Identification of Anastatica hierochuntica L. Methanolic-Leaf-Extract-Derived Metabolites Exhibiting Xanthine Oxidase Inhibitory Activities: In Vitro and In Silico Approaches
Abstract
1. Introduction
2. Results
2.1. Chemical Structures of the Metabolites Identified in Crude Methanolic Extracts of Leaves of A. hierochuntica
2.2. Molecular Docking of the Identified A. hierochuntica Methanolic-Leaf-Extract-Derived Metabolites with Crystal Structure of Bovine XO
2.3. Validation of XO Inhibitory Activity of Selected A. hierochuntica Methanolic-Leaf-Extract-Derived Metabolites Using an In Vitro Enzymatic Assay
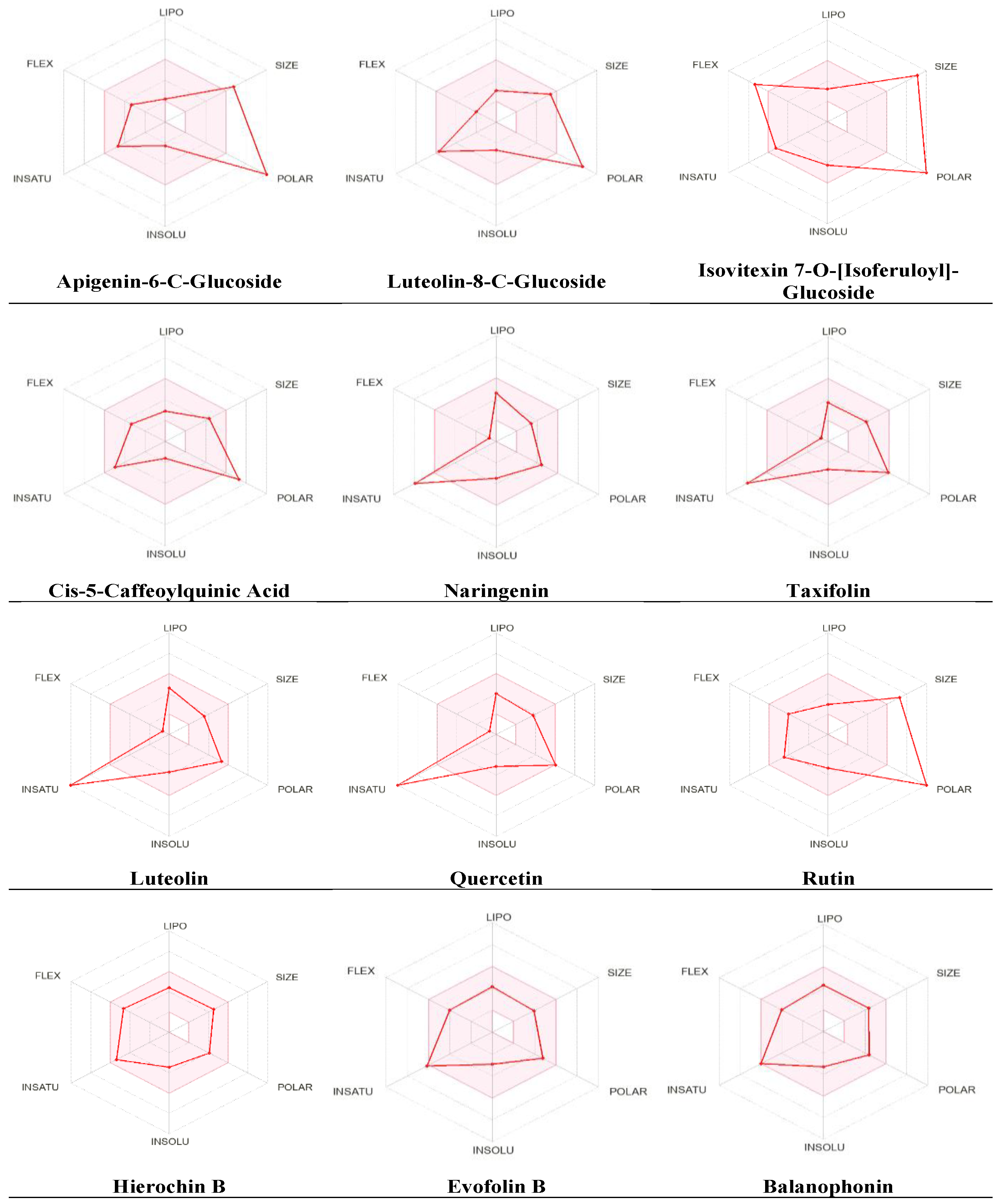
2.4. Physicochemical Parameter Evaluation of A. hierochuntica Methanolic-Leaf-Extract-Derived Metabolites
2.5. Predicted Toxicity Assessment of A. hierochuntica Methanolic-Leaf-Extract-Derived Metabolites
2.6. Estimation of Anticancer Activity Spectra of A. hierochuntica Methanolic-Leaf-Extract-Derived Metabolites
2.7. Evaluation of Endocrine Disruption Potential
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Extraction
4.3. Screening of A. hierochuntica Methanolic-Leaf-Extract-Derived Metabolites Using LC-QTOF-MS
4.4. Molecular Docking Study
4.5. Enzyme Inhibition Assay
4.6. Pharmacokinetic Properties Predictions
4.7. Organ and Endpoint Toxicity Assessment
4.8. Prediction of Anticancer Activity
4.9. Predictions of Endocrine Disruptors’ Properties
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhang, J.; Jin, C.; Ma, B.; Sun, H.; Chen, Y.; Zhong, Y.; Han, C.; Liu, T.; Li, Y. Global, regional and national burdens of gout in the young population from 1990 to 2019: A population-based study. RMD Open 2023, 9, e003025. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, R.; Barry, M.J.; Choi, H.K.; Hernandez, D.; Johnsen, B.; Labrador, M.; Reid, S.; Singh, J.A.; Terkeltaub, R.; Vazquez Mellado, J.; et al. Gout, hyperuricaemia and crystal-associated disease network (G-CAN) common language definition of gout. RMD Open 2021, 7, e001623. [Google Scholar] [CrossRef]
- Stamp, L.K.; Dalbeth, N. Prevention and treatment of gout. Nat. Rev. Rheumatol. 2019, 15, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase-derived reactive species: Physiological and pathological effects. Oxid. Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef]
- Kumar, R.; Joshi, G.; Kler, H.; Kalra, S.; Kaur, M.; Arya, R. Toward an understanding of structural insights of xanthine and aldehyde oxidases: An overview of their inhibitors and role in various diseases. Med. Res. Rev. 2018, 38, 1073–1125. [Google Scholar] [CrossRef]
- Yu, W.; Cheng, J.D. Uric acid and cardiovascular disease: An update from molecular mechanism to clinical perspective. Front. Pharmacol. 2020, 11, 582680. [Google Scholar] [CrossRef]
- Charles Seychell, B.; Vella, M.; James Hunter, G.; Hunter, T. The Good and the Bad: The Bifunctional Enzyme Xanthine Oxidoreductase in the Production of Reactive Oxygen Species [Internet]. In Reactive Oxygen Species—Advances and Developments; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Yiu, A.; Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Malmström, H.; Lambe, M.; Hammar, N.; Walldius, G.; Jungner, I.; Wulaningsih, W. Circulating uric acid levels and subsequent development of cancer in 493,281 individuals: Findings from the AMORIS Study. Oncotarget 2017, 8, 42332. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, C.M.; Paradisi, F. Looking back: A short history of the discovery of enzymes and how they became powerful chemical tools. ChemCatChem 2020, 12, 6082–6102. [Google Scholar] [CrossRef]
- Rullo, R.; Cerchia, C.; Nasso, R.; Romanelli, V.; Vendittis, E.D.; Masullo, M.; Lavecchia, A. Novel reversible inhibitors of xanthine oxidase targeting the active site of the enzyme. Antioxidants 2023, 12, 825. [Google Scholar] [CrossRef]
- Ramasamy, S.N.; Korb-Wells, C.S.; Kannangara, D.R.; Smith, M.W.; Wang, N.; Roberts, D.M.; Graham, G.G.; Williams, K.M.; Day, R.O. Allopurinol hypersensitivity: A systematic review of all published cases, 1950–2012. Drug Saf. 2013, 36, 953–980. [Google Scholar] [CrossRef]
- Bohm, M.; Vuppalanchi, R.; Chalasani, N. Febuxostat-induced acute liver injury. Hepatology 2016, 63, 1047–1049. [Google Scholar] [CrossRef]
- Kataoka, H.; Yang, K.; Rock, K.L. The xanthine oxidase inhibitor Febuxostat reduces tissue uric acid content and inhibits injury-induced inflammation in the liver and lung. Eur. J. Pharmacol. 2015, 746, 174–179. [Google Scholar] [CrossRef]
- Jordan, A.; Gresser, U. Side effects and interactions of the xanthine oxidase inhibitor febuxostat. Pharmaceuticals 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, M.F.S.; Wegermann, C.A.; Ceroullo, M.S.; Sant’Anna, I.G.M.; Lessa, R.C. Ten years milestones in xanthine oxidase inhibitors discovery: Febuxostat-based inhibitors trends, bifunctional derivatives, and automatized screening assays. Organics 2022, 3, 380–414. [Google Scholar] [CrossRef]
- Borges, F.; Fernandes, E.; Roleira, F. Progress towards the discovery of xanthine oxidase inhibitors. Curr. Med. Chem. 2002, 9, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Deniz, F.S. Natural products and extracts as xantine oxidase inhibitors—A hope for gout disease? Curr. Pharm. Des. 2021, 27, 143–158. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. The inhibitory activity of natural products to xanthine oxidase. Chem. Biodivers. 2023, 20, e202300005. [Google Scholar] [CrossRef]
- Saleh, J.; Machado, L. Rose of jericho: A word of caution. Oman Med. J. 2012, 27, 338. [Google Scholar] [CrossRef]
- Zin, S.R.M.; Kassim, N.M.; Alshawsh, M.A.; Hashim, N.E.; Mohamed, Z. Biological activities of Anastatica hierochuntica L.: A systematic review. Biomed. Pharmacother. 2017, 91, 611–620. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Xu, F.; Morikawa, T.; Ninomiya, K.; Matsuda, H. Anastatins A and B, new skeletal flavonoids with hepatoprotective activities from the desert plant Anastatica hierochuntica. Bioorg. Med. Chem. Lett. 2003, 13, 1045–1049. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Xu, F.; Ando, S.; Matsuda, H. (7R,8S) and (7S,8R) 8-5′ linked neolignans from Egyptian herbal medicine Anastatica hierochuntica and inhibitory activities of lignans on nitric oxide production. Heterocycles 2003, 60, 1787–1792. [Google Scholar] [CrossRef]
- AlGamdi, N.; Mullen, W.; Crozier, A. Tea prepared from Anastatica hirerochuntica seeds contains a diversity of antioxidant flavonoids, chlorogenic acids and phenolic compounds. Phytochemistry 2011, 72, 248–254. [Google Scholar] [CrossRef]
- El-Garawani, I.M.; Abd El-Gaber, A.S.; Algamdi, N.A.; Saeed, A.; Zhao, C.; Khattab, O.M.; AlAjmi, M.F.; Guo, Z.; Khalifa, S.A.M.; El-Seedi, H.R. In Vitro induction of apoptosis in isolated acute myeloid leukemia cells: The role of Anastatica hierochuntica methanolic Extract. Metabolites 2022, 12, 878. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.M.; Al-Nowaihi, A.S.M.; Kawashty, S.A.; Saleh, N.A.M. Chemosystematic studies on certain species of the family Brassicaceae (Cruciferae) in Egypt. Biochem. System Ecol. 2010, 38, 680–685. [Google Scholar] [CrossRef]
- Nakashima, S.; Matsuda, H.; Oda, Y.; Nakamura, S.; Xu, F.; Yoshikawa, M. Melanogenesis inhibitors from the desert plant Anastatica hierochuntica in B16 melanoma cells. Bioorg. Med. Chem. 2010, 18, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Enroth, C.; Eger, B.T.; Okamoto, K.; Nishino, T.; Nishino, T.; Pai, E.F. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: Structure-based mechanism of conversion. Proc. Natl. Acad. Sci. USA 2000, 97, 10723–10728. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Nakashima, Y.; Tsukamoto, S.; Kurohara, T.; Suzuki, M.; Sakae, Y.; Oda, M.; Okamoto, Y.; Suzuki, T. N+-C-H···O Hydrogen bonds in protein-ligand complexes. Sci. Rep. 2019, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Dhiman, P.; Khatkar, A. In silico design and synthesis of targeted rutin derivatives as xanthine oxidase inhibitors. BMC Chem. 2019, 13, 71. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary flavonoids as xanthine oxidase inhibitors: Structure–affinity and structure–activity relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, C.S.; Chen, C.T.; Liang, Y.C.; Lin, J.K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002, 294, 167–172. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, G.; Hu, Y.; Ma, Y. Effect of luteolin on xanthine oxidase: Inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem. 2013, 141, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.C.; Murugaiyah, V.; Chan, K.L. Flavonoids and phenylethanoid glycosides from Lippia nodiflora as promising antihyperuricemic agents and elucidation of their mechanism of action. J. Ethnopharmacol. 2015, 176, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T.; Poso, A. Binding affinity via docking: Fact and fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef]
- Zhao, L.; Ai, X.; Pan, F.; Zhou, N.; Zhao, L.; Cai, S.; Tang, X. Novel peptides with xanthine oxidase inhibitory activity identified from macadamia nuts: Integrated in silico and in vitro analysis. Eur. Food Res. Technol. 2022, 248, 2031–2042. [Google Scholar] [CrossRef]
- Xue, H.; Xu, M.; Gong, D.; Zhang, G. Mechanism of flavonoids inhibiting xanthine oxidase and alleviating hyperuricemia from structure–activity relationship and animal experiments: A review. Food Front. 2023, 4, 1643–1665. [Google Scholar] [CrossRef]
- Mehmood, A.; Li, J.; Rehman, A.U.; Kobun, R.; Llah, I.U.; Khan, I.; Althobaiti, F.; Albogami, S.; Usman, M.; Alharthi, F.; et al. Xanthine oxidase inhibitory study of eight structurally diverse phenolic compounds. Front. Nutr. 2022, 9, 966557. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Zhang, G.; Gong, D. Mechanistic insights into the inhibition of quercetin on xanthine oxidase. Int. J. Biol. Macromol. 2018, 112, 405–412. [Google Scholar] [CrossRef]
- Sayed, U.; Hudaib, M.; Issa, A.; Tawaha, K.; Bustanji, Y. Plant products and their inhibitory activity against xanthine oxidase. FARMACIA 2021, 69, 1042–1052. [Google Scholar] [CrossRef]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Kumari, L.; Choudhari, Y.; Patel, P.; Gupta, G.D.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Kurmi, B.D. Advancement in solubilization approaches: A step towards bioavailability enhancement of poorly soluble drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef]
- Chen, C.P.; Chen, C.C.; Huang, C.W.; Chang, Y.C. Evaluating molecular properties involved in transport of small molecules in stratum corneum: A quantitative structure-activity relationship for skin permeability. Molecules 2018, 23, 911. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N.; Lung, I. Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives. Molecules 2019, 24, 1505. [Google Scholar] [CrossRef]
- Effinger, A.; O’Driscoll, C.M.; McAllister, M.; Fotaki, N. Impact of gastrointestinal disease states on oral drug absorption–implications for formulation design–a PEARRL review. J. Pharm. Pharmacol. 2019, 71, 674–698. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and dietary polyphenols. Oxid. Med. Cell Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef]
- Choi, S.Y.; Koh, K.H.; Jeong, H. Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab. Dispos. 2013, 41, 263–269. [Google Scholar] [CrossRef]
- El-Sayed, M.; El-Sherif, F.; Elhassaneen, Y.; Abd El-Rahman, A. Potential therapeutic effects of some Egyptian plant parts on hepatic toxicity induced by carbon tetrachloride in rats. Life Sci. J. 2012, 9, 3747–3755. [Google Scholar]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Abou-Elella, F.; Ahmed, E.; Gavamukulya, Y. Determination of antioxidant and anti-inflammatory activities, as well as in vitro cytotoxic activities of extracts of Anastatica hierochuntica (Kaff Maryam) against HeLa cell lines. J. Med. Plants Res. 2016, 10, 77–87. [Google Scholar] [CrossRef]
- Mohammd, T.U.; Baker, R.K.; Al-Ameri, K.A.; Abd-Ulrazzaq, S.S. Cytotoxic effect of aqueous extract of Anastatica hierochuntica L. on AMN-3 cell line in vitro. Adv. Life Sci. Technol. 2015, 31, 59–63. [Google Scholar]
- Ali, M.A.; Abul Farah, M.; Al-Hemaid, F.M.; Abou-Tarboush, F.M. In vitro cytotoxicity screening of wild plant extracts from Saudi Arabia on human breast adenocarcinoma cells. Genet. Mol. Res. 2014, 13, 3981–3990. [Google Scholar] [CrossRef] [PubMed]
- Rameshbabu, S.; Messaoudi, S.A.; Alehaideb, Z.I.; Ali, M.S.; Venktraman, A.; Alajmi, H.; Al-Eidi, H.; Matou-Nasri, S. Anastatica hierochuntica (L.) methanolic and aqueous extracts exert antiproliferative effects through the induction of apoptosis in MCF-7 breast cancer cells. Saudi Pharm. J. 2020, 28, 985–993. [Google Scholar] [CrossRef]
- Md Zin, S.R.; Mohamed, Z.; Alshawsh, M.A.; Wong, W.F.; Kassim, N.M. Mutagenicity evaluation of Anastatica hierochuntica L. aqueous extract in vitro and in vivo. Exp. Biol. Med. 2018, 243, 375–385. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Comp. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Gokce, M.; Guler, E.M.A. hierchuntica extract exacerbates genotoxic, cytotoxic, apoptotic and oxidant effects in B16F10 melanoma cells. Toxicon 2021, 198, 73–79. [Google Scholar] [CrossRef]
- Hajjar, D.; Kremb, S.; Sioud, S.; Emwas, A.H.; Voolstra, C.R.; Ravasi, T. Anti-cancer agents in Saudi Arabian herbals revealed by automated high-content imaging. PLoS ONE 2017, 12, e0177316. [Google Scholar] [CrossRef] [PubMed]
- Nazeam, J.A.; El-Emam, S.Z. Middle Eastern plants with potent cytotoxic effect against lung cancer cells. J. Med. Food 2024, 27, 198–207. [Google Scholar] [CrossRef]
- Domańska, A.; Orzechowski, A.; Litwiniuk, A.; Kalisz, M.; Bik, W.; Baranowska-Bik, A. The beneficial role of natural endocrine disruptors: Phytoestrogens in Alzheimer’s disease. Oxid. Med. Cell Longev. 2021, 2021, 3961445. [Google Scholar] [CrossRef]
- Humfrey, C.D. Phytoestrogens and human health effects: Weighing up the current evidence. Nat. Toxins 1998, 6, 51–59. [Google Scholar] [CrossRef]
- Smeriglio, A.; Trombetta, D.; Marcoccia, D.; Narciso, L.; Mantovani, A.; Lorenzetti, S. Intracellular distribution and biological effects of phytochemicals in a sex steroid- sensitive model of human prostate adenocarcinoma. Anticancer. Agents Med. Chem. 2014, 14, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, G.; Gianquinto, E.; Rossetti, G.; Cruciani, G.; Lorenzetti, S.; Spyrakis, F. Binding of androgen-and estrogen-like flavonoids to their cognate (non) nuclear receptors: A comparison by computational prediction. Molecules 2021, 26, 1613. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, J.H.; Cho, H.J.; Ha, Y.J.; Kang, E.H.; Shin, K.; Byun, S.S.; Lee, E.Y.; Song, Y.W.; Lee, Y.J. Influence of androgen deprivation therapy on serum urate levels in patients with prostate cancer: A retrospective observational study. PLoS ONE 2018, 13, e0209049. [Google Scholar] [CrossRef]






| Peak No. | Rt (min) | [M+H]+ | [M−H]− | Err PPM | Molecular Formula | Tentative Identification | Literature Review of the Compounds |
|---|---|---|---|---|---|---|---|
| Peak A (M1) | (21.445–21.793) | 433.29 | 431.5 | −0.65 | C21H20O10 | Apigenin-6-C-glucoside (isovitexin) | [23,24] |
| Peak B (M2) | (36.628–36.694) | 449.29 | 447.3 | −0.50 | C21H20O11 | Luteolin-8-C-glucoside (orientin) | [23,24] |
| Peak C (M3) | (32.948–32.965) | 771.50 | 769.7 | −0.45 | C37H38O18 | Isovitexin 7-O-glucoside | [23] |
| Peak D (M4) | (31.058–31.257) | 355.24 | 353.5 | −0.65 | C16H18O9 | 5-Caffeoylquinic acid | [23] |
| Peak E (M5) | (27.893–28.125) | 273.2 | 271.3 | −0.45 | C15H12O5 | Naringenin | [21,24,25,26] |
| Peak F (M6) | (29.484–29.650) | 305.14 | 303.3 | −0.65 | C15H12O7 | Taxifolin | [26] |
| Peak G (M7) | (25.754–25.821) | 287.13 | 285.3 | −0.40 | C15H12O6 | Luteolin | [21,26] |
| Peak H (M8) | (28.804–29.268) | 303.13 | 301.9 | −0.70 | C15H10O7 | Quercetin | [23,25,26] |
| Peak I (M9) | (32.020–32.467) | 611.34 | 609.4 | −0.65 | C27H30O16 | Rutin | [21,26] |
| Peak J (M10) | (33.048–33.765) | 373.46 | 371.37 | −0.70 | C21H24O6 | Hierochin B | [21,26] |
| Peak K (M11) | (26.142–26.324) | 319.32 | 317.38 | −0.85 | C17H18O6 | Evofolin B | [21] |
| Peak L (M12) | (28.173–28.397) | 357.46 | 355.39 | −0.65 | C20H20O6 | Balanophonin | [21,26] |
| Metabolite Name | Glide Docking Score | Molecular Interactions |
|---|---|---|
| Apigenin-6-C-glucoside | −10.26 | H bonds: LEU 648 π–π: PHE 649, PHE 1013 |
| Luteolin-8-C-glucoside | −8.40 | H bonds: GLU 802, LYS 771 π–π: PHE 649, PHE 1013 |
| Isovitexin 7-O-[isoferuloyl]-glucoside | −4.49 | H bonds: THR 1010 π–π: PHE 649, LYS 771, PHE 914, PHE 1009, PHE 1013 |
| Cis-5-caffeoylquinic acid | −10.19 | H bonds: LEU 648, THR 1010 π–π: PHE 914, PHE 1009 |
| Naringenin | −9.96 | H bonds: GLU 802, THR 1010 π–π: PHE 914, PHE 1009 |
| Taxifolin | −7.88 | H bonds: GLU 802 π–π: PHE 649, PHE 1013 |
| Luteolin | −10.43 | H bonds: SER 876, ARG 880, THR 1010 π–π: PHE 914, PHE 1009 |
| Quercetin | −11.15 | H bonds: GLU 802, SER 876, ARG 880, THR 1010 π–π: PHE 914, PHE 1009 |
| Rutin | −12.39 | H bonds: GLU 802, GLU 879, SER 876, HIS 875 Pi-Pi: PHE 1013 π–cation: LYS 771 |
| Hierochin B | −7.75 | π–π: PHE 1009 |
| Evofolin B | −8.54 | H bonds: LYS771, SER 876 π–π: PHE 914, PHE 1009, PHE 1013 |
| Balanophonin | −8.53 | H bonds: LYS 771 π–π: PHE 914, PHE 1009 |
| Metabolite | Rutin | Quercetin | Luteolin | Allopurinol |
|---|---|---|---|---|
| IC50 value (µM) | 11.35 ± 2.09 | 11.1 ± 1.72 | 21.58 ± 2.41 | 4.3 ± 1.01 |
| Properties | Parameters | Apigenin-6-C-glucoside | Luteolin-8-C-glucoside | Isovitexin 7-O-[isoferuloyl]-glucoside | Cis-5-caffeoylquinic acid | Naringenin | Taxifolin | Luteolin | Quercetin | Rutin | Hierochin B | Evofolin B | Balanophonin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physico-chemical Properties | MW (g/mol) | 564.49 | 448.38 | 770.69 | 354.31 | 272.25 | 304.25 | 286.24 | 302.24 | 610.52 | 372.41 | 318.32 | 356.37 |
| HBA | 14 | 11 | 18 | 9 | 5 | 7 | 6 | 7 | 16 | 6 | 6 | 6 | |
| HBD | 10 | 8 | 10 | 6 | 3 | 5 | 4 | 5 | 10 | 2 | 3 | 2 | |
| Lipophilicity Log Po/w | iLOGP | 1.73 | 1.27 | 2.46 | 0.96 | 1.75 | 1.30 | 1.86 | 1.63 | 1.58 | 3.36 | 2.24 | 2.79 |
| XLOGP3 | −1.64 | −0.15 | 0.12 | −0.42 | 2.52 | 0.95 | 2.53 | 1.54 | −0.33 | 2.24 | 1.78 | 2.04 | |
| MLOGP | −3.97 | −2.51 | −3.42 | −1.05 | 0.71 | −0.64 | −0.03 | −0.56 | −3.89 | 1.31 | 0.78 | 1.01 | |
| Absorption | Water solubility (Log S) | −0.84 Soluble | −1.79 Soluble | −2.75 Soluble | 0.40 Soluble | −3.42 Soluble | −2.03 Soluble | −3.82 Soluble | −3.24 Soluble | −0.29 Soluble | −4.82 Moderate soluble | −3.83 Soluble | −4.25 Moderate soluble |
| GI | Low | Low | Low | Low | High | High | High | High | Low | High | High | High | |
| Log Kp (skin permeation) cm/s | −10.91 | −9.14 | −10.92 | −8.76 | −6.17 | −7.48 | −6.25 | −7.05 | −10.26 | −6.98 | −6.98 | −7.03 | |
| Distribution | BBB permeant | No | No | No | No | No | No | No | No | No | Yes | No | No |
| Metabolism | CYP1A2 inhibitor | No | No | No | No | Yes | No | Yes | Yes | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | |
| CYP2D6 inhibitor | No | No | No | No | No | No | Yes | Yes | No | Yes | No | No | |
| CYP3A4 inhibitor | No | No | No | No | Yes | No | Yes | Yes | No | No | Yes | No | |
| Drug-likeness | Lipinski | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Metabolite Name | Classification | ||||
|---|---|---|---|---|---|
| Organ Toxicity (%Probability) | Toxicity Endpoint (% Probability) | ||||
| Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity | |
| Apigenin-6-C-glucoside | Inactive (0.81) | Inactive (0.69) | Inactive (0.85) | Inactive (0.55) | Inactive (0.81) |
| Luteolin-8-C-glucoside | Inactive (0.81) | Inactive (0.72) | Active (0.52) | Active (0.52) | Inactive (0.87) |
| isovitexin 7-O-[isoferuloyl]-glucoside | Inactive (0.81) | Inactive (0.88) | Active (0.99) | Inactive (0.52) | Inactive (0.65) |
| Cis-5-Caffeoyl quinic acid | Inactive (0.72) | Inactive (0.68) | Active (0.99) | Inactive (0.93) | Inactive (0.80) |
| Naringenin | Inactive (0.67) | Inactive (0.62) | Inactive (0.88) | Inactive (0.83) | Active (0.59) |
| Taxifolin | Inactive (0.69) | Active (0.68) | Inactive (0.76) | Active (0.51) | Inactive (0.99) |
| Luteolin | Inactive (0.69) | Active (0.68) | Inactive (0.97) | Active (0.51) | Inactive (0.99) |
| Quercetin | Inactive (0.69) | Active (0.68) | Inactive (0.87) | Active (0.51) | Inactive (0.99) |
| Rutin | Inactive (0.80) | Inactive (0.91) | Active (0.98) | Inactive (0.88) | Inactive (0.64) |
| Hierochin B | Inactive (0.79) | Inactive (0.59) | Active (0.90) | Inactive (0.73) | Inactive (0.91) |
| Evofolin B | Inactive (0.82) | Inactive (0.74) | Inactive (0.94) | Inactive (0.70) | Inactive (0.98) |
| Balanophonin | Inactive (0.72) | Inactive (0.58) | Active (0.95) | Inactive (0.64) | Inactive (0.89) |
| Metabolite Name | Pa | Pi |
|---|---|---|
| Apigenin-6-C-glucoside | 0.831 | 0.004 |
| Luteolin-8-C-glucoside | 0.872 | 0.003 |
| Isovitexin 7-O-[isoferuloyl]-glucoside | 0.988 | 0.001 |
| Cis-5-caffeoylquinic Acid | 0.846 | 0.004 |
| Naringenin | 0.751 | 0.018 |
| Taxifolin | 0.821 | 0.005 |
| Luteolin | 0.783 | 0.014 |
| Quercetin | 0.797 | 0.012 |
| Rutin | 0.983 | 0.001 |
| Hierochin B | 0.636 | 0.038 |
| Evofolin B | 0.387 | 0.033 |
| Balanophonin | 0.552 | 0.056 |
| Metabolites | AR | ER α | ERβ | GR | LXR α | LXR β | MR | PPAR α | PPAR β | PPAR γ | PR | RXR α | TR α | TR β |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apigenin-6-C-glucoside | 5.7 | −7.1 | 3.1 | −10.2 | −7.9 | −9.4 | 2.1 | −6.3 | −8 | −7.6 | −2.5 | −6.2 | −5.9 | −7.6 |
| Luteolin-8-C-glucoside | 1.4 | −6.1 | −4.1 | −6.4 | −6.8 | −7.9 | −4.2 | −8.1 | −7.1 | −7.9 | −2.9 | −8.4 | −1.9 | −2.3 |
| Isovitexin 7-O-[isoferuloyl]-glucoside | 15.7 | −5.4 | 8.2 | −2.7 | −4.3 | −8.4 | 6.9 | −7.7 | −8 | −8.7 | −2.9 | 0.9 | 5.1 | 2.2 |
| Cis-5-caffeoyl quinic acid | −6.8 | −8.5 | −8.4 | −8.9 | −8.7 | −8.9 | −7.4 | −7.6 | −7.9 | −7.8 | −2.7 | −9.3 | −8.7 | −8.4 |
| Naringenin | −8.8 | −8.9 | −8.2 | −8.6 | −8.8 | −9.4 | −9.1 | −9.1 | −8.5 | −9.2 | −2.5 | −9.5 | −9.3 | −9.6 |
| Taxifolin | −9.3 | −8.3 | −7.8 | −8.7 | −9.3 | −9.4 | −9 | −7.8 | −8.6 | −9.1 | −2.7 | −8.4 | −8.6 | −9.5 |
| Luteolin | −9.0 | −8.6 | −7.6 | −9.2 | −9.0 | −9.6 | −9.3 | −9.0 | −8.5 | −9.2 | −2.5 | −9.7 | −9.4 | −9.5 |
| Quercetin | −8.7 | −8.3 | −7.2 | −9.5 | −9.1 | −9.2 | −9.1 | −8.0 | −8.5 | −9.2 | −2.8 | −8.6 | −8.9 | −9.1 |
| Rutin | 7.6 | −5.4 | 2.0 | −5.8 | −7.5 | −7.2 | 0.8 | −7.6 | −7.8 | −7.4 | −2.8 | −5.2 | 2.3 | −4.0 |
| Hierochin B | −0.6 | −5.1 | −1.4 | −8.8 | −8.7 | −9.1 | −5.1 | −7.2 | −7.8 | −7.7 | −2.3 | −6.8 | −7.5 | −8.6 |
| Evofolin B | −8.0 | −7.6 | −7.8 | −7.6 | −8.3 | −8.3 | −7.9 | −7.6 | −7.2 | −7.1 | −2.7 | −8.4 | −8.3 | −8.8 |
| Balanophonin | −3.1 | −5.9 | −1.8 | −8.9 | −9.1 | −9.3 | −5.5 | −7.7 | −7.8 | −7.4 | −2.3 | −8.3 | −7.2 | −9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rameshbabu, S.; Alehaideb, Z.; Alghamdi, S.S.; Suliman, R.S.; Almourfi, F.; Yacoob, S.A.M.; Venkataraman, A.; Messaoudi, S.; Matou-Nasri, S. Identification of Anastatica hierochuntica L. Methanolic-Leaf-Extract-Derived Metabolites Exhibiting Xanthine Oxidase Inhibitory Activities: In Vitro and In Silico Approaches. Metabolites 2024, 14, 368. https://doi.org/10.3390/metabo14070368
Rameshbabu S, Alehaideb Z, Alghamdi SS, Suliman RS, Almourfi F, Yacoob SAM, Venkataraman A, Messaoudi S, Matou-Nasri S. Identification of Anastatica hierochuntica L. Methanolic-Leaf-Extract-Derived Metabolites Exhibiting Xanthine Oxidase Inhibitory Activities: In Vitro and In Silico Approaches. Metabolites. 2024; 14(7):368. https://doi.org/10.3390/metabo14070368
Chicago/Turabian StyleRameshbabu, Saranya, Zeyad Alehaideb, Sahar S. Alghamdi, Rasha S. Suliman, Feras Almourfi, Syed Ali Mohamed Yacoob, Anuradha Venkataraman, Safia Messaoudi, and Sabine Matou-Nasri. 2024. "Identification of Anastatica hierochuntica L. Methanolic-Leaf-Extract-Derived Metabolites Exhibiting Xanthine Oxidase Inhibitory Activities: In Vitro and In Silico Approaches" Metabolites 14, no. 7: 368. https://doi.org/10.3390/metabo14070368
APA StyleRameshbabu, S., Alehaideb, Z., Alghamdi, S. S., Suliman, R. S., Almourfi, F., Yacoob, S. A. M., Venkataraman, A., Messaoudi, S., & Matou-Nasri, S. (2024). Identification of Anastatica hierochuntica L. Methanolic-Leaf-Extract-Derived Metabolites Exhibiting Xanthine Oxidase Inhibitory Activities: In Vitro and In Silico Approaches. Metabolites, 14(7), 368. https://doi.org/10.3390/metabo14070368






