Electrochemical Probing of Human Liver Subcellular S9 Fractions for Drug Metabolite Synthesis
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Microsomal and S9 Film Preparation
2.3. Direct Electron Transfer Measurements
2.4. Electrocatalytic Oxygen Reduction
2.5. Electrocatalytic Diclofenac Hydroxylation and the Detection of Metabolites
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
3. Results and Discussion
3.1. FTIR Characterization of HLM and S9 Films Immobilized on the HPG Electrodes
3.2. Electrochemical Probing of S9 Films and Electron Transfer Kinetics
3.3. Electrocatalytic Oxygen Reduction by the S9 Films
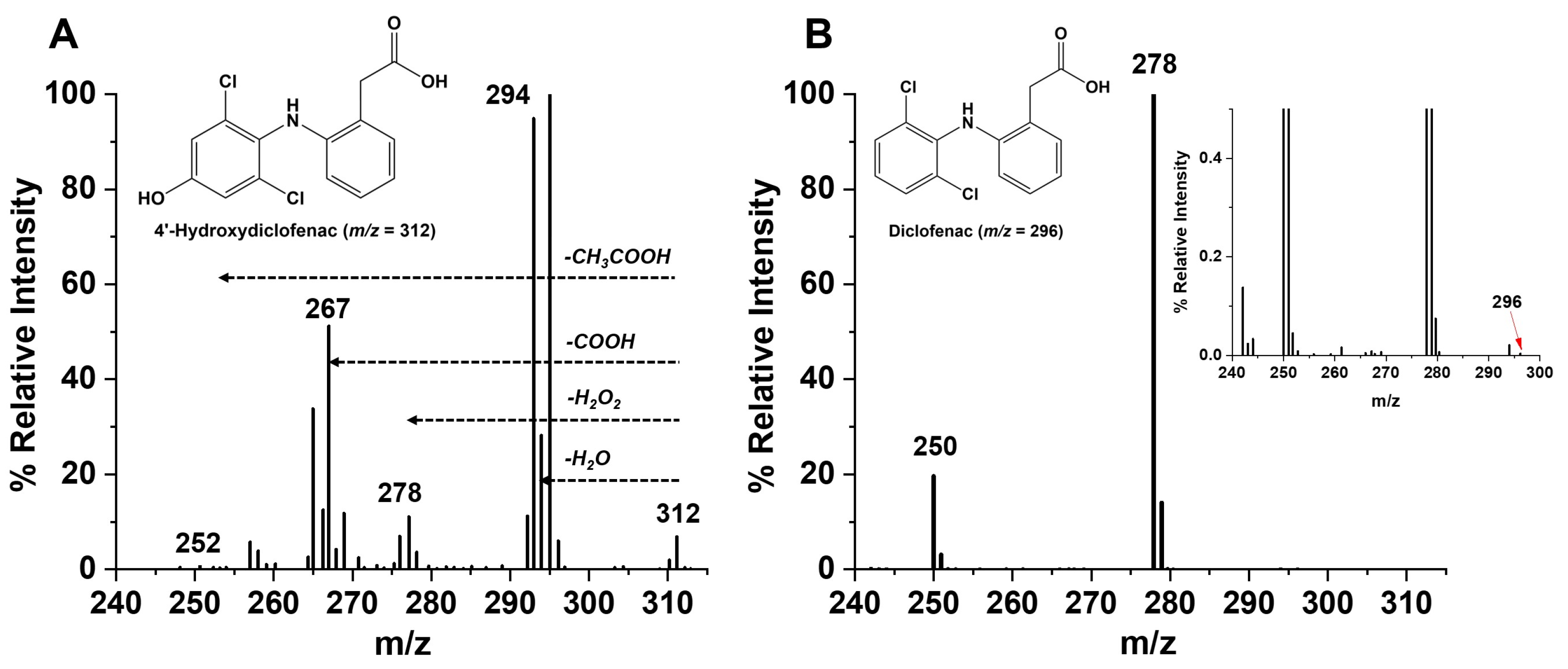
3.4. Electrocatalytic Diclofenac Hydroxylation and Metabolite Identification Using LCMS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berdigaliyev, N.; Aljofan, M. An overview of drug discovery and development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Loewa, A.; Feng, J.J.; Hedtrich, S. Human disease models in drug development. Nat. Rev. Bioeng. 2023, 1, 545–559. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, L.; Bernhardt, R. Redox Partners: Function Modulators of Bacterial P450 Enzymes. Trends Microbiol. 2020, 28, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, B.; Shi, J.; Li, Q.; Zhu, H.-J. Comparative Proteomics Analysis of Human Liver Microsomes and S9 Fractions. Drug Metab. Dispos. 2020, 48, 31. [Google Scholar] [CrossRef] [PubMed]
- Eilstein, J.; Grégoire, S.; Fabre, A.; Arbey, E.; Géniès, C.; Duplan, H.; Rothe, H.; Ellison, C.; Cubberley, R.; Schepky, A.; et al. Use of human liver and EpiSkin™ S9 subcellular fractions as a screening assays to compare the in vitro hepatic and dermal metabolism of 47 cosmetics-relevant chemicals. J. Appl. Toxicol. 2020, 40, 416–433. [Google Scholar] [CrossRef] [PubMed]
- Lassila, T.; Rousu, T.; Mattila, S.; Chesné, C.; Pelkonen, O.; Turpeinen, M.; Tolonen, A. Formation of GSH-trapped reactive metabolites in human liver microsomes, S9 fraction, HepaRG-cells, and human hepatocytes. J. Pharm. Biomed. Anal. 2015, 115, 345–351. [Google Scholar] [CrossRef]
- Tremmel, M.; Paetz, C.; Heilmann, J. In Vitro Liver Metabolism of Six Flavonoid C-Glycosides. Molecules 2021, 26, 6632. [Google Scholar] [CrossRef]
- Hrycay, E.G.; Bandiera, S.M. Monooxygenase, peroxidase and peroxygenase properties and reaction mechanisms of cytochrome P450 enzymes. Adv. Exp. Med. Biol. 2015, 851, 1–61. [Google Scholar]
- Butt, J.N.; Jeuken, L.J.C.; Zhang, H.; Burton, J.A.J.; Sutton-Cook, A.L. Protein film electrochemistry. Nat. Rev. Methods Prim. 2023, 3, 77. [Google Scholar] [CrossRef]
- Kumar, N.; He, J.; Rusling, J.F. Electrochemical transformations catalyzed by cytochrome P450s and peroxidases. Chem. Soc. Rev. 2023, 52, 5135–5171. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.; Deluca, G.; Bulko, T.; Carrara, S.; Nicolini, C.; Usanov, S.A.; Archakov, A. Cholesterol amperometric biosensor based on cytochrome P450scc. Biosens. Bioelectron. 2004, 19, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.R.; Ning, J.; Sun, G.B.; Wang, P.; Zhang, F.; Ma, H.Y.; Zou, L.W.; Hou, J.; Wu, J.J.; Ge, G.B.; et al. Cytochrome P450 3A Enzymes Are Key Contributors for Hepatic Metabolism of Bufotalin, a Natural Constitute in Chinese Medicine Chansu. Front. Pharmacol. 2019, 10, 52. [Google Scholar] [CrossRef]
- Krishnan, S.; Schenkman, J.B.; Rusling, J.F. Bioelectronic Delivery of Electrons to Cytochrome P450 Enzymes. J. Phys. Chem. B 2011, 115, 8371–8380. [Google Scholar] [CrossRef]
- Fantuzzi, A.; Fairhead, M.; Gilardi, G. Direct Electrochemistry of Immobilized Human Cytochrome P450 2E1. J. Am. Chem. Soc. 2004, 126, 5040–5041. [Google Scholar] [CrossRef]
- Mie, Y.; Suzuki, M.; Komatsu, Y. Electrochemically Driven Drug Metabolism by Membranes Containing Human. Cytochrome P450. J. Am. Chem. Soc. 2009, 131, 6646–6647. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Rusling, J.F. Thin film voltammetry of metabolic enzymes in rat liver microsomes. Electrochem. Commun. 2007, 9, 2359–2363. [Google Scholar] [CrossRef]
- Sultana, N.; Schenkman, J.B.; Rusling, J.F. Protein Film Electrochemistry of Microsomes Genetically Enriched in Human Cytochrome P450 Monooxygenases. J. Am. Chem. Soc. 2005, 127, 13460–13461. [Google Scholar] [CrossRef]
- Nerimetla, R.; Walgama, C.; Singh, V.; Hartson, S.D.; Krishnan, S. Mechanistic Insights into Voltage-Driven Biocatalysis of a Cytochrome P450 Bactosomal Film on a Self-Assembled Monolayer. ACS Catal. 2017, 7, 3446–3453. [Google Scholar] [CrossRef]
- Walgama, C.; Nerimetla, R.; Materer, N.F.; Schildkraut, D.; Elman, J.F.; Krishnan, S. A Simple Construction of Electrochemical Liver Microsomal Bioreactor for Rapid Drug Metabolism and Inhibition Assays. Anal. Chem. 2015, 87, 4712–4718. [Google Scholar] [CrossRef]
- Walker, A.; Walgama, C.; Nerimetla, R.; Habib Alavi, S.; Echeverria, E.; Harimkar, S.P.; McIlroy, D.N.; Krishnan, S. Roughened graphite biointerfaced with P450 liver microsomes: Surface and electrochemical characterizations. Coll. Surf. B Biointerfaces 2020, 189, 110790. [Google Scholar] [CrossRef]
- Premaratne, G.; Niroula, J.; Moulton, J.T.; Krishnan, S. Nanobioelectrocatalysis Using. Human. Liver Microsomes and Cytochrome P450 Bactosomes: Pyrenyl-Nanocarbon Electrodes. ACS Appl. Bio Mater. 2024, 7, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Nerimetla, R.; Krishnan, S. Electrocatalysis by subcellular liver fractions bound to carbon nanostructures for stereoselective green drug metabolite synthesis. Chem. Commun. 2015, 51, 11681–11684. [Google Scholar] [CrossRef]
- Nerimetla, R.; Premaratne, G.; Liu, H.; Krishnan, S. Improved electrocatalytic metabolite production and drug biosensing by human liver microsomes immobilized on amine-functionalized magnetic nanoparticles. Electrochim. Acta 2018, 280, 101–107. [Google Scholar] [CrossRef]
- Melin, A.-M.; Perromat, A.; Déléris, G. Pharmacologic application of Fourier transform IR spectroscopy: In vivo toxicity of carbon tetrachloride on rat liver. Biopolymers 2000, 57, 160–168. [Google Scholar] [CrossRef]
- Yoon, S.; Kazusaka, A.; Fujita, S. FTIR spectroscopic and HPLC chromatographic studies of carbon tetrachloride induced acute hepatitis in rats: Damage in liver phospholipid membrane. Biopolymers 2000, 57, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy: A Method of Choice for Studying Membrane Proteins and Lipids. Biochemistry 2003, 42, 11898–11907. [Google Scholar] [CrossRef] [PubMed]
- Arrondo, J.L.R.; Goñi, F.M. Structure and dynamics of membrane proteins as studied by infrared spectroscopy. Prog. Biophys. Mol. Biol. 1999, 72, 367–405. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley & Sons Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Wasalathanthri, D.P.; Malla, S.; Faria, R.C.; Rusling, J.F. Electrochemical Activation of the Natural Catalytic Cycle of Cytochrome P450s in Human Liver Microsomes. Electroanalysis 2012, 24, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Wasalathanthri, D.; Zhao, L.; Schenkman, J.B.; Rusling, J.F. Efficient Bioelectronic Actuation of the Natural Catalytic Pathway of Human Metabolic Cytochrome P450s. J. Am. Chem. Soc. 2011, 133, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, H.; Cui, D.; Zhang, Y.; Liu, S. Enhanced enzymatic reactivity for electrochemically driven drug metabolism by confining cytochrome P450 enzyme in TiO2 nanotube arrays. Anal. Chem. 2014, 86, 8003–8009. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Mi, L.; Xu, X.; Lu, J.; Qian, J.; Liu, S. Nanocomposites of Graphene and Cytochrome P450 2D6 Isozyme for Electrochemical-Driven Tramadol Metabolism. Langmuir 2014, 30, 11833–11840. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Kato, D.; Kamata, T.; Guo, Q.; You, T.; Niwa, O. Human cytochrome P450 3A4 and a carbon nanofiber modified film electrode as a platform for the simple evaluation of drug metabolism and inhibition reactions. Analyst 2013, 138, 6463–6468. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Martin, M.V.; Sohl, C.D.; Cheng, Q. Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat. Protoc. 2009, 4, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Montellano, P.R.; De Voss, J.J. Oxidizing species in the mechanism of cytochrome P450. Nat. Prod. Rep. 2002, 19, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- De Montellano, P.R.O. Cytochrome P450: Structure, Mechanism, and Biochemistry; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Guengerich, F.P. Common and Uncommon Cytochrome P450 Reactions Related to Metabolism and Chemical Toxicity. Chem. Res. Toxicol. 2001, 14, 611–650. [Google Scholar] [CrossRef]
- Dorado, P.; Berecz, R.; Cáceres, M.C.; Llerena, A. Analysis of diclofenac and its metabolites by high-performance liquid chromatography: Relevance of CYP2C9 genotypes in diclofenac urinary metabolic ratios. J. Chromatogr. B 2003, 789, 437–442. [Google Scholar] [CrossRef]
- Bort, R.; Macé, K.; Boobis, A.; Gómez-Lechón, M.a.-J.; Pfeifer, A.; Castell, J. Hepatic metabolism of diclofenac: Role of human CYP in the minor oxidative pathways. Biochem. Pharmacol. 1999, 58, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Bajrami, B.; Zhao, L.; Schenkman, J.B.; Rusling, J.F. Rapid LC-MS Drug Metabolite Profiling Using Microsomal Enzyme Bioreactors in a Parallel Processing Format. Anal. Chem. 2009, 81, 9921–9929. [Google Scholar] [CrossRef] [PubMed]


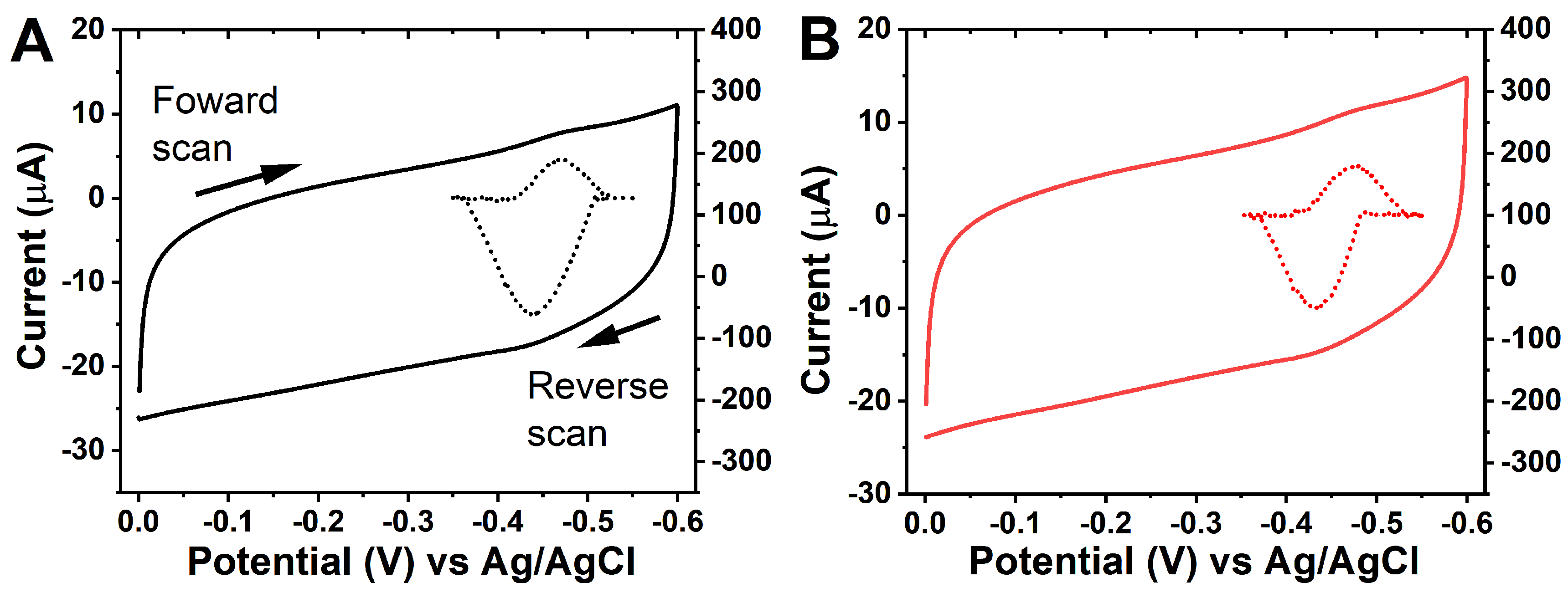
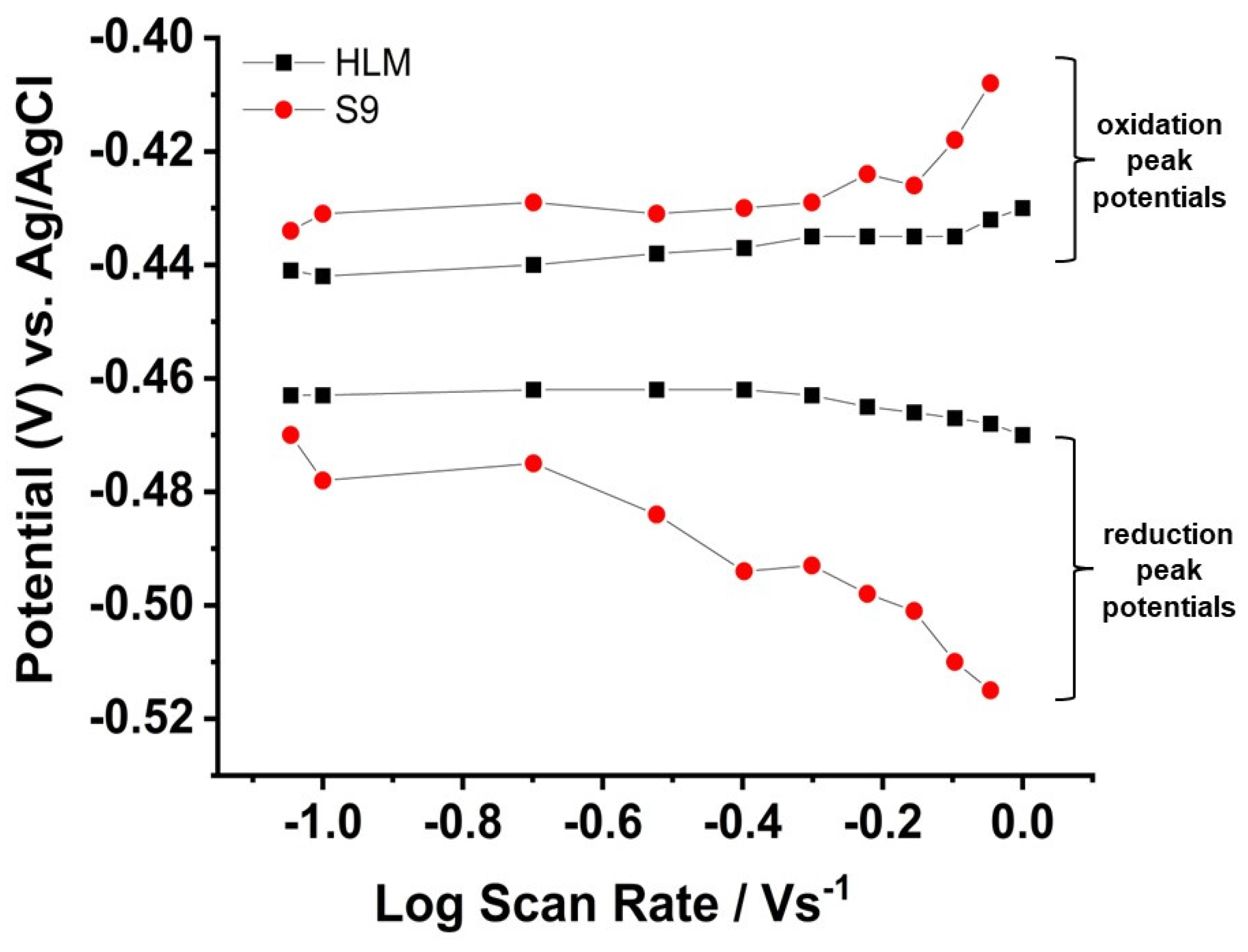
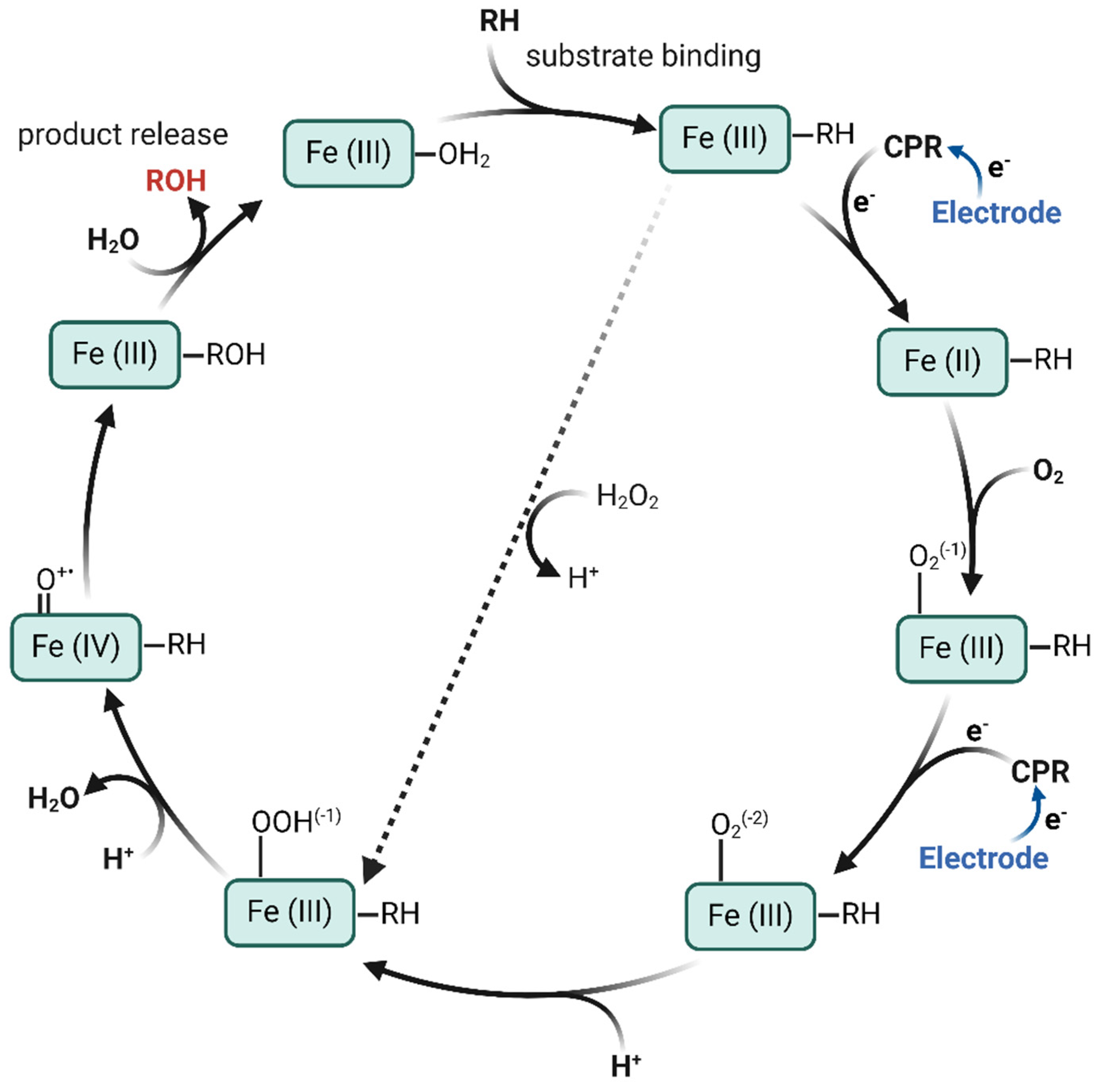

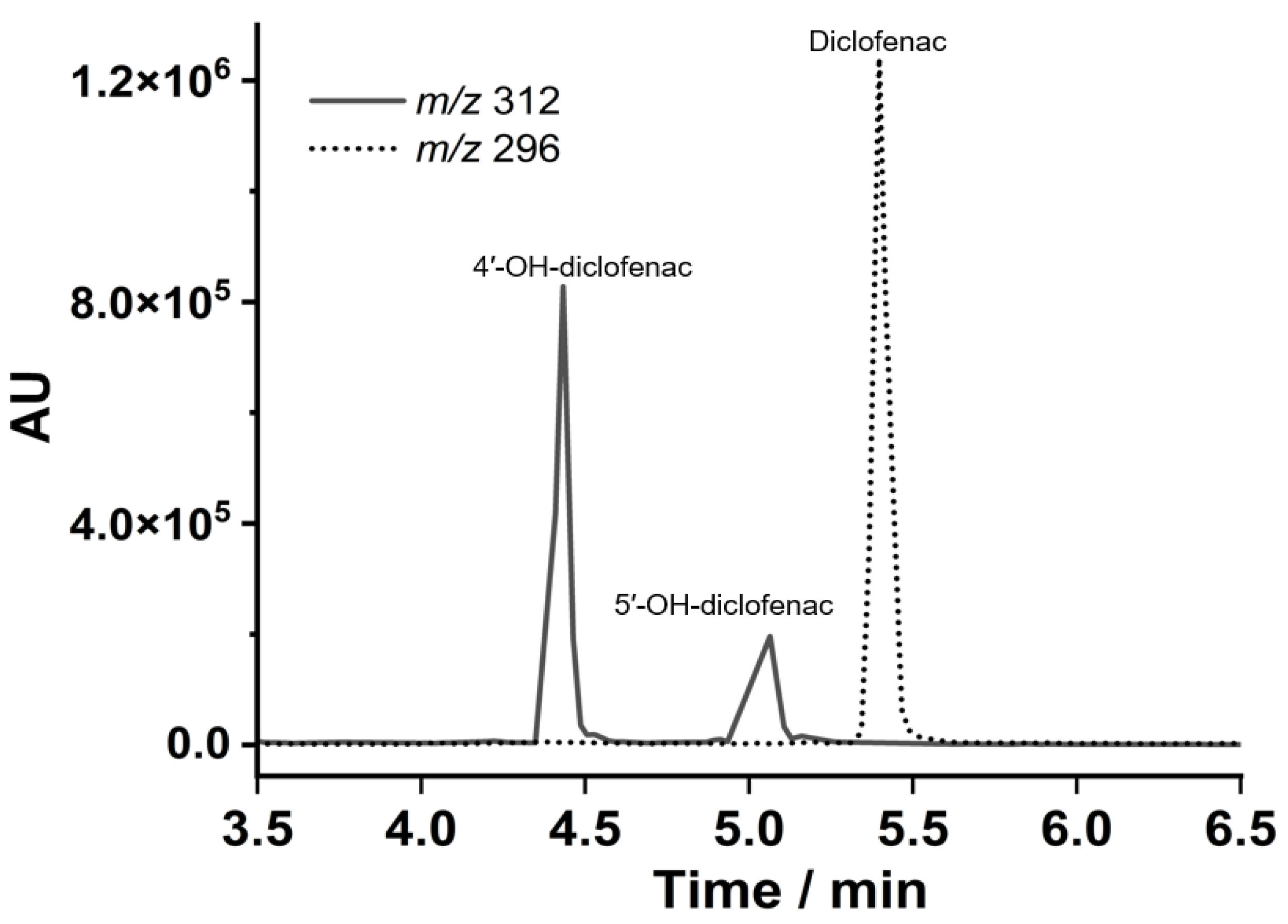
| Electrode Assembly | E°′/mV vs. Ag/AgCl | Q/nC | PWHM/mV | ks (s−1) |
|---|---|---|---|---|
| HPG/HLM | −453 ± 3 | 275 ± 12 | 66 ± 1 | 66 ± 10 |
| HPG/S9 | −451 ± 1 | 344 ± 14 | 59 ± 2 | 14 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, D.; Omanakuttan, B.; Nguyen, R.; Alwarsh, E.; Walgama, C. Electrochemical Probing of Human Liver Subcellular S9 Fractions for Drug Metabolite Synthesis. Metabolites 2024, 14, 429. https://doi.org/10.3390/metabo14080429
Medina D, Omanakuttan B, Nguyen R, Alwarsh E, Walgama C. Electrochemical Probing of Human Liver Subcellular S9 Fractions for Drug Metabolite Synthesis. Metabolites. 2024; 14(8):429. https://doi.org/10.3390/metabo14080429
Chicago/Turabian StyleMedina, Daphne, Bhavana Omanakuttan, Ricky Nguyen, Eman Alwarsh, and Charuksha Walgama. 2024. "Electrochemical Probing of Human Liver Subcellular S9 Fractions for Drug Metabolite Synthesis" Metabolites 14, no. 8: 429. https://doi.org/10.3390/metabo14080429
APA StyleMedina, D., Omanakuttan, B., Nguyen, R., Alwarsh, E., & Walgama, C. (2024). Electrochemical Probing of Human Liver Subcellular S9 Fractions for Drug Metabolite Synthesis. Metabolites, 14(8), 429. https://doi.org/10.3390/metabo14080429








