Association of Whole Blood Amino Acid and Acylcarnitine Metabolome with Anthropometry and IGF-I Serum Levels in Healthy Children and Adolescents in Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Study Design and Selection Criteria
2.3. Preanalytics and Analytics
2.4. Other Measures
2.5. Statistical Analysis
3. Results
3.1. Variance of Metabolites
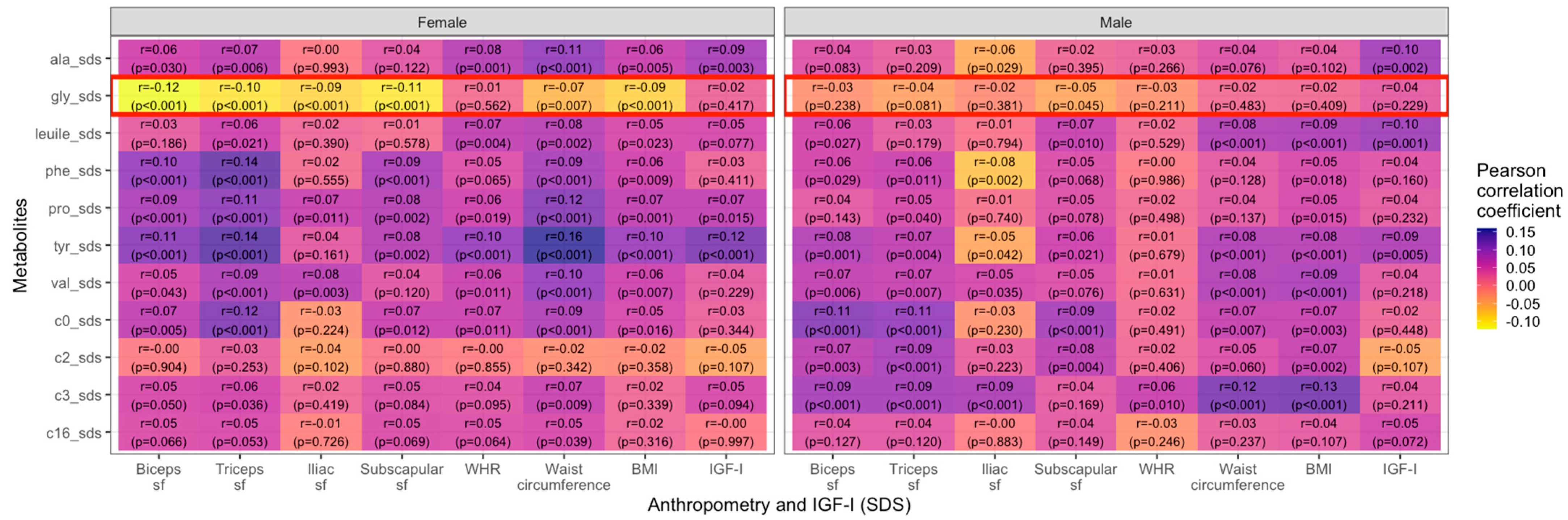
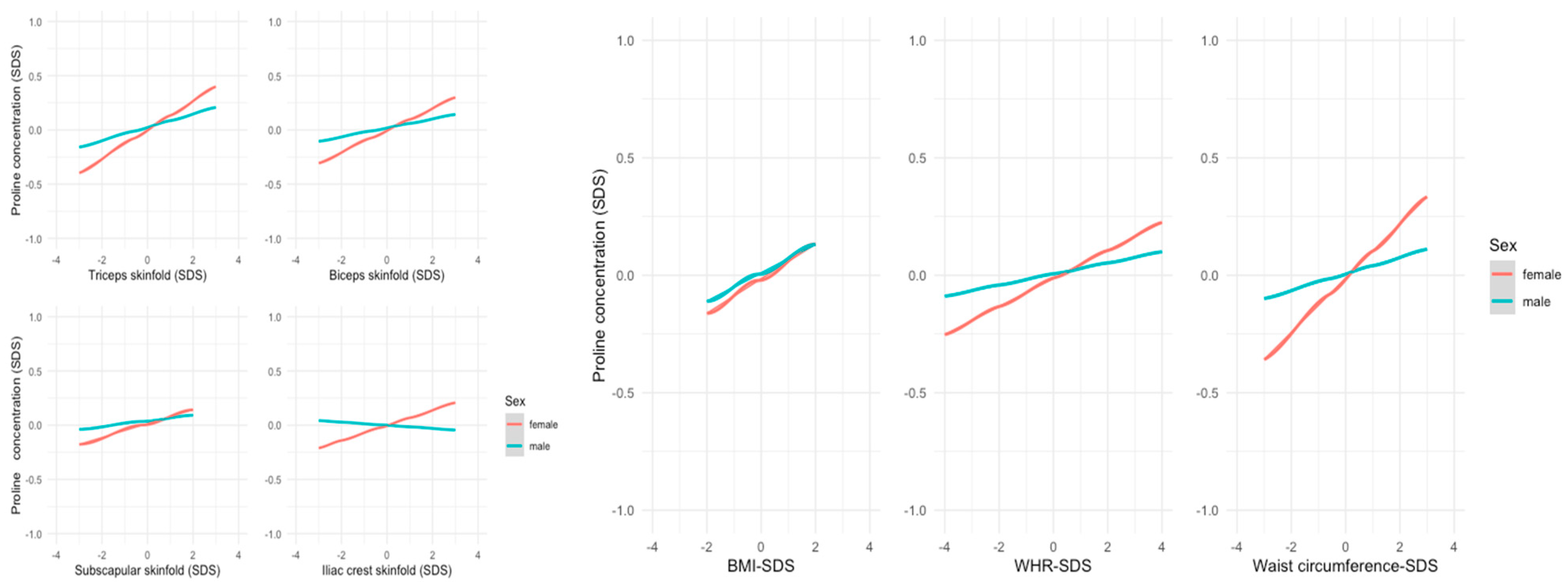
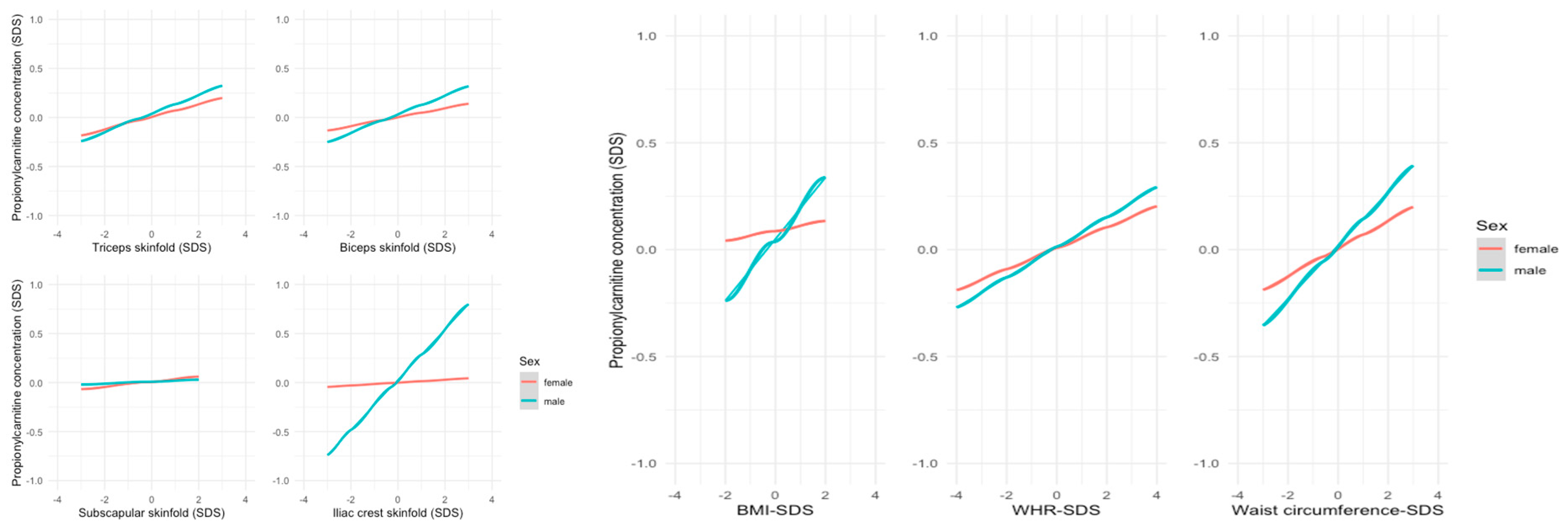
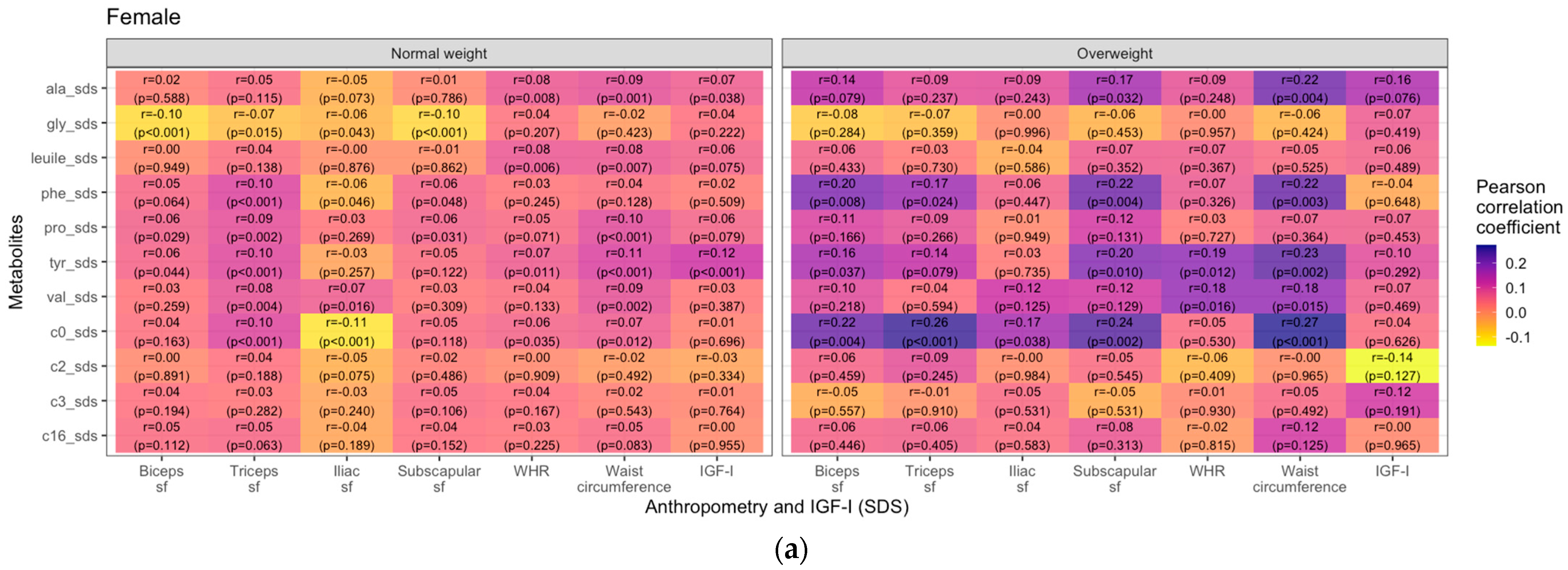
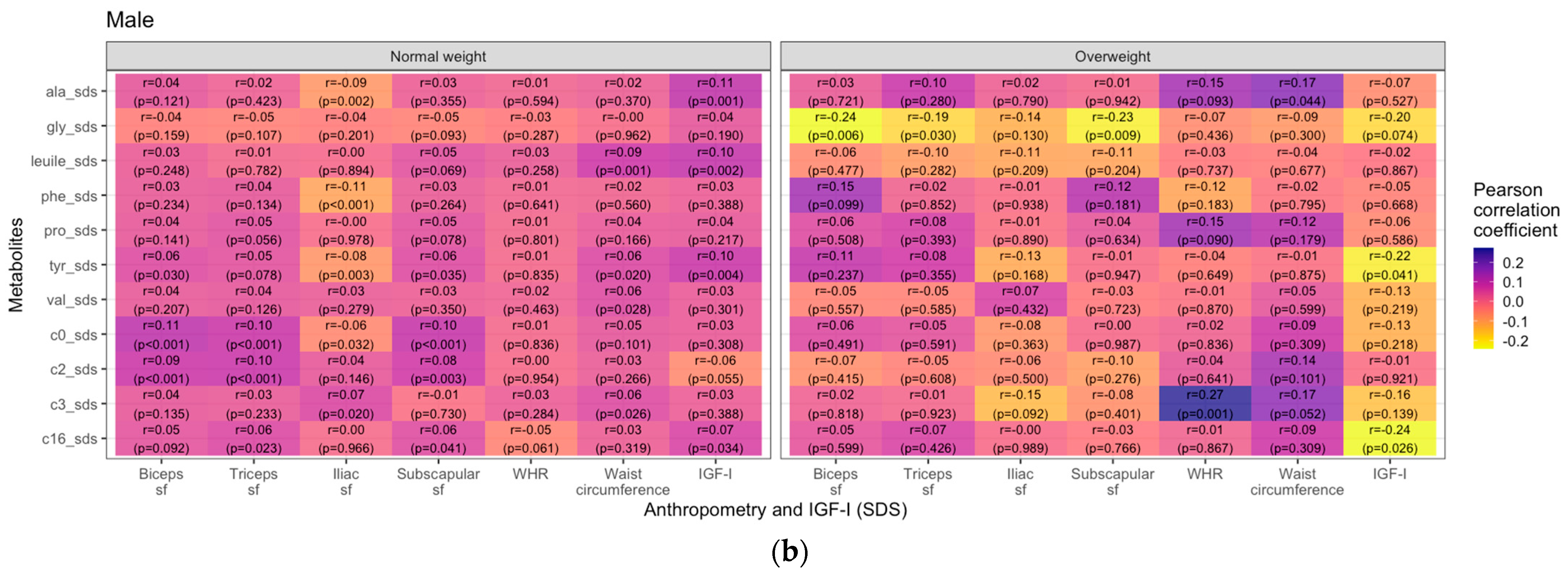
3.2. Metabolites and Anthropometric Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Spiekerkoetter, U.; Krude, H. Target Diseases for Neonatal Screening in Germany. Dtsch. Ärzteblatt Int. 2022, 119, 306–316. [Google Scholar] [CrossRef]
- Miller, M.J.; Cusmano-Ozog, K.; Oglesbee, D.; Young, S. Laboratory Analysis of Acylcarnitines, 2020 Update: A Technical Standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 249–258. [Google Scholar] [CrossRef]
- Holeček, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; DeLany, J.P. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Tong, L.; Tian, M.; Ma, X.; Bai, L.; Zhou, J.; Ding, W. Metabolome Profiling and Pathway Analysis in Metabolically Healthy and Unhealthy Obesity among Chinese Adolescents Aged 11–18 Years. Metabolites 2023, 13, 641. [Google Scholar] [CrossRef]
- Qu, H.-Q.; Glessner, J.; Qu, J.; Gilhool, S.; Mentch, F.; Campbell, I.; Sleiman, P.; Connolly, J.J.; Hakonarson, H.; IHCC Consortium. Metabolomic Profiling of Samples from Pediatric Patients with Asthma Unveils Deficient Nutrients in African Americans. iScience 2022, 25, 104650. [Google Scholar] [CrossRef]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating Branched-Chain Amino Acid Concentrations Are Associated with Obesity and Future Insulin Resistance in Children and Adolescents: Branched-Chain Amino Acids and IR in Children. Pediatr. Obes. 2013, 8, 52–61. [Google Scholar] [CrossRef]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.-E.; Oken, E. Metabolomic Profiles and Childhood Obesity. Obesity 2014, 22, 2570–2578. [Google Scholar] [CrossRef]
- Zhao, X.; Gang, X.; Liu, Y.; Sun, C.; Han, Q.; Wang, G. Using Metabolomic Profiles as Biomarkers for Insulin Resistance in Childhood Obesity: A Systematic Review. J. Diabetes Res. 2016, 2016, 8160545. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Jang, H.B.; Ra, M.; Choi, Y.; Lee, H.-J.; Park, J.Y.; Kang, J.H.; Park, K.-H.; Park, S.I.; Song, J. Prediction of Future Risk of Insulin Resistance and Metabolic Syndrome Based on Korean Boy’s Metabolite Profiling. Obes. Res. Clin. Pract. 2015, 9, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of Branched-Chain Amino Acid Metabolism in the Pathogenesis of Obesity and Type 2 Diabetes-Related Metabolic Disturbances BCAA Metabolism in Type 2 Diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ba Ssalamah, A.; Item, C.B.; De Gier, C.; Walleczek, N.; Metz, T.F.; Jakober, I.; Greber-Platzer, S.; et al. A Branched-chain Amino Acid-based Metabolic Score Can Predict Liver Fat in Children and Adolescents with Severe Obesity. Pediatr. Obes. 2021, 16, e12739. [Google Scholar] [CrossRef]
- Hirschel, J.; Vogel, M.; Baber, R.; Garten, A.; Beuchel, C.; Dietz, Y.; Dittrich, J.; Körner, A.; Kiess, W.; Ceglarek, U. Relation of Whole Blood Amino Acid and Acylcarnitine Metabolome to Age, Sex, BMI, Puberty, and Metabolic Markers in Children and Adolescents. Metabolites 2020, 10, 149. [Google Scholar] [CrossRef]
- Graf, G.H.-J.; Biroli, P.; Belsky, D.W. Critical Periods in Child Development and the Transition to Adulthood. JAMA Netw. Open 2021, 4, e2033359. [Google Scholar] [CrossRef]
- Wood, C.L.; Lane, L.C.; Cheetham, T. Puberty: Normal Physiology (Brief Overview). Best Pract Res. Clin. Endocrinol. Metab. 2019, 33, 101265. [Google Scholar] [CrossRef]
- Ong, K.; Kratzsch, J.; Kiess, W.; Dunger, D.; ALSPAC Study Team. Circulating IGF-I Levels in Childhood Are Related to Both Current Body Composition and Early Postnatal Growth Rate. J. Clin. Endocrinol. Metab. 2002, 87, 1041–1044. [Google Scholar] [CrossRef]
- Van Vught, A.J.A.H.; Nieuwenhuizen, A.G.; Brummer, R.-J.M.; Westerterp-Plantenga, M.S. Effects of Oral Ingestion of Amino Acids and Proteins on the Somatotropic Axis. J. Clin. Endocrinol. Metab. 2008, 93, 584–590. [Google Scholar] [CrossRef]
- Available online: https://home.uni-leipzig.de/lifechild/research-profile/ (accessed on 1 September 2024).
- Quante, M.; Hesse, M.; Döhnert, M.; Fuchs, M.; Hirsch, C.; Sergeyev, E.; Casprzig, N.; Geserick, M.; Naumann, S.; Koch, C.; et al. The LIFE Child Study: A Life Course Approach to Disease and Health. BMC Public Health 2012, 12, 1021. [Google Scholar] [CrossRef]
- The LIFE Child Study Team; Poulain, T.; Baber, R.; Vogel, M.; Pietzner, D.; Kirsten, T.; Jurkutat, A.; Hiemisch, A.; Hilbert, A.; Kratzsch, J.; et al. The LIFE Child Study: A Population-Based Perinatal and Pediatric Cohort in Germany. Eur. J. Epidemiol. 2017, 32, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Ceglarek, U.; Müller, P.; Stach, B.; Bührdel, P.; Thiery, J.; Kiess, W. Validation of the Phenylalanine/Tyrosine Ratio Determined by Tandem Mass Spectrometry: Sensitive Newborn Screening for Phenylketonuria. Clin. Chem. Lab. Med. 2002, 40, 693. [Google Scholar] [CrossRef]
- Hörenz, C.; Vogel, M.; Wirkner, K.; Ceglarek, U.; Thiery, J.; Pfäffle, R.; Kiess, W.; Kratzsch, J. BMI and Contraceptives Affect New Age-, Sex-, and Puberty-Adjusted IGF-I and IGFBP-3 Reference Ranges Across Life Span. J. Clin. Endocrinol. Metab. 2022, 107, e2991–e3002. [Google Scholar] [CrossRef]
- Rönnecke, E.; Vogel, M.; Bussler, S.; Grafe, N.; Jurkutat, A.; Schlingmann, M.; Koerner, A.; Kiess, W. Age- and Sex-Related Percentiles of Skinfold Thickness, Waist and Hip Circumference, Waist-to-Hip Ratio and Waist-to-Height Ratio: Results from a Population-Based Pediatric Cohort in Germany (LIFE Child). Obes. Facts 2019, 12, 25–39. [Google Scholar] [CrossRef]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Xi, B.; Zong, X.; Kelishadi, R.; Litwin, M.; Hong, Y.M.; Poh, B.K.; Steffen, L.M.; Galcheva, S.V.; Herter-Aeberli, I.; Nawarycz, T.; et al. International Waist Circumference Percentile Cutoffs for Central Obesity in Children and Adolescents Aged 6 to 18 Years. J. Clin. Endocrinol. Metab. 2020, 105, e1569–e1583. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, M.; Bokor, B.R. Tanner Stages. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wabitsch, M.; Moss, A. Evidence-Based (S3) Guideline of the Working Group on Childhood and Adolescent Obesity (AGA) of the German Obesity Society (DAG) and the German Society of Pediatrics and Adolescent Medicine (DGKJ). 2019. Available online: https://www.awmf.org/leitlinien/detail/ll/050-002.html (accessed on 1 September 2024).
- Faizi, N.; Alvi, Y. Correlation. In Biostatistics Manual for Health Research; Elsevier: Amsterdam, The Netherlands, 2023; pp. 109–126. ISBN 978-0-443-18550-2. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hardikar, S.; Albrechtsen, R.D.; Achaintre, D.; Lin, T.; Pauleck, S.; Playdon, M.; Holowatyj, A.N.; Gigic, B.; Schrotz-King, P.; Boehm, J.; et al. Impact of Pre-Blood Collection Factors on Plasma Metabolomic Profiles. Metabolites 2020, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Beuchel, C.; Becker, S.; Dittrich, J.; Kirsten, H.; Toenjes, A.; Stumvoll, M.; Loeffler, M.; Thiele, H.; Beutner, F.; Thiery, J.; et al. Clinical and Lifestyle Related Factors Influencing Whole Blood Metabolite Levels—A Comparative Analysis of Three Large Cohorts. Mol. Metab. 2019, 29, 76–85. [Google Scholar] [CrossRef]
- Newbern, D.; Gumus Balikcioglu, P.; Balikcioglu, M.; Bain, J.; Muehlbauer, M.; Stevens, R.; Ilkayeva, O.; Dolinsky, D.; Armstrong, S.; Irizzary, K.; et al. Sex Differences in Biomarkers Associated with Insulin Resistance in Obese Adolescents: Metabolomic Profiling and Principal Components Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 4730–4739. [Google Scholar] [CrossRef]
- Würtz, P.; Wang, Q.; Kangas, A.J.; Richmond, R.C.; Skarp, J.; Tiainen, M.; Tynkkynen, T.; Soininen, P.; Havulinna, A.S.; Kaakinen, M.; et al. Metabolic Signatures of Adiposity in Young Adults: Mendelian Randomization Analysis and Effects of Weight Change. PLoS Med. 2014, 11, e1001765. [Google Scholar] [CrossRef]
- Azab, S.M.; Shanmuganathan, M.; de Souza, R.J.; Kroezen, Z.; Desai, D.; Williams, N.C.; Morrison, K.M.; Atkinson, S.A.; Teo, K.K.; Azad, M.B.; et al. Early Sex-Dependent Differences in Metabolic Profiles of Overweight and Adiposity in Young Children: A Cross-Sectional Analysis. BMC Med. 2023, 21, 176. [Google Scholar] [CrossRef]
- Saner, C.; Harcourt, B.E.; Pandey, A.; Ellul, S.; McCallum, Z.; Kao, K.-T.; Twindyakirana, C.; Pons, A.; Alexander, E.J.; Saffery, R.; et al. Sex and Puberty-Related Differences in Metabolomic Profiles Associated with Adiposity Measures in Youth with Obesity. Metabolomics 2019, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Laamanen, S.E.; Eloranta, A.-M.; Haapala, E.A.; Sallinen, T.; Schwab, U.; Lakka, T.A. Associations of Diet Quality and Food Consumption with Serum Biomarkers for Lipid and Amino Acid Metabolism in Finnish Children: The PANIC Study. Eur. J. Nutr. 2024, 63, 623–637. [Google Scholar] [CrossRef]
- Kubo, H.; Sawada, S.; Satoh, M.; Asai, Y.; Kodama, S.; Sato, T.; Tomiyama, S.; Seike, J.; Takahashi, K.; Kaneko, K.; et al. Insulin-like Growth Factor-1 Levels Are Associated with High Comorbidity of Metabolic Disorders in Obese Subjects; a Japanese Single-Center, Retrospective-Study. Sci. Rep. 2022, 12, 20130. [Google Scholar] [CrossRef] [PubMed]
- Juiz-Valiña, P.; Pena-Bello, L.; Cordido, M.; Outeiriño-Blanco, E.; Pértega, S.; Varela-Rodriguez, B.; Garcia-Brao, M.J.; Mena, E.; Sangiao-Alvarellos, S.; Cordido, F. Altered GH-IGF-1 Axis in Severe Obese Subjects Is Reversed after Bariatric Surgery-Induced Weight Loss and Related with Low-Grade Chronic Inflammation. JCM 2020, 9, 2614. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Lee, E.; Kim, K.; Cha, B.; Song, Y.; Lim, S.; Lee, H.; Huh, K. Effect of Obesity on Total and Free Insulin-like Growth Factor (IGF)-1, and Their Relationship to IGF-Binding Protein (BP)-1, IGFBP-2, IGFBP-3, Insulin, and Growth Hormone. Int. J. Obes. 1997, 21, 355–359. [Google Scholar] [CrossRef]
- Knacke, H.; Pietzner, M.; Do, K.T.; Römisch-Margl, W.; Kastenmüller, G.; Völker, U.; Völzke, H.; Krumsiek, J.; Artati, A.; Wallaschofski, H.; et al. Metabolic Fingerprints of Circulating IGF-1 and the IGF-1/IGFBP-3 Ratio: A Multifluid Metabolomics Study. J. Clin. Endocrinol. Metab. 2016, 101, 4730–4742. [Google Scholar] [CrossRef]
- Staiano, A.E.; Katzmarzyk, P.T. Ethnic and Sex Differences in Body Fat and Visceral and Subcutaneous Adiposity in Children and Adolescents. Int. J. Obes. 2012, 36, 1261–1269. [Google Scholar] [CrossRef]
- Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008.
- Wahl, S.; Yu, Z.; Kleber, M.; Singmann, P.; Holzapfel, C.; He, Y.; Mittelstrass, K.; Polonikov, A.; Prehn, C.; Römisch-Margl, W.; et al. Childhood Obesity Is Associated with Changes in the Serum Metabolite Profile. Obes. Facts 2012, 5, 660–670. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Ocampo-Medina, E.; Gutierrez-Aguilar, R.; Macías-Kauffer, L.; Villamil-Ramírez, H.; López-Contreras, B.E.; León-Mimila, P.; Vega-Badillo, J.; Gutierrez-Vidal, R.; Villarruel-Vazquez, R.; et al. An Amino Acid Signature Associated with Obesity Predicts 2-Year Risk of Hypertriglyceridemia in School-Age Children. Sci. Rep. 2017, 7, 5607. [Google Scholar] [CrossRef]
- Taylor, R.W.; Jones, I.E.; Williams, S.M.; Goulding, A. Evaluation of Waist Circumference, Waist-to-Hip Ratio, and the Conicity Index as Screening Tools for High Trunk Fat Mass, as Measured by Dual-Energy X-Ray Absorptiometry, in Children Aged 3–19 y. Am. J. Clin. Nutr. 2000, 72, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.Y.; Kim, O.Y.; Ham, B.M.; Kim, H.-J.; Kwon, D.Y.; Jang, Y.; Lee, J.H. Metabolic Profiling of Plasma in Overweight/Obese and Lean Men Using Ultra Performance Liquid Chromatography and Q-TOF Mass Spectrometry (UPLC−Q-TOF MS). J. Proteome Res. 2010, 9, 4368–4375. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Michaliszyn, S.F.; de las Heras, J.; Bacha, F.; Lee, S.; Chace, D.H.; DeJesus, V.R.; Vockley, J.; Arslanian, S.A. Metabolomic Profiling of Fatty Acid and Amino Acid Metabolism in Youth with Obesity and Type 2 Diabetes: Evidence for Enhanced Mitochondrial Oxidation. Diabetes Care 2012, 35, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma Acylcarnitine Profiles Suggest Incomplete Long-Chain Fatty Acid β-Oxidation and Altered Tricarboxylic Acid Cycle Activity in Type 2 Diabetic African-American Women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Pinelli, L.; Schutz, Y. Increased Fat Oxidation in Prepubertal Obese Children: A Metabolic Defense against Further Weight Gain? J. Pediatr. 1995, 126, 15–20. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of Mitochondria in Human Skeletal Muscle in Type 2 Diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef]
- Butte, N.F.; Liu, Y.; Zakeri, I.F.; Mohney, R.P.; Mehta, N.; Voruganti, V.S.; Göring, H.; Cole, S.A.; Comuzzie, A.G. Global Metabolomic Profiling Targeting Childhood Obesity in the Hispanic Population. Am. J. Clin. Nutr. 2015, 102, 256–267. [Google Scholar] [CrossRef]
- Jois, M.; Hall, B.; Fewer, K.; Brosnan, J.T. Regulation of Hepatic Glycine Catabolism by Glucagon. J. Biol. Chem. 1989, 264, 3347–3351. [Google Scholar] [CrossRef]
- Del Prato, S.; Gallwitz, B.; Holst, J.J.; Meier, J.J. The Incretin/Glucagon System as a Target for Pharmacotherapy of Obesity. Obes. Rev. 2022, 23, e13372. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Müllner, E.; Röhnisch, H.E.; von Brömssen, C.; Moazzami, A.A. Metabolomics Analysis Reveals Altered Metabolites in Lean Compared with Obese Adolescents and Additional Metabolic Shifts Associated with Hyperinsulinaemia and Insulin Resistance in Obese Adolescents: A Cross-Sectional Study. Metabolomics 2021, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, J.; Chen, K.; Saito, R.; Gangoiti, J.; Mendez, E.; Nikita, M.E.; Barshop, B.A.; Natarajan, L.; Sharma, K.; Kim, J.J. Identification of Pathognomonic Purine Synthesis Biomarkers by Metabolomic Profiling of Adolescents with Obesity and Type 2 Diabetes. PLoS ONE 2020, 15, e0234970. [Google Scholar] [CrossRef] [PubMed]
- Hosking, J.; Pinkney, J.; Jeffery, A.; Cominetti, O.; Da Silva, L.; Collino, S.; Kussmann, M.; Hager, J.; Martin, F.-P. Insulin Resistance during Normal Child Growth and Development Is Associated with a Distinct Blood Metabolic Phenotype (Earlybird 72). Pediatr. Diabetes 2019, 20, 832–841. [Google Scholar] [CrossRef]
- Bervoets, L.; Massa, G.; Guedens, W.; Reekmans, G.; Noben, J.-P.; Adriaensens, P. Identification of Metabolic Phenotypes in Childhood Obesity by 1H NMR Metabolomics of Blood Plasma. Future Sci. OA 2018, 4, FSO310. [Google Scholar] [CrossRef] [PubMed]
- Gumus Balikcioglu, P.; Jachthuber Trub, C.; Balikcioglu, M.; Ilkayeva, O.; White, P.J.; Muehlbauer, M.; Bain, J.R.; Armstrong, S.; Freemark, M. Branched-Chain α-Keto Acids and Glutamate/Glutamine: Biomarkers of Insulin Resistance in Childhood Obesity. Endocrinol. Diabetes Metab. 2023, 6, e388. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Jang, H.-B.; Kim, H.-J.; Lee, H.-J. Identification of Biomarkers Related to Metabolically Unhealthy Obesity in Korean Obese Adolescents: A Cross-Sectional Study. Children 2023, 10, 322. [Google Scholar] [CrossRef]
- Jachthuber Trub, C.; Balikcioglu, M.; Freemark, M.; Bain, J.; Muehlbauer, M.; Ilkayeva, O.; White, P.J.; Armstrong, S.; Østbye, T.; Grambow, S.; et al. Impact of Lifestyle Intervention on Branched-Chain Amino Acid Catabolism and Insulin Sensitivity in Adolescents with Obesity. Endocrinol. Diabetes Metab. 2021, 4, e00250. [Google Scholar] [CrossRef]

| Follow-Up Visits | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Male (n) | 531 | 314 | 231 | 49 | 0 |
| Female (n) | 516 | 303 | 219 | 48 | 2 |
| All N = 2213 | Female N = 1088 | Male N = 1125 | |
|---|---|---|---|
| Mean age (years) ± SD | 8.11 (5.13) | 8.46 (5.21) | 7.77 (5.04) |
| Weight group, n% | |||
| Underweight | 200 (9.17%) | 91 (8.47%) | 109 (9.85%) |
| Normal weight | 1764 (80.8%) | 865 (80.5%) | 899 (81.2%) |
| Overweight | 139 (6.37%) | 80 (7.44%) | 59 (5.33%) |
| Obese | 79 (3.62%) | 39 (3.63%) | 40 (3.61%) |
| Puberty status | |||
| Prepubertal | 1215 (68.6%) | 577 (60.0%) | 638 (78.8%) |
| Pubertal | 556 (31.4%) | 384 (40.0%) | 172 (21.2%) |
| Anthropometry (mean ± SD) | |||
| WHR | 0.82 (0.07) | 0.80 (0.07) | 0.85 (0.06) |
| BMI | 17.66 (3.33) | 17.83 (3.54) | 17.49 (3.11) |
| Waist circumference (cm) | 60.96 (9.66) | 60.47 (9.48) | 61.45 (9.80) |
| Biceps skinfold thickness (mm) | 7.26 (3.91) | 8.20 (4.15) | 6.34 (3.42) |
| Triceps skinfold thickness (mm) | 12.30 (5.51) | 13.69 (5.67) | 10.96 (4.99) |
| Subscapular skinfold thickness (mm) | 8.64 (5.33) | 9.43 (5.72) | 7.86 (4.80) |
| Iliac crest skinfold thickness (mm) | 9.51 (6.78) | 10.81 (6.79) | 8.26 (6.54) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensch, R.; Baber, R.; Körner, A.; Kiess, W.; Ceglarek, U.; Garten, A.; Vogel, M. Association of Whole Blood Amino Acid and Acylcarnitine Metabolome with Anthropometry and IGF-I Serum Levels in Healthy Children and Adolescents in Germany. Metabolites 2024, 14, 489. https://doi.org/10.3390/metabo14090489
Jensch R, Baber R, Körner A, Kiess W, Ceglarek U, Garten A, Vogel M. Association of Whole Blood Amino Acid and Acylcarnitine Metabolome with Anthropometry and IGF-I Serum Levels in Healthy Children and Adolescents in Germany. Metabolites. 2024; 14(9):489. https://doi.org/10.3390/metabo14090489
Chicago/Turabian StyleJensch, Ricky, Ronny Baber, Antje Körner, Wieland Kiess, Uta Ceglarek, Antje Garten, and Mandy Vogel. 2024. "Association of Whole Blood Amino Acid and Acylcarnitine Metabolome with Anthropometry and IGF-I Serum Levels in Healthy Children and Adolescents in Germany" Metabolites 14, no. 9: 489. https://doi.org/10.3390/metabo14090489
APA StyleJensch, R., Baber, R., Körner, A., Kiess, W., Ceglarek, U., Garten, A., & Vogel, M. (2024). Association of Whole Blood Amino Acid and Acylcarnitine Metabolome with Anthropometry and IGF-I Serum Levels in Healthy Children and Adolescents in Germany. Metabolites, 14(9), 489. https://doi.org/10.3390/metabo14090489








