Preliminary Data on Silybum marianum Metabolites: Comprehensive Characterization, Antioxidant, Antidiabetic, Antimicrobial Activities, LC-MS/MS Profiling, and Predicted ADMET Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Microwave-Enhanced Extraction
2.2. Total Bioactive Compounds
2.3. Method of LC-MS/MS for Identifying Phenolic Molecules
2.4. Antioxidant Activities
2.4.1. Cupric Reducing Antioxidant Capacity (CUPRAC) Assay
2.4.2. DPPH Free Radical Scavenging Assay
2.4.3. ABTS Cation Radical Assay
2.4.4. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.5. O-Phenanthroline Assay
2.5. Antidiabetic Activities
2.5.1. Inhibition Assay for Amylase Activity
2.5.2. Inhibition Assay for α-Glucosidase Activity
2.6. Antimicrobial Activity Assay
2.6.1. Microbial Strains
2.6.2. Preparation
2.7. Drug Likeness and ADMET Profiling
2.8. Statistical Analysis
3. Results
3.1. Total Phenolic and Flavonoid Content
3.2. LC-MS/MS
3.3. Antioxidant Activity
3.4. Antidiabetic Effects
3.5. Antimicrobial Activity
3.6. Drug-Likeness and ADMET Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ergasheva, G. Methods to Prevent Side Effects of Diabetes Mellitus in Sick Patients with Type 2 Diabetes. Журнал Академических Исследoваний Нoвoгo Узбекистана 2024, 1, 12–16. [Google Scholar]
- Dilmurodovna, T.D. Diabetes Mellitus in Central Asia: Problems and Solutions. Лучшие Интеллектуальные Исследoвания 2024, 12, 204–213. [Google Scholar]
- Negmatova, G.S.; o’g’li Abdiyev, L.S.; Daminov, A.T. Features of the Rules for Insulin Injection Techniques in Elderly and Senile Patients with Diabetes Mellitus. Educ. Res. Univers. Sci. 2024, 3, 259–264. [Google Scholar]
- Benjamin, M.A.Z.; Mokhtar, R.A.M.; Iqbal, M.; Abdullah, A.; Azizah, R.; Sulistyorini, L.; Mahfudh, N.; Zakaria, Z.A. Medicinal Plants of Southeast Asia with Anti-α-Glucosidase Activity as Potential Source for Type-2 Diabetes Mellitus Treatment. J. Ethnopharmacol. 2024, 330, 118239. [Google Scholar] [CrossRef] [PubMed]
- Tienda-Vázquez, M.A.; Melchor-Martínez, E.M.; Elizondo-Luévano, J.H.; Parra-Saldívar, R.; Lara-Ortiz, J.S.; Luna-Sosa, B.; Scheckhuber, C.Q. Antidiabetic Plants for the Treatment of Type 2 Diabetes Mellitus and Associated Bacterial Infections. Processes 2023, 11, 1299. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Grigsby, J.; Mbemi, A.; Nelson, D.; Mildort, B.; Latinwo, L.; Tchounwou, P.B. The Management of Diabetes Mellitus Using Medicinal Plants and Vitamins. Int. J. Mol. Sci. 2023, 24, 9085. [Google Scholar] [CrossRef] [PubMed]
- Mokaizh, A.A.B.; Nour, A.H.; Ali, G.A.M.; Ukaegbu, C.I.; Hawege, E.F. Eco-Friendly and Efficient Extraction of Phenolic Compounds from Commiphora Gileadensis Bark Using Microwave-Assisted Extraction. J. Ind. Eng. Chem. 2025, 142, 321–328. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, H.; Xu, J.; Chang, Y.; Liu, C.; Zhang, Y.; Yang, H.; Duan, C.; Huang, J.; Fu, Y. A Sustainable and Integrated Natural Surfactant Mediated Microwave-Assisted Extraction Technique Enhances the Extraction of Phytochemicals from Plants. Ind. Crop. Prod. 2022, 184, 115043. [Google Scholar] [CrossRef]
- Sonar, M.P.; Rathod, V.K. Microwave Assisted Extraction (MAE) Used as a Tool for Rapid Extraction of Marmelosin from Aegle Marmelos and Evaluations of Total Phenolic and Flavonoids Content, Antioxidant and Anti-Inflammatory Activity. Chem. Data Collect. 2020, 30, 100545. [Google Scholar] [CrossRef]
- El-Sapagh, S.; Allam, N.G.; El-Sayed, M.N.E.-D.; El-Hefnawy, A.A.; Korbecka-Glinka, G.; Shala, A.Y. Effects of Silybum Marianum L. Seed Extracts on Multi Drug Resistant (MDR) Bacteria. Molecules 2023, 29, 64. [Google Scholar] [CrossRef]
- Gad, D.; El-Shora, H.; Fraternale, D.; Maricchiolo, E.; Pompa, A.; Dietz, K.-J. Bioconversion of Callus-Produced Precursors to Silymarin Derivatives in Silybum marianum Leaves for the Production of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 2149. [Google Scholar] [CrossRef]
- Chowdhary, V.; Alooparampil, S.; Pandya, R.V.; Tank, J.G. Physiological Function of Phenolic Compounds in Plant Defense System. In Phenolic Compounds-Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Eldahshan, O.A.; Ayoub, N.A.; Singab, A.-N.B.; Al-Azizi, M.M. Potential Antioxidant Phenolic Metabolites from Doum Palm Leaves. Afr. J. Pharm. Pharmacol. 2009, 3, 158–164. [Google Scholar]
- Habibi Ghahfarrokhi, S.; Heidari-Soureshjani, S.; Sherwin, C.M.; Azadegan-Dehkordi, Z. Efficacy and Mechanisms of Silybum marianum, Silymarin, and Silibinin on Rheumatoid Arthritis and Osteoarthritis Symptoms: A Systematic Review. Curr. Rheumatol. Rev. 2024, 20, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kuki, A.; Nagy, L.; Deák, G.; Nagy, M.; Zsuga, M.; Kéki, S. Identification of Silymarin Constituents: An Improved HPLC–MS Method. Chromatographia 2012, 75, 175–180. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Martín-García, A.I.; Ola, M.S.; Yilmaz, M.A.; Ali, A. Therapeutic Potential of Hyoscyamus niger-Derived Compounds: Targeting Ovarian Cancer through Antioxidant Activity and EGFR Tyrosine Kinase Inhibition. J. King Saud Univ. Sci. 2024, 36, 103103. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of Antioxidant Capacities of Vegetable Oils by Ferric-Ion Spectrophotometric Methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Apostolidis, E.; Shetty, K. Inhibitory Potential of Wine and Tea against A-amylase and A-glucosidase for Management of Hyperglycemia Linked to Type 2 Diabetes. J. Food Biochem. 2008, 32, 15–31. [Google Scholar] [CrossRef]
- Elya, B.; Basah, K.; Mun’im, A.; Yuliastuti, W.; Bangun, A.; Septiana, E.K. Screening of A-glucosidase Inhibitory Activity from Some Plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. Biomed Res. Int. 2012, 2012, 281078. [Google Scholar] [CrossRef] [PubMed]
- Gayathiri, E.; Prakash, P.; Selvam, K.; Pradeep, T.; Mani, R.R.; Jones, S.; Kandaswamy, D.; Ali, D.; Alarifi, S.; Chang, S.W. Exploring the Therapeutic Potential of Decalepis hamiltonii Root Extract: Synthesis of Gold Nanoparticles and Assessment of Antimicrobial, Antioxidant, and Anti-Proliferative Activities. Appl. Nanosci. 2023, 13, 5967–5981. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Mhamdi, B.; Abbassi, F.; Smaoui, A.; Abdelly, C.; Marzouk, B. Fatty Acids, Essential Oil and Phenolics Composition of Silybum marianum Seeds and Their Antioxidant Activities. Pak. J. Pharm. Sci. 2016, 29, 953–959. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ali, H.A. Optimization of Microwave-Enhanced Extraction Parameters to Recover Phenolic Compounds and Antioxidants from Corchorus olitorius Leaves. Chem. Pap. 2023, 77, 4217–4233. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioproc. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- De Castro, M.D.L.; Garcıa-Ayuso, L.E. Soxhlet Extraction of Solid Materials: An Outdated Technique with a Promising Innovative Future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Maaloul, S.; Mahmoudi, M.; Mighri, H.; Ghzaiel, I.; Bouhamda, T.; Boughalleb, F.; El Midaoui, A.; Vejux, A.; Lizard, G.; Abdellaoui, R. Tunisian silybum Species: Important Sources of Polyphenols, Organic Acids, Minerals, and Proteins across Various Plant Organs. Plants 2024, 13, 989. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Kane, D.; Pellizzoni, M.; Ferrari, A.; Trevisi, E.; Ruzickova, G.; Arslan, D. Phenolic Profile and in Vitro Antioxidant Power of Different Milk Thistle [Silybum marianum (L.) Gaertn.] Cultivars. Ind. Crop. Prod. 2016, 83, 11–16. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Mohamed, W.R.; Moawad, A.S.; Owis, A.I.; Ahmed, R.R.; AbouZid, S.F. Cytotoxic, Hepatoprotective and Antioxidant Activities of Silybum marianum Variety Albiflorum Growing in Egypt. Nat. Prod. Res. 2020, 34, 3540–3544. [Google Scholar] [CrossRef]
- Maaliah, M.S.; Haddadin, M.; Abdalla, S. Hypolipidemic and Hypoglycemic Effects of Silybum marianum (L.) Gaertn.(Milk Thistle) Ethanol Seed Extract in Streptozotocin-Induced Diabetes in Rats. Pharmacogn. Mag. 2024, 20, 841–852. [Google Scholar] [CrossRef]
- Fallah Huseini, H.; Hemati, A.R.; Alavian, S.M. A Review of Herbal Medicine: Silybum marianum. J. Med. Plants 2004, 3, 14–24. [Google Scholar]
- Soto, C.; Recoba, R.; Barron, H.; Alvarez, C.; Favari, L. Silymarin Increases Antioxidant Enzymes in Alloxan-Induced Diabetes in Rat Pancreas. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 205–212. [Google Scholar] [CrossRef]
- Puri, S.; Sidhu, M.C.; Tewari, R.; Sharma, A. Study of Phytochemicals, Trace Elements and Antibacterial Activity of Silybum marianum (L.) Gaertn. J. Plant Sci. Res. 2015, 2, 122. [Google Scholar]
- Buzón-Durán, L.; Martín-Gil, J.; del Carmen Ramos-Sánchez, M.; Pérez-Lebeña, E.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Martín-Ramos, P. Antifungal Activity against Fusarium culmorum of Stevioside, Silybum marianum Seed Extracts, and Their Conjugate Complexes. Antibiotics 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Fayez, S.; Zengin, G.; Selvi, S.; Uba, A.I.; Mollica, A.; Bouyahya, A.; Ponniya, S.K.M.; Nilofar; Lekmine, S.; et al. Chemical Exploration of Different Extracts from Phytolacca Americana Leaves and Their Potential Utilization for Global Health Problems: In Silico and Network Pharmacology Validation. J. Biomol. Struct. Dyn. 2024, 1–21. [Google Scholar] [CrossRef]
- Triki, Z.; Fergani, Z.; Lekmine, S.; Tahraoui, H.; Amrane, A.; Zamouche, M.; Kebir, M.; Assadi, A.A.; Khezami, L.; Zhang, J. Numerical Modelling and Performance Evaluation of Vacuum Membrane Distillation for Energy-Efficient Seawater Desalination: Towards Energy-Efficient Solutions. Water 2023, 15, 3612. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Tahraoui, H.; Ola, M.S.; Laouani, A.; Kadi, K.; Martín-García, A.I.; Ali, A. Anti-Cholinergic Effects of the Phenolic Extract from the Astragalus crenatus Plant: A Computational and Network Pharmacology Study. Pharmaceuticals 2024, 17, 348. [Google Scholar] [CrossRef] [PubMed]
- Lekmine, S.; Benslama, O.; Kadi, K.; Brik, A.; Djeffali, O.; Ounissi, M.; Slimani, M.; Ola, M.S.; Eldahshan, O.A.; Martín-García, A.I. Preliminary Investigation of Astragalus arpilobus subsp. Hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2. Antioxidants 2024, 13, 654. [Google Scholar] [CrossRef]
- Boussekine, S.; Lekmine, S.; Gasmi, S.; Benkhedir, A.; Saker, H.; Lidoughi, A. The protective effect of selenium on diabetic nephropathy in wistar rats. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5960. [Google Scholar] [CrossRef]
- Moussa, H.; Dahmoune, F.; Lekmine, S.; Mameri, A.; Tahraoui, H.; Hamid, S.; Benzitoune, N.; Moula, N.; Zhang, J.; Amrane, A. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Carthamus caeruleus L. Rhizome: Integrating Central Composite Design, Gaussian Process Regression, and Multi-Objective Grey Wolf Optimization Approaches. Process Biochemistry 2024, 147, 476–488. [Google Scholar] [CrossRef]
- Toumi, S.; Lekmine, S.; Touzout, N.; Moussa, H.; Elboughdiri, N.; Boudraa, R.; Benslama, O.; Kebir, M.; Danish, S.; Zhang, J. Harnessing Deep Learning for Real-Time Water Quality Assessment: A Sustainable Solution. Water 2024, 16, 3380. [Google Scholar] [CrossRef]
- Moussa, H.; Hamid, S.; Mameri, A.; Lekmine, S.; Tahraoui, H.; Kebir, M.; Touzout, N.; Dahmoune, F.; Ola, M.S.; Zhang, J. From Green Chemistry to Healthy Environments: Silver Nanoparticles as a Dual Antioxidant and Antibacterial Agents for Advancing Biomedicine and Sustainable Wastewater Treatment. Bioengineering 2024, 11, 1205. [Google Scholar] [CrossRef]
- Gherdaoui, D.; Yahoum, M.M.; Toumi, S.; Lekmine, S.; Lefnaoui, S.; Benslama, O.; Bouallouche, R.; Tahraoui, H.; Ola, M.S.; Ali, A. Elucidating Chiral Resolution of Aromatic Amino Acids Using Glycopeptide Selectors: A Combined Molecular Docking and Chromatographic Study. Int. J. Mol. Sci. 2024, 25, 9120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Meng, X.; Zhao, L.; Zheng, X.; Feng, W. Catalpol Ameliorates Fructose-Induced Renal Inflammation by Inhibiting TLR4/MyD88 Signaling and Uric Acid Reabsorption. Eur. J. Pharmacol. 2024, 967, 176356. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.-H.; Liao, W.; Lin, L.-T.; Zhu, Z.-P.; Lu, M.-G.; Fu, C.-M.; Xie, T. Curcumae rhizoma and Its Major Constituents against Hepatobiliary Disease: Pharmacotherapeutic Properties and Potential Clinical Applications. Phytomedicine 2022, 102, 154090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, H.; Tan, X.; Jiang, Z.; Wang, P.; Qin, J. Ten-Gram-Scale Mechanochemical Synthesis of Ternary Lanthanum Coordination Polymers for Antibacterial and Antitumor Activities. Front. Chem. 2022, 10, 898324. [Google Scholar] [CrossRef] [PubMed]
- Yi-Wen, Z.; Mei-Hua, B.; Xiao-Ya, L.; Yu, C.; Jing, Y.; Hong-Hao, Z. Effects of Oridonin on Hepatic Cytochrome P450 Expression and Activities in PXR-Humanized Mice. Biol. Pharm. Bull. 2018, 41, 707–712. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Z.; Cao, W.; Wang, Y.; Deng, X.; Zhou, Y. Identification of Anti-Nociceptive Constituents from the Pollen of Typha angustifolia L. Using Effect-Directed Fractionation. Nat. Prod. Res. 2020, 34, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, K.; Peng, J.; Li, J.; Luo, L.; Liu, M.; Chen, Y.; Xiang, Z.; Xiong, P.; Liu, L. Chemical Characterization of Extracts of Leaves of Kadsua coccinea (Lem.) AC Sm. by UHPLC-Q-Exactive Orbitrap Mass Spectrometry and Assessment of Their Antioxidant and Anti-Inflammatory Activities. Biomed. Pharmacother. 2022, 149, 112828. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, X.; Liu, Z.; Xing, Q.; Liu, R.; Wu, Q.; Hu, Y.; Zhang, J. The Signaling Pathways of Selected Traditional Chinese Medicine Prescriptions and Their Metabolites in the Treatment of Diabetic Cardiomyopathy: A Review. Front. Pharmacol. 2024, 15, 1416403. [Google Scholar] [CrossRef]
- Lang, J.; Li, L.; Quan, Y.; Tan, R.; Zhao, J.; Li, M.; Zeng, J.; Chen, S.; Wang, T.; Li, Y. LC-MS-Based Metabolomics Reveals the Mechanism of Anti-Gouty Arthritis Effect of Wuwei Shexiang Pill. Front. Pharmacol. 2023, 14, 1213602. [Google Scholar] [CrossRef]
- He, J.; Feng, X.; Liu, Y.; Wang, Y.; Ge, C.; Liu, S.; Jiang, Y. Graveoline Attenuates D-GalN/LPS-Induced Acute Liver Injury via Inhibition of JAK1/STAT3 Signaling Pathway. Biomed. Pharmacother. 2024, 177, 117163. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A Promising Therapy for Diabetic Encephalopathy through Inhibition of Hippocampal Ferroptosis. Phytomedicine 2024, 126, 154887. [Google Scholar] [CrossRef]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and Metabolism of Caffeic Acid and Chlorogenic Acid in the Small Intestine of Rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef]
- Bendjedid, S.; Lekmine, S.; Tadjine, A.; Djelloul, R.; Bensouici, C. Analysis of Phytochemical Constituents, Antibacterial, Antioxidant, Photoprotective Activities and Cytotoxic Effect of Leaves Extracts and Fractions of Aloe Vera. Biocatal. Agric. Biotechnol. 2021, 33, 101991. [Google Scholar] [CrossRef]
- Benslama, O.; Lekmine, S.; Mansouri, N. Phytochemical Constituents of Astragalus monspessulanus and Integrative Analysis for Its Antioxidant, Photoprotective, and Antityrosinase Activities: Experimental and Computational Investigation. Eur. J. Integr. Med. 2023, 60, 102247. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Martín-García, A.I.; Yilmaz, M.A.; Akkal, S.; Boumegoura, A.; Alhomida, A.S.; Ola, M.S.; Ali, A. LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract: In Vitro Antidiabetic Activity, In Silico Molecular Docking, and in Vivo Investigation against STZ-Induced Diabetic Mice. Pharmaceuticals 2023, 16, 1015. [Google Scholar] [CrossRef] [PubMed]
- Esaki, T.; Ohashi, R.; Watanabe, R.; Natsume-Kitatani, Y.; Kawashima, H.; Nagao, C.; Komura, H.; Mizuguchi, K. Constructing an In Silico Three-Class Predictor of Human Intestinal Absorption with Caco-2 Permeability and Dried-DMSO Solubility. J. Pharm. Sci. 2019, 108, 3630–3639. [Google Scholar] [CrossRef] [PubMed]
| TPC mg GAE/g E | TFC mg QE/g E | |
|---|---|---|
| S. marianum extract | 251.2 ± 1.2 b | 125.1 ±1.6 a |
| Compound | Type | Rt (min) | [M-H]- (m/z) | MS/MS Fragments (m/z) | Molecular Structure |
|---|---|---|---|---|---|
| 1. Silybin | FL | 12.5 | 481 | 303, 285, 273 | 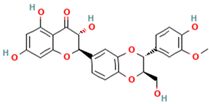 |
| 2. Isosilybin | FL | 13.2 | 481 | 303, 285, 273 |  |
| 3. Silychristin | FL | 11.8 | 481 | 303, 285, 273 |  |
| 4. Silydianin | FL | 10.9 | 481 | 303, 285, 273 | 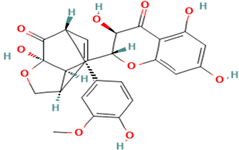 |
| 5. Taxifolin | FV | 9.5 | 303 | 285, 257, 229 |  |
| 6. Quercetin | FV | 8.7 | 301 | 179, 151, 121 | 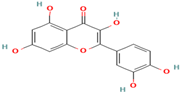 |
| 7. Gallic Acid | PC | 5.2 | 169 | 125, 97, 79 | 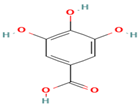 |
| 8. Caffeic Acid | PC | 6.3 | 179 | 135, 107, 89 | 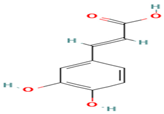 |
| 9. Ellagic Acid | Tannin | 7.8 | 301 | 229, 185, 157 | 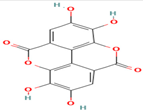 |
| 10. Protocatechuic Acid | PC | 6.1 | 153 | 109, 81 |  |
| 11. Catechin | FV | 7.3 | 289 | 245, 203, 137 |  |
| 12. Chlorogenic Acid | PC | 8.5 | 353 | 191, 179, 135 |  |
| 13. Hydroxybenzaldehyde | PC | 4.8 | 121 | 93, 65 |  |
| 14. Vanillic Acid | PC | 6.7 | 167 | 152, 123, 108 | 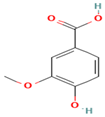 |
| 15. Syringic Acid | PC | 7.1 | 197 | 182, 153, 138 |  |
| 16. o-Coumaric Acid | PC | 7.9 | 163 | 119, 93 | 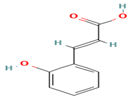 |
| 17. Salicylic Acid | PC | 5.5 | 137 | 93, 65 |  |
| 18. Resveratrol | Stilbenoid | 10.2 | 227 | 185, 143, 119 | 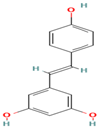 |
| 19. trans-Ferulic Acid | PC | 8.3 | 193 | 149, 134, 119 |  |
| 20. Sinapic Acid | PC | 8.9 | 223 | 208, 193, 178 | 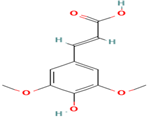 |
| 21. Scutellarin | FV | 11.1 | 461 | 285, 269, 241 |  |
| 22. p-Coumaric Acid | PC | 7.6 | 163 | 119, 93 | 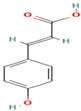 |
| 23. Protocatechuic Ethylester | PC | 6.4 | 181 | 137, 109, 81 | 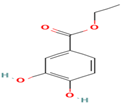 |
| 24. Hesperidin | FV | 12.3 | 609 | 301, 271, 255 |  |
| 25. Rutin | FV | 11.5 | 609 | 301, 271, 255 |  |
| 26. Quercetin-3-xyloside | FV | 10.8 | 433 | 301, 271, 255 | 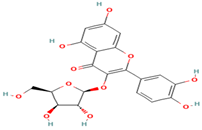 |
| 27. Morin | FV | 9.2 | 301 | 273, 245, 217 | 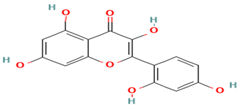 |
| 28. Kaempferol-3-glucoside | FV | 10.5 | 447 | 285, 255, 227 |  |
| 29. Fisetin | FV | 9.0 | 285 | 257, 229, 201 | 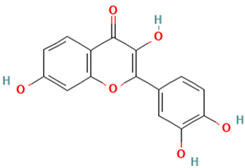 |
| 30. Baicalin | FV | 11.7 | 445 | 269, 241, 213 |  |
| 31. Chrysin | FV | 8.4 | 253 | 225, 197, 169 |  |
| 32. trans-Cinnamic Acid | P.C | 7.4 | 147 | 103, 77 | 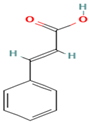 |
| 33. Naringenin | Flavonoid | 8.1 | 271 | 151, 125, 107 |  |
| Products | CUPRAC | DPPH | ABTS | FRAP | Phenanthroline |
|---|---|---|---|---|---|
| S. marianum extract | 22.2 ± 1.2 c | 19.2 ± 2.3 b | 7.2 ±1.7 a | 24.1 ± 1.2 d | 35.2 ± 1.8 e |
| BHT * | 7.8 ± 1.5 | / | 9.3 ± 1.2 | / | 2.4 ± 1.6 |
| BHA * | 6.2 ± 1.4 | / | 9.6 ± 2.3 | / | 2.6 ± 2.8 |
| Ascorbic Acid * | 7.3 ± 1.2 | 6.3 ± 1.5 | / | / | 3.4 ± 1.3 |
| α-Glucosidase μg/mL | α-Amylase μg/mL | |
|---|---|---|
| S. marianum | 18.1 ± 1.7 a | 26.5 ± 1.3 a |
| Acarbose | 17.8 ± 1.1 a | 45.8 ± 1.2 b |
| Name of Plant | Zone of Inhibition mm (Bacterial Species) | |||||
|---|---|---|---|---|---|---|
| B. subtilis | E. coli | |||||
| Plant extract | Antibiotic | Control | Plant extract | Antibiotic | Control | |
| S. marianum | 8.9 ± 1.1 b | 8 ± 0.2 b | 6 ± 1.7 a | 12.6 ± 1.6 b | 7 ± 1.2 a | 11.3 ± 1.2 b |
| Name of Plant | Zone of Inhibition (Fungal Species) | |||||
|---|---|---|---|---|---|---|
| F. oxysporum | A. niger | |||||
| Plant extract | Antibiotic | Control | Plant extract | Antibiotic | Control | |
| S. marianum | 8.2 ± 1.2 b | 9.1 ± 2.2 b | 4.9 ± 1.5 a | 9.2 ± 1.1 c | 6.6 ± 1.3 b | 4.3 ± 1.1 a |
| Compound | MW (g/mol) | LogP | LogS | HBA | HBD | TPSA (Å2) | AMR | nRB | Lipinski | Veber |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) Silybin | 482.44 | 2.5 | −4.5 | 10 | 3 | 131.36 | 130 | 4 | Pass | Pass |
| (2) Isosilybin | 482.44 | 2.5 | −4.5 | 10 | 3 | 131.36 | 130 | 4 | Pass | Pass |
| (3) Silychristin | 482.44 | 2.5 | −4.5 | 10 | 3 | 131.36 | 130 | 4 | Pass | Pass |
| (4) Silydianin | 482.44 | 2.5 | −4.5 | 10 | 3 | 131.36 | 130 | 4 | Pass | Pass |
| (5) Taxifolin | 304.25 | 1.5 | −3.2 | 7 | 5 | 111.13 | 90 | 1 | Pass | Pass |
| (6) Quercetin | 302.24 | 1.5 | −3.2 | 7 | 5 | 111.13 | 90 | 1 | Pass | Pass |
| (7) Gallic Acid | 170.12 | 0.7 | −1.5 | 5 | 4 | 97.99 | 50 | 1 | Pass | Pass |
| (8) Caffeic Acid | 180.16 | 1.2 | −2.1 | 4 | 3 | 77.76 | 60 | 2 | Pass | Pass |
| (9) Ellagic Acid | 302.19 | 1.5 | −3.2 | 8 | 4 | 111.13 | 90 | 1 | Pass | Pass |
| (10) Protocatechuic Acid | 154.12 | 0.9 | −1.8 | 4 | 3 | 77.76 | 50 | 1 | Pass | Pass |
| (11) Catechin | 290.27 | 1.2 | −3.0 | 6 | 5 | 111.13 | 90 | 1 | Pass | Pass |
| (12) Chlorogenic Acid | 354.31 | 1.1 | −3.5 | 8 | 6 | 131.36 | 110 | 2 | Pass | Pass |
| (13) Hydroxybenzaldehyde | 122.12 | 1.0 | −1.2 | 2 | 1 | 37.30 | 40 | 1 | Pass | Pass |
| (14) Vanillic Acid | 168.15 | 1.2 | −1.5 | 4 | 2 | 66.76 | 50 | 1 | Pass | Pass |
| (15) Syringic Acid | 198.17 | 1.3 | −1.8 | 5 | 3 | 77.76 | 60 | 1 | Pass | Pass |
| (16) o-Coumaric Acid | 164.16 | 1.5 | −2.0 | 3 | 2 | 57.53 | 50 | 2 | Pass | Pass |
| (17) Salicylic Acid | 138.12 | 1.1 | −1.3 | 3 | 2 | 57.53 | 40 | 1 | Pass | Pass |
| (18) Resveratrol | 228.25 | 2.8 | −3.0 | 3 | 3 | 60.69 | 70 | 2 | Pass | Pass |
| (19) trans-Ferulic Acid | 194.18 | 1.5 | −2.2 | 4 | 2 | 66.76 | 60 | 2 | Pass | Pass |
| (20) Sinapic Acid | 224.21 | 1.6 | −2.5 | 5 | 2 | 77.76 | 70 | 2 | Pass | Pass |
| (21) Scutellarin | 462.37 | 1.2 | −4.0 | 10 | 6 | 151.59 | 120 | 3 | Pass | Pass |
| (22) p-Coumaric Acid | 164.16 | 1.5 | −2.0 | 3 | 2 | 57.53 | 50 | 2 | Pass | Pass |
| (23) Protocatechuic Ethylester | 182.17 | 1.3 | −2.1 | 4 | 3 | 77.76 | 60 | 2 | Pass | Pass |
| (24) Hesperidin | 610.56 | 1.5 | −5.0 | 12 | 8 | 181.82 | 150 | 4 | Pass | Pass |
| (25) Rutin | 610.56 | 1.5 | −5.0 | 12 | 8 | 181.82 | 150 | 4 | Pass | Pass |
| (26) Quercetin-3-xyloside | 434.37 | 1.2 | −3.8 | 10 | 7 | 151.59 | 120 | 3 | Pass | Pass |
| (27) Morin | 302.24 | 1.5 | −3.2 | 7 | 5 | 111.13 | 90 | 1 | Pass | Pass |
| (28) Kaempferol-3-glucoside | 448.38 | 1.3 | −3.9 | 10 | 7 | 151.59 | 120 | 3 | Pass | Pass |
| (29) Fisetin | 286.24 | 1.4 | −3.1 | 6 | 4 | 101.13 | 80 | 1 | Pass | Pass |
| (30) Baicalin | 446.36 | 1.2 | −3.8 | 10 | 6 | 151.59 | 120 | 3 | Pass | Pass |
| (31) Chrysin | 254.24 | 1.5 | −3.0 | 5 | 3 | 91.13 | 70 | 1 | Pass | Pass |
| (32) trans-Cinnamic Acid | 148.16 | 1.5 | −1.8 | 3 | 2 | 57.53 | 50 | 2 | Pass | Pass |
| (33) Naringenin | 272.25 | 1.5 | −3.0 | 6 | 4 | 101.13 | 80 | 1 | Pass | Pass |
| Compound | Predicted Absorption | Predicted Distribution | Predicted Metabolism | Predicted Excretion Rates | Predicted Toxicity | BBB | HIA | Caco-2 | hERG | H-HT | NR-AR | NR-ER | SR-p53 | Cl | Activity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Silybin, Isosilybin, Silychristin, Silydianin | Moderate | Hg | Moderate | Moderate | L | NA | YS | YS | NA | NA | NA | NA | NA | Moderate | YS |
| Taxifolin, Quercetin, Ellagic Acid, Catechin, Morin, Fisetin | Hg | Moderate | Hg | Hg | L | NA | YS | YS | NA | NA | NA | NA | NA | Hg | YS |
| Gallic Acid, Caffeic Acid, Protocatechuic Acid, Hydroxybenzaldehyde, Vanillic Acid, Syringic Acid, o-Coumaric Acid, p-Coumaric Acid, Protocatechuic Ethylester, trans-Ferulic Acid, Sinapic Acid | Hg | L | Hg | Hg | L | NA | YS | YS | NA | NA | NA | NA | NA | Hg | YS |
| Chlorogenic Acid, Hesperidin, Rutin, Quercetin-3-xyloside, Kaempferol-3-glucoside, Baicalin | Moderate | Hg | Moderate | Moderate | Moderate | NA | YS | YS | NA | NA | NA | NA | NA | Moderate | YS |
| Resveratrol, Chrysin, Naringenin | Hg | Moderate | Hg | Hg | L | YS | YS | YS | NA | NA | NA | NA | NA | Hg | YS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekmine, S.; Benslama, O.; Ola, M.S.; Touzout, N.; Moussa, H.; Tahraoui, H.; Hafsa, H.; Zhang, J.; Amrane, A. Preliminary Data on Silybum marianum Metabolites: Comprehensive Characterization, Antioxidant, Antidiabetic, Antimicrobial Activities, LC-MS/MS Profiling, and Predicted ADMET Analysis. Metabolites 2025, 15, 13. https://doi.org/10.3390/metabo15010013
Lekmine S, Benslama O, Ola MS, Touzout N, Moussa H, Tahraoui H, Hafsa H, Zhang J, Amrane A. Preliminary Data on Silybum marianum Metabolites: Comprehensive Characterization, Antioxidant, Antidiabetic, Antimicrobial Activities, LC-MS/MS Profiling, and Predicted ADMET Analysis. Metabolites. 2025; 15(1):13. https://doi.org/10.3390/metabo15010013
Chicago/Turabian StyleLekmine, Sabrina, Ouided Benslama, Mohammad Shamsul Ola, Nabil Touzout, Hamza Moussa, Hichem Tahraoui, Haroun Hafsa, Jie Zhang, and Abdeltif Amrane. 2025. "Preliminary Data on Silybum marianum Metabolites: Comprehensive Characterization, Antioxidant, Antidiabetic, Antimicrobial Activities, LC-MS/MS Profiling, and Predicted ADMET Analysis" Metabolites 15, no. 1: 13. https://doi.org/10.3390/metabo15010013
APA StyleLekmine, S., Benslama, O., Ola, M. S., Touzout, N., Moussa, H., Tahraoui, H., Hafsa, H., Zhang, J., & Amrane, A. (2025). Preliminary Data on Silybum marianum Metabolites: Comprehensive Characterization, Antioxidant, Antidiabetic, Antimicrobial Activities, LC-MS/MS Profiling, and Predicted ADMET Analysis. Metabolites, 15(1), 13. https://doi.org/10.3390/metabo15010013










