Abstract
Metabolic reprogramming is a critical pathogenesis of colorectal cancer (CRC), referring to metabolic disorders that cancer cells make in response to the stimulating pressure. Metabolic reprogramming induces changes in genetic material and promotes CRC progression and has been proven to be an efficient target of CRC. As natural products have garnered interest due to notable pharmacological effects and potential in counteracting chemoresistance, an increasing body of research is delving into the impact of these natural products on the metabolic reprogramming associated with CRC. In this review, we collected published data from the Web of Science and PubMed, covering the period from January 1980 to October 2023. This article focuses on five central facets of metabolic alterations in cancer cells, glucose metabolism, mitochondrial oxidative phosphorylation (OXPHOS), amino acid metabolism, fatty acid synthesis, and nucleotide metabolism, to provide an overview of recent advancements in natural product interventions targeting metabolic reprogramming in CRC. Our analysis underscores the potential of natural products in disrupting the metabolic pathways of CRC, suggesting promising therapeutic targets for CRC and expanding treatment options for metabolic-associated ailments.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in the world, which accounts for approximately 10% of all cancer cases [1]. As the second leading cause of cancer-related mortality worldwide, CRC seriously threatens people’s health and places an enormous economic burden on the world [2]. With the introduction and in-depth study of the tumor metabolism hypothesis that tumor cells preferentially utilize glycolysis even in the presence of oxygen, known as the Warburg effect, metabolic dysregulation is recognized as one of the fundamental hallmarks of cancer, in alignment with genomic instability, tumor immune evasion, and the tumor microenvironment [3,4]. Tumor cells experience various metabolic alterations to meet their energy needs for rapid growth and multiplication, thereby adjusting to the tumor microenvironment, like hypoxia [5,6]. In contrast to the main energy supply mode of normal cells—mitochondrial respiration and OXPHOS—the metabolic pattern of tumor cells is significantly regulated, including but not limited to aerobic glycolysis, increased de novo biosynthesis of fatty acids (FA), massive consumption of glutamine and hypermetabolism of nucleotides [7]. Targeting the metabolic heterogeneity of CRC cells is a promising therapeutic approach. 5-Fluorouracil (5-FU) targets nucleotide metabolism thymidylate synthase (TS) with excellent clinical results of CRC [8]. Although various metabolic enzymes inhibitors are currently under development, issues such as unstable efficacy and adverse events have limited their progress [9]. Therefore, the search for safe and effective alternative drugs has become imperative.
Natural products not only possess various advantages, such as abundant sources, multiple bioactivities, and diverse varieties, but also have demonstrated potential in CRC therapeutics [10,11]. In addition, natural products can regulate metabolism in various diseases such as obesity and diabetes [12,13,14]. Therefore, natural products serve as a valuable reservoir of candidate compounds for developing targeted metabolic enzyme inhibitors. Advancing research continues to substantiate the role of these natural products in remodeling the metabolic landscape of CRC cells, influencing both the metabolic flux and the enzymatic activities which are pivotal to the neoplastic phenotype, thereby refining their relevance in antitumor strategies [15,16].Therefore, this review outlines principal metabolic enzymes regulated in CRC and provides an overview of the impact of natural compounds on these metabolic enzymes, associated proteins, oncogenes, and intertwined pathways specific to CRC. In summary, we hope this article gives a reference for the screening of antitumor natural compounds with significant antimetabolic effects
2. Natural Products Regulate Multiple Metabolisms in CRC
Natural products regulate the synthesis and catabolism of diverse substances, such as glucose, amino acids, lipids, and nucleotides, thereby reducing CRC proliferation and invasion. The metabolic pathways include various metabolic enzymes, of which activities regulate substance metabolism in cells and determine the rate and direction of the entire metabolic pathway. This section seeks to encapsulate the impact of natural products on metabolic enzymes and metabolic products (Figure 1).
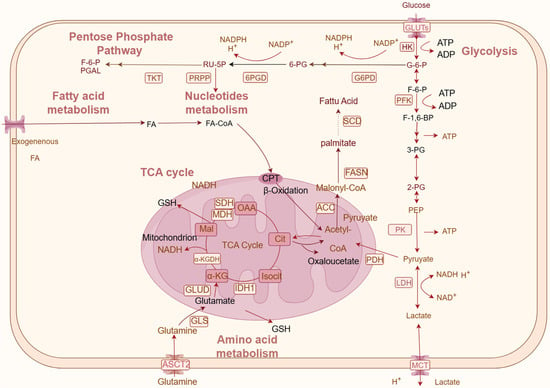
Figure 1.
Schematic of natural products regulating key metabolism factors and metabolism pathways in CRC. Metabolic pathways mainly consist of glucose metabolism (glycolysis, the PPP pathway, and the TCA cycle), lipid metabolism, amino acid metabolism, and nucleotide metabolism. Rectangular boxes indicate metabolic enzymes; arrows represent metabolic directions. OAA: oxaloacetate; Cit: citric acid; isocit: citric acid; α-KG: alpha-ketoglutarate; Mal: malic acid; 3-PG: 3-phosphoglyceric acid; RU-5P: ribulose 5-phosphate; 6-PG: 6-phosphogluconic acid (created on www.figdraw.com).
2.1. Natural Products Regulate Glucose Metabolism
In CRC, abnormal glucose metabolism is a common alteration, characterized by a preference for increased glycolysis for energy production, which disrupts the balance of the tricarboxylic acid (TCA) cycle [17,18]. Furthermore, the pentose phosphate pathway (PPP), which supplies ribonucleotide and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) as required, was also enhanced in CRC cells [19]. Such a metabolic reconfiguration not only satiates the energy demands of proliferating cancer cells but also provides essential macromolecules including lipids, amino acids, nucleic acids, and abundant biosynthetic intermediates for biosynthesis [20]. Lactate, another metabolite of glycolysis, is excreted to the extracellular matrix by MCT. The extracellular accumulation of lactate results in the tumor microenvironment being characterized by hypoxia and acidity, propelling tumor growth, invasion, and metastasis [21].
The control of glucose metabolism is contingent on the activities of enzymes critical to glucose metabolism, including key glucose transporter (GLUT) and monocarboxylate transporter (MCTS) glycolysis enzymes, such as phosphofructokinase (PFK), hexokinase (HK), lactate dehydrogenase (LDHA), pyruvate kinase 1/2 (PKM1/2), and isocitrate dehydrogenase (IDH) (Figure 1). Inhibition of glycolytic enzyme activity can reduce glycolytic flux, diminish glucose uptake, and lactate production in CRC cells, thereby slowing CRC progression (Table 1).
2.1.1. Natural Products Regulate Glycolysis
One molecule of glucose is metabolized into two molecules of pyruvate in glycolysis, serving as the common initial step for both anaerobic and aerobic glucose oxidation [22]. HK is a key enzyme that catalyzes the first rate-limiting step in the phosphorylation of glucose to generate glucose-6-phosphate (G-6-P). There are five distinct isoforms within the mammalian HK family: HKI, HKII, HKIII, HKIV, and HK domain-containing protein 1 (HKDC1). Among them, the isoenzyme HK2 is most relevant for energy metabolism in tumor cells, which is closely associated with the initiation and progression of tumorigenesis [23,24,25]. PFK1 acts as a critical glycolytic agent regulating the second rate-limiting step of glycolysis, transforming fructose-6-phosphate (F-6-P) into fructose-1, 6-bisphosphate (F-1,6-BP) [26]. PK is a key controller in the process of glycolysis, and catalyzes the formation of pyruvate from the substrate, among which the M2 subtype PKM2 confers a growth advantage to tumor cells, allowing them to prosper and adjust in their surrounding environment [27].
The GLUT is the critical rate-determining enzyme in glucose metabolism, orchestrating the transmembrane transport of glucose [28]. Thirteen distinct GLUT isoforms have been identified. Structural variances among the GLUT isoforms give rise to distinct functional attributes and distribution patterns [29]. Notably, GLUT1-5 have been extensively characterized in terms of their structural and functional nuances. GLUT1 and GLUT3, which exhibit a heightened affinity for glucose, serve as the foundational transporters mediating cellular glucose uptake [30,31]. These transporters are ubiquitously expressed throughout the organism and exhibit a profound association with neoplastic cells.
LDHA plays a crucial role as an active enzyme in the glycolytic cascade, catalyzing the conversion of pyruvate to lactate under anaerobic conditions [32]. Lactate not only emerges as an anaerobic byproduct but also acts as a catalyst within the tumor microenvironment, where an acidic milieu confers a proliferative advantage to neoplastic cells [33]. The MCT represents a significant molecular entity crucial for the maintenance of glycolytic metabolism, exhibiting dual functionality as both a lactate transporter and a pH regulator [34]. The MCT family encompasses a total of 14 members, with the initial four (MCT1-4) identified as facilitators of proton-coupled transport of monocarboxylic acids across the plasma membrane [35]. Inhibition of MCT disrupts cellular and extracellular balance; that is, it affects pH homeostasis, induces apoptosis, and reduces tumor angiogenesis, invasion, and metastasis [36].
Wogonin, a flavonoid isolated from Scutellaria baicalensis Georgi., has shown potent antitumor effects [37]. Wogonin downregulates the expression of GLUT1 and the activities of key glycolytic enzymes (PGM, HK2, GLUT1, and LDHA) and PDHK1 [38]. Although PDHK itself is not involved in the chemistry of glycolysis, it is linked to glycolysis because it regulates the subsequent metabolic pathway of pyruvate produced from glycolysis. PDHK acts as a regulator, influencing the fate of pyruvate after glycolysis, rather than being directly involved in its breakdown [39]. Saponin monomer 13 of the dwarf lilyturf tuber (DT-13) is the major steroidal saponin in Liriope muscari (Decne). DT-13 significantly downregulates GLUT1 to inhibit glycolysis and alter energy homeostasis in cancer cells [15]. DT13 showed better activity against two CRC cells (SW480, COLO205) compared to 5-FU (Table 1). Diosgenin (DSG) mediates GLUT proteins (GLUT3 and GLUT4) to reduce aerobic glycolysis, and downregulates pyruvate carboxylase (PC), increasing the proximity of CRC cells to hypoxic glycolysis and OXPHOS [40]. DSG exhibits better drug toxicity against SW1116 cells compared to 5-FU, while demonstrating weaker toxicity towards normal cells (Table 1). The apple polyphenol phloretin (Ph) inhibits tumor cell invasion by inhibiting the activity of GLUT2 and inducing HNF6-mediated p53 activation [41]. Sophora flavescens Aiton(also known as “Ku Shen”) is a Chinese traditional medicinal herb, with its cold nature and bitter taste, and traditionally efficacy are heat-clearing and dampness-drying. It can be utilized for the treatment of symptoms associated with CRC, including rectal bleeding [42]. Matrine, an alkaloid extracted from the root of Kushen, exhibits outstanding pharmacological activity, including cardiovascular and cerebrovascular protection, anticancer, and anti-inflammatory activity [43,44,45,46]. Matrine reversed the Warburg effect by downregulating Hypoxia-inducible factor 1-alpha (HIF-1α) and inhibiting the expression of its downstream target (GLUT1, HK2, and LDHA) [47]. Additionally, it demonstrates excellent anti-CRC activity comparable to that of 5-FU (Table 1). Oxymatrine inhibits cell metastasis possibly by blocking aerobic glycolysis, which depends on dual inhibition of PKM2 and downregulation of GLUT1 expression [48].
Parthenolide (PT), an efficacious compound isolated from medicinal plants, has profound anti-inflammatory properties [49]. Derivatives of PT impeded the nuclear translocation of PKM2, inducing a change in metabolic pattern from aerobic glycolysis to oxidative phosphorylation, and thereby exerting antiproliferative effects on HT29 and SW480 cells [50]. PT exhibits lower IC50 values compared to 5-FU in HCT116, HT29, and SW480 cell lines, as well as in normal cells. This suggests high cytotoxicity and potential off-target effects. Kaempferol (KP) is a flavonol that is extracted from many medicinal plants. It is recognized for diversiform and outstanding pharmacological activities [51,52]. Kaempferol promotes the expression of miR-339-5p, which in turn, directly targets hnRNPA1 and PTBP1, diminishing the expression of PKM2 and inducing the expression of PKM1, thus inhibiting glycolysis and the growth of colorectal cancer [53].
Tannic acid (TA), is a natural polyphenolic acid found predominantly in grapes and green tea, that possesses potent antioxidant and anticancer properties [54]. TA inhibits CRC cell proliferation by binding to lysine 433, triggering PKM2 tetramer dissociation and blocking PKM2 metabolic activity [55]. Berberine, an isoquinoline alkaloid extracted from the herb Coptis chinensis Franch., displays antiproliferative and anticancer effects [56]. Berberine suppress the enzymatic activity of PKM2 and downregulated glycolysis [57]. Among other plants, apigenin (AP), which was identified in celery, inhibits glycolysis in cancer cells by inhibiting the activity and expression of PKM2. It reduces the PKM2/PKM1 ratio in HCT116 cells by interrupting the β-catenin/c-Myc/PTBP1 signaling pathway [58]. Resveratrol is a naturally occurring antimicrobial polyphenol (phytoalexin) present in a variety of plants. The principal sources of natural resveratrol include grapes, especially specific cultivars such as Vitis amurensis and certain Rootstock varieties, as well as the medicinal plant Polygonum cuspidatum [59,60,61]. Resveratrol downregulates PKM2 expression by upregulating micro RNA326 (miR-326), thereby exerting antitumor effects through the induction of endoplasmic reticulum stress and mitochondrial fission [62]. Additionally, Resveratrol decreases glycolytic activity by inhibiting the activity of pyruvate kinase (PK) and LDH in Caco2 cells [63]. IC50 of berberine, AP and resveratrol are higher than 5FU, suggesting the capacity of these compounds to inhibit the proliferation of CRC cells may not related to cell toxicity, but regulation of metabolism.
Dioscin is a natural steroidal saponin extracted from a variety of herbs. Dioscin inhibits glycolysis by inducing Skp2 ubiquitination and inhibiting HK2 in tumor tissues, which is dependent on the Skp2-Akt-HK2 axis [64]. Wu found that dioscin promotes the interaction of c-Myc and FBW7 to promote c-myc ubiquitination, which in turn inhibits the expression of HK2 to reduce tumor glycolysis and induce cell apoptosis [65]. Curcumin inhibits the glycolytic activity of tumor cells by inhibiting the expression and activity of HK2 in HCT116 and HT29 cells, and induces the dissociation of mitochondrial HK2 through AKT phosphorylation, leading to mitochondria-mediated apoptosis [24]. Xanthohumol, a polyphenol chalcone from Humulus lupulus L., has been proven to have good antitumor activity [66]. Xanthohumol inhibits glycolytic activity by mediating Akt and promotes apoptosis through the downregulation of HK2 expression [67]. Thymoquinone, an important bioactive phytochemical component of Nigella sativa L., has antioxidation, antimicrobial, anti-inflammatory, metabolic syndrome, and other effects [68,69,70]. It inhibits the rate-limiting HK2 of glycolysis by regulating the PI3K/AKT axis [71], and shows greater cell toxicity to SW480 cell.
Oridonin, a diterpenoid extracted from Isodon rubescens (Hemsl.), which exhibits excellent activity of suppress multiple CRC cells (Table 1), regulates AMPK-related GLUT1 and MCT1 to affect glucose supply and lactate production, thereby affecting glucose metabolism and inducing autophagy through metabolic pathways [72]. Triterpenoids (TER) of Rhus chinensis Mill., with unsignificant cell toxicity (Table 1), dose-dependently inhibited the expression of the glucose metabolism enzymes GLUT1, LDHA, PKM2, and MCT1, which mediate lactate transport, and affect the growth and proliferation of tumor cells by affecting the ASIC2-mediated calcineurin/NFAT1 pathway [73]. Lycorine is a crystalline alkaloid extracted from the Lycoris radiata Herb. that has multiple pharmacological effects, including anti-inflammatory, antifungal, antiviral, and antitumor activities [74,75,76]. Lycorine interferes with the interaction between IDH1 and the deacetylase SIRT1, thereby significantly promoting the acetylation of IDH1 and driving the oxidative stress-dependent imbalance of mitochondrial dynamics to exert an anti-CRC effect [77]. Xanthatin is a bioactive sesquiterpene lactone isolated from Xanthium strumarium L. The inhibition of glycolysis by xanthatin may be related to the reduction in GLUT1 and MCT4 mRNA and protein levels. It promotes complex II activity and OXPHOS, leading to mitochondrial damage and resulting in cell death. Xanthatin inhibited the phosphorylation of mTOR, 4E-binding protein 1 (4E-BP1), and c-myc in HT-29 cells [78]. β-Caryophyllene (BCP) is a natural sesquiterpene extracted from various plants, such as cloves and cinnamon. BCP inhibits ART1-induced glycolysis and increases ATP and lactate production through the AKT/mTOR pathway, thereby increasing the expression of PKD1 and LDHA and affecting the proliferation and apoptosis of CT26 cells under high-glucose conditions [79]. Water extracts from the seeds of Myristica fragrans Houtt. (MF) inhibits the Warburg effect and cell growth by downregulating LDH enzyme activity in HT29 cells [80].
2.1.2. Natural Products Regulate the TCA Cycle and Oxidative Phosphorylation
The TCA cycle represents the terminal metabolic pathway for the three principal nutrients—carbohydrates, lipids, and amino acids—and serves as the central hub interlinking the metabolism of these macromolecules. The TCA cycle is instrumental in generating metabolic intermediates and NADH to support cellular proliferation [81]. Pyruvate produced from glycolysis is converted into acetyl-CoA by the catalytic action of the pyruvate dehydrogenase (PDH) complex, after which it enters the TCA cycle. Except for the reactions catalyzed by citrate synthase and the oxoglutarate dehydrogenase (OGDH) complex, the majority of reactions within the cycle are reversible, with most intermediates capable of anaplerotic reactions. Fluxes through the TCA cycle are of paramount importance in metabolism and are frequently dysregulated in cancers, including CRC [82]. The investigation into enhancing pyruvate-mediated mitochondrial oxidation, modulating TCA cycle enzymes, and perturbing respiratory chain complexes has identified potential therapeutic targets [83]. The pyruvate dehydrogenase complex (PDC) is crucial for converting pyruvate to acetyl-CoA, a key substrate for the TCA cycle, de novo lipogenesis, and protein acetylation [84]. Notably, this complex is subject to negative regulation by pyruvate dehydrogenase kinases (PDKs), which are frequently overexpressed in cancer cells [85].
Following resveratrol intervention, a decrease in PDH activity has been observed in Caco-2 and HCT116 colorectal cancer cell lines, indicating that resveratrol-induced glycolytic reprogramming may occur through the modulation of PDP1 gene expression [86]. Withania somnifera is a traditional medicinal plant, of which main active compounds include Withanolides (Withaferin A, Withanolide A, Withanone), sitoindosides, Withanosides, and other alkaloids [87], and they have significant anticancer activity, antimicrobial, anti-inflammatory, and cardioprotective effects [88,89,90,91]. Alcoholic extract of withania somnifera increased the activities of key TCA cycle enzymes, including isocitrate dehydrogenase (ICDH), succinatede hydrogenase (SDH), malate dehydrogenase (MDH), Alpha-ketoglutarate dehydrogenase complex (α-KGDH) and four complex electron transport chain enzymes to normalize the levels of these enzymes in azoxymethane-induced experimental mice [92]. Pyruvate dehydrogenase kinase 1(PDK1) phosphorylates the PDH complex to inactive it, which is the critical point connecting aerobic glycolysis and the diaminobutoxy and TCA cycle (Figure 1). Hemistepsin A (HsA) is a sesquiterpene lactone isolated from the medical herb Hemisteptia lyrata (Bunge) Fisch. & C.A.Mey. HsA exhibits significant cytotoxicity against CRC cells while demonstrating relatively low toxicity towards normal cells (Table 1). HsA inhibits the activity of PDK1 by interfering with the interaction between PDK1 and the L2 lipoamide domain of PDH-E2, thus switching the metabolic pattern from glycolysis to OXPHOS. Therefore, the growth of CRC cells is suppressed, and mitochondrial ROS-mediated apoptosis is induced [93]. Diaminobutoxy-substituted isoflavonoid (DBI-1), a complex I inhibitor, has good synergy with the GLUT1 inhibitor BAY-876 to inhibit the survival of CRC cells both in vitro and in vivo [94]. DBT-1 exhibits superior inhibitory effects on CRC cell proliferation compared to 5-FU.
2.1.3. Natural Products Regulate Pentose Phosphate Pathway
In addition to the aerobic and anaerobic decomposition of glucose, PPP also occurs in tissues involved in active lipid synthesis and proliferation (such as liver, adipose tissue, bone marrow, and tumor tissues), and is an important pathway for glucose oxidative decomposition [95]. The PPP starts from the intermediate product of glycolysis, G-6-P, to bypass G-6-P through two stages—oxidation and group transfer—ultimately producing F-6-P and glyceraldehyde 3-phosphate, thus returning to glycolysis. The PPP does not produce ATP, but it produces the phosphonic ribose and NADPH, which are essential for nucleic acid biosynthesis and various metabolic pathways. This pathway provides a carbon source and a hydrogen donor for a multitude of anabolic reactions within the body. [96]. In addition, NAPDH is not only involved in lipid cholesterol amino acid metabolism, but also an essential substance for maintaining the glutathione reduction state, which is highly important for ROS clearance [97]. Thus, the PPP is necessary to promote the growth of CRC cell growth and to produce NADPH to overcome oxidative stress.
6-phosphogluconate dehydrogenase (G6PD), 6-phosphogluconate lactonase (PGLS), and 6-phosphogluconate dehydrogenase (6PGD) are key enzymes involved in the oxidation of the PPP (Figure 1). Transketolase (TKT) and transaldolase (TAL) are the key enzymes involved in radical metastasis, and are closely related to tumor growth [98]. Sánchez-Tena demonstrated that the catechin derivative epicatechin gallate (ECG) significantly decreased glucose uptake and glutamate accumulation while concurrently augmenting glutamine consumption. The activity of critical enzymes associated with the PPP, namely, TKT and G6PD, was found to be inhibited [99]. At a concentration of 50 μM, resveratrol targeted the PPP and the talin–pFAK axis, effectively inhibiting CRC cell proliferation and inducing apoptosis. This inhibitory effect was concomitant with a decrease in the activity and/or protein expression levels of G6PD and TKT, culminating in the suppression of the PPP. Moreover, the impact of resveratrol was notably associated with alterations in both glycolytic and PPP activities [100]. Halofuginone (HF), a febrifugine derivative sourced from the traditional medicinal plant Dichroa febrifuga Lour., which significantly inhibited the viability of CRC cells, was observed to inactivate G6PD within the PPP posttreatment. This led to a reduction in NADPH levels, a decrease in glycolytic flux, and a decrease in the rate of glucose-derived TCA flux. Furthermore, CRC cells treated with HF exhibited downregulation of GLUT1 and HK2, ultimately resulting in the inhibition of cell proliferation and the induction of reactive oxygen species production [101].

Table 1.
Natural products regulate glucose metabolism enzymes in CRC.
Table 1.
Natural products regulate glucose metabolism enzymes in CRC.
| Chemical Class | Bioactive Compounds | Medicinal Plant | Cancer Model | IC50 | Metabolic Regulation | Targets | Potential Mechanisms | References |
|---|---|---|---|---|---|---|---|---|
| Flavonoids | Wogonin | Scutellaria baicalensis Georgi. | HT-29, HCT116 | - | Glycolysis | GLUT1, PGM, HK2, PDHK1, LDHA | Increased the expression of p53 and downregulates key glycolytic enzymes to inhibit colorectal cancer glycolysis | [38] |
| Flavonoids | Apple Polyphenol Phloretin | Malus pumila Mill. | HT29, COLO205, xenograft mouse model | - | Glucose metabolism | GLUT2 | Inhibited GLUT2 protein expression and induces Hepatocyte nuclear factor 6 (HNF6) to activate GLUT2 and p53 | [41] |
| Flavonoids | Silybin | Silybum marianum(L.) Gaertn. | LoVo WT LoVo DOX | - | Glucose metabolism | GLUT1 | Targeted GLUTs to increase doxorubicin sensitivity and elude drug resistance | [102] |
| Flavonoids | Kaempferol | Kaempferia galanga L. | HCT116, DLD1 | HCT116: 63.0 ± 12.9 μM DLD1: 98.3 ± 15.9 μM | Glycolysis | PKM2, hnRNPA1 | Inhibited glycolysis and colon cancer growth by modulating miR-339-5p-hnRNPA1/PTBP1-PKM2 axis | [53] |
| Flavonoids | Apigenin | Thymus mongolicus Ronniger | HCT116, HT29, DLD1 | HCT116: 27.9 ± 2.45 μM HT29: 48.2 ± 3.01 μM DLD1: 89.5 ± 4.89 μM | Glycolysis | PKM2 | Bonded to PKM2 and inhibited PKM2 activity, and reduced PKM2/PKM1 by blocking β-catenin/c-Myc/PTBP1 signaling pathway | [58] |
| Flavonoids | Xanthohumol | Humulus lupulus L. | HCT116, HT29, SW620, SW480, LOVO, Xenograft mouse model | Glycolysis | HK2 | Reduced Akt activity, and inhibited HK2 expression and glycolysis | [67] | |
| Flavonoids | Quercetin | Crataegus pinnatifida Bge. | HCT15, RKO | HCT15:142.7 μM RKO:121.9 μM NCM460: >200 μM | Glycolysis | MCT1, MCT4 | Decreased glucose consumption and lactate acid generation by inhibiting MCT, and enhanced the cytotoxicity of 5-FU | [103] |
| Isoflavonoid | Diaminobutoxy-substituted Isoflavonoid (DBI-1) | semisynthetic derivatives | LS174T Pt2377 Xenograft mouse model | LS174T: 1.2 μM Pt2377: 1.4 μM | TCA cycle | GLUT1 mitochondrial complex I | Inhibits mitochondrial complex I, and combined with GLUT1 inhibitor, BAY-876, synergistically inhibited colorectal cancer | [94] |
| Steroidal saponins | Saponin monomer 13 of the dwarf lilyturf tuber (DT13) | Liriope muscari (Decne.) | HCT15,HT29, COLO205, HCT116, SW480,SW620 Orthotopic implantation mouse model, C57BL/6J APCmin mice model | HCT15: 7.53 ± 0.15 μM HT29: 9.05 ± 1.65 μM COLO205: 8.36 ± 0.04 μM HCT116: 8.75 ± 1.58 μM SW480: 27.72 ± 10.96 μM SW620: 22.39 ± 15.17 μM | Glycolysis | GLUT1 | Downregulated GLUT1 and activated AMPK to inhibit glycolysis, and inhibited the phosphorylation of p-mTOR, p-P70S6K, and p-4EBP1 | [15] |
| Steroidal saponins | Diosgenin | Dioscorea opposita Thunb. | SW1116, RKO, xenograft mouse model | SW1116: 21.15 ± 0.43 μM RKO: 24.06 ± 1.37 μM NCM460: 69.76 ± 1.28 μM | Glycolysis | GLUT2,3,4, PC | Disturbed the aerobic glycolysis and reduce ATP generation | [40] |
| Steroidal saponins | Dioscin | Dioscorea opposita Thunb. | HT29, HCT116, SW620, xenograft mouse model | - | Glycolysis | HK2 | Induced Cdh1-mediated Skp2 degradation, thereby inhibiting HK2 expression and glycolysis | [64] |
| Steroidal saponins | Dioscin | Dioscorea opposita Thunb. | HCT116, HT29 | - | Glycolysis | HK2 | Inhibited glycolysis by restraining HK2, which relates to FBW-7-mediated c-myc degradation | [65] |
| Alkaloids | Matrine | Sophora flavescens Aiton | HCT116, SW620, xenograft mouse model | HCT116: 6.1 μM SW620: 4.9 μM | Glycolysis | GLUT1, HK2, LDHA | Inhibited expression of HIF-1α and its downstream targets to downregulate glycolysis | [47] |
| Alkaloids | Oxymatrine | Sophora flavescens Aiton | HT29, HCT116, hepatic metastasis mouse model of colorectal cancer | - | Glycolysis | PKM2, GLUT1 | Double inhibition of PKM2 and downregulation of GLUT1 expression to block aerobic glycolysis | [48] |
| Alkaloids | Berberine | Coptis chinensis Franch. | HCT116 | 63.6 ± 3 μM | Glycolysis | PKM2 | Inhibited PKM2 enzyme activity | [57] |
| Alkaloids | Lycorine | Lycoris radiata (L’Hér.) Herb. | HCT116, HT29, xenograft mouse model | - | TCA cycle | IDH1 | Promoted the acetylation of IDH1 to drive the imbalance of mitochondrial dynamics | [77] |
| Alkaloid derivatives | Halofuginone | Dichroa febrifuga Lour. | SW480, HCT116, xenograft mouse model | SW480: 24.83 nM HCT116: 5.82 nM | PPP | G6PD | Inhibited the activity of G6PD and reduced the level of NADPH by regulating Akt/mTORC1 signaling pathway, thereby inhibiting glucose uptake and glycolysis in CRC cells | [101] |
| Quinones | Curcumin | Curcuma longa L. | HCT116, HT29 | - | Glycolysis | HK2 | Inhibited glycolysis and induces mitochondrial-mediated apoptosis through the regulation of HK2 | [24] |
| Polyphenols | Tannic acid | Rhus chinensis Mill. | HCT116, DLD1 | DLD1: 53.6 μM HCT116: 43.1 μM FHC: >100 μM | Glycolysis | PKM2 | Selectively inhibited the pyruvate kinase activity of PKM2 | [55] |
| Non-flavonoid phenolic compounds | Resveratrol | Polygonum cuspidatum Siebold & Zucc. | DLD1 | DLD1: 75 ± 4.54 μM | Glycolysis | PKM2 | Inhibited PKM2 expression by upregulating miR-326 | [62] |
| Non-flavonoid phenolic compounds | Resveratrol | Polygonum cuspidatum Siebold & Zucc. | HCT116, Caco2 | HCT116: 50 μM Caco2: 131 μM | Glycolysis | PK | Decreased glucose consumption and the expression of glycolytic enzymes to induce cell apoptosis | [63] |
| Non-flavonoid phenolic compounds | Resveratrol | Polygonum cuspidatum Siebold & Zucc. | Caco2, HCT116 | - | TCA cycle | PDH | Targeted the PDH complex, leading to enhanced PDH activity | [86] |
| Non-flavonoid phenolic compounds | Resveratrol | Polygonum cuspidatum Siebold & Zucc. | HT29 | - | PPP | G6PD, TKT, PGD | Regulated TKT, G6PD to inhibit pentose phosphate pathway | [100] |
| Polyphenols | Epicatechin gallate(ECG) | Acacia catechu (L. f.) Willd. | HT29 | - | PPP, fatty acid metabolism | TKT, G6PD | Regulated de novo synthesis of fatty acids and the PPP way. Inhibited key enzymes | [99] |
| Sesquiterpenes | Parthenolide derivatives | Tanacetum parthenium (L.) | HT29, SW480, HCT116 | HT29: 0.66 μM SW480: 0.22 μM HCT116: 1 μM NCM460: 2 μM | Glycolysis | PKM2 | Impeded the nuclear translocation of PKM2, fostering a metabolic shift from aerobic glycolysis to oxidative phosphorylation | [50] |
| Sesquiterpene lactones | Xanthatin | Xanthium strumarium L. | HT29, HCT116 | - | Glycolysis | GLUT1, MCT4 | Reduced Glut1 and MCT4 mRNA and protein levels by inhibiting the phosphorylation of mTOR, 4E-binding protein 1 (4E-BP1) and c-myc | [78] |
| Sesquiterpene lactones | Hemistepsin A e | Hemisteptia lyrata (Bunge) Fisch. & C.A.Mey. | DLD1, CT26, murine allograft model | DLD1: 10.31 ± 0.5536 μM CT26: 9.27 ± 1.497 μM Detroit 551: 52.72 ± 9.042 μM | TCA cycle | PDK1 | Inhibited PDK1 activity and decreased lactate production, thus promoting and switching metabolic patterns from glycolysis to OXPHOS | [93] |
| Diterpenes | Oridonin | Isodon rubescens (Hemsl.) | HCT15, COLO205, HCT116, RKO, SW480, SW620, xenograft mouse model | HCT15: 14.105 μM COLO205: 10.272 μM HCT116: 32.977 μM RKO:20.552 μM SW480: 13.373 μM SW620: 11.774 μM | Glycolysis | GLUT1, MCT1 | Altered energy homeostasis in cancer cells through downregulating GLUT1 and MCT1 by inhibiting AMPK | [72] |
| Triterpenoids | Rhus chinensis triterpenoids extract | Rhus chinensis Mill. | SW620, HCT116 | SW620: 112.3 μg/mL HCT116: 89.6 μg/ml | Glycolysis | GLUT1, LDHA, PKM2, MCT1 | Inhibited the expression of glucose metabolism enzymes to mediate lactate transport, and affect the ASIC2-mediated calcineurin/NFAT1 pathway | [73] |
| Bicyclic sesquiterpenoids | β-caryophyllene | yzygium aromaticum (L.) Merr. & L.M.Perry | CT26, xenograft mouse model | - | Glycolysis | ART1 | Inhibited ART1-induced glycolysis through AKT/mTOR pathway | [79] |
| Quinones | Thymoquinone | Nigella sativa L. | HCT116, SW480 | HCT116: 21.71 μM SW480: 20.53 μM | Glycolysis | HK2 | Inhibited HK2-mediated glycolytic metabolism via the PI3K-AKT/HK2 pathway | [71] |
| water extracts | Myristica fragrans Houtt. | HT29 | 31.8 ± 1.8 μg/mL | Glycolysis | LDHA | Downregulated LDHA enzyme activity, thereby decreasing lactate production and glucose uptake | [80] | |
| Alcoholic extract | Withania somnifera (L.) Dunal | Azoxymethane-induced colon cancer animals | - | TCA cycle | ICDH, SDH, MDH,α-KGDH | Increased activities of TCA cycle key enzymes and four electron transport chain complex enzymes | [92] |
Colorectal cancer cell: SW480, SW620, HCT116, HT29, HCT15, COLO205, RKO, CT26, DLD1, SW1116, LS174T, and Pt2377. Normal human fibroblast cells: Detroit 551. Normal human colon mucosal epithelial cell: NCM460. IC50 of 5-fluorouracil (5FU): colorectal cancer cell IC50:HCT116 2.5 μg/mL; HT29: 0.23 μM, DLD1: 6.5 μM; SW480: 113.10 μg/mL; SW620: 4 μM; HCT8: 52.30 ± 6.80 μM; HCT15: 9.49 ± 2.43 μg/mL; COLO205: 68.1 ± 4.8 μg/mL; SW1116: >400 μg/Ml; LS174T: 3.436 μM. Normal human colon mucosal epithelial cell NCM460: >100 μg/mL.
2.2. Natural Products Regulate Amino Acid Metabolism
Amino acids are one of the main energy supply substances in the body. The energy and substances produced in the metabolic process of synthesis and decomposition of amino acids are necessary to maintain body activity and constitute the body. In addition, amino acid metabolism is one of the three major metabolic processes of the body, and involves glutamine metabolism, serine and glycine metabolism, cysteine metabolism, and other pathways [104]. In addition to meeting the energy requirements, tumor cells have more vigorous nutritional requirements than normal cells, because they require more amino acids for biosynthesis. In addition, amino acids can maintain the REDOX state, maintain tumor cell survival, and prevent apoptosis. Studies have shown that abnormal amino acid metabolism plays a crucial role in tumor growth, the tumor microenvironment, and immunity [105].
Glutamine is a conditionally essential amino acid and an important substance for the growth and metabolism of tumor cells, which is required for tumor cells survival in vitro [106]. Glutaminase (GLS) catalyzes the conversion of glutamate and ammonia into other intermediate products to supply nitrogen for other amino acids, nucleic acids and hexosamines. Furthermore, it is metabolized through the TCA cycle to replenish depleted intermediates and facilitate energy production [107] In addition, it is an alternative carbon donator for the synthesis of lipid, and it participates in the synthesis of Glutathione (GSH), a major cellular antioxidant. Glutamine is one of the main energy sources for some tumor cells, and tumor cells consume large amounts of glutamine [108]. Thus, glutamine metabolism or glutaminolysis is equally important for reprogramming CRC cell metabolism by supporting ATP, glutamine metabolism is a promising therapeutic target, and current drug strategies specifically target the inhibition of glutaminase [109].
Epicatechin gallate (ECG) exerted antitumor effects by reducing glucose uptake, increasing glutamine consumption, and decreasing glutamate enrichment, which changes glutamate metabolism [99]. Curcumol has been identified as a potent agent that promotes the degradation of HIF1α, effectively inhibiting the epithelial-mesenchymal transition (EMT) and consequently reducing the invasive and migratory capabilities of CRC cells. Moreover, it impeded the GLS1-mediated metabolism of glutamine, a critical pathway that fuels the proliferation of CRC cells [110].
The MDR in SW620/Ad300 cells is related to the upregulation of spermine synthesis, and D-glutamine metabolism. Curcumin can inhibit spermine and spermidine biosynthesis by decreasing ornithine decarboxylase (ODC) expression, thus decreasing the antioxidative stress and P-gp transport monomer activity of SW620/Ad300 cells [111]. The antitumor mechanism of triptolide may depend on the correction of branched-chain amino acid metabolism disorders, serine/glycine/methionine biosynthesis, and ketone body metabolism disorders [112]. Lobetyolin induces apoptosis and inhibits glutamine metabolism through the ASCT2 signaling pathway in HCT116 cells, leading to a reduction in glutamine-related biomarkers and enhancement of the apoptotic process [113] (Table 2).

Table 2.
Natural products regulate amino acid metabolism enzymes in CRC.
2.3. Natural Products Regulate Lipid Metabolism
Lipids serve as one of the principal energy sources for the human body and play key roles in both energy and synthetic metabolism for energy supply and storage. Owing to their vast diversity and intricate molecular structures, lipids are important substances that constitute cells and maintain life activities [114]. Lipid metabolism, encompassing the mechanisms of lipid uptake, biosynthesis, and catabolism, is critical for cellular homeostasis. Dysregulation of these processes, manifesting as augmented lipid assimilation, escalated endogenous de novo fatty acid synthesis, intensified fatty acid oxidation, and excessive cholesterol accumulation, has been intimately associated with tumorigenesis. Such metabolic aberrations are known to foster neoplastic growth and advancement [115]. In neoplastic cells facing augmented metabolic demands, surplus lipids and cholesterol are sequestered within lipid droplets. These cells catalyze the de novo biosynthesis of fatty acids (FAs) via enzymatic activation, ensuring an uninterrupted supply requisite for membrane biogenesis, energy generation, and posttranslational protein modifications. Throughout oncogenesis, the fluctuating availability of nutrients necessitates the interplay between oncogenic signaling and lipid metabolic pathways, orchestrating a synergistic modulation that sustains cancer cells’ rapid proliferation, survival, migration, invasion, and metastatic potential. This adaptive metabolic reprogramming is intricately linked to the upregulation of specific signaling cascades, in addition to alterations in pertinent metabolic enzymes and transcription factors [116]. Currently, the molecular constituents of lipid metabolism, including enzymes and transcription factors, are recognized as prospective pharmacological targets in oncology.
FAs constitute the principal components of lipids and are synthesized de novo by an ensemble of enzymes including fatty acid synthase (FASN), ATP-citrate lyase (ACLY), and acetyl-CoA carboxylase (ACC) [117]. Lipid metabolic pathways and related enzymes are potential targets for tumor diagnosis and treatment [118]. ASN, which is the critical target of various natural products that inhibit fatty acid synthesis and CRC progression, plays a crucial role in the synthesis of FAs. Oridonin can effectively inhibit the mRNA and protein expression of FAS and SREBP1 in human CRC cells, subsequently reducing the transcriptional activity of the FAS promoter, which contains the sterol regulatory element binding protein 1 (SREBP1) binding site. This inhibitions reduces cellular fatty acid levels, and increase the apoptosis of CRC cells [119]. RA-XII downregulated the expression of FASN and SCD, thereby inhibiting fatty acid synthesis and reducing fatty acid levels [120]. Berberine downregulated the expression of key lipogenesis enzymes (ACC, ACL, FASN) and inhibited lipid synthesis, which is related to the Wnt/β-catenin pathway [121]. PLA2G16, an adipocyte-specific phospholipase A2, commands the critical regulation of lipid metabolism, and serves as an essential rate-limiting enzyme in the biosynthesis of free fatty acids. Notably, PLA2G16 is implicated in the malignant progression of CRC, predominantly through the suppression of the Hippo signaling conduit—a guardian of cellular growth and organ size. Ginsenoside compound K (GCK), a saponin derived from traditional Chinese medicinal roots such as ginseng and Panax notoginseng, has proved that it has good pharmacological activities such as anti-inflammatory, antiatherosclerosis, neuroprotection, and antitumor effects [122,123,124]. GCK exhibited significant inhibitory effects on LOVO cells (Table 3). The anticarcinogenic potential of GCK is intrinsically linked to its inhibitory impact on PLA2G16 protein expression [125].
Fatty acids are dehydrogenated to form unsaturated fatty acids, and polyunsaturated fatty acid derivatives have important physiological functions. Trichothecin (TCN), a sesquiterpenoid found in the endophytic fungus herbaceous Maytenus hookeri Loes, reduces the production of unsaturated FAs by blocking SCD-1 activity and impairing tumor cell invasion and metastasis [126]. Short-chain fatty acids (SCFAs) are saturated fatty acids with a chain length of 1–6 carbon atoms that can maintain the integrity of the intestinal wall barrier and prevent intestinal inflammation. EPS-1 is a natural polysaccharide composed of glucose, mannose, galactose, and fructose, that has good antitumor and immune-enhancing effects [127]. EPS-1 changed the composition of the fecal microbiota and increased the concentration of total SCFAs in azomethane/dextran sulfate sodium (AOM/DSS) -induced colorectal cancer mice to inhibit the progression of CRC [128].
Mirabilite, a sulfate class mineral traditionally utilized in clinical settings as Chinese herbal medicine, is recognized for its cathartic and laxative properties. Contemporary research has substantiated its pharmacological activities, including anti-inflammatory effects, immunomodulation, and the facilitation of small intestinal motility [129,130]. Lipidomic studies have revealed that extracts of mirabilite modulate retinol metabolism, propionate metabolism, and glycerophospholipid metabolism, with seven significant biomarkers identified. This evidence suggested that mirabilite extracts may exert anti- CRC effects by influencing lipid metabolism pathways [131].
CRC cells absorb fatty acids from surrounding fat cells and promote oxidative metabolism, consequently providing raw materials for the growth of CRC cells. Increasing fatty acid oxidative breakdown to reduce fatty acid levels in CRC cells is an approach to inhibit CRC progression. Oroxylin A inactivates HIF-1α and modulates fatty acid metabolism, resulting in the downregulation of lipid uptake and synthesis. Moreover, it enhances fatty acid oxidation, consequently reducing intracellular fatty acid levels [132].
The bulb of Allium cepa L., commonly known as onion, is replete with an array of phytoconstituents such as volatile oils, phenylpropanoids, flavonoid complexes, and steroidal saponins. Flavonoids constitute a principal bioactive cohort within onions and have multifaceted pharmacological properties, including antineoplastic, and anti-inflammatory effects [133,134]. Related studies have shown that these flavonoid compounds enhance lipid metabolic pathways, substantively decreasing the levels of apolipoprotein B— a critical modifier of low-density lipoprotein (LDL) and total cholesterol (TC)—thereby inhibiting the progression of CRC [135]. In ApcMin/+ mice, kaempferol may reverse the reduction in BA levels by increasing the CYP27A1 and CYP8B1 protein levels in the liver, upregulates the expression of BSEP and promotes hepatic efflux of BA, thereby regulating BA homeostasis, increasing FXR protein expression, and inhibiting Wnt/β-catenin pathway activation [52].

Table 3.
Natural products regulate lipid metabolism enzymes in CRC.
Table 3.
Natural products regulate lipid metabolism enzymes in CRC.
| Medicinal Plant | Bioactive Compounds | Medicinal Plant | Cancer Model | IC50 | Metabolic Regulation | Targets | Potential Mechanisms | References |
|---|---|---|---|---|---|---|---|---|
| Flavonoids | Oroxylin A | Scutellaria baicalensis Georgi | HCT116, Xenograft mouse model | - | Lipid metabolism | HIF1α, FASN, SREBP, CPT | Inactivated HIF1α and regulates fatty acid metabolism, by blocking the Wnt signaling pathway | [132] |
| Flavonoids | Onion Flavonoids | Allium cepa L. | Hyperlipidemia-subcutaneously heterotopic colorectal cancer orthotopic transplant model | - | Cholesterol metabolism | apoB TC | Regulated lipid metabolism, and decreased levels of apoB and TC | [135] |
| Flavonoids | Kaempferol | Kaempferia galanga L. | ApcMin/+ mice | - | BAs metabolism | CYP27A1, CYP8B1 | Increased the protein content of liver CYP27A1 and CYP8B1. Upregulated the expression of BSEP to regulate the BA homeostasis, and inhibited the Wnt/β-catenin pathway by increasing the protein expression of FXR. | [52] |
| Alkaloids | Berberine | Coptis chinensis Franch. | DLD-1, Caco-2, Xenograft mouse model | - | Fatty acid synthesis | ACC, ACL, FASN | Downregulated the expression of key enzymes of lipogenesis and inhibited lipid synthesis through the SCAP/SREBP-1 pathway, which is related to the Wnt/β-catenin pathway. | [121] |
| Saponins | Ginsenoside Compound K(GCK) | Panax ginseng C. A. Mey. | SW480, HT29, HCT116, LOVO, Xenograft mouse model | SW480: 46.07 μM HT29: 43.8 μM LOVO: 19.72 μM | Lipid metabolism | PLA2G16 | Inhibited the protein expression of PLA2G16 to correct the abnormal lipid metabolism, and regulated the biosynthesis of FFA | [125] |
| Sesquiterpenes | Trichothecin (TCN) | endophytic fungus of Maytenus hookeri Loes | HCT116, LOVO, Xenograft mouse model | - | monounsaturated FA (MUFA) biosynthesis | SCD-1, SREBP1, FAs | Reduced production of unsaturated FAs by blocking SCD-1 activity | [126] |
| Diterpenoids | Oridonin | Isodon rubescens (Hemsl.) H.Hara | SW480, SW620 | SW480: 20.79 μM SW620: 37.02 μM | Fatty acid synthesis | FAS | Inhibited the mRNA and protein expression of FAS and SREBP1 and reduced the level of cellular fatty acids | [119] |
| Glycosides | RA-XII | Rubia yunnanensis Diels | HCT116 | - | Fatty acid synthesis | SREBP1, FASN, SCD | Reduced fatty acid levels by decreasing the expression of SREBP1 and inhibiting the expressions of de novo fatty acid synthesis proteins FASN and SCD | [120] |
| Polysaccharides | EPS1-1 | Rhizopus stolonifer (Ehrenb.) Vuill. | AOM/DSS-induced mice | - | Lipid metabolism | SCFAs | Modulated gut microbiota, increased the concentration of total SCFAs | [128] |
| Mirabilite extract | Mirabilite | APCmin/+ mice model | - | Lipid metabolism | Mirabilite influences six lipid metabolic pathways | [131] |
2.4. Natural Products Regulate Nucleotide Metabolism
Nucleotides include purine nucleotides and pyrimidine nucleotides, which are essential raw materials for the synthesis of DNA and RNA. Nucleotides participate in metabolism and physiological regulation and play crucial roles in vital biological processes [136]. Nucleotide metabolism includes anabolism and catabolism. In tumor cells, enhanced nucleotide synthesis is induced to satisfy the energy requirement [137]. Anabolism involving de novo synthesis and salvage pathways. De novo synthesis utilizes newly synthesized nitrogen-containing heterocycles derived from amino acids, one-carbon units, CO2, and other sources. The salvage pathway utilizes free nucleobases within the cell. Notably, substrates for nucleotide biosynthesis are derived from glycolysis, the PPP, the serine-glycine pathway, the TCA cycle, and glutamine transaminase reactions. Affecting other metabolic pathways often results in simultaneous changes in nucleotide metabolism.
Hydroxycamptothecin (SN38) is a natural plant extract isolated from Camptotheca acuminata Decne, which has a cell viability inhibitory effect comparable to that of 5-FU (Table 4). SN38 induces DNA damage, further disrupting ribonucleotide (RNP) and deoxyribonucleotide (dRN) metabolism to change the synthesis of DNA and RNA. Moreover, SN38 regulates the expression of ribonucleotide reductase (RNR) to change the levels of ATP, UTP, dATP, and TTP, which are identified as critical metabolites of nucleotide metabolism [138]. A dimeric metabolite of 3,3′-diindolylmethane (DIM), has been obtained from cruciferous vegetables. DIM inhibited the expression of pyrimidine synthesis-related genes (CAD, DHODH, UMPS, NME1, RNR, and CTPS), and increased the expression of the pyrimidine-catabolism-associated gene UPP1. In addition, DIM decreases the total contents of UTP and CTP, as well as blocks DHODH to disrupt pyrimidine synthesis, thus inhibiting the malignant progression of CRC [139]. Rabdosianone I is a bitter diterpene from the herbIsodon japonicus (Burm. f.) H. Hara, which inhibits the mRNA and protein expression of TS by directly binding to the mitochondrial inner proteins ANT2 and PHB2, thereby reducing the proliferation of CRC cells [140].

Table 4.
Natural products regulate nucleotide metabolism enzymes in CRC.
2.5. Natural Products Regulate Multiple Metabolism
Natural products regulate a variety of metabolic pathways, with multitarget and multipathway characteristics. Peiminine is an isosteroidal alkaloid derived from a variety of plants, such as Fritillaria Liliaceae. Peiminine regulates oxidative stress to change carbohydrate, amino acid and lipid metabolism in CRC cells by activating the PI3K/AKT/mTOR pathway, thereby inducing apoptosis and autophagy in cancer cells [141]. TER induces the apoptosis of CRC cells and affect eight key points, namely, ENO1, ALDOA, PFKFB3, PKM2, and LDHA, thereby exerting anti-CRC effects through key glycolysis and glutamine hydrolysis pathways [142]. Morin and esculetin inhibit aerobic glycolysis and glutaminolysis in colon cancer cells promoted by c-Myc through the suppression of β-catenin [143]. Flexibilide, isolated from the soft coral Sinularia flexibilis Quoy & Gaimard, regulates sphingolipid, alanine, aspartate, glutamate metabolism, d-glutamine and d-glutamate metabolism in HCT116 cells. Flexibility impedes the biosynthesis of sphingolipids and glycerophospholipids in phosphatidylcholine (PC) metabolism. This inhibition may be attributable to the blockage of the TCA cycle, concurrent with the activation of TNF receptor-associated factor 2 (TRAF2) and caspase-8, culminating in apoptosis [144].
The chemopreventive effect of Panax quinquefolius L. was associated with the alleviation of impaired amino acid, carbohydrate, and lipid metabolism. Arachidonic acid, linoleic acid, glutamic acid, docosahexaenoic acid, tryptophan, and fructose were significantly altered by American ginseng, all of which are associated with inflammation and oxidation [145]. Another study showed that the levels of 28 metabolites related to tryptophan metabolism, glycerophosphatidylcholine metabolism, and unsaturated fatty acid biosynthesis were affected in APCmin/+ mice, and mirabite protected against CRC mainly by regulating tryptophan metabolism and glycerophosphatidylcholine metabolism [146]. Evodiamine (EVO), an indole alkaloid extracted from the plant Evodia rutaecarpa (Juss.) Benth., confers a protective effect against AOM/DSS-induced CRC in mice, promoting the enrichment of SCFA-producing bacteria, and facilitating an alteration in microbial metabolism, with a particular emphasis on tryptophan metabolism [147]. Honokiol (HNK), an active polyphenolic compound, was extracted from Houpoea officinalis (Rehder & E. H. Wilson) N. H. Xia & C. Y. Wu. In APCmin/+ mice, metabolomics results showed that HNK regulates tryptophan metabolism, the TCA cycle, and the PPP [148].
3. Natural Products Regulate Metabolism Related Genes and Pathways
3.1. p53
The p53 gene, an important tumor suppressor, is the most frequently mutated gene in human cancers and often undergoes functional impairment during the progression of CRC [149]. Dysregulation of p53 triggers a cascade of metabolic disruptions, constituting a critical mechanism in CRC progression. Oroxin A, a flavonoid derivative isolated from Scutellaria baicalensis Georgi., exerts its anticancer effects on CRC by mediating aerobic glycolysis in a p53-dependent manner both in vivo and in vitro. Investigations into oroxin A have revealed its potential to inhibit CRC progression by repressing Sirt3-regulated MDM2 transcription, thereby preventing MDM2-mediated p53 degradation and consequentially diminishing aerobic glycolysis [150]. Furthermore, wogonin induces p53 phosphorylation at Ser15 and Ser20 and acetylation at Lys382, inhibiting MDM2 expression and enhancing p53 stability, to upregulate the expression of p53 and inhibit glycolysis, while concurrently downregulating the mRNA and protein expression of GLUT1, PGM, HK2, GLUT1, PDHK1, and LDHA [38]. Additionally, p53 moulates the impact of lobetyolin on glutamine metabolism in HCT116 CRC cells by influencing ASCT2 transcription and protein expression, thereby regulating apoptosis [113] (Figure 2).
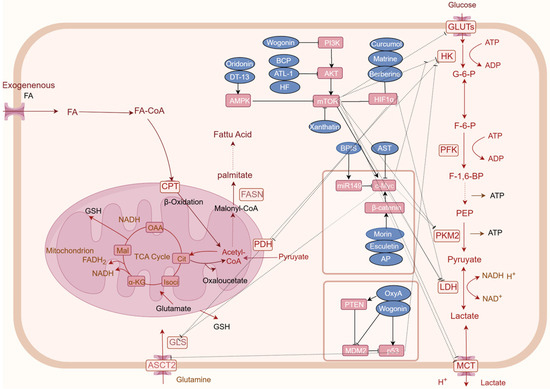
Figure 2.
Natural products regulating key factors and signaling pathways that are involved in CRC cancer metabolism reprogramming. Natural products may regulate oncogenes genes and related signaling pathways to regulate tumor cell metabolism, such as HIF-1α, MYC, and p53, PI3K-AKT-mTOR, and AMPK pathway. Rectangular boxes indicate altered glucose metabolism enzymes and targets; Blue boxes represent natural products; arrows represent metabolic directions (created on www.figdraw.com).
3.2. c-Myc
MYC is a proto-oncogene encoding a protein that plays a crucial role in cell growth, differentiation, and apoptosis. As a transcription factor, c-Myc regulates gene expression, influencing cell cycle progression and metabolism. Dysregulation of c-Myc, through overexpression or mutation, promotes tumorigenesis by enhancing anabolic metabolism. This aberrant upregulation of synthetic metabolic pathways drives tumor growth [151]. Astragaloside IV (AST), a principal active component of the traditional Chinese medicinal herb Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. AST has been demonstrated to reduce the mRNA expression levels of c-Myc and glycolytic enzymes (LDH-A, Glut-1, and HK2) in a DSS-induced mouse model, suggesting its potential for combating tumor-associated inflammation and maintaining normal glucose homeostasis [152]. Furthermore, compounds such as morin and esculetin impede aerobic glycolysis and glutaminolysis in colon cancer cells promoted by c-Myc, via the inhibition of β-catenin [143]. Bound Polyphenols compounds (BPIS) inhibits aerobic glycolysis mediated by PKM2 through the upregulation of miR-149 expression, which directly targets the 3’-UTR of c-Myc, thereby suppressing the proliferation of HT-29 cells [153]. AP impedes cellular glycolysis in colorectal cancer cells by obstructing the β-catenin/c-Myc/PTBP1 signaling pathway, thereby inhibiting the activity and expression of tumor-specific PKM2 [58] (Figure 2).
3.3. HIF1α
HIF1α is one of the key regulatory factors for cell cycle adaptation in hypoxia. In the tumor microenvironment, it becomes activated and simultaneously functions as a modulator of genes associated with glucose transport proteins, glycolytic enzymes, and vascular endothelial growth factors. Such regulation can lead to metabolic reprogramming, indirectly fostering cancer progression [154]. HIF1α is known to activate the expression of target genes involved in glycolysis, which impacts CRC cell growth, proliferation, cell cycle progression, and the Warburg effect. Notably, matrine, downregulates HIF1α-mediated expression of downstream glycolytic targets such as GLUT1, HK2, and LDHA, suggesting its potential to suppress CRC cell growth by inhibiting the Warburg effect [47]. Worenine, an alkaloid from Coptis chinensis Franch., targets HIF1α to decrease glycolytic activity and downregulate the expression of glycolytic enzymes (PFK-L, HK2, and PKM2), thereby inhibiting colorectal cancer cell growth, proliferation, and cell cycle progression [155]. In addition to its potential to curtail glucose uptake in colon cancer cells and attenuate the transcription of glucose metabolism-related genes (GLUT1, LDHA, and HK2), berberine be identified its potential by suppressing mTOR-dependent HIF1α protein synthesis, thus inhibiting heightened glucose metabolism in colorectal cancer cells [156]. Curcumin promotes the degradation of HIF1α, inhibits EMT, curtails CRC cell invasion and migration, and suppresses GLS1-mediated glutamine breakdown [110] (Figure 2).
3.4. PI3K/AKT/mTOR Signaling Pathway
Phosphatidylinositol 3-kinases (PI3Ks), which include three classes of lipid kinases, are a family of signal transduction enzymes. PI3Ks plays a key role in activating serine/threonine kinase (AKT) within its downstream signaling pathways, which regulates a number of cellular functions including metabolism, growth, proliferation, survival, transcription, and protein synthesis [157]. AKT, also known as protein kinase B (PKB), is overexpressed in a variety of cancers, including CRC, where it plays a critical role in numerous cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration. AKT modulates cellular metabolism and tumorigenic metabolic reprogramming by phosphorylating an array of downstream targets, thus facilitating tumor proliferation [158]. The phosphorylation of AKT catalyzes the activation of the mechanistic target of rapamycin complex 1 (mTORC1), underscoring the significant role of the PI3K/AKT/mTOR signaling pathway in the metabolic reprogramming of colorectal cancer [159] (Figure 2).
Atractylenolide I (ATL-1) is one of the principal active constituents of Atractylodes macrocephala Koidz [16,160]. ATL-1 impeded tumor proliferation by modulating glycolysis, stemness maintenance, and cellular apoptosis through its effects on the AKT/mTOR signaling pathway [16]. Wogonin restricted the growth of xenograft tumors in nude mice by downregulating HIF-1α expression and glycolysis via inhibition of the PI3K/Akt pathway, concurrently suppressing the expression of HIF-1α, glycolytic proteins, and PI3K/Akt [161]. Xanthatin attenuates the phosphorylation of mTOR, eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), and c-Myc in HT29 cells, and its impact on energy metabolism is likely linked to the inhibition of the mTOR signaling pathway [78]. Peiminine induced apoptosis and autophagy in CRC cells by activating PI3K/Akt/mTOR signaling and modulating oxidative stress, subsequently altering carbohydrate, amino acid, and lipid metabolism [141]. β-Caryophyllene inhibits ART1-induced glycolysis and increases ATP and lactate production through the AKT/mTOR pathway [79]. Studies indicate that Halofuginone (HF) downregulates the Akt/mTORC1 signaling pathway, mediating the metabolic regulation of HF through aerobic glycolysis and inducing autophagy [101].
3.5. AMP-Activated Protein Kinase (AMPK) Signaling Pathway
AMPK functions not only as a pivotal enzyme in the regulation of energy homeostasis but also as a central modulator of eukaryotic cellular and organismal metabolism, serving as a key protein in multiple signaling pathways [162]. Under conditions of nutrient deprivation, HF inactivates ULK1 via the LKB1/AMPK signaling pathway by downregulating ULK1 at Ser317 and Ser777, thereby suppressing autophagy [163]. DT-13 inhibits the growth of CRC by suppressing GLUT1 expression via activation of the AMPK/mTOR pathway [15]. Oridonin deubiquitinated p-AMPK, causing GLUT1 to be downregulated and autophagy induced in cancer cells [72] (Figure 2).
4. Synergistic Effect of Natural Products and Chemotherapy Medicine
Although clinical chemotherapy has demonstrated notable efficacy in the control of CRC, significant resistance to chemotherapeutic drugs severely impedes therapeutic outcomes. The molecular mechanisms underlying drug resistance are intricate and remain to be elucidated. Cancer cells exhibit pronounced alterations in glucose metabolism, lipid metabolism, and glutamine metabolism, which strongly differentiate them from normal cells. Current research indicates that the development of drug resistance is associated with metabolic dysregulation in cancer cells. The metabolic pattern of doxorubicin-resistant human colorectal adenocarcinoma cells (Lovo-DOX) transitions from OXPHOS to the glycolytic pathway, as evidenced by the marked increase in GLUT1 mRNA and protein expression levels. Silybin, the primary bioactive component extracted from the seeds of milk thistles, acts as a competitive inhibitor of GLUTs [164]. A study demonstrated that silybin reduces glucose uptake in resistant cells by downregulating GLUT1 protein expression. The combination of doxorubicin and silybin has synergistic effects on Lovo-DOX cells and result in selective cytotoxicity toward resistant cells, confirming the heightened dependency of these cells on glycolysis [102]. In combination with doxorubicin, curcumin micelle-started GLUT1 significantly suppressed the growth of CRC cells and increased the therapeutic effect. The GLUT1-targeted CUR and DOX-loaded combination micelles decreased glucose uptake by blocking the GLUT1 transport protein, thereby helping increase treatment effectiveness [165]. The expression of SLC6A14 (ATB0+), a Na+/Cl− coupled amino acid transporter, is upregulated in CRC cells to meet their demand for additional amino acids. Targeting ATB0+ can reduce acid uptake and enhance amino acid deprivation. Oxaliplatin (O) and berbamine (B)-coloaded tryptophan (Trp)-conjugated NPs, can inhibit autophagy and induce apoptosis to reverse OXA resistance [166].
5-FU is one of the most commonly used chemotherapeutic agents for the treatment of CRC and is a critical targeted drug of the nucleotide metabolism thymidylate synthase (TS). However, drug resistance limits the effectiveness of 5-FU. In 5-FU-resistant CRC cells, TS mRNA and protein are overexpressed, which is related to the mechanism of drug resistance. Gambogic acid (GA), Coptidis Rhizoma Extract (CRE) and its main active compound berberine reduce TS protein expression either alone or in combination with 5-FU, and CRE and GA significantly reduce the IC50 of 5FU [167,168]. The dry roots of Rauvolfia vomitoria Afzel (RVA), exhibit pharmacological effects such as neuroprotective, antitumor, and anti-inflammatory effects [169,170,171]. Comparative metabolomic analysis between the 5-FU treatment group and the high-dose combination therapy group revealed 25 distinct metabolites, that predominantly affect lipid and fatty acid metabolic pathways. These findings suggest that the concomitant use of RVA and 5-FU may inhibit cancer cell proliferation by impacting membrane stability and energy supply in carcinogenic cells. These findings imply that RVA could potentiate the efficacy of the chemotherapeutic drug 5-FU, enhancing its antitumor activity through modulation of lipid metabolism and cellular energy homeostasis [172]. Additionally, quercetin is a flavonoid found in many vegetables and fruits, that has been proven to possess many pharmacological activities, such as antioxidant, antibacterial, antidiabetic, and anticancer effects [173,174,175,176]. Quercetin decreases glucose consumption and lactate acid generation in HCT-15 cells by inhibiting MCT. Cytotoxicity of 5-FU in CRC cells was enhanced, which reduced the viability of CRC cells, and promoting cell apoptosis [103]. Cinnamaldehyde suppressed mRNA expression of BRCA1, TOPO1, ERCC1, and TS, and upregulated OPRT mRNA levels in CRC HT-29 and LoVo cells, to enhance the efficacy of either 5-FU or OXA [177].
Multidrug resistance (MDR) is a phenomenon in which cancer cells develop cross-resistance with different functions and structures to different anticancer medicines. Membrane transport-mediated drug efflux is considered the principal mode of resistance. P-glycoprotein (P-gp), which is encoded by MDR1 gene, is a typical member of the ATP-binding box (ABC) transport protein, which is known to mediate the delivery of chemotherapeutic agents in ATP-dependent fashion [178]. The MDR phenotype of SW620/Ad300 cells was closely related to the upregulation of spermidine and spermine synthesis as well as D-glutamine metabolism. Curcumin inhibited spermidine and spermine biosynthesis by decreasing ornithine decarboxylase (ODC) expression and inhibiting the metabolism of D-glutamine, which in turn reduced the ability of antioxidative stress and the transport activity of P-gp in SW620/Ad300 cells, resulting in the reversal of the MDR [111]. The prophylactic effects of American ginseng are linked to the mitigation of disturbances in amino acid, carbohydrate, and lipid metabolism [145].
5. Classification and Role of Natural Products Targeting CRC Metabolic Reprogramming
Natural products, due to their abundant sources and significant bioactivity, have shown promising effects on CRC metabolic reprogramming. This paper discusses natural products derived from plants, minerals, and microorganisms. Plant-derived components include polyphenols, alkaloids, quinones, and terpenoids. Polyphenolic compounds, such as quercetin, apigenin, and kaempferol, are diverse, widely available, and easily obtainable. These compounds are abundant in foods and have demonstrated activity in regulating glucose and lipid metabolism, with a good safety profile [58,103]. However, natural polyphenols face challenges in drug development due to poor solubility and stability [179]. Alkaloids, a large category of complex cyclic compounds containing nitrogen, generally exhibit basic properties. For instance, the bioactive compound berberine has multiple pharmacological activities, including anti-inflammatory and antioxidant effects [180]. Berberine targets PKM2 to regulate glycolysis and interferes with ACC to reduce lipid synthesis, but its poor oral bioavailability limits drug development [57,181]. Steroid saponins, which exhibit good pharmacological activity in this study, target HK2 and GLUT to inhibit glycolysis [15,64], but have high cytotoxicity and low bioavailability. Terpenoids, typically possessing cyclic structures, are the most diverse class of compounds [182]. Terpenoids, which intervene in the activities of multiple enzymes such as PKM2, GLUT, MCT1, GLS1, SCD1, and TS, effectively regulate the four major energy metabolism pathways [50,72,110]. Among them, TCN, derived from endophytic fungi, specifically regulates fatty acid metabolism [126]. Mineral-based components, like mirabilite (sodium sulfate), a traditional Chinese medicine, have been shown to regulate lipid metabolism, but their low solubility and poor safety profile pose challenges for research.
6. Conclusions and Prospects
Metabolic reprogramming is a hallmark of CRC. Metabolism is primarily facilitated by metabolic pathways comprising enzymatic reactions, with the activity of these metabolic enzymes playing a crucial role in determining the direction and rate of the metabolic processes. In addition to their canonical role in catalyzing metabolites and producing the nutrients needed, metabolic enzymes are also involved in regulating gene expression, DNA repair, cell apoptosis, and the tumor environment in cancers [183]. Therefore, metabolic enzymes are the main target in the discovery of metabolism-targeted drugs of all time. This review delineates the key metabolic enzymes (HK2, GLUTs, PKM2, LDH, PDH, G6PD) modulated in CRC and provides an overview of the effects of 39 natural compounds derived from three species on metabolic enzymes, related proteins, oncogenes, and the interconnected pathways specific to colorectal carcinogenesis.
Except for rich sources, and well-defined pharmacological activities, natural products have several advantages for metabolic drug development [184,185]. Firstly, natural products, with their multitarget and multipathway characteristics, are ideally suited to addressing the complex metabolic alterations in tumors. The metabolism pathways of glucose, amino acids, lipids, and nucleotides are linked to each other through the common intermediate, the TCA cycle, and oxidative phosphorylation [186]. It is suggested that natural products should not be limited to the metabolism of a single substance but may be achieved through multiple pathways and targets. Curcumin regulates a variety of key enzymes involved in glucose metabolism and amino acid metabolism. Natural products modulate multiple substance metabolism, through directly binding to enzymes, changing the expression of genetic material, influencing the activity of enzymes, changing epigenetics, and influencing upstream or downstream regulators [187,188], therefore inhibiting cellular proliferation, inducing apoptosis, activating antitumoral immune responses, and modulating the tumor microenvironment. Secondly, the abundant variety and diverse scaffolds of natural products make them ideal candidates for the screening and development of drugs targeting tumor metabolism. Moreover, natural products, when used in combination with other drugs, exhibit significant synergistic and anti-resistance effects.
However, there are still challenges that hinder the development of metabolic inhibitors derived from natural products. Natural products often suffer from instability, low bioavailability, poor solubility, and suboptimal selectivity, limiting their therapeutic application in oncology [189]. The plasticity of metabolic pathways and the heterogeneity of tumors should be taken into consideration, which complicates the assurance of drug safety. Most natural products cannot precisely target tumor metabolic changes and may affect the metabolism of normal cells. In addition, some natural products exhibit hepatotoxicity and nephrotoxicity. To overcome these challenges, a series of actions must be taken. Integrating multiomics, single-cell, and spatial detection technologies to identify the precise mechanisms of CRC metabolic reprogramming that differ from normal cells is of paramount importance. Further, drug delivery systems, novel materials, and structural modification offer solutions to limitations, thereby expanding the utility of natural compounds [190]. Taken together, development and modification of natural products to target the metabolism of malignant cells are a novel and promising field of drug discovery for the treatment of CRC.
Author Contributions
Conceptualization and methodology, B.Y.K.L. and X.F.; software, validation, and formal analysis, L.Q.; investigation and resources, L.Q. and X.D.; data curation, P.S.; writing—original draft preparation and writing—review and editing, M.W.; visualization, J.P.L.N.; supervision, V.K.W.W. and B.Y.K.L.; project administration, B.Y.K.L.; funding acquisition, X.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NATCM’s Project of High-level Construction of Key TCM Disciplines (Marine Traditional Chinese Medicine; No. zyyzdxk-2023124), the Key R&D Program of Shandong Province, China (No. 2021CXGC010510), the Shandong Provincial Natural Science Foundation (No. ZR2022LZY026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The main figure were assembled in Figdraw https://www.figdraw.com/ (accessed on 4 September 2024).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Shi, R.; Tang, Y.-Q.; Miao, H. Metabolism in Tumor Microenvironment: Implications for Cancer Immunotherapy. MedComm 2020, 1, 47–68. [Google Scholar] [CrossRef]
- Brown, R.E.; Short, S.P.; Williams, C.S. Colorectal Cancer and Metabolism. Curr. Color. Cancer Rep. 2018, 14, 226–241. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of Cancer Metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Noordhuis, P.; Holwerda, U.; Van der Wilt, C.L.; Van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil Incorporation into RNA and DNA in Relation to Thymidylate Synthase Inhibition of Human Colorectal Cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef]
- Choe, S.; Wang, H.; DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Watts, J.M.; Pollyea, D.A.; et al. Molecular Mechanisms Mediating Relapse Following Ivosidenib Monotherapy in IDH1-Mutant Relapsed or Refractory AML. Blood Adv. 2020, 4, 1894–1905. [Google Scholar] [CrossRef]
- Chen, X.-X.; Khyeam, S.; Zhang, Z.-J.; Zhang, K.Y.-B. Granatin B and Punicalagin from Chinese Herbal Medicine Pomegranate Peels Elicit Reactive Oxygen Species-Mediated Apoptosis and Cell Cycle Arrest in Colorectal Cancer Cells. Phytomedicine 2022, 97, 153923. [Google Scholar] [CrossRef]
- Chen, S.; Nishi, M.; Morine, Y.; Shimada, M.; Tokunaga, T.; Kashihara, H.; Takasu, C.; Yamada, S.; Wada, Y. Epigallocatechin-3-gallate Hinders Metabolic Coupling to Suppress Colorectal Cancer Malignancy through Targeting Aerobic Glycolysis in Cancer-associated Fibroblasts. Int. J. Oncol. 2022, 60, 19. [Google Scholar] [CrossRef]
- Castro, M.; Preto, M.; Vasconcelos, V.; Urbatzka, R. Obesity: The Metabolic Disease, Advances on Drug Discovery and Natural Product Research. Curr. Top. Med. Chem. 2016, 16, 2577–2604. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, Z.; Wang, Y.; Zhang, B.; Liu, G.; Liu, J. Network Pharmacology and Metabolomics Study on the Intervention of Traditional Chinese Medicine Huanglian Decoction in Rats with Type 2 Diabetes Mellitus. J. Ethnopharmacol. 2020, 258, 112842. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, S.; Ramdas; Khare, S.; Shukla, A.; Shanker, K.; Pal, A.; Khan, F.; Darokar, M.P. Quebrachitol from Putranjiva roxburghii Wall. (Putranjivaceae) a Potent Antimalarial: Pre-Clinical Efficacy and Its Interaction with PfLDH. Parasitol. Int. 2023, 92, 102675. [Google Scholar] [CrossRef]
- Wei, X.; Mao, T.; Li, S.; He, J.; Hou, X.; Li, H.; Zhan, M.; Yang, X.; Li, R.; Xiao, J.; et al. DT-13 Inhibited the Proliferation of Colorectal Cancer via Glycolytic Metabolism and AMPK/mTOR Signaling Pathway. Phytomedicine 2019, 54, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, W.; Sang, X.; Wu, X.; Shan, Q.; Tang, D.; Xu, X.; Cao, G. Atractylenolide I Inhibits Colorectal Cancer Cell Proliferation by Affecting Metabolism and Stemness via AKT/mTOR Signaling. Phytomedicine 2020, 68, 153191. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Yin, P.; Xu, K.; Wang, Y.; Shi, F.; Gao, J.; Fu, X. New Strategies for Targeting Glucose Metabolism-Mediated Acidosis for Colorectal Cancer Therapy. J. Cell. Physiol. 2019, 234, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Devic, S. Warburg Effect—A Consequence or the Cause of Carcinogenesis? J. Cancer 2016, 7, 817–822. [Google Scholar] [CrossRef]
- Ghanem, N.; El-Baba, C.; Araji, K.; El-Khoury, R.; Usta, J.; Darwiche, N. The Pentose Phosphate Pathway in Cancer: Regulation and Therapeutic Opportunities. Chemotherapy 2021, 66, 179–191. [Google Scholar] [CrossRef]
- Paul, S.; Ghosh, S.; Kumar, S. Tumor Glycolysis, an Essential Sweet Tooth of Tumor Cells. Semin. Cancer Biol. 2022, 86, 1216–1230. [Google Scholar] [CrossRef]
- Kooshki, L.; Mahdavi, P.; Fakhri, S.; Akkol, E.K.; Khan, H. Targeting Lactate Metabolism and Glycolytic Pathways in the Tumor Microenvironment by Natural Products: A Promising Strategy in Combating Cancer. Biofactors 2022, 48, 359–383. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Blanco, G. (Eds.) Chapter 14—Carbohydrate Metabolism. In Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2017; pp. 283–323. ISBN 978-0-12-803550-4. [Google Scholar]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase II: Cancer’s Double-Edged Sword Acting as Both Facilitator and Gatekeeper of Malignancy When Bound to Mitochondria. Oncogene 2006, 25, 4777–4786. [Google Scholar] [CrossRef]
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin Inhibits Aerobic Glycolysis and Induces Mitochondrial-Mediated Apoptosis through Hexokinase II in Human Colorectal Cancer Cells in Vitro. Anticancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D. Insunn Muscle Glucos Uptake, and Hexokinase Revisiting Road Not Taken. Physiology 2022, 37, 115–127. [Google Scholar] [CrossRef]
- Webb, B.A.; Forouhar, F.; Szu, F.-E.; Seetharaman, J.; Tong, L.; Barber, D.L. Structures of Human Phosphofructokinase-1 and Atomic Basis of Cancer-Associated Mutations. Nature 2015, 523, 111–114. [Google Scholar] [CrossRef]
- Li, T.; Han, J.; Jia, L.; Hu, X.; Chen, L.; Wang, Y. PKM2 Coordinates Glycolysis with Mitochondrial Fusion and Oxidative Phosphorylation. Protein Cell 2019, 10, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Ancey, P.-B.; Contat, C.; Meylan, E. Glucose Transporters in Cancer—From Tumor Cells to the Tumor Microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Long, W.; Cheeseman, C.I. Structure of, and Functional Insight into the GLUT Family of Membrane Transporters. Cell Health Cytoskelet. 2015, 7, 167–183. [Google Scholar] [CrossRef]
- Schwartz, L.; Supuran, C.T.; Alfarouk, K.O. The Warburg Effect and the Hallmarks of Cancer. Anticancer Agents Med. Chem. 2017, 17, 164–170. [Google Scholar] [CrossRef]
- Gould, G.W.; Holman, G.D. The Glucose Transporter Family: Structure, Function and Tissue-Specific Expression. Biochem. J. 1993, 295 Pt 2, 329–341. [Google Scholar] [CrossRef]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate Dehydrogenase A: A Key Player in Carcinogenesis and Potential Target in Cancer Therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef] [PubMed]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef]
- Halestrap, A.P. The SLC16 Gene Family—Structure, Role and Regulation in Health and Disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate Transporters in Cancer. Mol. Metab. 2019, 33, 48–66. [Google Scholar] [CrossRef]
- He, L.; Lu, N.; Dai, Q.; Zhao, Y.; Zhao, L.; Wang, H.; Li, Z.; You, Q.; Guo, Q. Wogonin Induced G1 Cell Cycle Arrest by Regulating Wnt/β-Catenin Signaling Pathway and Inactivating CDK8 in Human Colorectal Cancer Carcinoma Cells. Toxicology 2013, 312, 36–47. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Wu, Y.; Dai, Q.; Zhou, Y.; Li, Z.; Yang, L.; Guo, Q.; Lu, N. Selective Anti-Tumor Activity of Wogonin Targeting the Warburg Effect through Stablizing P53. Pharmacol. Res. 2018, 135, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, X.; Yan, Y.; Li, H. Pyruvate Dehydrogenase Kinases (PDKs): An Overview toward Clinical Applications. Biosci. Rep. 2021, 41, BSR20204402. [Google Scholar] [CrossRef]
- Li, S.-Y.; Shang, J.; Mao, X.-M.; Fan, R.; Li, H.-Q.; Li, R.-H.; Shen, D.-Y. Diosgenin Exerts Anti-Tumor Effects through Inactivation of cAMP/PKA/CREB Signaling Pathway in Colorectal Cancer. Eur. J. Pharmacol. 2021, 908, 174370. [Google Scholar] [CrossRef]
- Lin, S.-T.; Tu, S.-H.; Yang, P.-S.; Hsu, S.-P.; Lee, W.-H.; Ho, C.-T.; Wu, C.-H.; Lai, Y.-H.; Chen, M.-Y.; Chen, L.-C. Apple Polyphenol Phloretin Inhibits Colorectal Cancer Cell Growth via Inhibition of the Type 2 Glucose Transporter and Activation of P53-Mediated Signaling. J. Agric. Food Chem. 2016, 64, 6826–6837. [Google Scholar] [CrossRef]
- Lanmao Materia Medica of South Yunnan. Yunnan Science and Technology Press: Kunming, China, 2004; p. 977. ISBN 7-5416-1497-1.
- Yu, J.; Yang, S.; Wang, X.; Gan, R. Matrine Improved the Function of Heart Failure in Rats via Inhibiting Apoptosis and Blocking Β3-Adrenoreceptor/Endothelial Nitric Oxide Synthase Pathway. Mol. Med. Rep. 2014, 10, 3199–3204. [Google Scholar] [CrossRef]
- Pu, J.; Fang, F.-F.; Li, X.-Q.; Shu, Z.-H.; Jiang, Y.-P.; Han, T.; Peng, W.; Zheng, C.-J. Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses. Int. J. Mol. Sci. 2016, 17, 1410. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Wang, Y.; Hou, Y.; Zhang, J.; Wu, J.; Zhang, F.; Sui, M.; Luo, J.; Yang, M. Zearalenone-14-Glucoside Is Hydrolyzed to Zearalenone by β-Glucosidase in Extracellular Matrix to Exert Intracellular Toxicity in KGN Cells. Toxins 2022, 14, 458. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-D.; Zhang, H.-P.; Cao, J.; Li, G.-Z.; Wang, S.-R. Effects of Matrine on ATP Binding Cassette Transporter A1 and Cholesterol in Monocyte Cells. Basic Clin. Med. 2007, 27, 996–1000. [Google Scholar] [CrossRef]
- Hong, X.; Zhong, L.; Xie, Y.; Zheng, K.; Pang, J.; Li, Y.; Yang, Y.; Xu, X.; Mi, P.; Cao, H.; et al. Matrine Reverses the Warburg Effect and Suppresses Colon Cancer Cell Growth via Negatively Regulating HIF-1α. Front. Pharmacol. 2019, 10, 1437. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Xu, Q.; Duan, W.; Yang, L.; Wu, X.; Lu, G.; Zhang, L.; Zheng, Y. Oxymatrine Inhibits Colorectal Cancer Metastasis via Attenuating PKM2-Mediated Aerobic Glycolysis. Cancer Manag. Res. 2020, 12, 9503–9513. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q. Parthenolide Could Become a Promising and Stable Drug with Anti-Inflammatory Effects. Nat. Prod. Res. 2015, 29, 1092–1101. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Li, S.; Qu, L.; Yin, F.; Lu, D.; Luo, H.; Chen, X.; Luo, Z.; Cui, N.; et al. Parthenolide Derivatives as PKM2 Activators Showing Potential in Colorectal Cancer. J. Med. Chem. 2021, 64, 17304–17325. [Google Scholar] [CrossRef]
- Choi, J.-B.; Kim, J.-H.; Lee, H.; Pak, J.-N.; Shim, B.S.; Kim, S.-H. Reactive Oxygen Species and P53 Mediated Activation of P38 and Caspases Is Critically Involved in Kaempferol Induced Apoptosis in Colorectal Cancer Cells. J. Agric. Food Chem. 2018, 66, 9960–9967. [Google Scholar] [CrossRef]
- Li, X.; Khan, I.; Huang, G.; Lu, Y.; Wang, L.; Liu, Y.; Lu, L.; Hsiao, W.L.W.; Liu, Z. Kaempferol Acts on Bile Acid Signaling and Gut Microbiota to Attenuate the Tumor Burden in ApcMin/+ Mice. Eur. J. Pharmacol. 2022, 918, 174773. [Google Scholar] [CrossRef]
- Wu, H.; Cui, M.; Li, C.; Li, H.; Dai, Y.; Cui, K.; Li, Z. Kaempferol Reverses Aerobic Glycolysis via miR-339-5p-Mediated PKM Alternative Splicing in Colon Cancer Cells. J. Agric. Food Chem. 2021, 69, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological Effects and Mechanisms of Tannic Acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ding, G.-B.; Liu, W.; Fu, R.; Sajid, A.; Li, Z. Tannic Acid Directly Targets Pyruvate Kinase Isoenzyme M2 to Attenuate Colon Cancer Cell Proliferation. Food Funct. 2018, 9, 5547–5559. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Sarvarian, P.; Gholipour, E.; Asenjan, K.S.; Aghebati-Maleki, L.; Motavalli, R.; Hojjat-Farsangi, M.; Yousefi, M. Berberine: A Novel Therapeutic Strategy for Cancer. IUBMB Life 2020, 72, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Lu, Y.; Yang, P.; Li, Z. Berberine Inhibited the Proliferation of Cancer Cells by Suppressing the Activity of Tumor Pyruvate Kinase M2. Nat. Prod. Commun. 2017, 12, 1934578X1701200909. [Google Scholar] [CrossRef]
- Shan, S.; Shi, J.; Yang, P.; Jia, B.; Wu, H.; Zhang, X.; Li, Z. Apigenin Restrains Colon Cancer Cell Proliferation via Targeted Blocking of Pyruvate Kinase M2-Dependent Glycolysis. J. Agric. Food Chem. 2017, 65, 8136–8144. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Wang, L.; Li, S. Extractable Amounts of Trans-Resveratrol in Seed and Berry Skin in Vitis Evaluated at the Germplasm Level. J. Agric. Food Chem. 2006, 54, 8804–8811. [Google Scholar] [CrossRef]
- Ji, M.; Li, Q.; Ji, H.; Lou, H. Investigation of the Distribution and Season Regularity of Resveratrol in Vitis Amurensis via HPLC–DAD–MS/MS. Food Chem. 2014, 142, 61–65. [Google Scholar] [CrossRef]
- Amiri, A.; Mahjoubin-Tehran, M.; Asemi, Z.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; Mirzaei, H.; Mirzaei, H. Role of Resveratrol in Modulating microRNAs in Human Diseases: From Cancer to Inflammatory Disorder. Curr. Med. Chem. 2021, 28, 360–376. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Wu, C.; Yang, P.; Li, H.; Li, Z. Resveratrol Induces Cancer Cell Apoptosis through MiR-326/PKM2-Mediated ER Stress and Mitochondrial Fission. J. Agric. Food Chem. 2016, 64, 9356–9367. [Google Scholar] [CrossRef]
- Fouad, M.; Agha, A.; Merzabani, M.A.; Shouman, S. Resveratrol Inhibits Proliferation, Angiogenesis and Induces Apoptosis in Colon Cancer Cells: Calorie Restriction Is the Force to the Cytotoxicity. Hum. Exp. Toxicol. 2013, 32, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, X.; Li, M.; Gong, G.; Liu, W.; Li, T.; Zuo, H.; Li, W.; Gao, F.; Liu, H. Cdh1-Mediated Skp2 Degradation by Dioscin Reprogrammes Aerobic Glycolysis and Inhibits Colorectal Cancer Cells Growth. EBioMedicine 2020, 51, 102570. [Google Scholar] [CrossRef]
- Wu, Z.; Han, X.; Tan, G.; Zhu, Q.; Chen, H.; Xia, Y.; Gong, J.; Wang, Z.; Wang, Y.; Yan, J. Dioscin Inhibited Glycolysis and Induced Cell Apoptosis in Colorectal Cancer via Promoting C-Myc Ubiquitination and Subsequent Hexokinase-2 Suppression. Onco-Targets Ther. 2020, 13, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, L.; Ma, Y.; Jiang, Z.; Tang, H.; Tong, X. Xanthohumol Inhibits Non-Small Cell Lung Cancer by Activating PUMA-Mediated Apoptosis. Toxicology 2022, 470, 153141. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Liu, H.; Yu, X. Xanthohumol Inhibits Colorectal Cancer Cells via Downregulation of Hexokinases II-Mediated Glycolysis. Int. J. Biol. Sci. 2019, 15, 2497–2508. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Anti-Inflammatory Effects of Thymoquinone and Its Protective Effects against Several Diseases. Biomed. Pharmacother. 2021, 138, 111492. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone Antioxidant/pro-Oxidant Effect as Potential Anticancer Remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.; Reeta, K.H.; Maulik, S.K.; Dinda, A.K.; Gupta, Y.K. Protective Effect of Thymoquinone against High-Fructose Diet-Induced Metabolic Syndrome in Rats. Eur. J. Nutr. 2015, 54, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Burzangi, A.S.; Ahmad, A.; Siddiqui, N.A.; Ibrahim, I.M.; Sharma, P.; Abualsunun, W.A.; Gabr, G.A. PI3K-AKT Pathway Modulation by Thymoquinone Limits Tumor Growth and Glycolytic Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 2305. [Google Scholar] [CrossRef]
- Yao, Z.; Xie, F.; Li, M.; Liang, Z.; Xu, W.; Yang, J.; Liu, C.; Li, H.; Zhou, H.; Qu, L.-H. Oridonin Induces Autophagy via Inhibition of Glucose Metabolism in P53-Mutated Colorectal Cancer Cells. Cell Death Dis. 2017, 8, e2633. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.-Z.; Yu, Y.; Wang, J.-J. Inhibitory ASIC2-Mediated Calcineurin/NFAT against Colorectal Cancer by Triterpenoids Extracted from Rhus Chinensis Mill. J. Ethnopharmacol. 2019, 235, 255–267. [Google Scholar] [CrossRef]
- Saltan Çitoğlu, G.; Bahadır Acıkara, Ö.; Sever Yılmaz, B.; Özbek, H. Evaluation of Analgesic, Anti-Inflammatory and Hepatoprotective Effects of Lycorine from Sternbergia Fisheriana (Herbert) Rupr. Fitoterapia 2012, 83, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Z.; Wang, R.; Zhang, Z.-R.; Zhang, Y.-N.; Deng, C.-L.; Zhang, B.; Shang, L.-Q.; Ye, H.-Q. In Vitro Inhibition of Alphaviruses by Lycorine. Virol. Sin. 2021, 36, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, S.; Zhou, C.; Sun, Z.; Wang, Q.; Sun, C. In Vitro and in Vivo Anticancer Activity of Lycorine in Prostate Cancer by Inhibiting NF-κB Signaling Pathway. J. Cancer 2022, 13, 3151–3159. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, F.; Li, L.; Liu, T.; Liang, X.; Yang, Z.; Zheng, Y.; Luo, Q.; Lu, J.; Liu, D.; Zeng, K.; et al. Lycorine Promotes IDH1 Acetylation to Induce Mitochondrial Dynamics Imbalance in Colorectal Cancer Cells. Cancer Lett. 2023, 573, 216364. [Google Scholar] [CrossRef]
- Li, L.; Liu, P.; Xie, Y.; Liu, Y.; Chen, Z.; Geng, Y.; Zhang, L. Xanthatin Inhibits Human Colon Cancer Cells Progression via mTOR Signaling Mediated Energy Metabolism Alteration. Drug Dev. Res. 2022, 83, 119–130. [Google Scholar] [CrossRef]
- Zhou, L.; Zhan, M.; Tang, Y.; Xiao, M.; Li, M.; Li, Q.; Yang, L.; Li, X.; Chen, W.; Wang, Y. Effects of β-Caryophyllene on Arginine ADP-Ribosyltransferase 1-Mediated Regulation of Glycolysis in Colorectal Cancer under High-Glucose Conditions. Int. J. Oncol. 2018, 53, 1613–1624. [Google Scholar] [CrossRef]
- Kim, E.; Choi, H.; Park, M.; Jung, Y.; Lee, S.; Kim, K.; Choi, J.; Chung, T.; Ha, K. Myristica Fragrans Suppresses Tumor Growth and Metabolism by Inhibiting Lactate Dehydrogenase A. Am. J. Chin. Med. 2016, 44, 1063–1079. [Google Scholar] [CrossRef]
- Kang, W.; Suzuki, M.; Saito, T.; Miyado, K. Emerging Role of TCA Cycle-Related Enzymes in Human Diseases. Int. J. Mol. Sci. 2021, 22, 13057. [Google Scholar] [CrossRef]
- Grimm, M.; Calgéer, B.; Teriete, P.; Biegner, T.; Munz, A.; Reinert, S. Targeting Thiamine-Dependent Enzymes for Metabolic Therapies in Oral Squamous Cell Carcinoma? Clin. Transl. Oncol. 2016, 18, 196–205. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The Assembly, Regulation and Function of the Mitochondrial Respiratory Chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; McCall, C.E. The Pyruvate Dehydrogenase Complex: Life’s Essential, Vulnerable and Druggable Energy Homeostat. Mitochondrion 2023, 70, 59–102. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Tao, K.; Cai, X. Pan-Cancer Analysis Reveals PDK Family as Potential Indicators Related to Prognosis and Immune Infiltration. Sci. Rep. 2024, 14, 5665. [Google Scholar] [CrossRef] [PubMed]
- Saunier, E.; Antonio, S.; Regazzetti, A.; Auzeil, N.; Laprévote, O.; Shay, J.W.; Coumoul, X.; Barouki, R.; Benelli, C.; Huc, L.; et al. Resveratrol Reverses the Warburg Effect by Targeting the Pyruvate Dehydrogenase Complex in Colon Cancer Cells. Sci. Rep. 2017, 7, 6945. [Google Scholar] [CrossRef]
- Bhat, J.A.; Akther, T.; Najar, R.A.; Rasool, F.; Hamid, A. Withania somnifera (L.) Dunal (Ashwagandha); Current Understanding and Future Prospect as a Potential Drug Candidate. Front. Pharmacol. 2022, 13, 1029123. [Google Scholar] [CrossRef]
- Dar, P.A.; Mir, S.A.; Bhat, J.A.; Hamid, A.; Singh, L.R.; Malik, F.; Dar, T.A. An Anti-Cancerous Protein Fraction from Withania Somnifera Induces ROS-Dependent Mitochondria-Mediated Apoptosis in Human MDA-MB-231 Breast Cancer Cells. Int. J. Biol. Macromol. 2019, 135, 77–87. [Google Scholar] [CrossRef]
- Bisht, P.; Rawat, V. Antibacterial Activity of Withania Somnifera against Gram-Positive Isolates from Pus Samples. AYU (Int. Q. J. Res. Ayurveda) 2014, 35, 330–332. [Google Scholar] [CrossRef]
- Pawar, P.; Gilda, S.; Sharma, S.; Jagtap, S.; Paradkar, A.; Mahadik, K.; Ranjekar, P.; Harsulkar, A. Rectal Gel Application of Withania Somnifera Root Extract Expounds Anti-Inflammatory and Muco-Restorative Activity in TNBS-Induced Inflammatory Bowel Disease. BMC Complement. Altern. Med. 2011, 11, 34. [Google Scholar] [CrossRef]
- Ojha, S.K.; Arya, D.S. Withania Somnifera Dunal (Ashwagandha): A Promising Remedy for Cardiovascular Diseases. World J. Med. Sci. 2009, 4, 156–158. [Google Scholar]
- Muralikrishnan, G.; Amanullah, S.; Basha, M.I.; Dinda, A.K.; Shakeel, F. Modulating Effect of Withania Somnifera on TCA Cycle Enzymes and Electron Transport Chain in Azoxymethane-Induced Colon Cancer in Mice. Immunopharmacol. Immunotoxicol. 2010, 32, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Kim, E.; Chung, T.; Han, C.; Park, S.; Han, J.; Bae, S.; Lee, J.; Kim, Y.; Jang, S.; et al. Hemistepsin A Suppresses Colorectal Cancer Growth through Inhibiting Pyruvate Dehydrogenase Kinase Activity. Sci. Rep. 2020, 10, 21940. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, W.; Xie, Y.; Chen, X.; Olmstead, E.E.; Lian, M.; Zhang, B.; Zaytseva, Y.Y.; Evers, B.M.; Spielmann, H.P.; et al. Diaminobutoxy-Substituted Isoflavonoid (DBI-1) Enhances the Therapeutic Efficacy of GLUT1 Inhibitor BAY-876 by Modulating Metabolic Pathways in Colon Cancer Cells. Mol. Cancer Ther. 2022, 21, 740–750. [Google Scholar] [CrossRef]
- Rashida, Z.; Laxman, S. The Pentose Phosphate Pathway and Organization of Metabolic Networks Enabling Growth Programs. Curr. Opin. Syst. Biol. 2021, 28, 100390. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The Return of Metabolism: Biochemistry and Physiology of the Pentose Phosphate Pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, L.; Liu, S.; Li, Y.; Xia, S.; Chen, D.; Wang, M.; Wu, S.; Dai, Q.; Vu, H.; et al. γ-6-Phosphogluconolactone, a Byproduct of the Oxidative Pentose Phosphate Pathway, Contributes to AMPK Activation through Inhibition of PP2A. Mol. Cell 2019, 76, 857–871. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Alcarraz-Vizán, G.; Marín, S.; Torres, J.L.; Cascante, M. Epicatechin Gallate Impairs Colon Cancer Cell Metabolic Productivity. J. Agric. Food Chem. 2013, 61, 4310–4317. [Google Scholar] [CrossRef]
- Vanamala, J.; Radhakrishnan, S.; Reddivari, L.; Bhat, V.B.; Ptitsyn, A. Resveratrol Suppresses Human Colon Cancer Cell Proliferation and Induces Apoptosis via Targeting the Pentose Phosphate and the Talin-FAK Signaling Pathways-A Proteomic Approach. Proteome Sci. 2011, 9, 49. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Tang, C.-F.; Shi, X.-K.; Lin, C.-Y.; Fatima, S.; Pan, X.-H.; Yang, D.-J.; Zhang, G.; Lu, A.-P.; Lin, S.-H.; et al. Halofuginone Inhibits Colorectal Cancer Growth through Suppression of Akt/mTORC1 Signaling and Glucose Metabolism. Oncotarget 2015, 6, 24148–24162. [Google Scholar] [CrossRef]
- Catanzaro, D.; Gabbia, D.; Cocetta, V.; Biagi, M.; Ragazzi, E.; Montopoli, M.; Carrara, M. Silybin Counteracts Doxorubicin Resistance by Inhibiting GLUT1 Expression. Fitoterapia 2018, 124, 42–48. [Google Scholar] [CrossRef]
- Amorim, R.; Pinheiro, C.; Miranda-Gonçalves, V.; Pereira, H.; Moyer, M.P.; Preto, A.; Baltazar, F. Monocarboxylate Transport Inhibition Potentiates the Cytotoxic Effect of 5-Fluorouracil in Colorectal Cancer Cells. Cancer Lett. 2015, 365, 68–78. [Google Scholar] [CrossRef]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New Aspects of Amino Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Shao, N.; Liu, J.; Zhao, J.; Chen, C.; Li, Q.; He, Z.; Zhao, X.; Xu, L. Amino Acid Metabolism in Tumor: New Shine in the Fog? Clin. Nutr. 2023, 42, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and Cancer: Cell Biology, Physiology, and Clinical Opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef]
- Le, A.; Lane, A.N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Zhang, H.; et al. Glucose-Independent Glutamine Metabolism via TCA Cycling for Proliferation and Survival in B Cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef]
- Kodama, M.; Oshikawa, K.; Shimizu, H.; Yoshioka, S.; Takahashi, M.; Izumi, Y.; Bamba, T.; Tateishi, C.; Tomonaga, T.; Matsumoto, M.; et al. A Shift in Glutamine Nitrogen Metabolism Contributes to the Malignant Progression of Cancer. Nat. Commun. 2020, 11, 1320. [Google Scholar] [CrossRef]
- Abu Aboud, O.; Habib, S.L.; Trott, J.; Stewart, B.; Liang, S.; Chaudhari, A.J.; Sutcliffe, J.; Weiss, R.H. Glutamine Addiction in Kidney Cancer Suppresses Oxidative Stress and Can Be Exploited for Real-Time Imaging. Cancer Res. 2017, 77, 6746–6758. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Yin, G.; Wang, G.; Liu, T.; Liang, L.; Yang, X.; Zhang, W.; Tang, D. Degradation of HIF-1α Induced by Curcumol Blocks Glutaminolysis and Inhibits Epithelial-Mesenchymal Transition and Invasion in Colorectal Cancer Cells. Cell Biol. Toxicol. 2023, 39, 1957–1978. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, M.; Wang, Z.; Zhang, J.; Cui, W.; Li, J.; Zhu, X.; Zhang, H.; Yang, D.; Xu, X. Curcumin Reverses Doxorubicin Resistance in Colon Cancer Cells at the Metabolic Level. J. Pharm. Biomed. Anal. 2021, 201, 114129. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Zhang, T.; Wei, P.; Li, N.; Zhang, W.; Ding, X.; Li, J. 1H NMR-Based Metabolomics Reveals the Antitumor Mechanisms of Triptolide in BALB/c Mice Bearing CT26 Tumors. Front. Pharmacol. 2019, 10, 1175. [Google Scholar] [CrossRef]
- He, W.; Tao, W.; Zhang, F.; Jie, Q.; He, Y.; Zhu, W.; Tan, J.; Shen, W.; Li, L.; Yang, Y.; et al. Lobetyolin Induces Apoptosis of Colon Cancer Cells by Inhibiting Glutamine Metabolism. J. Cell. Mol. Med. 2020, 24, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, S. Mammalian Lipids: Structure, Synthesis and Function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, X.; Yan, P.; Yang, C.; Li, Y.; Han, L.; Ren, X. Lipid Metabolism Reprogramming in Colorectal Cancer. J. Cell Biochem. 2023, 124, 3–16. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid Metabolism in Cancer: New Perspectives and Emerging Mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, L.; Qiu, Z.; Deng, W.; Wang, W. Key Molecules of Fatty Acid Metabolism in Gastric Cancer. Biomolecules 2022, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Alannan, M.; Fayyad-Kazan, H.; Trézéguet, V.; Merched, A. Targeting Lipid Metabolism in Liver Cancer. Biochemistry 2020, 59, 3951–3964. [Google Scholar] [CrossRef]
- Kwan, H.-Y.; Yang, Z.; Fong, W.-F.; Hu, Y.-M.; Yu, Z.-L.; Hsiao, W.-L.W. The Anticancer Effect of Oridonin Is Mediated by Fatty Acid Synthase Suppression in Human Colorectal Cancer Cells. J. Gastroenterol. 2013, 48, 182–192. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, D.; He, J.; Song, L.; Chen, H.; Zhang, Z.; Tan, N. Inhibition of Fatty Acid Synthesis Arrests Colorectal Neoplasm Growth and Metastasis: Anti-Cancer Therapeutical Effects of Natural Cyclopeptide RA-XII. Biochem. Biophys. Res. Commun. 2019, 512, 819–824. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, W.; Li, Y.; Xian, X.; Zhao, Z.; Liu, C.; Zou, J.; Li, J.; Fang, X.; Zhu, Y. Berberine Suppresses Colon Cancer Cell Proliferation by Inhibiting the SCAP/SREBP-1 Signaling Pathway-Mediated Lipogenesis. Biochem. Pharmacol. 2020, 174, 113776. [Google Scholar] [CrossRef]
- Park, E.-K.; Shin, Y.-W.; Lee, H.-U.; Kim, S.-S.; Lee, Y.-C.; Lee, B.-Y.; Kim, D.-H. Inhibitory Effect of Ginsenoside Rbl and Compound K on NO and Prostaglandin E2 Biosyntheses of RAW264.7 Cells Induced by Lipopolysaccharide. Biol. Pharm. Bull. 2005, 28, 652–656. [Google Scholar] [CrossRef]
- Dong, X.; Han, H.; Shi, H.; Wang, T.; Zhao, J. Compound K, a Metabolite of Ginseng Saponin, Induces Apoptosis of Hepatocellular Carcinoma Cells through the Mitochondria-Mediated Caspase-Dependent Pathway. Int. J. Clin. Exp. Med. 2017, 10, 11146–11156. [Google Scholar]
- Park, J.-S.; Shin, J.A.; Jung, J.-S.; Hyun, J.-W.; Van Le, T.K.; Kim, D.-H.; Park, E.-M.; Kim, H.-S. Anti-Inflammatory Mechanism of Compound K in Activated Microglia and Its Neuroprotective Effect on Experimental Stroke in Mice. J. Pharmacol. Exp. Ther. 2012, 341, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, L.; Xie, X.; Luo, J.; Yu, J.; Zhang, L.; Meng, W.; Zhou, Y.; Chen, L.; Ouyang, D.; et al. Targeting PLA2G16, a Lipid Metabolism Gene, by Ginsenoside Compound K to Suppress the Malignant Progression of Colorectal Cancer. J. Adv. Res. 2022, 36, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, M.; Li, X.; Li, N.; Zhao, X.; Wang, X.; Song, Y.; Quan, J.; Cheng, C.; Liu, J.; et al. Trichothecin Inhibits Invasion and Metastasis of Colon Carcinoma Associating with SCD-1-Mediated Metabolite Alteration. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158540. [Google Scholar] [CrossRef]
- Chu, G.; Miao, Y.; Huang, K.; Song, H.; Liu, L. Role and Mechanism of Rhizopus Nigrum Polysaccharide EPS1-1 as Pharmaceutical for Therapy of Hepatocellular Carcinoma. Front. Bioeng. Biotechnol. 2020, 8, 509. [Google Scholar] [CrossRef]
- Yu, Z.; Song, G.; Liu, J.; Wang, J.; Zhang, P.; Chen, K. Beneficial Effects of Extracellular Polysaccharide from Rhizopus Nigricans on the Intestinal Immunity of Colorectal Cancer Mice. Int. J. Biol. Macromol. 2018, 115, 718–726. [Google Scholar] [CrossRef]
- Cai, H.; Du, J.; Luo, C.; Li, S. External Application of Mirabilite before Surgery Can Reduce the Inflammatory Response and Accelerate Recovery in Mild Acute Biliary Pancreatitis. BMC Gastroenterol. 2023, 23, 264. [Google Scholar] [CrossRef]
- Pan, W.; Cai, S.; Latour, J.M.; Zhong, M.; Lv, M.; Li, J.; Zhang, X.; Zhang, Y. External Use of Mirabilite Combined with Lactulose Improves Postoperative Gastrointestinal Mobility among Older Patients Undergoing Abdominal Surgery. J. Adv. Nurs. 2021, 77, 755–762. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, A.; Zhou, X.; Sun, H.; Wang, X.; Liang, L.; Wang, X. High-Throughput Lipidomics Reveal Mirabilite Regulating Lipid Metabolism as Anticancer Therapeutics. RSC Adv. 2018, 8, 35600–35610. [Google Scholar] [CrossRef]
- Ni, T.; He, Z.; Dai, Y.; Yao, J.; Guo, Q.; Wei, L. Oroxylin A Suppresses the Development and Growth of Colorectal Cancer through Reprogram of HIF1α-Modulated Fatty Acid Metabolism. Cell Death Dis. 2017, 8, e2865. [Google Scholar] [CrossRef]
- Galeone, C.; Pelucchi, C.; Levi, F.; Negri, E.; Franceschi, S.; Talamini, R.; Giacosa, A.; La Vecchia, C. Onion and Garlic Use and Human Cancer. Am. J. Clin. Nutr. 2006, 84, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Mai, Y.; Li, H.; Wang, Z.; Xu, J.; He, X. Health Benefits of the Flavonoids from Onion: Constituents and Their Pronounced Antioxidant and Anti-Neuroinflammatory Capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jin, H.; Gong, W.; Zhang, C.; Zhou, A. Effect of Onion Flavonoids on Colorectal Cancer with Hyperlipidemia: An in Vivo Study. Onco Targets Ther. 2014, 7, 101–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lane, A.N.; Fan, T.W.-M. Regulation of Mammalian Nucleotide Metabolism and Biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Mullen, N.J.; Singh, P.K. Nucleotide Metabolism: A Pan-Cancer Metabolic Dependency. Nat. Rev. Cancer 2023, 23, 275–294. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Zhang, H.; Wang, C.; Yu, C.; Jiang, Z.; Zhang, W. Antitumor Mechanism of Hydroxycamptothecin via the Metabolic Perturbation of Ribonucleotide and Deoxyribonucleotide in Human Colorectal Carcinoma Cells. Molecules 2021, 26, 4902. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, S.; Zhang, Y.; Yang, Z.; Wang, W.; Meng, M.; Feng, J.; Zhang, P.; Xiao, L.; Lee, M.; et al. 3,3′-Diindolylmethane Enhances Fluorouracil Sensitivity via Inhibition of Pyrimidine Metabolism in Colorectal Cancer. Metabolites 2022, 12, 410. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamada, Y.; Kurumida, Y.; Kameda, T.; Sukeno, M.; Iizuka-Ohashi, M.; Sowa, Y.; Iizumi, Y.; Takakura, H.; Miyamoto, S.; et al. Rabdosianone I, a Bitter Diterpene from an Oriental Herb, Suppresses Thymidylate Synthase Expression by Directly Binding to ANT2 and PHB2. Cancers 2021, 13, 982. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, L.; Zhang, S.; Li, W.; Tou, F.; He, Q.; Rao, J.; Shen, Q. Peiminine Inhibits Colorectal Cancer Cell Proliferation by Inducing Apoptosis and Autophagy and Modulating Key Metabolic Pathways. Oncotarget 2017, 8, 47619–47631. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yu, Y.; Wang, J.; Yin, P.; Xu, K. Triterpenoids Extracted from Rhus Chinensis Mill Act Against Colorectal Cancer by Inhibiting Enzymes in Glycolysis and Glutaminolysis: Network Analysis and Experimental Validation. Nutr. Cancer-Int. J. 2020, 72, 293–319. [Google Scholar] [CrossRef]
- Sharma, S.H.; Thulasingam, S.; Chellappan, D.R.; Chinnaswamy, P.; Nagarajan, S. Morin and Esculetin Supplementation Modulates C-Myc Induced Energy Metabolism and Attenuates Neoplastic Changes in Rats Challenged with the Procarcinogen 1,2-Dimethylhydrazine. Eur. J. Pharmacol. 2017, 796, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wang, Y.; Xie, W.; Yang, T.; Jiang, Y.; Guo, Y.; Guan, J.; Liu, H. Metabolomics Study on the Antitumor Effect of Marine Natural Compound Flexibilide in HCT-116 Colon Cancer Cell Line. J. Chromatogr. B 2016, 1014, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, C.; Yu, C.; Qiu, Y.; Wen, X.; Zhang, C.; Yuan, C.; Jia, W. Metabonomic Profiling Reveals Cancer Chemopreventive Effects of American Ginseng on Colon Carcinogenesis in ApcMin/+ Mice. J. Proteome Res. 2015, 14, 3336–3347. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, A.; Zhang, H.; Zhou, X.; Wang, X.; Liu, L.; Wang, X. Ultra-Performance Liquid Chromatography/Mass Spectrometry Technology and High-Throughput Metabolomics for Deciphering the Preventive Mechanism of Mirabilite on Colorectal Cancer via the Modulation of Complex Metabolic Networks. RSC Adv. 2019, 9, 35356–35363. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, B.; Cong, W.; Zhang, M.; Li, Z.; Li, Y.; Liang, S.; Chen, K.; Yang, D.; Wu, Z. Amelioration of AOM/DSS-Induced Murine Colitis-Associated Cancer by Evodiamine Intervention Is Primarily Associated with Gut Microbiota-Metabolism-Inflammatory Signaling Axis. Front. Pharmacol. 2021, 12, 797605. [Google Scholar] [CrossRef]
- Chen, X.; Shi, B.; Qi, R.; Chang, X.; Zheng, H. Ultra-Performance Liquid Chromatography/Mass Spectrometry-Based Metabolomics for Discovering Potential Biomarkers and Metabolic Pathways of Colorectal Cancer in Mouse Model (ApcMin/plus) and Revealing the Effect of Honokiol. Front. Oncol. 2021, 11, 671014. [Google Scholar] [CrossRef]
- Jansson, A.; Gentile, M.; Sun, X.F. P53 Mutations Are Present in Colorectal Cancer with Cytoplasmic P53 Accumulation. Int. J. Cancer 2001, 92, 338–341. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, Y.; Qiao, C.; Ni, T.; Li, Z.; Wang, X.; Guo, Q.; Lu, N.; Wei, L. Oroxylin A Promotes PTEN-Mediated Negative Regulation of MDM2 Transcription via SIRT3-Mediated Deacetylation to Stabilize P53 and Inhibit Glycolysis in Wt-P53 Cancer Cells. J. Hematol. Oncol. 2015, 8, 41. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Goldman, A.R.; Zhang, X.; Speicher, D.W.; Dang, C.V. Measuring MYC-Mediated Metabolism in Tumorigenesis. Methods Mol. Biol. 2021, 2318, 231–239. [Google Scholar] [CrossRef]
- Guo, H.; Wan, B.; Wang, J.; Zhang, J.; Yao, W.; Shen, Z. Astragalus Saponins Inhibit Cell Growth, Aerobic Glycolysis and Attenuate the Inflammatory Response in a DSS-Induced Colitis Model. Int. J. Mol. Med. 2019, 43, 1041–1048. [Google Scholar] [CrossRef]
- Shi, J.; Shan, S.; Zhou, G.; Li, H.; Song, G.; Li, Z.; Yang, D. Bound Polyphenol from Foxtail Millet Bran Exhibits an Antiproliferative Activity in HT-29 Cells by Reprogramming miR-149-Mediated Aerobic Glycolysis. J. Funct. Foods 2019, 56, 246–254. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yokota, A.; Harada, H.; Huang, G. Hypoxia/Pseudohypoxia-Mediated Activation of Hypoxia-Inducible Factor-1 in Cancer. Cancer Sci. 2019, 110, 1510–1517. [Google Scholar] [CrossRef]
- Ji, L.; Shen, W.; Zhang, F.; Qian, J.; Jiang, J.; Weng, L.; Tan, J.; Li, L.; Chen, Y.; Cheng, H.; et al. Worenine Reverses the Warburg Effect and Inhibits Colon Cancer Cell Growth by Negatively Regulating HIF-1α. Cell Mol. Biol. Lett. 2021, 26, 19. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Chen, Q.; Gong, K.; Xu, X.; Xie, Y.; Zhang, W.; Cao, H.; Hu, T.; Hong, X.; Zhan, Y.-Y. Berberine Decelerates Glucose Metabolism via Suppression of mTOR-dependent HIF-1α Protein Synthesis in Colon Cancer Cells. Oncol. Rep. 2018, 39, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Noorolyai, S.; Shajari, N.; Baghbani, E.; Sadreddini, S.; Baradaran, B. The Relation between PI3K/AKT Signalling Pathway and Cancer. Gene 2019, 698, 120–128. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in Cancer: Mediator and More. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Long, F.; Lin, H.; Zhang, X.; Zhang, J.; Xiao, H.; Wang, T. Atractylenolide-I Suppresses Tumorigenesis of Breast Cancer by Inhibiting Toll-Like Receptor 4-Mediated Nuclear Factor-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 598939. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Zhu, L.-T.; Wang, Y.; Pan, D.; Yao, J.; You, Q.-D.; Guo, Q.-L. Wogonin Reverses Hypoxia Resistance of Human Colon Cancer HCT116 Cells via Downregulation of HIF-1α and Glycolysis, by Inhibiting PI3K/Akt Signaling Pathway. Mol. Carcinog. 2014, 53 (Suppl. S1), E107–E118. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Gong, R.-H.; Yang, D.-J.; Zhang, G.; Lu, A.-P.; Yan, S.-C.; Lin, S.-H.; Bian, Z.-X. Halofuginone Dually Regulates Autophagic Flux through Nutrient-Sensing Pathways in Colorectal Cancer. Cell Death Dis. 2017, 8, e2789. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Digel, M.; Küch, E.-M.; Stremmel, W.; Füllekrug, J. Silybin and Dehydrosilybin Decrease Glucose Uptake by Inhibiting GLUT Proteins. J. Cell. Biochem. 2011, 112, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Abouzeid, A.H.; Patel, N.R.; Rachman, I.M.; Senn, S.; Torchilin, V.P. Anti-Cancer Activity of Anti-GLUT1 Antibody-Targeted Polymeric Micelles Co-Loaded with Curcumin and Doxorubicin. J. Drug Target. 2013, 21, 994–1000. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Huang, L.; Duan, B.; Dai, S.; Cai, W.; Sun, M.; Jiang, Z.; Lu, R.; Jiang, Y.; et al. ATB0,+-Targeted Nanoparticles Initiate Autophagy Suppression to Overcome Chemoresistance for Enhanced Colorectal Cancer Therapy. Int. J. Pharm. 2023, 641, 123082. [Google Scholar] [CrossRef]
- Yh, K.; Js, L.; Nh, L.; Sh, K.; Cs, S.; Cg, S. Coptidis Rhizoma Extract Reverses 5-Fluorouracil Resistance in HCT116 Human Colorectal Cancer Cells via Modulation of Thymidylate Synthase. Molecules 2021, 26, 1856. [Google Scholar] [CrossRef]
- Wei, J.; Yang, P.; Li, W.; Hei, F.; Zeng, S.; Zhang, T.; Zhong, J.; Huang, D.; Chen, Z.; Wang, C.; et al. Gambogic Acid Potentiates the Chemosensitivity of Colorectal Cancer Cells to 5-Fluorouracil by Inhibiting Proliferation and Inducing Apoptosis. Exp. Ther. Med. 2017, 13, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Oyeniran, O.H.; Ademiluyi, A.O.; Oboh, G. Phenolic Constituents and Inhibitory Effects of the Leaf of Rauvolfia Vomitoria Afzel on Free Radicals, Cholinergic and Monoaminergic Enzymes in Rat’s Brain in Vitro. J. Basic. Clin. Physiol. Pharmacol. 2020, 32, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Miao, R.; Zhang, F.; Hao, Y.; Zhang, Y.; Zhang, Y.; Khurm, M.; Zhang, X.; Guo, Z. Peraksine Derivatives with Potential Anti-Inflammatory Activities from the Stems of Rauvolfia Vomitoria. Fitoterapia 2020, 146, 104704. [Google Scholar] [CrossRef]
- Cui, W.-L.; Guo, D.-X.; Wang, N.; Wang, Z.-F.; Ji, J.-B.; Wang, X.; Yang, C.-G.; Lin, Y.-Q.; Wang, S.-Q. Identification of Chemosensitizing Agents of Colorectal Cancer in Rauvolfia Vomitoria Using an NMR-Based Chemometric Approach. Front. Chem. 2022, 10, 1069591. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, W.; Wang, N.; You, Y.; Wang, J.; Wang, S. A Serum Metabolomics Study Based on LC-MS: Chemosensitization Effects of Rauvolfia Vomitoria Afzel. Combined with 5-Fluorouracil on Colorectal Cancer Mice. J. Pharm. Biomed. Anal. 2022, 221, 115074. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Zhang, F.; Zhang, J.; Shi, T.; Zeng, Z. Antioxidant Effect of Quercetin against Acute Spinal Cord Injury in Rats and Its Correlation with the p38MAPK/iNOS Signaling Pathway. Life Sci. 2013, 92, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, J.; Li, X.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.; Lu, G.; Zhou, J. Quercetin Induces P53-independent Cancer Cell Death through Lysosome Activation by the Transcription Factor EB and Reactive Oxygen Species-dependent Ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.-L.; Qi, M.-H.; Zou, X. Cinnamaldehyde/Chemotherapeutic Agents Interaction and Drug-Metabolizing Genes in Colorectal Cancer. Mol. Med. Rep. 2014, 9, 669–676. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. Recent Progress in Understanding the Mechanism of P-Glycoprotein-Mediated Drug Efflux. J. Membr. Biol. 2005, 206, 173–185. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Paini, M.; Casazza, A.A.; Perego, P. Effect of Encapsulating Agent on Physical-Chemical Characteristics of Olive Pomace Polyphenols-Rich Extracts. Chem. Eng. Trans. 2015, 43, 97–102. [Google Scholar] [CrossRef]
- Shakeri, F.; Kiani, S.; Rahimi, G.; Boskabady, M.H. Anti-Inflammatory, Antioxidant, and Immunomodulatory Effects of Berberis Vulgaris and Its Constituent Berberine, Experimental and Clinical, a Review. Phytother. Res. 2024, 38, 1882–1902. [Google Scholar] [CrossRef]
- Tan, X.-S.; Ma, J.-Y.; Feng, R.; Ma, C.; Chen, W.-J.; Sun, Y.-P.; Fu, J.; Huang, M.; He, C.-Y.; Shou, J.-W.; et al. Tissue Distribution of Berberine and Its Metabolites after Oral Administration in Rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Wang, F.; Liang, L.; Yu, M.; Wang, W.; Badar, I.H.; Bao, Y.; Zhu, K.; Li, Y.; Shafi, S.; Li, D.; et al. Advances in Antitumor Activity and Mechanism of Natural Steroidal Saponins: A Review of Advances, Challenges, and Future Prospects. Phytomedicine 2024, 128, 155432. [Google Scholar] [CrossRef]
- Xu, D.; Shao, F.; Bian, X.; Meng, Y.; Liang, T.; Lu, Z. The Evolving Landscape of Noncanonical Functions of Metabolic Enzymes in Cancer and Other Pathologies. Cell Metab. 2021, 33, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Smit, T.; Calitz, C.; Willers, C.; Svitina, H.; Hamman, J.; Fey, S.J.; Gouws, C.; Wrzesinski, K. Characterization of an Alginate Encapsulated LS180 Spheroid Model for Anti-Colorectal Cancer Compound Screening. ACS Med. Chem. Lett. 2020, 11, 1014–1021. [Google Scholar] [CrossRef]
- Ekawati, M.M.; Nasution, M.A.F.; Siregar, S.; Rizki, I.F.; Tambunan, U.S.F. Pharmacophore-Based Virtual Screening and Molecular Docking Simulation of Terpenoid Compounds as the Inhibitor of Sonic Hedgehog Protein for Colorectal Cancer Therapy. In Proceedings of the 13th Joint Conference on Chemistry (13th JCC), Semarang, Indonesia, 7–8 September 2018; IoP Publishing Ltd.: Bristol, UK, 2019; Volume 509, p. 012075. [Google Scholar]
- Martínez-Reyes, I.; Cardona, L.R.; Kong, H.; Vasan, K.; McElroy, G.S.; Werner, M.; Kihshen, H.; Reczek, C.R.; Weinberg, S.E.; Gao, P.; et al. Mitochondrial Ubiquinol Oxidation Is Necessary for Tumour Growth. Nature 2020, 585, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Kaweesa, E.N.; Padhi, A.; Davis, G.N.; Mcmillan, R.P.; Brown, D.A.; Nain, A.S.; Loesgen, S. Combination of the Natural Product Mensacarcin with Vemurafenib (Zelboraf) Combats BRAF Mutant and Chemo-Resistant Melanoma in Vitro by Affecting Cell Metabolism and Cellular Migration. Adv. Cancer Biol.-Metastasis 2022, 6, 100070. [Google Scholar] [CrossRef]
- Jain, H.; Chella, N. Methods to Improve the Solubility of Therapeutical Natural Products: A Review. Environ. Chem. Lett. 2021, 19, 111–121. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).