Light Spectral-Ranged Specific Metabolisms of Plant Pigments
Abstract
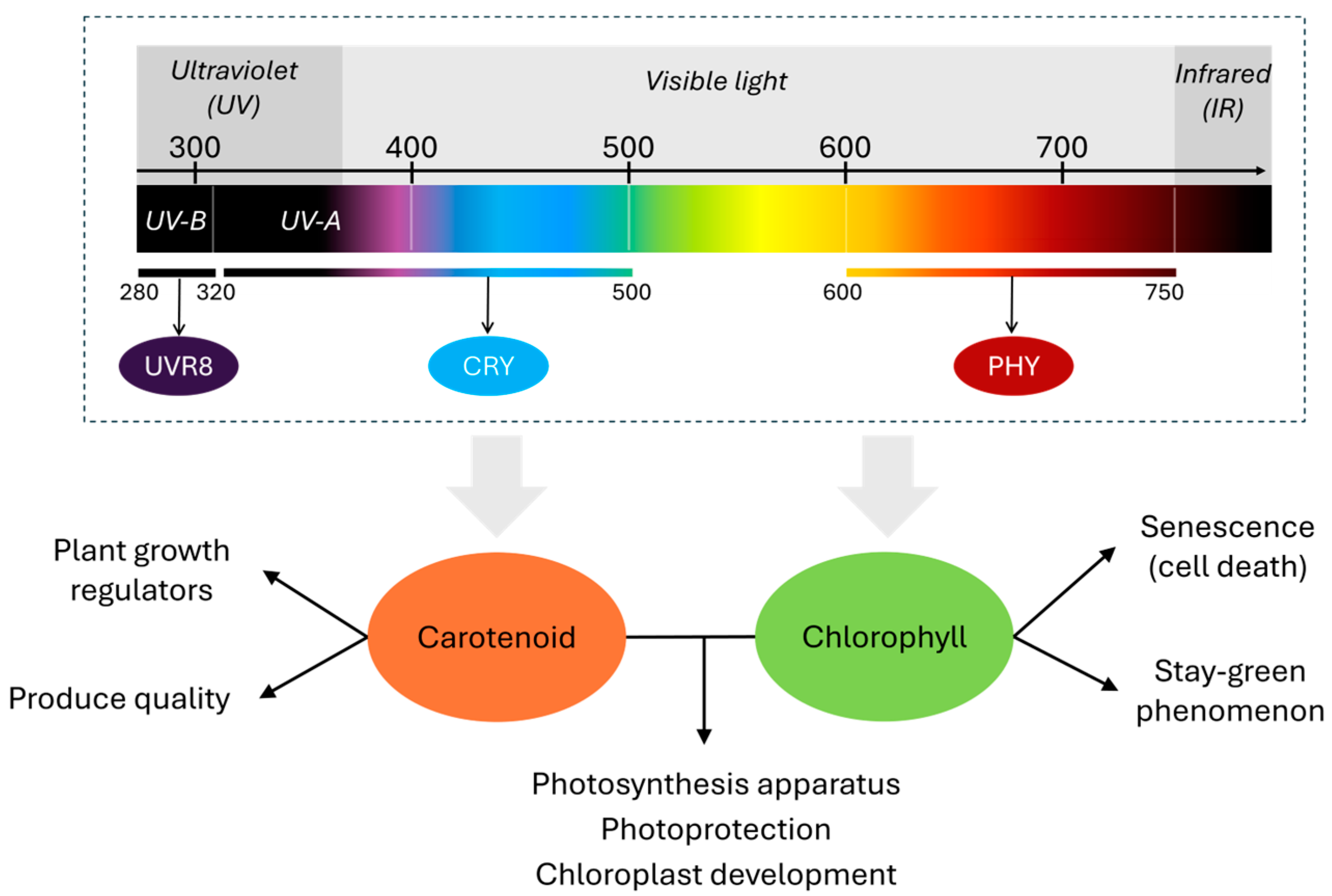
:1. Introduction
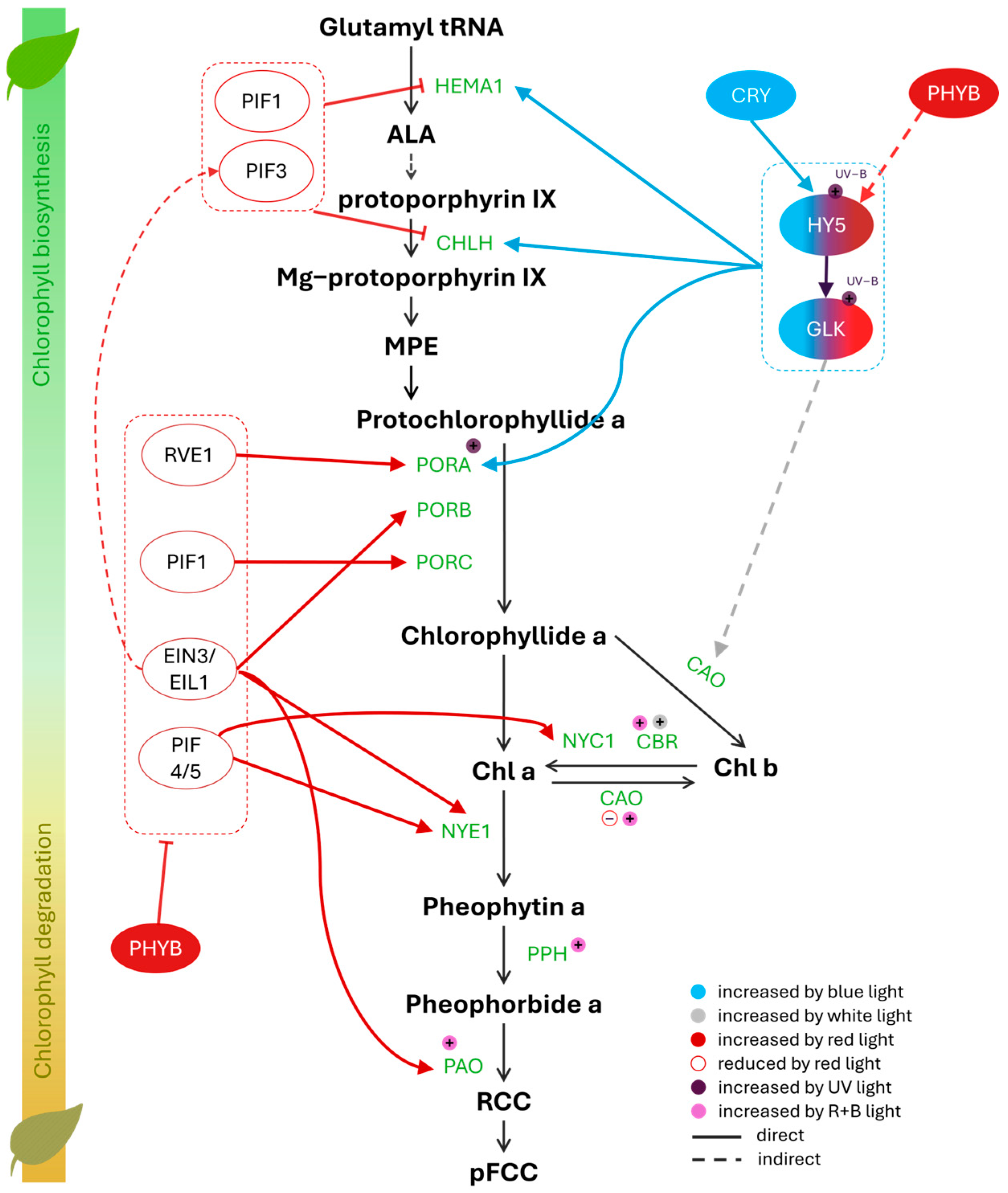
2. Chlorophyll Pathway Under Different Light Spectral Bands
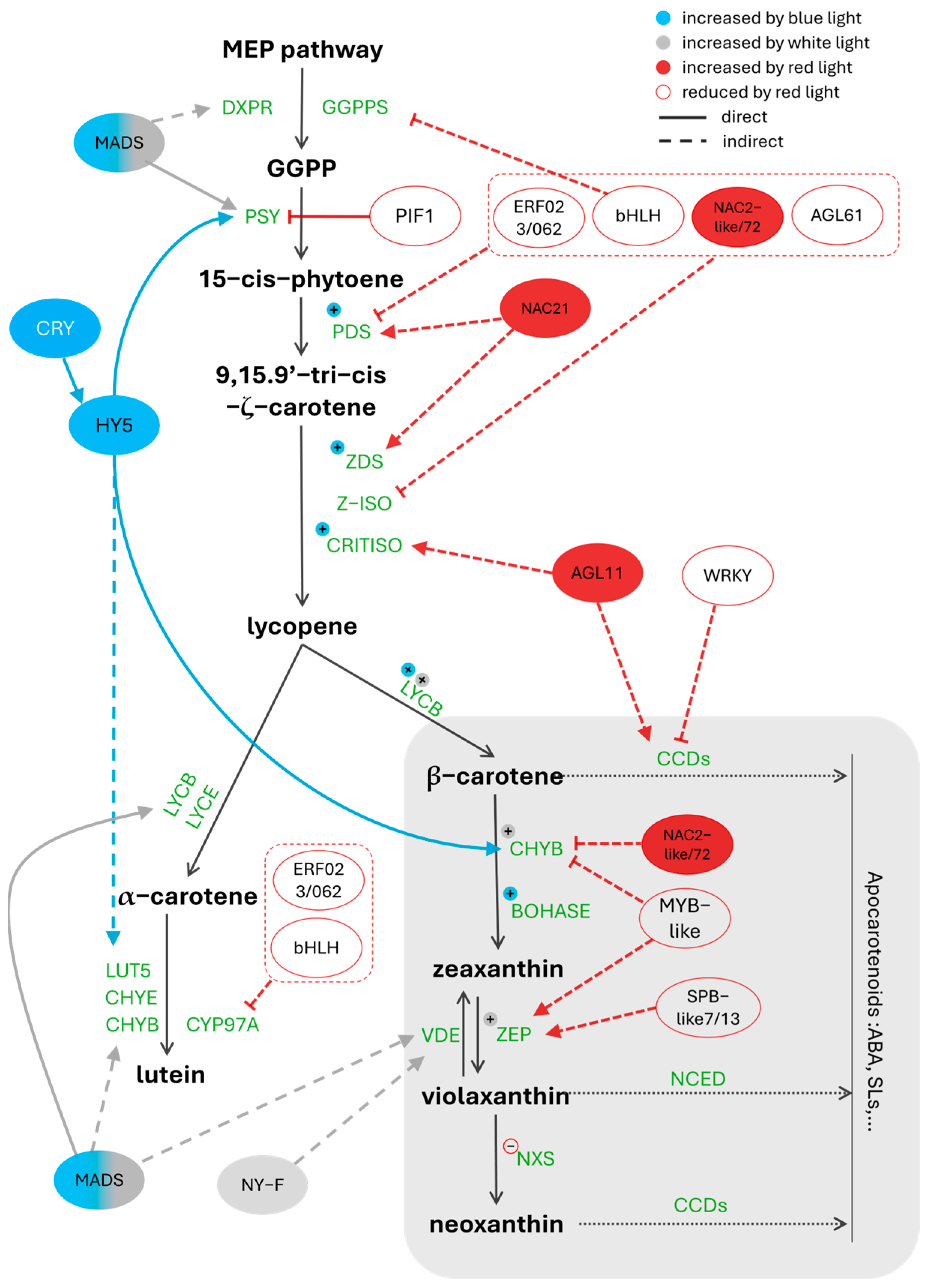
3. Carotenoid Pathway Under Different Light Spectral Bands
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathy, B.C.; Pattanayak, G.K. Chlorophyll Biosynthesis in Higher Plants. In Photosynthesis; Eaton-Rye, J.J., Tripathy, B.C., Sharkey, T.D., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2012; Volume 34, pp. 63–94. ISBN 978–94–007–1578–3. [Google Scholar]
- Reinbothe, C.; El Bakkouri, M.; Buhr, F.; Muraki, N.; Nomata, J.; Kurisu, G.; Fujita, Y.; Reinbothe, S. Chlorophyll Biosynthesis: Spotlight on Protochlorophyllide Reduction. Trends Plant Sci. 2010, 15, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, J.; Qiu, K.; Kuai, B. Phytohormone and Light Regulation of Chlorophyll Degradation. Front. Plant Sci. 2017, 8, 1911. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tanaka, R. Chlorophyll Metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Quian-Ulloa, R.; Stange, C. Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light. Int. J. Mol. Sci. 2021, 22, 1184. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, J.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Krishnamurthy, N.P. Carotenoids. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 259–296. ISBN 9780128052570. [Google Scholar]
- Stanley, L.; Yuan, Y.-W. Transcriptional Regulation of Carotenoid Biosynthesis in Plants: So Many Regulators, So Little Consensus. Front. Plant Sci. 2019, 10, 1017. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Kong, S.-G.; Okajima, K. Diverse Photoreceptors and Light Responses in Plants. J. Plant Res. 2016, 129, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Wei, Z.; Teo, N.; Shen, L.; Yu, H. Seeing the Lights for Leafy Greens in Indoor Vertical Farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light Signaling and Plant Responses to Blue and UV Radiations—Perspectives for Applications in Horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Xiang, N.; Zhao, Y.; Wang, S.; Guo, X. The Modulation of Light Quality on Carotenoids in Maize (Zea mays L.) Sprouts. Food Chem. Mol. Sci. 2022, 5, 100128. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, L.; Stange, C. Light-Dependent Regulation of Carotenoid Biosynthesis in Plants. Cienc. Investig. Agrar. 2009, 36, 143–162. [Google Scholar] [CrossRef]
- Mohanty, B.; Lakshmanan, M.; Lim, S.-H.; Kim, J.K.; Ha, S.-H.; Lee, D.-Y. Light-Specific Transcriptional Regulation of the Accumulation of Carotenoids and Phenolic Compounds in Rice Leaves. Plant Signal Behav. 2016, 11, e1184808. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.; Martinez-garcia, J.F.; Stange, C.; Rodriguez-concepcion, M. Illuminating Colors: Regulation of Carotenoid Biosynthesis and Accumulation by Light. Curr. Opin. Plant Biol. 2017, 37, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chu, L.; Zhang, Y.; Bian, Y.; Xiao, J.; Xu, D. HY5: A Pivotal Regulator of Light-Dependent Development in Higher Plants. Front. Plant Sci. 2022, 12, 800989. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Y.; Li, M.; Fu, D.; Wu, S.; Li, J.; Gong, Z.; Liu, H.; Yang, S. The CRY2–COP1–HY5–BBX7/8 Module Regulates Blue Light-Dependent Cold Acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, R.; Zeng, X.-Y.; Lee, S.; Ye, L.-H.; Tian, S.-L.; Zhang, Y.-J.; Busch, W.; Zhou, W.-B.; Zhu, X.-G.; et al. GLK Transcription Factors Accompany ELONGATED HYPOCOTYL5 to Orchestrate Light-Induced Seedling Development in Arabidopsis. Plant Physiol. 2024, 194, 2400–2421. [Google Scholar] [CrossRef] [PubMed]
- McCormac, A.C.; Terry, M.J. Light-signalling Pathways Leading to the Co-ordinated Expression of HEMA1 and Lhcb during Chloroplast Development in Arabidopsis Thaliana. Plant J. 2002, 32, 549–559. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Xie, X.; ABM, K.; ATAK, A.; Zhong, C.; Li, D. Effect of Light on Growth and Chlorophyll Development in Kiwifruit Ex Vitro and in Vitro. Sci. Hortic. 2022, 291, 110599. [Google Scholar] [CrossRef]
- Stephenson, P.G.; Fankhauser, C.; Terry, M.J. PIF3 Is a Repressor of Chloroplast Development. Proc. Natl. Acad. Sci. USA 2009, 106, 7654–7659. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Zhong, S. Interplay between Light and Plant Hormones in the Control of Arabidopsis Seedling Chlorophyll Biosynthesis. Front. Plant Sci. 2017, 8, 1433. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, G.; Jing, Y.; Tang, W.; Lin, R. Phytochrome B and REVEILLE1/2-Mediated Signalling Controls Seed Dormancy and Germination in Arabidopsis. Nat. Commun. 2016, 7, 12377. [Google Scholar] [CrossRef]
- Shi, H.; Shen, X.; Liu, R.; Xue, C.; Wei, N.; Deng, X.W.; Zhong, S. The Red Light Receptor Phytochrome B Directly Enhances Substrate-E3 Ligase Interactions to Attenuate Ethylene Responses. Dev. Cell 2016, 39, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhao, M.; Shi, T.; Shi, H.; An, F.; Zhao, Q.; Guo, H. EIN3/EIL1 Cooperate with PIF1 to Prevent Photo-Oxidation and to Promote Greening of Arabidopsis Seedlings. Proc. Natl. Acad. Sci. USA 2009, 106, 21431–21436. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Jeong, J.; Kang, M.-Y.; Kim, J.; Paek, N.-C.; Choi, G. Phytochrome-Interacting Transcription Factors PIF4 and PIF5 Induce Leaf Senescence in Arabidopsis. Nat. Commun. 2014, 5, 4636. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t Ignore the Green Light: Exploring Diverse Roles in Plant Processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, X.; Cao, J.; Zhu, W.; Wan, X.; Liu, L. GOLDEN 2-LIKE Transcription Factors Regulate Chlorophyll Biosynthesis and Flavonoid Accumulation in Response to UV-B in Tea Plants. Hortic. Plant J. 2023, 9, 1055–1066. [Google Scholar] [CrossRef]
- Huang, X.; Hu, L.; Kong, W.; Yang, C.; Xi, W. Red Light-Transmittance Bagging Promotes Carotenoid Accumulation of Grapefruit during Ripening. Commun. Biol. 2022, 5, 1–10. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Rodríguez-Concepción, M. Direct Regulation of Phytoene Synthase Gene Expression and Carotenoid Biosynthesis by Phytochrome-Interacting Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.x.; Wei, J.j.; Zhang, Y.t.; Song, S.w.; Su, W.; Sun, G.w.; Hao, Y.w.; Liu, H. cheng Supplemental Blue and Red Light Promote Lycopene Synthesis in Tomato Fruits. J. Integr. Agric. 2019, 18, 590–598. [Google Scholar] [CrossRef]
- Fu, C.; Han, Y.; Kuang, J.; Chen, J.; Lu, W. Papaya CpEIN3a and CpNAC2 Co-Operatively Regulate Carotenoid Biosynthesis-Related Genes CpPDS2/4, CpLCY-e and CpCHY-b During Fruit Ripening. Plant Cell Physiol. 2017, 58, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, M.; Cao, L.; Yuan, W.; Dong, M.; Wang, X.; Chen, W.; Shang, F. Characterization of OfWRKY3, a Transcription Factor That Positively Regulates the Carotenoid Cleavage Dioxygenase Gene OfCCD4 in Osmanthus Fragrans. Plant Mol. Biol. 2016, 91, 485–496. [Google Scholar] [CrossRef]
- Lu, S.; Ye, J.; Zhu, K.; Zhang, Y.; Zhang, M.; Xu, Q.; Deng, X. A Fruit Ripening-Associated Transcription Factor CsMADS5 Positively Regulates Carotenoid Biosynthesis in Citrus. J. Exp. Bot. 2021, 72, 3028–3043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Chen, H.; Mei, X.; Lu, S.; Xie, H.; Liu, J.; Chai, L.; Xu, Q.; Wurtzel, E.T.; Ye, J.; et al. Transcription Factor CsMADS3 Coordinately Regulates Chlorophyll and Carotenoid Pools in Citrus Hesperidium. Plant Physiol. 2023, 193, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N.; Raghavan, V. Plant Carotenoids Evolution during Cultivation, Postharvest Storage, and Food Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1561–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, S.; Tian, S.; Ma, T.; Wang, W. Light Regulates Chlorophyll Biosynthesis via ELIP1 during the Storage of Chinese Cabbage. Sci. Rep. 2022, 12, 11098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.N.P.; Sung, J. Light Spectral-Ranged Specific Metabolisms of Plant Pigments. Metabolites 2025, 15, 1. https://doi.org/10.3390/metabo15010001
Nguyen TNP, Sung J. Light Spectral-Ranged Specific Metabolisms of Plant Pigments. Metabolites. 2025; 15(1):1. https://doi.org/10.3390/metabo15010001
Chicago/Turabian StyleNguyen, The Ngoc Phuong, and Jwakyung Sung. 2025. "Light Spectral-Ranged Specific Metabolisms of Plant Pigments" Metabolites 15, no. 1: 1. https://doi.org/10.3390/metabo15010001
APA StyleNguyen, T. N. P., & Sung, J. (2025). Light Spectral-Ranged Specific Metabolisms of Plant Pigments. Metabolites, 15(1), 1. https://doi.org/10.3390/metabo15010001







