholar] [CrossRef] [PubMed]
- van de Merbel, N.C.; Hendriks, G.; Imbos, R.; Tuunainen, J.; Rouru, J.; Nikkanen, H. Quantitative determination of free and total dopamine in human plasma by LC-MS/MS: The importance of sample preparation. Bioanalysis 2011, 3, 1949–1961. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Ji, C.; McDonald, T.; Walton, J.; Groeber, E.A.; Steenwyk, R.C.; Lin, Z. Ultra sensitive measurement of endogenous epinephrine and norepinephrine in human plasma by semi-automated SPE-LC–MS/MS. J. Chromatogr. B 2012, 895–896, 186–190. [Google Scholar] [CrossRef]
- Greer, B.; Chevallier, O.; Quinn, B.; Botana, L.M.; Elliott, C.T. Redefining dilute and shoot: The evolution of the technique and its application in the analysis of foods and biological matrices by liquid chromatography mass spectrometry. TrAC Trends Anal. Chem. 2021, 141, 116284. [Google Scholar] [CrossRef]
- Deventer, K.; Pozo, O.J.; Verstraete, A.G.; Van Eenoo, P. Dilute-and-shoot-liquid chromatography-mass spectrometry for urine analysis in doping control and analytical toxicology. TrAC Trends Anal. Chem. 2014, 55, 1–13. [Google Scholar] [CrossRef]
- Xie, Z.; Lorkiewicz, P.; Riggs, D.W.; Bhatnagar, A.; Srivastava, S. Comprehensive, robust, and sensitive UPLC-MS/MS analysis of free biogenic monoamines and their metabolites in urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1099, 83–91. [Google Scholar] [CrossRef]
- Clark, Z.D.; Cutler, J.M.; Pavlov, I.Y.; Strathmann, F.G.; Frank, E.L. Simple dilute-and-shoot method for urinary vanillylmandelic acid and homovanillic acid by liquid chromatography tandem mass spectrometry. Clin. Chim. Acta 2017, 468, 201–208. [Google Scholar] [CrossRef]
- Chan, E.C.; Wee, P.Y.; Ho, P.C. Evaluation of degradation of urinary catecholamines and metanephrines and deconjugation of their sulfoconjugates using stability-indicating reversed-phase ion-pair HPLC with electrochemical detection. J. Pharm. Biomed. Anal. 2000, 22, 515–526. [Google Scholar] [CrossRef]
- Lynch, K.L. CLSI C62-A: A New Standard for Clinical Mass Spectrometry. Clin. Chem. 2016, 62, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P. CLSI. Liquid Chromatography-Mass Spectrometry Methods; Approved Guideline. CLSI C62-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Taylor, R.L.; Singh, R.J. Validation of Liquid Chromatography–Tandem Mass Spectrometry Method for Analysis of Urinary Conjugated Metanephrine and Normetanephrine for Screening of Pheochromocytoma. Clin. Chem. 2002, 48, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, S.A.; O’Kane, D.J.; Singh, R.J. Measurement of plasma free metanephrine and normetanephrine by liquid chromatography-tandem mass spectrometry for diagnosis of pheochromocytoma. Clin. Chem. 2004, 50, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Peitzsch, M.; McWhinney, B.C. Impact of LC-MS/MS on the laboratory diagnosis of catecholamine-producing tumors. TrAC Trends Anal. Chem. 2016, 84, 106–116. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Approved Guideline—Second Edition EP9-A3—Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013; p. 33. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Chan, E.C.; Ho, P.C. High-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometric method for the analysis of catecholamines and metanephrines in human urine. Rapid Commun. Mass Spectrom. 2000, 14, 1959–1964. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Goldstein, D.S.; Sullivan, P.; Csako, G.; Brouwers, F.M.; Lai, E.W.; Adams, K.T.; Pacak, K. Biochemical and clinical manifestations of dopamine-producing paragangliomas: Utility of plasma methoxytyramine. J. Clin. Endocrinol. Metab. 2005, 90, 2068–2075. [Google Scholar] [CrossRef]
- Peitzsch, M.; Prejbisz, A.; Kroiss, M.; Beuschlein, F.; Arlt, W.; Januszewicz, A.; Siegert, G.; Eisenhofer, G. Analysis of plasma 3-methoxytyramine, normetanephrine and metanephrine by ultraperformance liquid chromatography-tandem mass spectrometry: Utility for diagnosis of dopamine-producing metastatic phaeochromocytoma. Ann. Clin. Biochem. 2013, 50, 147–155. [Google Scholar] [CrossRef]

| Analyte | Precursor Ion | Quantifier Transition | Qualifier Transition | Ion Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Product Ion | CE | DP | CXP | Product Ion | CE | DP | CXP | |||
| Metanephrine | 180.1 | 148.1 | 23 | 50 | 13 | 165.1 | 23 | 50 | 13 | 1.24 |
| Normetanephrine | 166.1 | 134.1 | 23 | 50 | 13 | 149.1 | 23 | 50 | 13 | 0.38 |
| 3-Methoxytyramine | 151.2 | 91.2 | 25 | 108 | 13 | 119.1 | 19 | 108 | 13 | 1.53 |
| Epinephrine | 184.2 | 166.2 | 15 | 20 | 3 | 107.2 | 20 | 20 | 13 | 0.11 |
| Norepinephrine | 152.2 | 135.2 | 15 | 50 | 13 | __ | __ | __ | __ | NA * |
| Dopamine | 154.1 | 91.1 | 31 | 20 | 3 | 137.1 | 30 | 20 | 3 | 0.18 |
| Metanephrine-d3 | 183.1 | 151.1 | 23 | 50 | 13 | 168.1 | 23 | 50 | 13 | 0.99 |
| Normetanephrine-d3 | 169.1 | 137.1 | 23 | 50 | 13 | 109.1 | 23 | 50 | 13 | 0.56 |
| 3-Methoxytyramine-d4 | 155.1 | 95.1 | 25 | 108 | 13 | 123.1 | 19 | 108 | 13 | 1.9 |
| Epinephrine-d6 | 190.2 | 172.1 | 15 | 20 | 3 | 113.2 | 30 | 20 | 13 | 0.04 |
| Norepinephrine-d6 | 158.2 | 139.2 | 15 | 50 | 13 | __ | __ | __ | __ | NA * |
| Dopamine-d4 | 158.1 | 95.1 | 31 | 20 | 3 | 141.1 | 30 | 20 | 3 | 2.07 |
| Compound | Measuring Interval (nmol/L) | Slope (a) | Intercept (b) | Coefficient of Determination (r2) | Carryover (%) |
|---|---|---|---|---|---|
| Metanephrine | 79.2–10,100 | 0.0008 | +0.0122 | 1.0000 | 0.0 |
| Normetanephrine | 85.3–10,917 | 0.0009 | −0.0323 | 0.9998 | 0.8 |
| 3-Methoxytyramine | 93.5–11,962 | 0.0014 | −0.0004 | 1.0000 | 0.0 |
| Epinephrine | 85.3–10,917 | 0.0007 | +0.0274 | 1.0000 | 0.0 |
| Norepinephrine | 92.3–11,820 | 0.0011 | −0.0481 | 0.9997 | 1.7 |
| Dopamine | 101.9–13,055 | 0.0011 | −0.0233 | 0.9999 | 0.0 |
| Compound | CV (%) Intra-Day (n = 10) | CV (%) Inter-Day (n = 20) | ||||||
|---|---|---|---|---|---|---|---|---|
| LLMI | QCL | QCM | QCH | LLMI | QCL | QCM | QCH | |
| Metanephrine | 5.38 | 2.74 | 3.48 | 4.84 | 11.79 | 3.80 | 3.58 | 2.63 |
| Normetanephrine | 7.66 | 3.36 | 3.50 | 3.70 | 12.06 | 3.22 | 4.96 | 3.04 |
| 3-Methoxytyramine | 2.15 | 2.48 | 2.44 | 3.07 | 4.85 | 3.13 | 4.22 | 4.23 |
| Epinephrine | 3.50 | 3.01 | 2.39 | 3.45 | 13.23 | 4.09 | 3.62 | 3.33 |
| Norepinephrine | 5.94 | 7.58 | 7.19 | 3.09 | 11.25 | 6.61 | 3.87 | 3.83 |
| Dopamine | 2.06 | 3.34 | 5.30 | 5.17 | 7.00 | 5.77 | 3.91 | 2.83 |
| MN | NMN | 3-MT | ||||||||||||
| Level 1 | Level 2 | Level 3 | Level 4 | Level 1 | Level 2 | Level 3 | Level 4 | Level 1 | Level 2 | Level 3 | Level 4 | |||
| Target conc. [nM] | 79.2 | 475 | 3800 | 7610 | Target conc. [nM] | 85.3 | 512 | 4090 | 8190 | Target conc. [nM] | 92.3 | 554 | 4430 | 8870 |
| Avg. measured conc. [nM] (n = 4) | 78.0 | 489.5 | 3787.5 | 7525.0 | Avg. measured conc. [nM] (n = 4) | 86.4 | 505.0 | 3985.0 | 8177.5 | Avg. measured conc. [nM] (n = 4) | 87.2 | 556.3 | 4412.5 | 8777.5 |
| Recovery % | 97.6 | 103.9 | 98.9 | 99.3 | Recovery % | 100.7 | 97.9 | 97.1 | 99.5 | Recovery % | 95.1 | 101.6 | 99.2 | 100.3 |
| Recovery % (avg.) | 100.02 | Recovery % (avg.) | 99.30 | Recovery % (avg.) | 98.40 | |||||||||
| EP | NE | DA | ||||||||||||
| Level 1 | Level 2 | Level 3 | Level 4 | Level 1 | Level 2 | Level 3 | Level 4 | Level 1 | Level 2 | Level 3 | Level 4 | |||
| Target conc. [nM] | 85.3 | 512 | 4090 | 8190 | Target conc. [nM] | 102 | 612 | 4900 | 9790 | Target conc. [nM] | 93.5 | 561 | 4490 | 8970 |
| Avg. measured conc. [nM] (n = 4) | 83.6 | 524.0 | 4302.5 | 8235.0 | Avg. measured conc. [nM] (n = 4) | 87.2 | 556.3 | 4412.5 | 8777.5 | Avg. measured conc. [nM] (n = 4) | 92.2 | 616.0 | 5002.5 | 10,177.5 |
| Recovery % | 102.3 | 102.9 | 106.4 | 100.0 | Recovery % | 86.0 | 91.9 | 89.7 | 90.9 | Recovery % | 96.4 | 108.4 | 111.7 | 113.4 |
| Recovery % (avg.) | 101.50 | Recovery % (avg.) | 89.00 | Recovery % (avg.) | 108.30 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qasrawi, D.O.; Pimenta, A.M.C.; Petrotchenko, E.V.; Eintracht, S.; Borchers, C.H. Multiplexed Dilute-and-Shoot Liquid Chromatography–Multiple-Reaction Monitoring Mass Spectrometry Clinical Assay for Metanephrines and Catecholamines in Human Urine. Metabolites 2025, 15, 30. https://doi.org/10.3390/metabo15010030
Qasrawi DO, Pimenta AMC, Petrotchenko EV, Eintracht S, Borchers CH. Multiplexed Dilute-and-Shoot Liquid Chromatography–Multiple-Reaction Monitoring Mass Spectrometry Clinical Assay for Metanephrines and Catecholamines in Human Urine. Metabolites. 2025; 15(1):30. https://doi.org/10.3390/metabo15010030
Chicago/Turabian StyleQasrawi, Deema O., Adriano M. C. Pimenta, Evgeniy V. Petrotchenko, Shaun Eintracht, and Christoph H. Borchers. 2025. "Multiplexed Dilute-and-Shoot Liquid Chromatography–Multiple-Reaction Monitoring Mass Spectrometry Clinical Assay for Metanephrines and Catecholamines in Human Urine" Metabolites 15, no. 1: 30. https://doi.org/10.3390/metabo15010030
APA StyleQasrawi, D. O., Pimenta, A. M. C., Petrotchenko, E. V., Eintracht, S., & Borchers, C. H. (2025). Multiplexed Dilute-and-Shoot Liquid Chromatography–Multiple-Reaction Monitoring Mass Spectrometry Clinical Assay for Metanephrines and Catecholamines in Human Urine. Metabolites, 15(1), 30. https://doi.org/10.3390/metabo15010030







