Sarcosine, Trigonelline and Phenylalanine as Urinary Metabolites Related to Visceral Fat in Overweight and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Anthropometric Measurements
2.3. Bioimpedance
2.4. Laboratory Assessment
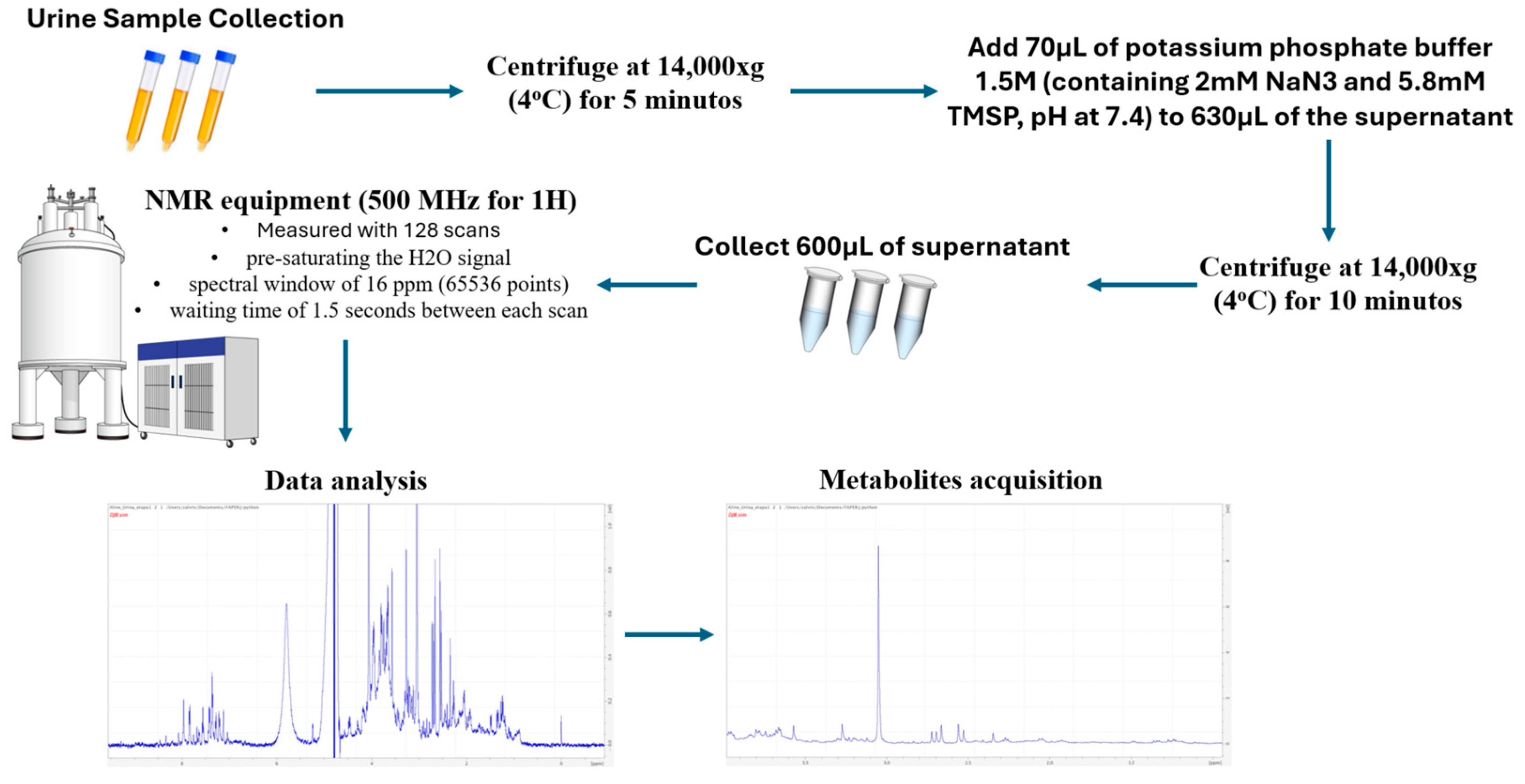
2.5. Acquisition of Urinary Samples, Measurement of Metabolites and Metabolome Analysis by Magnetic Resonance and Selection of Metabolites of Interest
2.6. Statistical Analysis
3. Results
3.1. Analysis of Body Composition, Demographic Data, Serum Biochemistry and Urinary Metabolites of Patients with Obesity or Overweight
3.2. Analysis of BMI Categories in Relation to Anthropometric, Biochemical, Demographic and Urinary Metabolite Parameters of Patients with Obesity or Overweight
3.3. Analysis of Abdominal Circumference Categories in Relation to Anthropometric, Biochemical, Demographic and Urinary Metabolic Parameters
3.4. Analysis of Visceral Fat Categories in Relation to Anthropometric, Biochemical, Demographic and Urinary Metabolic Parameters
3.5. Analysis of Fat Percentile Categories in Relation to Demographic, Anthropometric, Biochemical and Urinary Metabolic Parameters
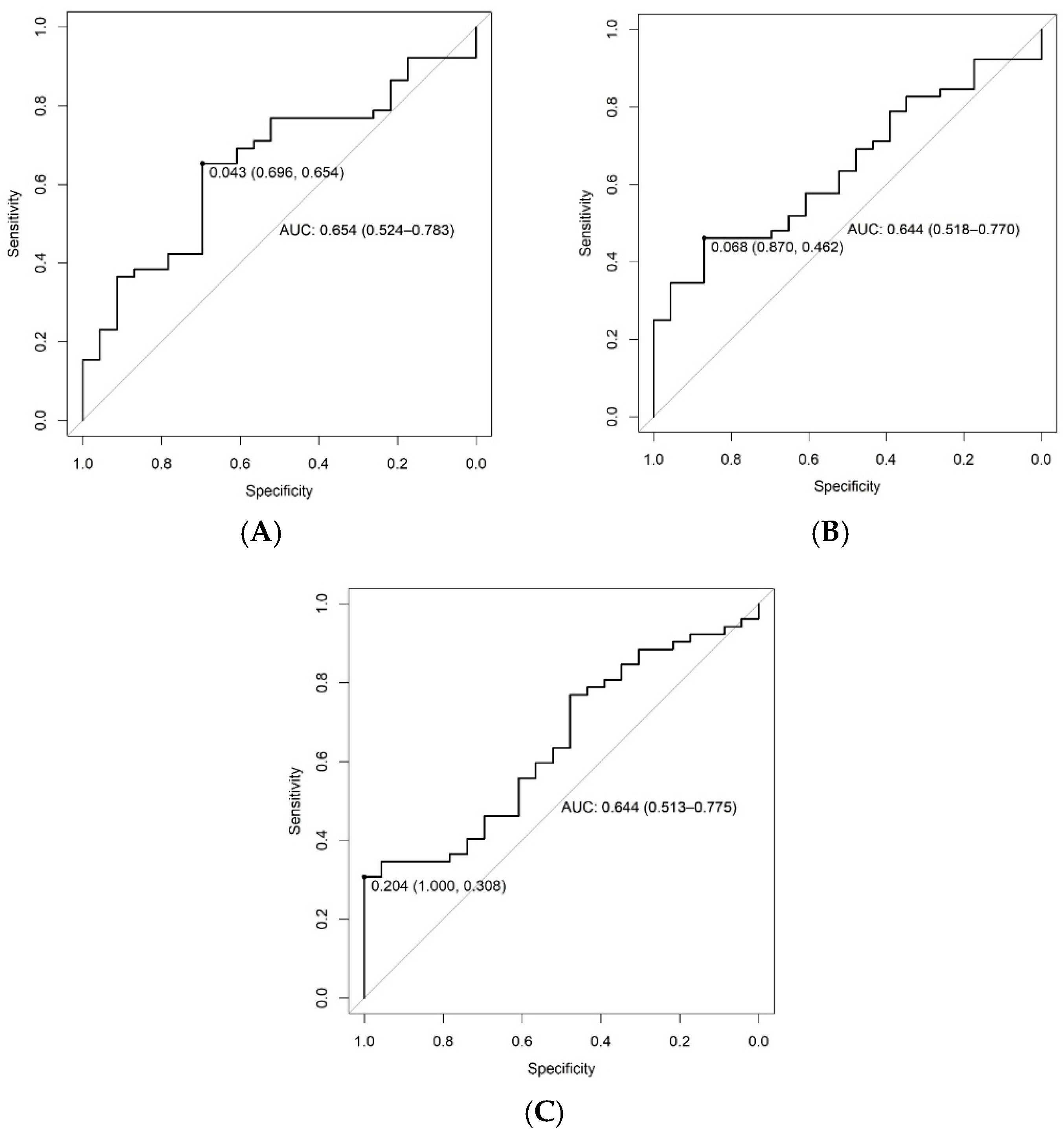
3.6. Discrimination of Visceral Fat from 9 to 16 kg or ≥16 kg by Trigonelline, Sarcosine and Phenylalanine Urinary Concentrations (ROC Curve)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 25, 1–8. [Google Scholar]
- Cava, E.; Neri, B.; Carbonelli, M.G.; Riso, S.; Carbone, S. Obesity pandemic during COVID-19 outbreak: Narrative review and future considerations. Clin Nutr. 2021, 40, 1637–1643. [Google Scholar] [CrossRef]
- Matheus, J.O.; Estivaleti, J.; Guzmán-Habinger, J.; Lobos, A.; Azeredo, C.M.; de Rezende, L.F.M. Time Trends and Projected Obesity Epidemic in Brazilian Adults between 2006 and 2030. Brazilian Obesity Panel 2022. Available online: https://painelobesidade.com.br/biblioteca/time-trends-and-projected-obesity-epidemic-in-brazilian-adults-between-2006-and-2030/ (accessed on 23 April 2024).
- Alston, J.M.; Okrent, A.M.; Alston, J.M.; Okrent, A.M. Causes of Obesity: Individual Physiology and Consumption Choices. In The Effects of Farm and Food Policy on Obesity in the United States; Palgrave Studies in Agricultural Economics and Food Policy; Palgrave Macmillan: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Masood, B.; Moorthy, M. Causes of obesity: A review. Clin. Med. 2023, 23, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Collier, D.; Kassotis, C.; Roepke, T.A.; Kim, M.J.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M.C.; Chatterjee, S.; et al. Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 199, 115012. [Google Scholar] [CrossRef]
- Shen, S. How Food Causes Obesity. Theor. Nat. Sci. 2023, 3, 745–750. [Google Scholar] [CrossRef]
- Alston, J.M.; Okrent, A.M. Causes of Obesity: External Influences. In The Effects of Farm and Food Policy on Obesity in the United States; Palgrave Studies in Agricultural Economics and Food Policy; Research Papers in Economics; Palgrave Macmillan: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Hou, W.; Arcan, C. Obesity Trends and Associations with Types of Physical Activity and Sedentary Behavior in US Adults: National Health and Nutrition Examination Survey, 2007–2016. Obesity 2020, 29, 240–250. [Google Scholar] [CrossRef]
- Hamberger, U. Physical Activity and Obesity—Underlying Mechanisms, Practical Actions. Ther. Umschau. Rev. Ther. 2024, 81, 74–82. [Google Scholar] [CrossRef]
- Bora, N.; Vaishali, K.; Verma, A.; Bharti, A.K.; Sinha, M.K. Physical activity and sedentary behavior perceptions in overweight and obese adults: A systematic review of qualitative study. F1000Research 2024, 13, 787. [Google Scholar] [CrossRef]
- Jones, S.A.; Wen, F.; Herring, A.H.; Evenson, K.R. Correlates of US adult physical activity and sedentary behavior patterns. J. Sci. Med. Sport 2016, 19, 1020–1027. [Google Scholar] [CrossRef]
- Voss, J.D.; Dhurandhar, N.V. Viral Infections and Obesity. Curr. Obes. Rep. 2017, 6, 28–37. [Google Scholar] [CrossRef]
- Cervantes-Echeverría, M.; Gallardo-Becerra, L.; Cornejo-Granados, F.; Leyva, A.O. A loss of crAssphage stability in the human gut virome is associated with obesity and metabolic syndrome. bioRxiv 2022. [Google Scholar] [CrossRef]
- Neeland, I.J.; Yokoo, T.; Leinhard, O.D.; Lavie, C.J. 21st Century Advances in Multimodality Imaging of Obesity for Care of the Cardiovascular Patient. JACC Cardiovasc. Imaging 2021, 14, 482–494. [Google Scholar] [CrossRef]
- Nurieva, A.R.; Parve, S.D.; Sineglazova, A.V. Heterogeneous Comorbidity in Individuals With Different Phenotypes of Obesity. Curēus 2023, 15, e38995. [Google Scholar] [CrossRef] [PubMed]
- Perez-Campos, E.; Mayoral, L.-C.; Andrade, G.; Mayoral, E.-C.; Huerta, T.; Canseco, S.; Rodal Canales, F.; Cabrera-Fuentes, H.; Cruz, M.; Pérez Santiago, A.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11. [Google Scholar] [CrossRef]
- Lin, Z.; Feng, W.; Liu, Y.; Ma, C.; Arefan, D.; Zhou, D.; Cheng, X.; Yu, J.; Gao, L.; Du, L.; et al. Machine Learning to Identify Metabolic Subtypes of Obesity: A Multi-Center Study. Front Endocrinol. 2021, 12, 7845. [Google Scholar] [CrossRef]
- Gowda, G.N.; Zhang, S.; Gu, H.; Asiago, V.; Shanaiah, N.; Raftery, D. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 2008, 8, 617–633. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Zhong, P.; Tan, S.; Zhu, Z.; Bulloch, G.; Long, E.; Huang, W.; He, M.; Wang, W. Metabolomic phenotyping of obesity for profiling cardiovascular and ocular diseases. J. Transl. Med. 2023, 21, 384. [Google Scholar] [CrossRef]
- Ottosson, F.; Smith, E.; Ericson, U.; Brunkwall, L.; Orho-Melander, M.; Di Somma, S.; Antonini, P.; Nilsson, P.M.; Fernandez, C.; Melander, O. Metabolome-Defined Obesity and the Risk of Future Type 2 Diabetes and Mortality. Diabetes Care 2022, 45, 1260–1267. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Michael, C.; Ju, L.Y.; Heck, D.E.; Duck, K.H. Sensor of molecular imbalance in metabolic disorder: Determination of molecular behavior wired in disease utilizing metabolomics. J. Addict. Med. Ther. Sci. 2020, 6, 61–63. [Google Scholar] [CrossRef]
- Zhong, F.; Xu, M.; Bruno, R.S.; Ballard, K.D.; Zhu, J. Targeted High Performance Liquid Chromatography Tandem Mass Spectrometry-based Metabolomics differentiates metabolic syndrome from obesity. Exp. Biol. Med. 2017, 242, 773–780. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 1–31. [Google Scholar] [CrossRef]
- Martinez, P.J.; Agudiez, M.; Molero, D.; Martin-Lorenzo, M.; Baldan-Martin, M.; Santiago-Hernandez, A.; García-Segura, J.M.; Madruga, F.; Cabrera, M.; Calvo, E.; et al. Urinary metabolic signatures reflect cardiovascular risk in the young, middle-aged, and elderly populations. J. Mol. Med. 2020, 98, 1603–1613. [Google Scholar] [CrossRef]
- Pawelzik, S.-C.; Avignon, A.; Idborg, H.; Boegner, C.; Stanke-Labesque, F.; Jakobsson, P.-J.; Sultan, A.; Bäck, M. Urinary prostaglandin D2 and E2 metabolites associate with abdominal obesity, glucose metabolism, and triglycerides in obese subjects. Prostaglandins Other Lipid Mediat. 2019, 145, 106361. [Google Scholar] [CrossRef] [PubMed]
- Payab, M.; Tayanloo-Beik, A.; Falahzadeh, K.; Mousavi, M.; Salehi, S.; Djalalinia, S.; Ebrahimpur, M.; Rezaei, N.; Rezaei-Tavirani, M.; Larijani, B.; et al. Metabolomics prospect of obesity and metabolic syndrome; a systematic review. J. Diabetes Metab. Disord. 2021, 21, 889–917. [Google Scholar] [CrossRef]
- Gowda, G.A.N.; Raftery, D. NMR Based Metabolomics. Adv. Exp. Med. Biol. 2021, 1280, 19–37. [Google Scholar]
- Wu, Q.; Huang, Q.-X.; Zeng, H.-L.; Ma, S.; Lin, H.-D.; Xia, M.-F.; Tang, H.-R.; Gao, X. Prediction of Metabolic Disorders Using NMR-Based Metabolomics: The Shanghai Changfeng Study. Phenomics 2021, 1, 186–198. [Google Scholar] [CrossRef]
- Bervoets, L.; Massa, G.; Guedens, W.; Reekmans, G.; Noben, J.-P.; Adriaensens, P. Identification of metabolic phenotypes in childhood obesity by 1H NMR metabolomics of blood plasma. Future Sci. OA 2018, 4, FSO310. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Sun, Y.; Fu, Y.; Shen, W.; Cai, L.; Tan, X.; Cai, Y.; Wang, N.; Lu, Y.; et al. Nuclear magnetic resonance-based metabolomics with machine learning for predicting progression from prediabetes to diabetes. medRxiv 2024. [Google Scholar] [CrossRef]
- Htun, K.T.; Pan, J.; Pasanta, D.; Tungjai, M.; Udomtanakunchai, C.; Petcharoen, T.; Chamta, N.; Kosicharoen, S.; Chukua, K.; Lai, C.; et al. Advanced Molecular Imaging (MRI/MRS/1H NMR) for Metabolic Information in Young Adults with Health Risk Obesity. Life 2021, 11, 1035. [Google Scholar] [CrossRef]
- Htun, K.T.; Jaikumkao, K.; Pan, J.; Moe, A.T.M.; Intachai, N.; Promsan, S.; Lungkaphin, A.; Tapanya, M.; Pasanta, D.; Tungjai, M.; et al. Noninvasive NMR/MRS Metabolic Parameters to Evaluate Metabolic Syndrome in Rats. Diagnostics 2022, 12, 1621. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Effects of metabolic syndrome on bone mineral density, histomorphometry and remodelling markers in male rats. PLoS ONE 2018, 13, e0192416. [Google Scholar] [CrossRef]
- Htun, K.T.; Pan, J.; Pasanta, D.; Tungjai, M.; Udomtanakunchai, C.; Chancharunee, S.; Kaewjaeng, S.; Kim, H.J.; Kaewkhao, J.; Kothan, S. Identification of Metabolic Phenotypes in Young Adults with Obesity by 1H NMR Metabolomics of Blood Serum. Life 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Goodrich, J.A.; Chen, W.; Qiu, C.; Chen, J.C.; Costello, E.; Alderete, T.L.; Chatzi, L.; Gilliland, F.; Chen, Z. Cardiometabolic profiles and proteomics associated with obesity phenotypes in a longitudinal cohort of young adults. Dent. Sci. Rep. 2024, 14, 7384. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Posma, J.M.; Chan, Q.; Garcia-Perez, I.; Wijeyesekera, A.; Bictash, M.; Ebbels, T.M.D.; Ueshima, H.; Zhao, L.; van Horn, L.; et al. Urinary metabolic signatures of human adiposity. Sci. Transl. Med. 2015, 7, 285ra62. [Google Scholar] [CrossRef] [PubMed]
- Geach, T. Obesity: Mapping metabolites--specific metabolic signatures in urine are associated with adiposity. Nat. Rev. Endocrinol. 2015, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, E.T.; Guo, L.; Swisher, C.L.; Shah, N.; Huang, L.; Napier, L.A.; Kirkness, E.F.; Spector, T.D.; Caskey, C.T.; Thorens, B.; et al. Profound Perturbation of the Metabolome in Obesity Is Associated with Health Risk. Cell Metab. 2019, 29, 488–500.e2. [Google Scholar] [CrossRef]
- Conover, W.J.; Iman, R.L. Practical Nonparametric Statistics. Rank Transformations as a Bridge Between Parametric and Nonparametric Statistics. Am. Stat. 1981, 35, 124–129. [Google Scholar] [CrossRef]
- Thiele, C.; Hirschfeld, G. Cutpoint: Improved estimation and validation of optimal Cutpoints in R. J. Stat. Softw. 2021, 98, 1–27. [Google Scholar] [CrossRef]
- Hubert, K.; Kolb, B. Obese visceral fat tissue inflammation: From protective to detrimental? BMC Med. 2022, 20, 494. [Google Scholar] [CrossRef]
- Tong, L.; Tian, M.; Ma, X.; Bai, L.; Zhou, J.; Ding, W. Metabolome Profiling and Pathway Analysis in Metabolically Healthy and Unhealthy Obesity among Chinese Adolescents Aged 11–18 Years. Metabolites 2023, 13, 641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stanislava, M.; Matejová, M.; Fikselov, M.; Buňka, F. Health safety aspects of foodstuffs intended for phenylketonurics. Potravinarstvo 2015, 9, 132–137. [Google Scholar] [CrossRef]
- van Vliet, K.; Melis, E.S.; de Blaauw, P.; van Dam, E.; Maatman, R.G.H.J.; Abeln, D.; van Spronsen, F.J.; Heiner-Fokkema, M.R. Aspartame and Phe-Containing Degradation Products in Soft Drinks across Europe. Nutrients 2020, 12, 1887. [Google Scholar] [CrossRef]
- Gao, X.; Lin, S.-H.; Ren, F.; Li, J.-T.; Chen, J.-J.; Yao, C.-B.; Yang, H.-B.; Jiang, S.-X.; Yan, G.-Q.; Wang, D.; et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 2016, 7, 11960. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishizaka, Y.; Mitushima, T.; Tani, M.; Toda, A.; Toda, E.-I.; Okada, M.; Yamamoto, H.; Yamakado, M. Plasma amino acid profile is altered by visceral fat accumulation and is a predictor of visceral obesity in humans. Nat. Preced. 2011. [Google Scholar] [CrossRef]
- Schlecht, I.; Gronwald, W.; Behrens, G.; Baumeister, S.E.; Hertel, J.; Hochrein, J.; Zacharias, H.U.; Fischer, B.; Oefner, P.J.; Leitzmann, M.F. Visceral adipose tissue but not subcutaneous adipose tissue is associated with urine and serum metabolites. PLoS ONE 2017, 12, e0175133. [Google Scholar] [CrossRef]
- Vazquez-Agra, N.; Fernandez-Crespo, S.; Marques-Afonso, A.-T.; Cruces-Sande, A.; Barbosa-Gouveia, S.; Martinez-Olmos, M.-A.; Hermida-Ameijeiras, A. The correlation of lipid profile and waist circumference with phenylalanine levels in adult patients with classical phenylketonuria. Med. Clínica 2023, 160, 385–391. [Google Scholar] [CrossRef]
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Trigonelline in Coffee and Coffee By-Products. Molecules 2013, 28, 3460. [Google Scholar] [CrossRef]
- Membrez, M.; Migliavacca, E.; Christen, S.; Yaku, K.; Trieu, J.; Lee, A.K.; Morandini, F.; Giner, M.P.; Stiner, J.; Makarov, M.V.; et al. Trigonelline is an NAD+ precursor that improves muscle function during aging and is reduced in human sarcopenia. Nat Metab 2024, 6, 433–447. [Google Scholar] [CrossRef]
- Aytekin, N.; Mileva, K.N.; Cunliffe, A.D. Selected B vitamins and their possible link to the aetiology of age-related sarcopenia: Relevance of UK dietary recommendations. Nutr. Res. Rev. 2018, 31, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Lone, N.A.; Knott, R.M.; Hassan, A.; Abdullah, T. Trigonelline prevents high cholesterol and high fat diet induced hepatic lipid accumulation and lipo-toxicity in C57BL/6J mice, via restoration of hepatic autophagy. Food Chem. Toxicol. 2018, 121, 283–296. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, X.; Cao, Y.; Wang, X.; Lu, J.; Xie, L.; Liu, K.; Li, X. The neuroprotective and antidiabetic effects of trigonelline: A review of signaling pathways and molecular mechanisms. Biochimie 2022, 206, 93–104. [Google Scholar] [CrossRef]
- Walvekar, M.V.; Jadhav, N.A.; Daunde, J.A.; Potphode, N.D.; Desai, S.S. Trigonelline: An Emerging Paradigm for Effective Therapy in Diabetes Mellitus. J. Endocrinol. Reprod. 2023, 27, 15–28. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; He, Q.; Huang, X.; Ren, Y.; Dong, Z. Changes in Isoleucine, Sarcosine, and Dimethylglycine During OGTT as Risk Factors for Diabetes. J. Clin. Endocrinol. Metab. 2024, 109, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Rodrigues, C.E.; Floegel, A.; Ahrens, W. Omics Biomarkers in Obesity: Novel Etiological Insights and Targets for Precision Prevention. Curr Obes Rep 2020, 9, 219–230. [Google Scholar] [CrossRef]
- Schartum-Hansen, H.; Ueland, P.M.; Pedersen, E.R.; Meyer, K.; Ebbing, M.; Bleie, Ø.; Svingen, G.F.T.; Seifert, R.; Vikse, B.E.; Nygård, O. Assessment of Urinary Betaine as a Marker of Diabetes Mellitus in Cardiovascular Patients. PLoS ONE 2013, 8, e69454. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hu, Y.; Bao, B. Relationship of body mass index and visceral fat area combination with arterial stiffness and cardiovascular risk in cardiovascular disease-free people: NHANES (2011–2018). Endocr. Connect. 2023, 12, e230291. [Google Scholar] [CrossRef]
- Silveira, E.A.; Vaseghi, G.; Santos, A.S.d.C.; Kliemann, N.; Masoudkabir, F.; Noll, M.; Mohammadifard, N.; Sarrafzadegan, N.; de Oliveira, C. Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments. Int. J. Mol. Sci. 2020, 21, 9042. [Google Scholar] [CrossRef]
- Raheem, J.; Sliz, E.; Shin, J.; Holmes, M.V.; Pike, G.B.; Richer, L.; Gaudet, D.; Paus, T.; Pausova, Z. Visceral adiposity is associated with metabolic profiles predictive of type 2 diabetes and myocardial infarction. Commun. Med. 2022, 2, 81. [Google Scholar] [CrossRef]
- Li, S.; Feng, L.; Sun, X.; Ding, J.; Zhou, W. Association between serum uric acid and measures of adiposity in Chinese adults: A cross-sectional study. BMJ Open 2023, 13, e072317. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Allan, J.; Gamble, G.D.; Horne, A.; Woodward, O.M.; Stamp, L.K.; Merriman, T.R. Effect of body mass index on serum urate and renal uric acid handling responses to an oral inosine load: Experimental intervention study in healthy volunteers. Arthritis Res Ther. 2020, 22, 259. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, L.; Yu, F.; Cai, Y.; Wang, J.; Gao, Y.; Ping, Z. The causal association between body mass index and type 2 diabetes mellitus-evidence based on regression discontinuity design. Diabetes Metab Res Rev. 2021, 37, e3455. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Liu, M.; Ji, N.; Zeng, X.Y.; Dong, W.L.; Mao, F.; Liu, S.W.; Dong, J.Q.; Zhou, M.G. Disease burden of diabetes attributable to high body mass index in China, 1990–2016. Chin. J Epidemiol. 2019, 40, 46–51. [Google Scholar] [CrossRef]
- Lal, M. To Determine the level of association of BMI with lipid profile and HbA1c in Type II Diabetes Mellitus Patients. J. Med. Sci. Clin. Res. 2020, 8, 535–548. [Google Scholar] [CrossRef]
- Mi, T.; Min, Y.; Kang, J.; Ok, S.; Eun, S.; Park, C. Effects of BMI and LDL-cholesterol change pattern on cardiovascular disease in normal adults and diabetics. BMJ Open Diabetes Res. Care 2020, 8, e001340. [Google Scholar] [CrossRef]
- Pramesti, A.R.; Kusumaningati, W. Body Mass Index and Waist Circumferences Related to Uric Acid Level among Adults. Muhammadiyah J. Nutr. Food Sci. 2020, 1, 31–34. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Hansel, B.; Bonnefont-Rousselot, D.; Bittar, R.; Girerd, X.; Girerd, P.; Bruckert, E. Waist circumference is a strong and independent determinant of the distribution of HDL subfractions in overweight patients with cardiovascular risk factors. Diabetes Vasc. Dis. Res. 2012, 9, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Labban, L.; Malek, Z. The Association between Visceral Fat, Dietary Patterns, and Comorbidities. Open Access Libr. J. 2018, 5, 85972. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, X.; Shen, Y.; Hao, Y.; Hu, Y.; Xiao, Y.; Bao, Y.; Jia, W. Positive relationship between serum low-density lipoprotein cholesterol levels and visceral fat in a Chinese nondiabetic population. PLoS ONE 2014, 9, e112715. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Zampino, M.; Moaddel, R.; Chen, T.K.; Tian, Q.; Ferrucci, L.; Semba, R.D. Plasma metabolites associated with chronic kidney disease and renal function in adults from the Baltimore Longitudinal Study of Aging. Metabolomics 2021, 17, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Variable | BMI 25–30 kg/m2 (n = 19) | BMI 30–35 kg/m2 (n = 26) | BMI ≥ 35 kg/m2 (n = 30) | p-Value |
|---|---|---|---|---|
| Valine | 0.00061546 ± 0.00505177 | 0.00538768 ± 0.01269377 | 0.00014959 ± 0.01684119 | 0.1033 |
| 2-Methylglutarate | 0.00165881 ± 0.01655289 | 0.00538768 ± 0.01269377 | 0.00014959± 0.01684119 | 0.1179 |
| PropyleneGlycol | 0.00090099 ± 0.03005212 | 0.01640011 ± 0.03826574 | −0.00205988 ± 0.05979712 | 0.1313 |
| 3-Hydroxybutyrate | 0.02010601 ± 0.06175205 | 0.08954448 ± 0.32246036 | −0.00302254 ± 0.15196923 | 0.4294 |
| Methylmalonate | 0.01264350 ± 0.11264410 | 0.14365927 ± 0.36298013 | −0.00404615 ± 0.40640139 | 0.0891 |
| 3-Hydroxyisovalerate | 0.00390361 ± 0.02444776 | 0.02586556 ± 0.05433844 | −0.00296892 ± 0.08594212 | 0.1105 |
| Lactate | 0.00937613 ± 0.06128961 | 0.06418234± 0.17184152 | −0.01809144 ± 0.23604109 | 0.0963 |
| 2-Hydroxyisobutyrate | 0.00281533 ± 0.01369690 | 0.01522823 ± 0.03859131 | −0.00273186 ± 0.05487979 | 0.0998 |
| Alanine | 0.01237974 ± 0.06928748 | 0.06809089 ± 0.18076744 | −0.00930114 ± 0.25718270 | 0.1789 |
| Acetate | 0.00437516 ± 0.03796163 | 0.04344184 ± 0.09805174 | −0.01301485 ± 0.15391431 | 0.1407 |
| Nacetylglucosamine | 0.02468230 ± 0.11157969 | 0.11868568 ± 0.26663178 | −0.03213317 ± 0.51034783 | 0.1360 |
| Lipids | 0.07097576 ± 0.32677337 | 0.35051343 ± 0.77455655 | −0.09941926 ± 1.51964602 | 0.1204 |
| Citrate | 0.03605499 ± 0.30676713 | 0.30029714 ± 0.59024590 | 0.02023172 ± 0.93709287 | 0.2556 |
| Piruvate | 0.00552071 ± 0.16038939 | 0.13064686 ± 0.28647585 | −0.07837194 ± 0.55049093 | 0.2342 |
| Carnitine | 0.00218021 ± 0.02079422 | 0.02055738 ± 0.04867125 | −0.00508360 ± 0.07319585 | 0.2123 |
| Sarcosine | 0.02019644 ± 0.09732506 | 0.12127916 ± 0.30305130 | −0.03881738 ± 0.47332956 | 0.1137 |
| Creatinine | 0.38869472 ± 3.01703968 | 3.25117597 ± 6.96024323 | 0.38856177 ± 10.0466937 | 0.1344 |
| AcetylcholineCholine | 0.00455496 ± 0.02257989 | 0.02897809 ± 0.07532024 | −0.00892429 ± 0.11170803 | 0.1127 |
| Methanol | 0.00361544 ± 0.01424125 | 0.02033866 ± 0.05404970 | −0.00490569 ± 0.07154278 | 0.3840 |
| Creatine | 0.10509202 ± 0.45258149 | 0.63520803 ± 1.25169756 | 0.00504035 ± 1.68803007 | 0.1385 |
| Lactate | 0.01789560 ± 0.09689388 | 0.11237576 ± 0.30115361 | −0.02013265 ± 0.38551300 | 0.1893 |
| Trigonelline | 0.02536680 ± 0.09299115 | 0.13292601 ± 0.36331211 | −0.00443190 ± 0.50497876 | 0.1871 |
| Glucose | 0.01127453 ± 0.03706481 | 0.11815990 ± 0.41238564 | 0.05508906 ± 0.42044034 | 0.3838 |
| Urea | 0.33765854 ± 1.62787013 | 2.19163044 ± 6.64806586 | −0.63307152 ± 6.84119576 | 0.4810 |
| Phenylalanine | 0.02472187 ± 0.38385305 | 0.27918801 ± 0.59437347 | −0.19283182 ± 1.16757047 | 0.2072 |
| Hippurate | 0.16583012 ± 0.33340075 | 0.72081954 ± 1.75203825 | −0.27248268 ± 2.54214070 | 0.4637 |
| Formate | 0.00566661 ± 0.01631842 | 0.01752892 ± 0.03717082 | −0.00544484 ± 0.08135147 | 0.3593 |
| Variable | Abdominal Circumference | p-Value | ||
|---|---|---|---|---|
| 81–93 cm (n = 15) | 94–104 cm (n = 18) | ≥104 cm (n = 42) | ||
| Valine | −0.00003918 ± 0.0059266 | 0.00640189 ± 0.0153453 | 0.00099083 ± 0.0141434 | 0.1816 |
| 2-Methylglutarate | −0.00099108 ± 0.0207314 | 0.01975488 ± 0.0455552 | 0.00131910 ± 0.0503638 | 0.2003 |
| PropyleneGlycol | −0.00110667 ± 0.0204604 | 0.02010409 ± 0.0607330 | 0.00517802 ± 0.05418077 | 0.0740 |
| 3-Hydroxybutyrate | 0.00370326 ± 0.11563711 | 0.11983077 ± 0.3755580 | 0.00969027 ± 0.13129364 | 0.7392 |
| Methylmalonate | 0.00037540 ± 0.13141282 | 0.17016515 ± 0.4431974 | 0.01869951 ± 0.33984214 | 0.1442 |
| 3-Hydroxyisovalerate | −0.00005216 ± 0.02897823 | 0.02996702 ± 0.0654515 | 0.00283289 ± 0.07252166 | 0.1495 |
| Lactate | −0.00863083 ± 0.10494395 | 0.07997944 ± 0.1903446 | −0.00014341 ± 0.20084090 | 0.2650 |
| 2-Hydroxyisobutyrate | −0.00016439 ± 0.02021745 | 0.01935372 ± 0.0456693 | 0.00051353 ± 0.04591141 | 0.2219 |
| Alanine | −0.00506805 ± 0.10705881 | 0.09296386 ± 0.2108559 | 0.00307655 ± 0.21384420 | 0.1934 |
| Acetate | 0.00092327 ± 0.04023385 | 0.04615098 ± 0.1121220 | −0.00053325 ± 0.13371478 | 0.1854 |
| Nacetylglucosamine | 0.00092327 ± 0.04023385 | 0.14632220 ± 0.3265442 | −0.00476474 ± 0.42717293 | 0.3087 |
| Lipids | 0.03263438 ± 0.40939744 | 0.42623953 ± 0.9511242 | −0.01625039 ± 1.27312614 | 0.3189 |
| Citrate | −0.01137573 ± 0.36231502 | 0.35305569 ± 0.6987732 | 0.06941323 ± 0.79118337 | 0.1543 |
| Piruvate | −0.00053166 ± 0.15408531 | 0.13135252 ± 0.3456552 | −0.02870993 ± 0.47428649 | 0.2558 |
| Carnitine | 0.00029096 ± 0.02298804 | 0.02438315 ± 0.0592505 | −0.00047270 ± 0.06177039 | 0.3534 |
| Sarcosine | 0.00464012 ± 0.13274367 | 0.15163795 ± 0.3620950 | −0.01015752 ± 0.39831673 | 0.2057 |
| Creatinine | 0.07774829 ± 3.44967844 | 4.23075194 ± 9.0271122 | 0.62506830 ± 8.10561314 | 0.2485 |
| AcetylcholineCholine | −0.00027200 ± 0.03279491 | 0.03616655 ± 0.0889678 | −0.00177790 ± 0.09425045 | 0.2140 |
| Methanol | 0.00157884 ± 0.01582618 | 0.02694244 ± 0.0662672 | −0.00138854 ± 0.05957149 | 0.3289 |
| Creatine | 0.07819098 ± 0.51246949 | 0.78868285 ± 1.4908866 | 0.07843361 ± 1.42436138 | 0.1827 |
| Lactate | 0.00264651 ± 0.12198857 | 0.14613636 ± 0.3651196 | −0.00029394 ± 0.32134385 | 0.1617 |
| Trigonelline | 0.00700087 ± 0.12808419 | 0.18859049 ± 0.4574680 | 0.00727254 ± 0.41000676 | 0.1667 |
| Glucose | −0.00235783 ± 0.05865739 | 0.17549902 ± 0.4900429 | 0.04322453 ± 0.35253559 | 0.2527 |
| Urea | −0.08895897 ± 2.38083542 | 2.84288549 ± 7.7893955 | −0.1293284 ± 5.82640239 | 0.2457 |
| Phenylalanine | 0.05580632 ± 0.30766599 | 0.26810350 ± 0.7645377 | −0.08855496 ± 1.00291494 | 0.1296 |
| Hippurate | 0.09461949 ± 0.36748316 | 0.84006634 ± 2.0311798 | −0.06721158 ± 2.19399656 | 0.2275 |
| Formate | 0.00308861 ± 0.01812276 | 0.02268495 ± 0.0447608 | −0.00129967 ± 0.06854088 | 0.2712 |
| Variable | Visceral Fat 9–16 kg n = 23 | Visceral Fat ≥ 16 kg n = 52 | p-Value |
|---|---|---|---|
| Valine | 0.00079868 ± 0.00485752 | 0.00265176 ± 0.01570310 | 0.0858 |
| 2-Methylglutarate | 0.00219207 ± 0.01581101 | 0.00664819 ± 0.05322867 | 0.0557 |
| PropyleneGlycol | 0.00110070 ± 0.02794615 | 0.01033527 ± 0.05850736 | 0.0858 |
| 3-Hydroxybutyrate | 0.01715363 ± 0.05389605 | 0.04278771 ± 0.25776019 | 0.1697 |
| Methylmalonate | 0.02620161 ± 0.13164189 | 0.06252589 ± 0.40224233 | 0.0817 |
| 3-Hydroxyisovalerate | 0.00521253 ± 0.02460903 | 0.01034072 ± 0.07633910 | 0.1922 |
| Lactate | 0.01016681 ± 0.05763499 | 0.01034072 ± 0.07633910 | 0.1222 |
| 2-Hydroxyisobutyrate | 0.00309513 ± 0.01308270 | 0.00569772 ± 0.05023104 | 0.1115 |
| Alanine | 0.01072919 ± 0.06342359 | 0.02845716 ± 0.23490839 | 0.1065 |
| Acetate | 0.00402614 ± 0.03489568 | 0.01403017 ± 0.13794045 | 0.0572 |
| Nacetylglucosamine | 0.02092272 ± 0.10046501 | 0.04056872 ± 0.43489233 | 0.0759 |
| Lipids | 0.06373849 ± 0.29764104 | 0.11564087 ± 1.28901603 | 0.0740 |
| Citrate | 0.04883437 ± 0.30544026 | 0.15339484 ± 0.82747618 | 0.1526 |
| Piruvate | 0.00095599 ± 0.14409099 | 0.02170318 ± 0.47383020 | 0.1090 |
| Carnitine | 0.00245187 ± 0.01990206 | 0.00705797 ± 0.06609374 | 0.1251 |
| Sarcosine | 0.01791004 ± 0.09199581 | 0.03770266 ± 0.42314367 | 0.0350 |
| Creatinine | 0.73891369 ± 3.26887661 | 1.66495410 ± 9.06680192 | 0.3092 |
| AcetylcholineCholine | 0.00519861 ± 0.02148845 | 0.00870534 ± 0.10124550 | 0.0923 |
| Methanol | 0.00323570 ± 0.01373972 | 0.00722897 ± 0.06711590 | 0.1733 |
| Creatine | 0.13589880 ± 0.50873213 | 0.29880183 ± 1.56824870 | 0.1308 |
| Trigonelline | 0.01992715 ± 0.08161980 | 0.06436085 ± 0.46340115 | 0.0488 |
| Glucose | 0.00899314 ± 0.03565325 | 0.09100390 ± 0.42928575 | 0.4245 |
| Urea | 0.35799561 ± 1.56831964 | 0.69561268 ± 7.09422371 | 0.1922 |
| Phenylalanine | −0.00766327 ± 0.30258184 | 0.04076740 ± 1.01042900 | 0.0488 |
| Hippurate | 0.12361041 ± 0.31564652 | 0.20912617 ± 2.33303284 | 0.1222 |
| Formate | 0.00532253 ± 0.01497627 | 0.00533950 ± 0.06768999 | 0.3038 |
| Variable | Total Fat Percentile | p-Value | ||
|---|---|---|---|---|
| ≤36% (n = 10) | 36–46% (n = 39) | ≥46% (n = 26) | ||
| Valine | 0.00101265 ± 0.00512192 | 0.00450994 ± 0.01139156 | −0.00114435 ± 0.01728648 | 0.7945 |
| 2-Methylglutarate | 0.00314260 ± 0.01611409 | 0.01467480 ± 0.03594132 | −0.00798537 ± 0.06027488 | 0.8306 |
| PropyleneGlycol | 0.00409456 ± 0.01609466 | 0.01783636 ± 0.05067407 | −0.00668513 ± 0.05786045 | 0.8664 |
| Ethano1 | 0.00000032 ± 0.00000065 | 0.00000290 ± 0.00001346 | 0.00000711 ± 0.00002287 | 0.5312 |
| 3-Hydroxybutyrate | 0.02643885 ± 0.06423856 | 0.06514708 ± 0.26499481 | −0.00713964 ± 0.16430558 | 0.6539 |
| Methylmalonate | 0.04090139 ± 0.16637206 | 0.12306473 ± 0.32330034 | −0.05209827 ± 0.39764965 | 0.8468 |
| 3-Hydroxyisovalerate | 0.00617340 ± 0.02663063 | 0.02253102 ± 0.05056713 | −0.01087839 ± 0.08715801 | 0.9368 |
| Lactate | 0.01293351± 0.05698275 | 0.05047227 ± 0.14923777 | −0.03052344 ± 0.24814429 | 0.8900 |
| 2-Hydroxyisobutyrate | 0.00348871 ± 0.01485912 | 0.01355455 ± 0.03461765 | −0.00754019 ± 0.05587989 | 0.9319 |
| Alanine | 0.01249510 ± 0.06293919 | 0.06211563 ± 0.16490216 | −0.03157372 ± 0.26081422 | 0.9454 |
| Acetate | 0.00491930 ± 0.03334036 | 0.03467889 ± 0.08651634 | −0.02228845 ± 0.16165477 | 0.6730 |
| Nacetylglucosamine | 0.02486789 ± 0.10438933 | 0.11057482 ± 0.25199513 | −0.07578080 ± 0.52229981 | 0.8673 |
| Lipids | 0.07362487 ± 0.30757253 | 0.32482659 ± 0.73261361 | −0.22789135 ± 1.55834702 | 0.8430 |
| Citrate | 0.06499638 ± 0.32964008 | 0.25071499 ± 0.56684142 | −0.05108179 ± 0.94872898 | 0.6722 |
| Piruvate | 0.00393686 ± 0.15424157 | 0.08876121 ± 0.25078126 | −0.09040393 ± 0.59355393 | 0.5918 |
| Carnitine | 0.00364686 ± 0.02018337 | 0.01692661 ± 0.04392245 | −0.01050764 ± 0.07565114 | 0.7559 |
| Citrate | 0.06745977 ± 0.33477574 | 0.24284605 ± 0.55647571 | −0.02256917 ± 0.90172737 | 0.7119 |
| Sarcosine | 0.02231522 ± 0.10276815 | 0.10630231 ± 0.26981221 | −0.07678743 ± 0.48738792 | 0.8166 |
| Creatinine | 0.60808788 ± 2.88463903 | 3.17411222 ± 7.16106132 | −1.01148568 ± 9.24674203 | 0.8177 |
| AcetylcholineCholine | 0.00565693 ± 0.02326015 | 0.02494170 ± 0.06599811 | −0.01757883 ± 0.11567202 | 0.8204 |
| Methanol | 0.00350903 ± 0.01478470 | 0.01822024 ± 0.04783386 | −0.01135970 ± 0.07252683 | 0.7426 |
| Creatine | 0.08155832 ± 0.30586861 | 0.44641898 ± 1.02869271 | 0.01682478 ± 1.86859026 | 0.7239 |
| Lactate | 0.01928254 ± 0.08798780 | 0.09675085 ± 0.26694490 | −0.05031931 ± 0.39399175 | 0.7291 |
| Trigonelline | 0.02374965 ± 0.08399346 | 0.13526169 ± 0.34584082 | −0.06567746 ± 0.48509884 | 0.7405 |
| Glucose | 0.00491625 ± 0.04189189 | 0.03958419 ± 0.09995621 | 0.12869613 ± 0.59905361 | 0.5960 |
| Urea | 0.25569148 ± 1.79448506 | 1.81532837 ± 5.54362472 | −1.11342165 ± 7.18541052 | 0.6830 |
| Phenylalanine | 0.00579840 ± 0.23872775 | 0.16557466 ± 0.52906930 | −0.17583641 ± 1.28293793 | 0.3153 |
| Hippurate | 0.12922105 ± 0.22979078 | 0.53681284 ± 1.45850899 | −0.32731966 ± 2.73430691 | 0.5870 |
| Formate | 0.00496132 ± 0.01400679 | 0.01507905 ± 0.03273125 | −0.00913938 ± 0.08648353 | 0.8986 |
| Metabolites | Accuracy AUC | CLAUC | Cutoff Point | Sensibility | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|---|---|
| Sarcosine (mg/mL) | 0.654 | 0.524; 0.783 | 0.043 | 65% | 69% | 83% | 47% |
| Trigonelline (mg/mL) | 0.644 | 0.518; 0.770 | 0.068 | 46% | 87% | 89% | 42% |
| Phenylalanine (mg/mL) | 0.644 | 0.513; 0.775 | 0.204 | 31% | 100% | 100% | 39% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurgel, A.M.C.; Batista, A.L.; Cavalcanti, D.M.L.d.P.; Magalhães, A.; Zantut-Wittmann, D.E. Sarcosine, Trigonelline and Phenylalanine as Urinary Metabolites Related to Visceral Fat in Overweight and Obesity. Metabolites 2024, 14, 491. https://doi.org/10.3390/metabo14090491
Gurgel AMC, Batista AL, Cavalcanti DMLdP, Magalhães A, Zantut-Wittmann DE. Sarcosine, Trigonelline and Phenylalanine as Urinary Metabolites Related to Visceral Fat in Overweight and Obesity. Metabolites. 2024; 14(9):491. https://doi.org/10.3390/metabo14090491
Chicago/Turabian StyleGurgel, Aline Maria Cavalcante, Aline Lidiane Batista, Diogo Manuel Lopes de Paiva Cavalcanti, Alviclér Magalhães, and Denise Engelbrecht Zantut-Wittmann. 2024. "Sarcosine, Trigonelline and Phenylalanine as Urinary Metabolites Related to Visceral Fat in Overweight and Obesity" Metabolites 14, no. 9: 491. https://doi.org/10.3390/metabo14090491
APA StyleGurgel, A. M. C., Batista, A. L., Cavalcanti, D. M. L. d. P., Magalhães, A., & Zantut-Wittmann, D. E. (2024). Sarcosine, Trigonelline and Phenylalanine as Urinary Metabolites Related to Visceral Fat in Overweight and Obesity. Metabolites, 14(9), 491. https://doi.org/10.3390/metabo14090491





