Propionic Acidemia, Methylmalonic Acidemia, and Cobalamin C Deficiency: Comparison of Untargeted Metabolomic Profiles
Abstract
1. Introduction
2. Experimental Design
Patients
3. Procedure
3.1. Sample Preparation
3.2. UHPLC and HRMS
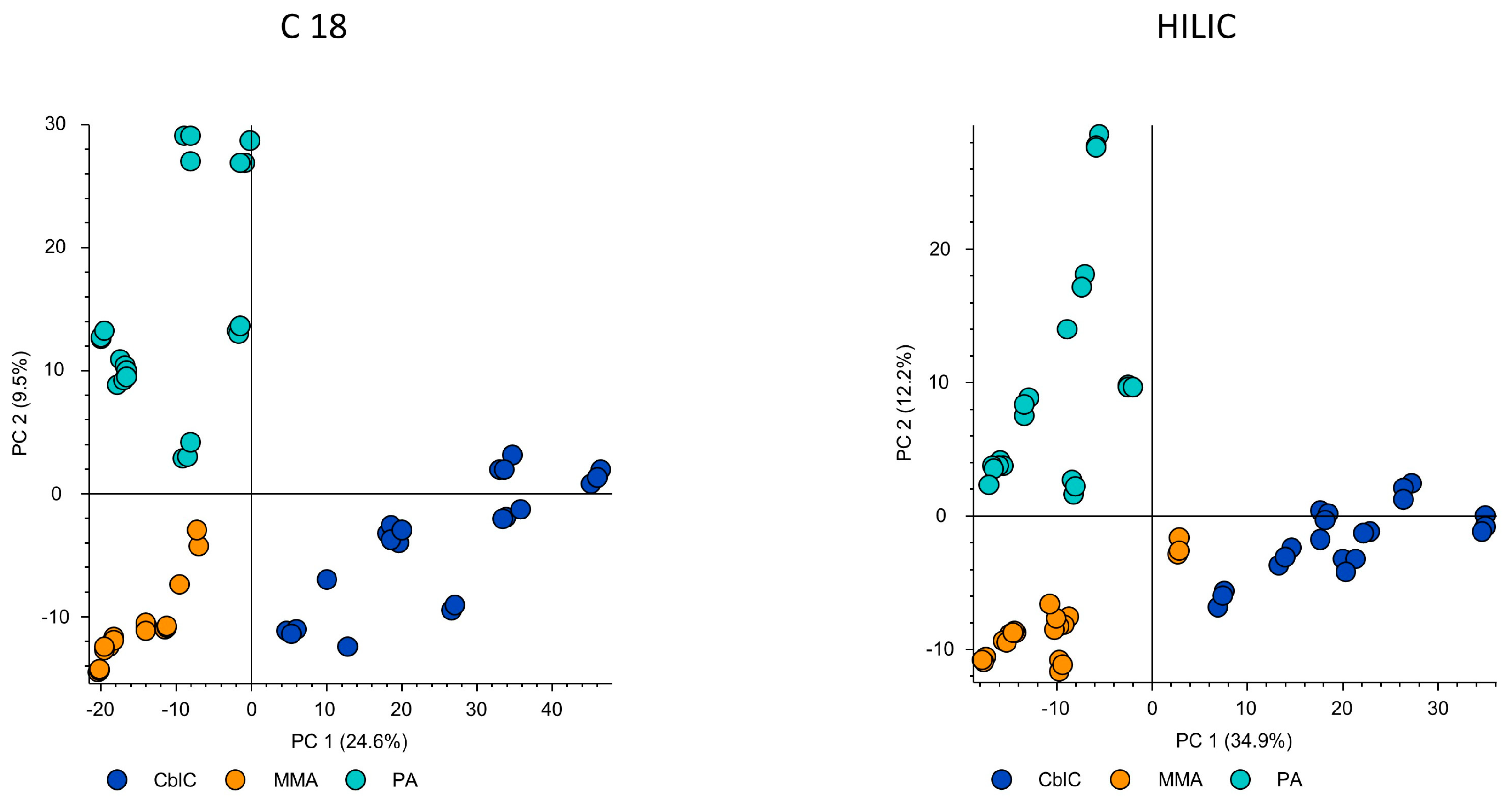
3.3. Metabolomic Data Analysis
4. Results
4.1. Significantly Different Metabolites
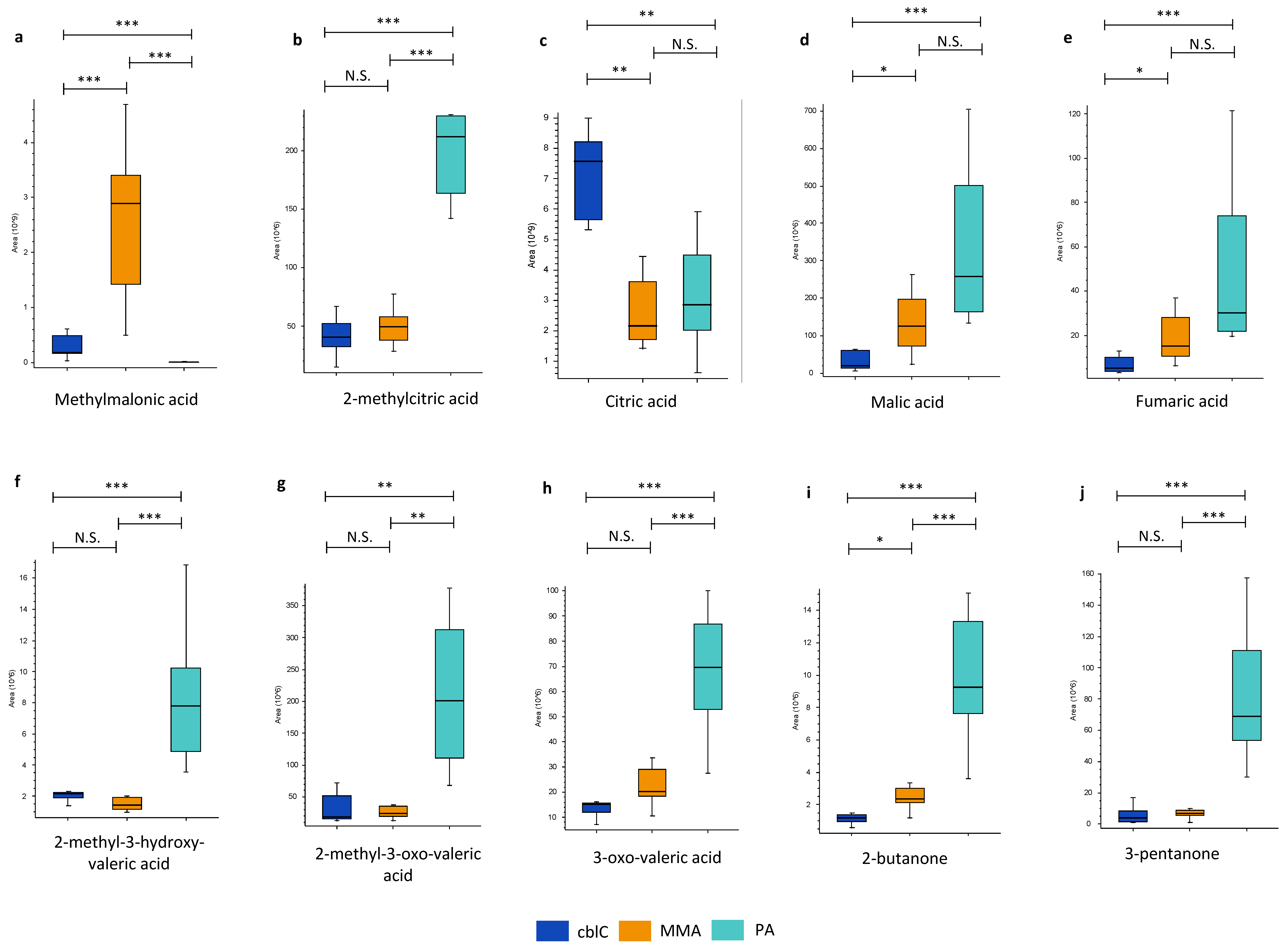
4.1.1. Organic Acids and Ketones
4.1.2. Amino Acids and Peptides
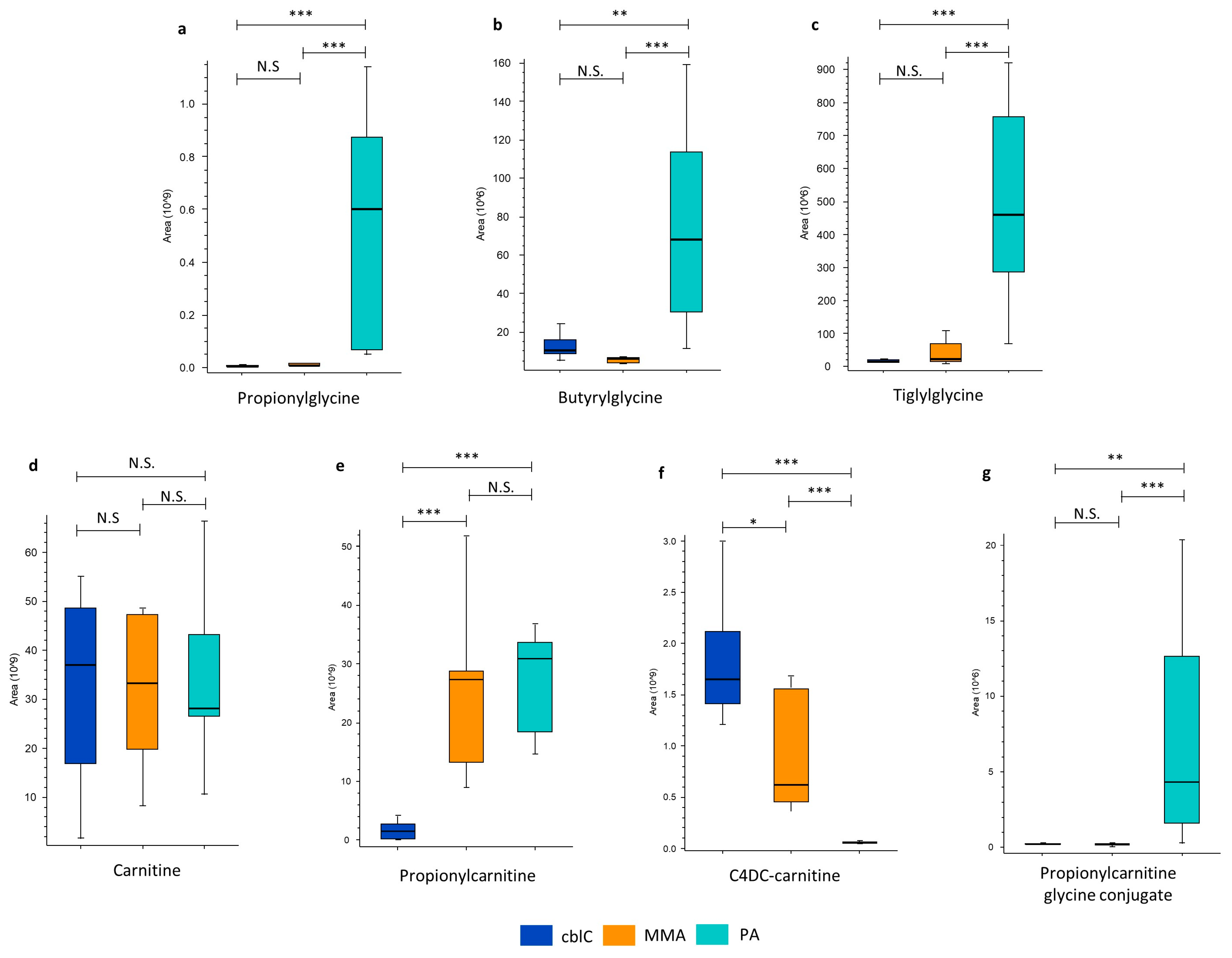
4.1.3. Glycine and Carnitine Conjugates
4.1.4. Transsulfuration Pathway Metabolites
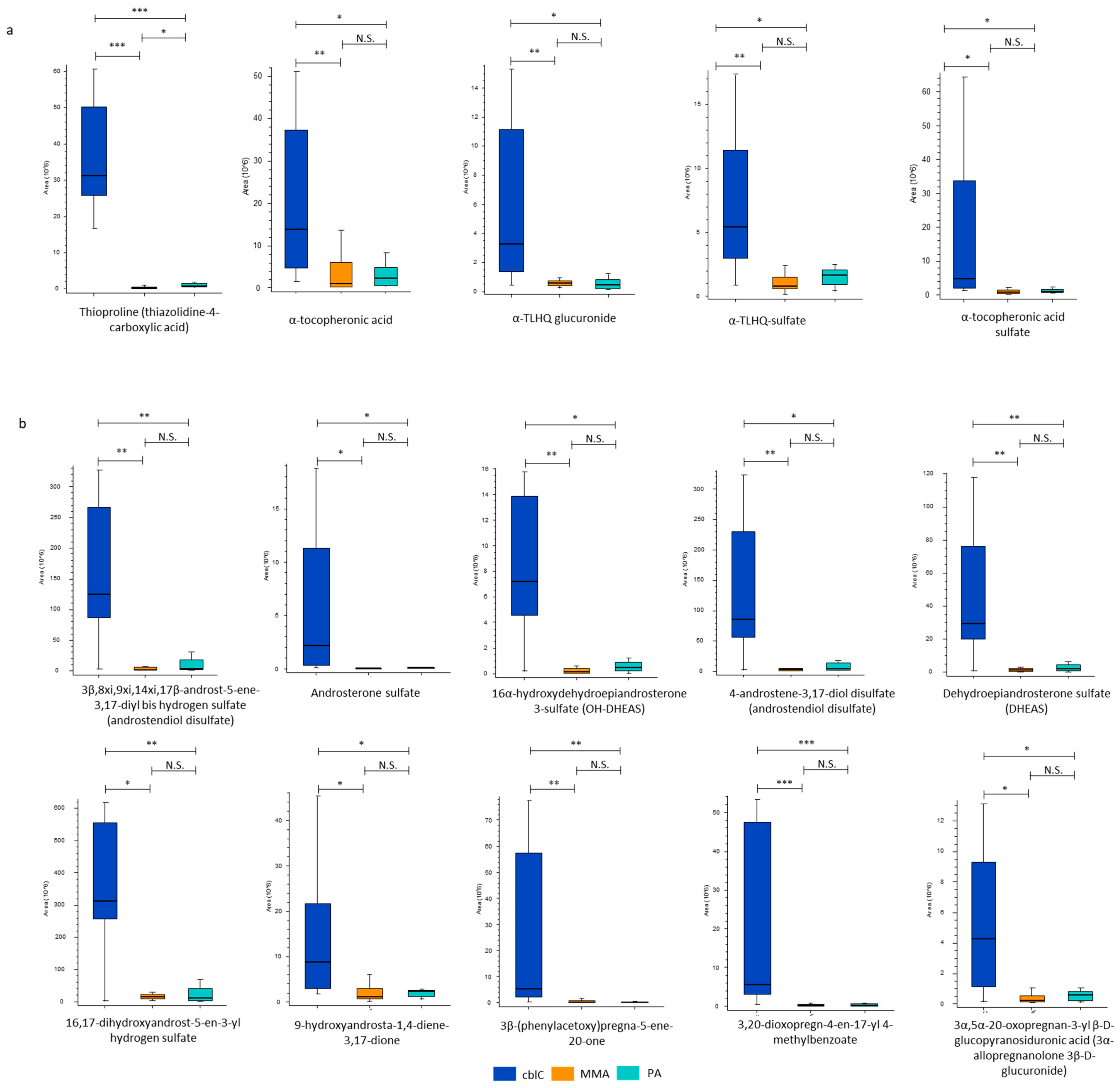
4.1.5. New Characteristic Compounds of cblC Disease
Biomarkers of Oxidative Damage
Steroid Hormones
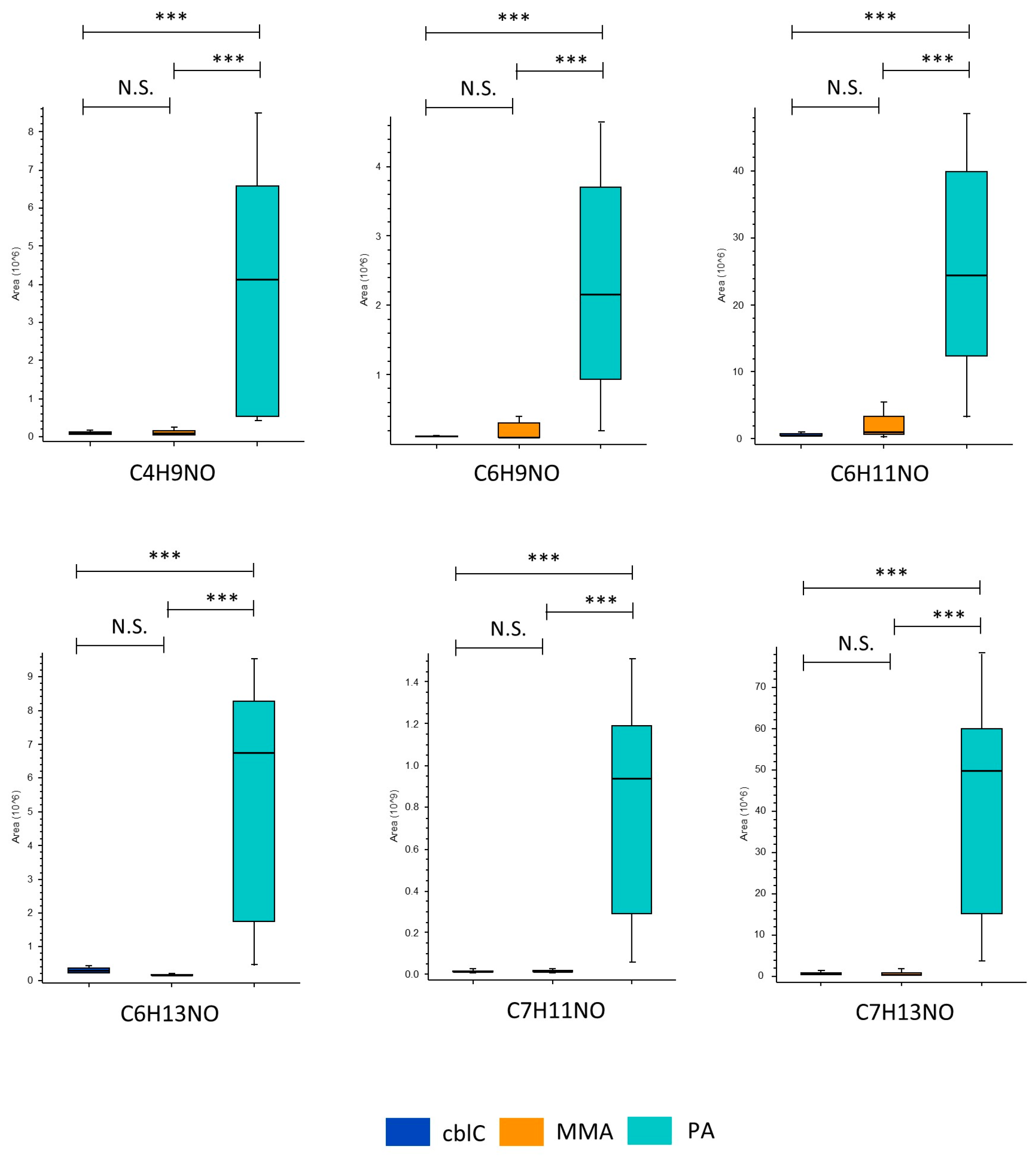
4.1.6. Non-Annotated Metabolites CxHyNO in PA
4.1.7. The Most Dysregulated Non-Annotated Features
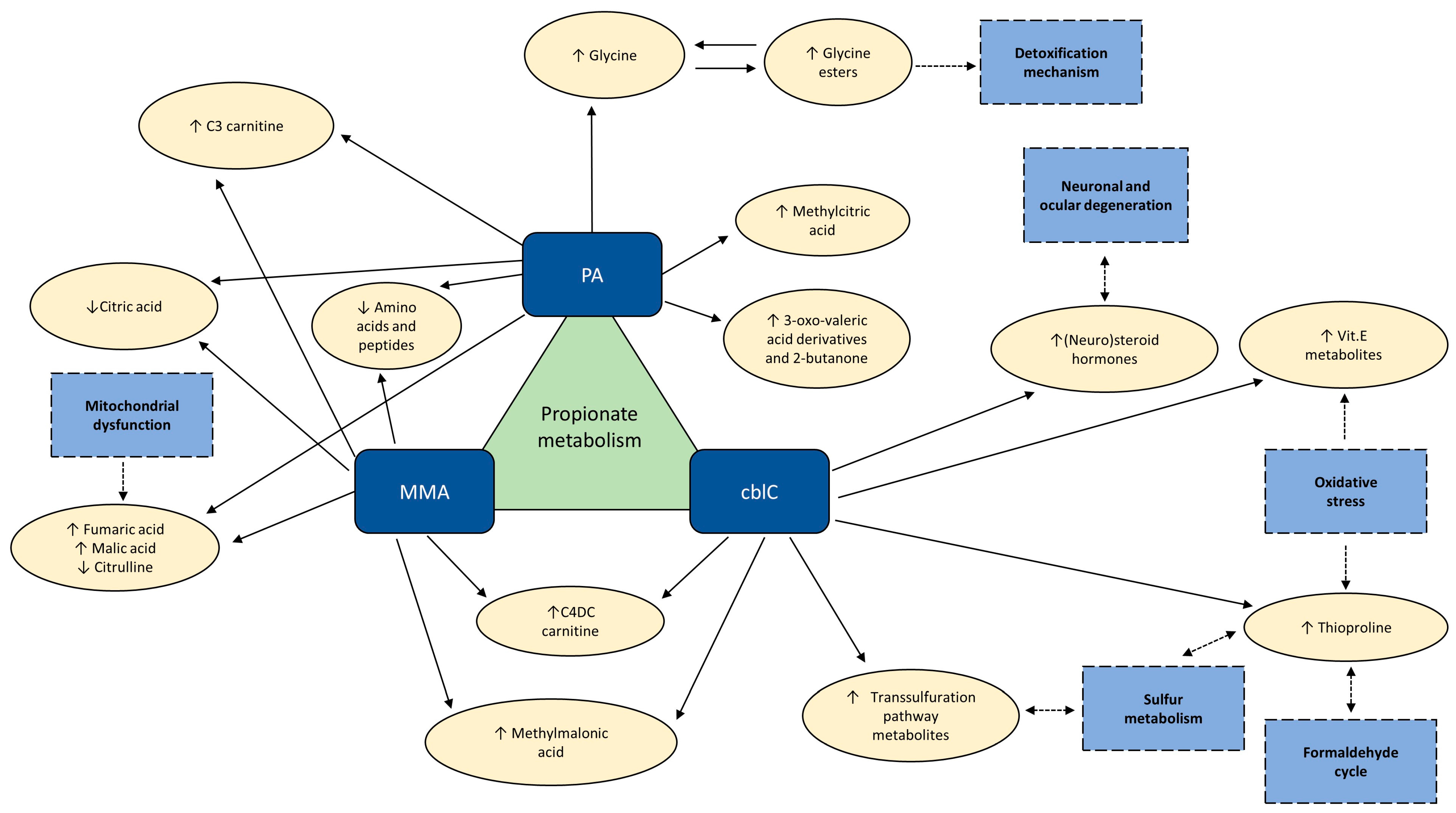
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deodato, F.; Boenzi, S.; Santorelli, F.M.; Dionisi-Vici, C. Methylmalonic and propionic aciduria. Am. J. Med. Genet. Part C Semin. Med. Genet. 2006, 142C, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Diodato, D.; Schwahn, B.; Schiff, M.; Bandeira, A.; Benoist, J.; Burlina, A.; Cerone, R.; Couce, M.L.; Garcia-Cazorla, A.; et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J. Inherit. Metab. Dis. 2017, 40, 21–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huemer, M.; Diodato, D.; Martinelli, D.; Olivieri, G.; Blom, H.; Gleich, F.; Kölker, S.; Kožich, V.; Morris, A.A.; Seifert, B.; et al. Phenotype, treatment practice and outcome in the cobalamin-dependent remethylation disorders and MTHFR deficiency: Data from the E-HOD registry. J. Inherit. Metab. Dis. 2019, 42, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Sass, J.O.; Jurecka, A.; Vockley, J. Biomarkers for drug development in propionic and methylmalonic acidemias. J. Inherit. Metab. Dis. 2022, 45, 132–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maines, E.; Catesini, G.; Boenzi, S.; Mosca, A.; Candusso, M.; Strologo, L.D.; Martinelli, D.; Maiorana, A.; Liguori, A.; Olivieri, G.; et al. Plasma methylcitric acid and its correlations with other disease biomarkers: The impact in the follow up of patients with propionic and methylmalonic acidemia. J. Inherit. Metab. Dis. 2020, 43, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Forny, P.; Hörster, F.; Ballhausen, D.; Chakrapani, A.; Chapman, K.A.; Dionisi-Vici, C.; Dixon, M.; Grünert, S.C.; Grunewald, S.; Haliloglu, G.; et al. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: First revision. J. Inherit. Metab. Dis. 2021, 44, 566–592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manoli, I.; Gebremariam, A.; McCoy, S.; Pass, A.R.; Gagné, J.; Hall, C.; Ferry, S.; Van Ryzin, C.; Sloan, J.L.; Sacchetti, E.; et al. Biomarkers to predict disease progression and therapeutic response in isolated methylmalonic acidemia. J. Inherit. Metab. Dis. 2023, 46, 554–572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wurth, R.; Turgeon, C.; Stander, Z.; Oglesbee, D. An evaluation of untargeted metabolomics methods to characterize inborn errors of metabolism. Mol. Genet. Metab. 2024, 141, 108115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anzmann, A.F.; Pinto, S.; Busa, V.; Carlson, J.; McRitchie, S.; Sumner, S.; Pandey, A.; Vernon, H.J. Multi-omics studies in cellular models of methylmalonic acidemia and propionic acidemia reveal dysregulation of serine metabolism. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 165538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wikoff, W.R.; Gangoiti, J.A.; Barshop, B.A.; Siuzdak, G. Metabolomics identifies perturbations in human disorders of propionate metabolism. Clin. Chem. 2007, 53, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Forny, P.; Bonilla, X.; Lamparter, D.; Shao, W.; Plessl, T.; Frei, C.; Bingisser, A.; Goetze, S.; van Drogen, A.; Harshman, K.; et al. Integrated multi-omics reveals anaplerotic rewiring in methylmalonyl-CoA mutase deficiency. Nat. Metab. 2023, 5, 80–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haijes, H.A.; Jans, J.J.M.; van der Ham, M.; van Hasselt, P.M.; Verhoeven-Duif, N.M. Understanding acute metabolic decompensation in propionic and methylmalonic acidemias: A deep metabolic phenotyping approach. Orphanet J. Rare Dis. 2020, 15, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, N.; Xiao, J.; Gijavanekar, C.; Pappan, K.L.; Glinton, K.E.; Shayota, B.J.; Kennedy, A.D.; Sun, Q.; Sutton, V.R.; Elsea, S.H. Comparison of Untargeted Metabolomic Profiling vs Traditional Metabolic Screening to Identify Inborn Errors of Metabolism. JAMA Netw. Open 2021, 4, e2114155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, M.J.; Kennedy, A.D.; Eckhart, A.D.; Burrage, L.C.; Wulff, J.E.; Miller, L.A.; Milburn, M.V.; Ryals, J.A.; Beaudet, A.L.; Sun, Q.; et al. Untargeted metabolomic analysis for the clinical screening of inborn errors of metabolism. J. Inherit. Metab. Dis. 2015, 38, 1029–1039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warrack, B.M.; Hnatyshyn, S.; Ott, K.-H.; Reily, M.D.; Sanders, M.; Zhang, H.; Drexler, D.M. Normalization strategies for metabonomic analysis of urine samples. J. Chromatogr. B 2009, 877, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A.K.R.; Rattray, N.J.W.; Cai, Y.; Santos-Neto, J.; Deziel, N.C.; Jukic, A.M.Z.; Johnson, C.H. Normalizing Untargeted Periconceptional Urinary Metabolomics Data: A Comparison of Approaches. Metabolites 2019, 9, 198. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xia, Y. Pretreating and normalizing metabolomics data for statistical analysis. Genes Dis. 2023, 11, 100979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallagher, E.M.; Rizzo, G.M.; Dorsey, R.; Dhummakupt, E.S.; Moran, T.S.; Mach, P.M.; Jenkins, C.C. Normalization of organ-on-a-Chip samples for mass spectrometry based proteomics and metabolomics via Dansylation-based assay. Toxicol. Vitr. 2023, 88, 105540. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, R.C.; Brindle, E.; Holman, D.J.; Shofer, J.; Klein, N.A.; Soules, M.R.; O’connor, K.A. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin. Chem. 2004, 50, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.E.; Iles, R.A.; Stacey, T.E.; Chalmers, R.A. Creatine metabolism during metabolic perturbations in patients with organic acidurias. Clin. Chim. Acta 1990, 194, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Younessi, D.; Moseley, K.; Yano, S. Creatine metabolism in combined methylmalonic aciduria and homocystinuria disease revisited. Ann. Neurol. 2009, 65, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P.; Rosa, R.; Pettenuzzo, L.; Sitta, A.; Wannmacher, C.; Wyse, A.; Wajner, M. Inhibition of mitochondrial creatine kinase activity from rat cerebral cortex by methylmalonic acid. Neurochem. Int. 2004, 45, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Shchelochkov, O.A.; Manoli, I.; Sloan, J.L.; Ferry, S.; Pass, A.; Van Ryzin, C.; Myles, J.; Schoenfeld, M.; McGuire, P.; Rosing, D.R.; et al. Chronic kidney disease in propionic acidemia. Anesthesia Analg. 2019, 21, 2830–2835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fowler, B.; Leonard, J.V.; Baumgartner, M.R. Causes of and diagnostic approach to methylmalonic acidurias. J. Inherit. Metab. Dis. 2008, 31, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, E.P.; Kitchens, R.L. A simplified and rapid quantitative assay for propionic and methylmalonic acids in urine. J. Lab. Clin. Med. 1975, 85, 487–496. [Google Scholar] [PubMed]
- Frenkel, E.P.; Kitchens, R.L. Applicability of an enzymatic quantitation of methylmalonic, propionic, and acetic acids in normal and megaloblastic states. Blood 1977, 49, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J.W.; Pullin, C.J.; Halpern, B.; Hammond, J.; Haan, E.; Danks, D.M. The identification of 3-keto-2-methylvaleric acid and 3-hydroxy-2-methylvaleric acid in a patient with propionic acidemia. J. Mass Spectrom. 1979, 6, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.A.; Lawson, A.M. Organic Acids in Man. The Analytical Chemistry, Biochemistry and Diagnosis of the Organic Acidurias; Chapman & Hall: London, UK, 1982. [Google Scholar]
- Wongkittichote, P.; Cunningham, G.; Summar, M.L.; Pumbo, E.; Forny, P.; Baumgartner, M.R.; Chapman, K.A. Tricarboxylic acid cycle enzyme activities in a mouse model of methylmalonic aciduria. Mol. Genet. Metab. 2019, 128, 444–451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Longo, N.; Price, L.B.; Gappmaier, E.; Cantor, N.L.; Ernst, S.L.; Bailey, C.; Pasquali, M. Anaplerotic therapy in propionic acidemia. Mol. Genet. Metab. 2017, 122, 51–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parfait, B.; de Lonlay, P.; von Kleist-Retzow, J.C.; Cormier-Daire, V.; Chrétien, D.; Rötig, A.; Rabier, D.; Saudubray, J.M.; Rustin, P.; Munnich, A. The neurogenic weakness, ataxia and retinitis pigmentosa (NARP) syndrome mtDNA mutation (T8993G) triggers muscle ATPase deficiency and hypocitrullinaemia. Eur. J. Pediatr. 1999, 158, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, C.; Frank, M.W.; Tangallapally, R.; Yun, M.-K.; Edwards, A.; White, S.W.; Lee, R.E.; Rock, C.O.; Jackowski, S. Pantothenate kinase activation relieves coenzyme A sequestration and improves mitochondrial function in mice with propionic acidemia. Sci. Transl. Med. 2021, 13, eabf5965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almannai, M.; El-Hattab, A.W. Nitric Oxide Deficiency in Mitochondrial Disorders: The Utility of Arginine and Citrulline. Front. Mol. Neurosci. 2021, 14, 682780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zecchini, V.; Paupe, V.; Herranz-Montoya, I.; Janssen, J.; Wortel, I.M.N.; Morris, J.L.; Ferguson, A.; Chowdury, S.R.; Segarra-Mondejar, M.; Costa, A.S.H.; et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature 2023, 615, 499–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Head, P.E.; Myung, S.; Chen, Y.; Schneller, J.L.; Wang, C.; Duncan, N.; Hoffman, P.; Chang, D.; Gebremariam, A.; Gucek, M.; et al. Aberrant methylmalonylation underlies methylmalonic acidemia and is attenuated by an engineered sirtuin. Sci. Transl. Med. 2022, 14, eabn4772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haijes, H.A.; van Hasselt, P.M.; Jans, J.J.M.; Verhoeven-Duif, N.M. Pathophysiology of propionic and methylmalonic acidemias. Part 2: Treatment strategies. J. Inherit. Metab. Dis. 2019, 42, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.; Bohan, T. L-carnitine therapy in propionicacidaemia. Lancet 1982, 319, 1411–1412. [Google Scholar] [CrossRef] [PubMed]
- Krieger, I.; Tanaka, K. Therapeutic effects of glycine in isovaleric acidemia. Pediatr. Res. 1976, 10, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Boenzi, S.; Inglese, R.; la Marca, G.; Muraca, M.; Martinez, T.B.; Johnson, D.W.; Zelli, E.; Dionisi-Vici, C. Measurement of succinyl-carnitine and methylmalonyl-carnitine on dried blood spot by liquid chromatography-tandem mass spectrometry. Clin. Chim. Acta 2014, 429, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.L.; Achilly, N.P.; Arnold, M.L.; Catlett, J.L.; Blake, T.; Bishop, K.; Jones, M.; Harper, U.; English, M.A.; Anderson, S.; et al. The vitamin B12 processing enzyme, mmachc, is essential for zebrafish survival, growth and retinal morphology. Hum. Mol. Genet. 2020, 29, 2109–2123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoss, G.R.W.; Poloni, S.; Blom, H.J.; Schwartz, I.V.D. Three main causes of homocystinuria: CBS, cblC and MTHFR deficiency. What do they have in common? J. Inborn Errors Metab. Screen. 2019, 7, e20190007. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mc Guire, P.J.; Parikh, A.; Diaz, G.A. Profiling of oxidative stress in patients with inborn errors of metabolism. Mol. Genet. Metab. 2009, 98, 173–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rivera-Barahona, A.; Alonso-Barroso, E.; Pérez, B.; Murphy, M.P.; Richard, E.; Desviat, L.R. Treatment with antioxidants ameliorates oxidative damage in a mouse model of propionic acidemia. Mol. Genet. Metab. 2017, 122, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Villar, L.; Rivera-Barahona, A.; Cuevas-Martín, C.; Guenzel, A.; Pérez, B.; Barry, M.; Murphy, M.; Logan, A.; Gonzalez-Quintana, A.; Martín, M.; et al. In vivo evidence of mitochondrial dysfunction and altered redox homeostasis in a genetic mouse model of propionic acidemia: Implications for the pathophysiology of this disorder. Free Radic. Biol. Med. 2016, 96, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Zhang, X.; Cai, H.; Liu, J.; Fang, S.; Yu, B. The Regulation and Characterization of Mitochondrial-Derived Methylmalonic Acid in Mitochondrial Dysfunction and Oxidative Stress: From Basic Research to Clinical Practice. Oxidative Med. Cell. Longev. 2022, 2022, 7043883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richard, E.; Jorge-Finnigan, A.; Garcia-Villoria, J.; Merinero, B.; Desviat, L.R.; Gort, L.; Briones, P.; Leal, F.; Pérez-Cerdá, C.; Ribes, A.; et al. Genetic and cellular studies of oxidative stress in methylmalonic aciduria (MMA) cobalamin deficiency type C (cblC) with homocystinuria (MMACHC). Hum. Mutat. 2009, 30, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Martinelli, D.; Piemonte, F.; Tozzi, G.; Boenzi, S.; Di Giovamberardino, G.; Petrillo, S.; Bertini, E.; Dionisi-Vici, C. Glutathione metabolism in cobalamin deficiency type C (cblC). J. Inherit. Metab. Dis. 2014, 37, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hashimoto, Y.; Martens, L.G.; Meulmeester, F.L.; Ashrafi, N.; Mook-Kanamori, D.O.; Rosendaal, F.R.; Jukema, J.W.; van Dijk, K.W.; Mills, K.; et al. Associations of metabolomic profiles with circulating vitamin E and urinary vitamin E metabolites in middle-aged individuals. Nutrition 2022, 93, 111440. [Google Scholar] [CrossRef] [PubMed]
- Wallert, M.; Schmölz, L.; Galli, F.; Birringer, M.; Lorkowski, S. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. 2014, 2, 495–503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, G.; Muller, D.P.; O’riordan, S.M.; Bryan, S.; Dattani, M.T.; Hindmarsh, P.C.; Mills, K. Urinary conjugated α-tocopheronolactone—A biomarker of oxidative stress in children with type 1 diabetes. Free Radic. Biol. Med. 2013, 55, 54–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Ham, Y.-H.; Chan, K.-K.J.; Chan, W. Thioproline Serves as an Efficient Antioxidant Protecting Human Cells from Oxidative Stress and Improves Cell Viability. Chem. Res. Toxicol. 2020, 33, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Morellato, A.E.; Umansky, C.; Pontel, L.B. The toxic side of one-carbon metabolism and epigenetics. Redox Biol. 2020, 40, 101850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barroso, M.; Handy, D.E.; Castro, R. The link between hyperhomocysteinemia and hypomethylation: Implications for cardiovascular disease. J. Inborn Errors Metab. Screen. 2019, 5, e160024. [Google Scholar] [CrossRef]
- Pietzke, M.; Burgos-Barragan, G.; Wit, N.; Tait-Mulder, J.; Sumpton, D.; Mackay, G.M.; Patel, K.J.; Vazquez, A. Amino acid dependent formaldehyde metabolism in mammals. Commun. Chem. 2020, 3, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tulpule, K.; Dringen, R. Formaldehyde in brain: An overlooked player in neurodegeneration? J. Neurochem. 2013, 127, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, H.; Ubuka, T.; Soma, K.K.; Tsutsui, K. Editorial: Recent Progress and Perspectives in Neurosteroid Research. Front. Endocrinol. 2022, 13, 951990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strac, D.S.; Konjevod, M.; Perkovic, M.N.; Tudor, L.; Erjavec, G.N.; Pivac, N. Dehydroepiandrosterone (DHEA) and its Sulphate (DHEAS) in Alzheimer’s Disease. Curr. Alzheimer Res. 2020, 17, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef] [PubMed]
- Chik, M.W.; Hazalin, N.A.M.N.; Singh, G.K.S. Regulation of phase I and phase II neurosteroid enzymes in the hippocampus of an Alzheimer’s disease rat model: A focus on sulphotransferases and UDP-glucuronosyltransferases. Steroids 2022, 184, 109035. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Evans, E.; Waller-Evans, H. Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease. Essays Biochem. 2020, 64, 591–606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bianchi, V.E.; Rizzi, L.; Bresciani, E.; Omeljaniuk, R.J.; Torsello, A. Androgen Therapy in Neurodegenerative Diseases. J. Endocr. Soc. 2020, 4, bvaa120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naylor, J.C.; Hulette, C.M.; Steffens, D.C.; Shampine, L.J.; Ervin, J.F.; Payne, V.M.; Massing, M.W.; Kilts, J.D.; Strauss, J.L.; Calhoun, P.S.; et al. Cerebrospinal fluid dehydroepiandrosterone levels are correlated with brain dehydroepiandrosterone levels, elevated in alzheimer’s disease, and related to neuropathological disease stage. J. Clin. Endocrinol. Metab. 2008, 93, 3173–3178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marx, C.E.; Trost, W.T.; Shampine, L.J.; Stevens, R.D.; Hulette, C.M.; Steffens, D.C.; Ervin, J.F.; Butterfield, M.I.; Blazer, D.G.; Massing, M.W.; et al. The neurosteroid allopregnanolone is reduced in prefrontal cortex in alzheimer’s disease. Biol. Psychiatry 2006, 60, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Landolfi, A.; Vitale, C.; Longo, K.; Cozzolino, A.; Squillante, M.; Savanelli, M.C.; Barone, P.; Amboni, M. A metabolomic signature of treated and drug-naïve patients with Parkinson’s disease: A pilot study. Metabolomics 2019, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol. Neurodegener. 2021, 16, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nuzzi, R.; Caselgrandi, P. Sex Hormones and Their Effects on Ocular Disorders and Pathophysiology: Current Aspects and Our Experience. Int. J. Mol. Sci. 2022, 23, 3269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishikawa, Y.; Morishita, S.; Horie, T.; Fukumoto, M.; Sato, T.; Kida, T.; Oku, H.; Sugasawa, J.; Ikeda, T.; Nakamura, K. A comparison of sex steroid concentration levels in the vitreous and serum of patients with vitreoretinal diseases. PLoS ONE 2017, 12, e0180933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schellevis, R.L.; Altay, L.; Kalisingh, A.; Mulders, T.W.F.; Sitnilska, V.; Hoyng, C.B.; Boon, C.J.F.; Groenewoud, J.M.M.; de Jong, E.K.; Hollander, A.I.D. Elevated Steroid Hormone Levels in Active Chronic Central Serous Chorioretinopathy. Investig. Opthalmology Vis. Sci. 2019, 60, 3407–3413. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Yu, H.G.; Ku, S.Y. Sex Steroid Hormone and Ophthalmic Disease. Korean J. Reprod. Med. 2010, 37, 89–98. [Google Scholar]
- Nuzzi, R.; Scalabrin, S.; Becco, A.; Panzica, G. Gonadal Hormones and Retinal Disorders: A Review. Front. Endocrinol. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onal, H.; Kutlu, E.; Aydın, B.; Ersen, A.; Topal, N.; Adal, E.; Güneş, H.; Doktur, H.; Tanıdır, C.; Pirhan, D.; et al. Assessment of retinal thickness as a marker of brain masculinization in children with congenital adrenal hyperplasia: A pilot study. J. Pediatr. Endocrinol. Metab. 2019, 32, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.K.; Chen, Y.; Barazanji, M.; Jeffries, K.A.; Cameroamortegui, F.; Merkler, D.J. Primary fatty acid amide metabolism: Conversion of fatty acids and an ethanolamine in N18TG2 and SCP cells. J. Lipid Res. 2012, 53, 247–256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Šebela, M.; Rašková, M. Polyamine-Derived Aminoaldehydes and Acrolein: Cytotoxicity, Reactivity and Analysis of the Induced Protein Modifications. Molecules 2023, 28, 7429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Patient | Onset | Cardiac Involvement | Pancreatitis | Renal Disease | Visual Dysfunction | Epilepsy | Abnormal MRI | Developmental Delay/ Intellectual Disablity | Deafness | Age at Urine Sampling (Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| PA 1 | Neonatal | - | - | - | - | - | + | + | + | 2.1 |
| PA 2 | 7 months | + | - | - | - | - | + | + | - | 9.9 |
| PA 3 | 3 months | + | - | - | - | - | - | - | - | 20.4 |
| PA 4 | Neonatal | + | - | - | - | - | - | +/- | + | 15.2 |
| PA 5 | Neonatal (NBS symptomatic) | - | - | - | - | - | - | +/- | - | 0.4 |
| PA 6 | Neonatal (NBS symptomatic) | - | - | - | - | - | + | + | - | 0.8 |
| PA 7 | Neonatal | + | - | - | - | - | - | - | - | 7.0 |
| MMA 1 | Neonatal | - | - | ++ | - | - | +/- | - | - | 0.6 |
| MMA 2 | Neonatal | - | + | +/- | - | - | +/- | + | + | 1.2 |
| MMA 3 | Neonatal | - | - | - | - | - | ++ | + | NA | 10.6 |
| MMA 4 | 17 months | - | - | ++ | + | - | - | - | - | 20.6 |
| MMA 5 | NBS (asymptomatic) | - | - | - | - | - | - | - | - | 0.3 |
| MMA 6 | Neonatal (NBS symptomatic) | - | - | - | - | - | - | - | NA | 3.7 |
| MMA 7 | 6 months | - | + | ++ | + | - | ++ | - | - | 22.2 |
| cblC 1 | Neonatal (NBS symptomatic) | + | - | - | + | - | - | +/- | - | 0.4 |
| cblC 2 | Neonatal | - | - | + | + | + | + | + | - | 3.7 |
| cblC 3 | 2 months | + | - | + | + | + | ++ | ++ | - | 2.8 |
| cblC 4 | Neonatal | - | - | - | + | + | + | + | - | 3.3 |
| cblC 5 | 5 months | - | - | - | + | ++ | + | ++ | - | 13.7 |
| cblC 6 | Neonatal | - | - | -/+ | + | - | + | +/- | - | 13.2 |
| cblC 7 | 3 months | ++ | - | + | - | + | +/- | +/- | - | 17.1 |
| Metabolite | cblC/MMA | cblC/PA | PA/MMA | KEGG Pathway |
|---|---|---|---|---|
| Organic Acids and Ketones | ||||
| Citric and isocitric acids | 3.3 ** | 2.5 ** | ns |
|
| 2-Butanone | 0.5 * | 0.13 *** | 3.9 *** | |
| Fumaric acid | 0.3 * | 0.2 *** | ns |
|
| Malic acid | 0.2 * | 0.08 *** | ns |
|
| Methylmalonic acid | 0.06 *** | 19 *** | 0.003 *** |
|
| MW 74.036 (propionic acid isobar) | 0.05 *** | 12 *** | 0.004 *** | |
| 2-Methylcitric acid | ns | 0.2 *** | 4.3 *** |
|
| 2-Methyl-3-hydroxy-valeric acid | ns | 0.3 *** | 5.4 *** | |
| 3-Oxo-valeric acid | ns | 0.21 *** | 3.4 *** | |
| 2-Methyl-3-oxo-valeric acid | ns | 0.09 ** | 8.3 ** | |
| 3-Pentanone | ns | 0.06 *** | 10 *** | |
| Glycine and Carnitine Conjugates | ||||
| C4DC-carnitine | 2.1 * | 348 *** | 0.006 *** | |
| Propionylcarnitine | 0.06 *** | 0.05 *** | ns | |
| Propionylglycine | ns | 0.01 *** | 60 *** | |
| Propionylcarinitine glycine conjugate | ns | 0.05 ** | 23 *** | |
| Tiglylglycine | ns | 0.07 *** | 21 *** | |
| Butyrylglycine | ns | 0.2 ** | 11 *** | |
| Amino Acids and Peptides | ||||
| Threonine | 83 *** | 36 *** | ns |
|
| Butyl-α-aspartyl-allothreoninate | 28 *** | 20 *** | ns | |
| Isoleucine | 24 ** | 50 *** | ns |
|
| Dimethylglycine | 21 *** | 36 *** | ns |
|
| Isoleucylalanine | 15 *** | 3.4 ** | ns | |
| Aspartylphenylalanine | 9.6 * | 23.4 ** | ns | |
| Glutamylisoleucine | 9.4 *** | 19 * | ns | |
| Prolylproline | 8.0 ** | 4.4 * | ns | |
| Valylvaline | 7.7 ** | 3.6 * | ns | |
| Glycylglycyl-alanyl-2-methylalanine | 6.7 * | 11 ** | ns | |
| Isoleucylvaline | 5.0 ** | 4.9 ** | ns | |
| Lysine | 4.1 ** | ns | ns |
|
| Citrulline | 3.9 ** | 2.3 * | ns |
|
| Valine | 3.8 *** | 2.4 *** | 1.6 * |
|
| Methionine | ns | 0.07 ** | 16 ** |
|
| Glycine | ns | 0.04 *** | 33 *** |
|
| Transsulfuration Pathway Metabolites | ||||
| Cystathionine | 78 *** | 20 *** | 3.8 ** |
|
| Homocysteine | 37 *** | 39 *** | ns |
|
| Thiosulfuric acid | 17 * | 18 ** | ns |
|
| Cysteine | 5.7 ** | 3.5 * | ns |
|
| 2-Hydroxybutyric acid | 5.0 *** | 3.2 * | ns |
|
| Sulfuric acid | 4.2 *** | 2.5 ** | ns |
|
| Biomarkers of Oxidative Damage | ||||
| Thioproline | 96 *** | 45 *** | 2.1 * | |
| α-Tocopheronic acid | 14 ** | 5.9 * | ns | |
| α-TLHQ glucuronide | 8.0 ** | 4.7 * | ns | |
| α-TLHQ sulfate | 6.8 ** | 3.3 * | ns | |
| α-Tocopheronic acid sulfate | 5.9 * | 4.9 * | ns | |
| CxHyNO Features | ||||
| C4 H9 N O | ns | 0.02 *** | 48 *** | |
| C6 H9 N O | ns | 0.05 *** | 21 *** | |
| C6 H11 N O | ns | 0.06 *** | 30 *** | |
| C6 H13 N O | ns | 0.04 *** | 44 *** | |
| C7 H11 N O | ns | 0.01 *** | 72 *** | |
| C7 H13 N O | ns | 0.01 *** | 142 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorina, A.; Catesini, G.; Sacchetti, E.; Rizzo, C.; Dionisi-Vici, C. Propionic Acidemia, Methylmalonic Acidemia, and Cobalamin C Deficiency: Comparison of Untargeted Metabolomic Profiles. Metabolites 2024, 14, 428. https://doi.org/10.3390/metabo14080428
Sidorina A, Catesini G, Sacchetti E, Rizzo C, Dionisi-Vici C. Propionic Acidemia, Methylmalonic Acidemia, and Cobalamin C Deficiency: Comparison of Untargeted Metabolomic Profiles. Metabolites. 2024; 14(8):428. https://doi.org/10.3390/metabo14080428
Chicago/Turabian StyleSidorina, Anna, Giulio Catesini, Elisa Sacchetti, Cristiano Rizzo, and Carlo Dionisi-Vici. 2024. "Propionic Acidemia, Methylmalonic Acidemia, and Cobalamin C Deficiency: Comparison of Untargeted Metabolomic Profiles" Metabolites 14, no. 8: 428. https://doi.org/10.3390/metabo14080428
APA StyleSidorina, A., Catesini, G., Sacchetti, E., Rizzo, C., & Dionisi-Vici, C. (2024). Propionic Acidemia, Methylmalonic Acidemia, and Cobalamin C Deficiency: Comparison of Untargeted Metabolomic Profiles. Metabolites, 14(8), 428. https://doi.org/10.3390/metabo14080428








