The Application of Resveratrol Derivatives in Oral Cells Reduces the Oxidative Stress Induced by Glucocorticoids
Abstract
1. Introduction
2. Materials and Methods
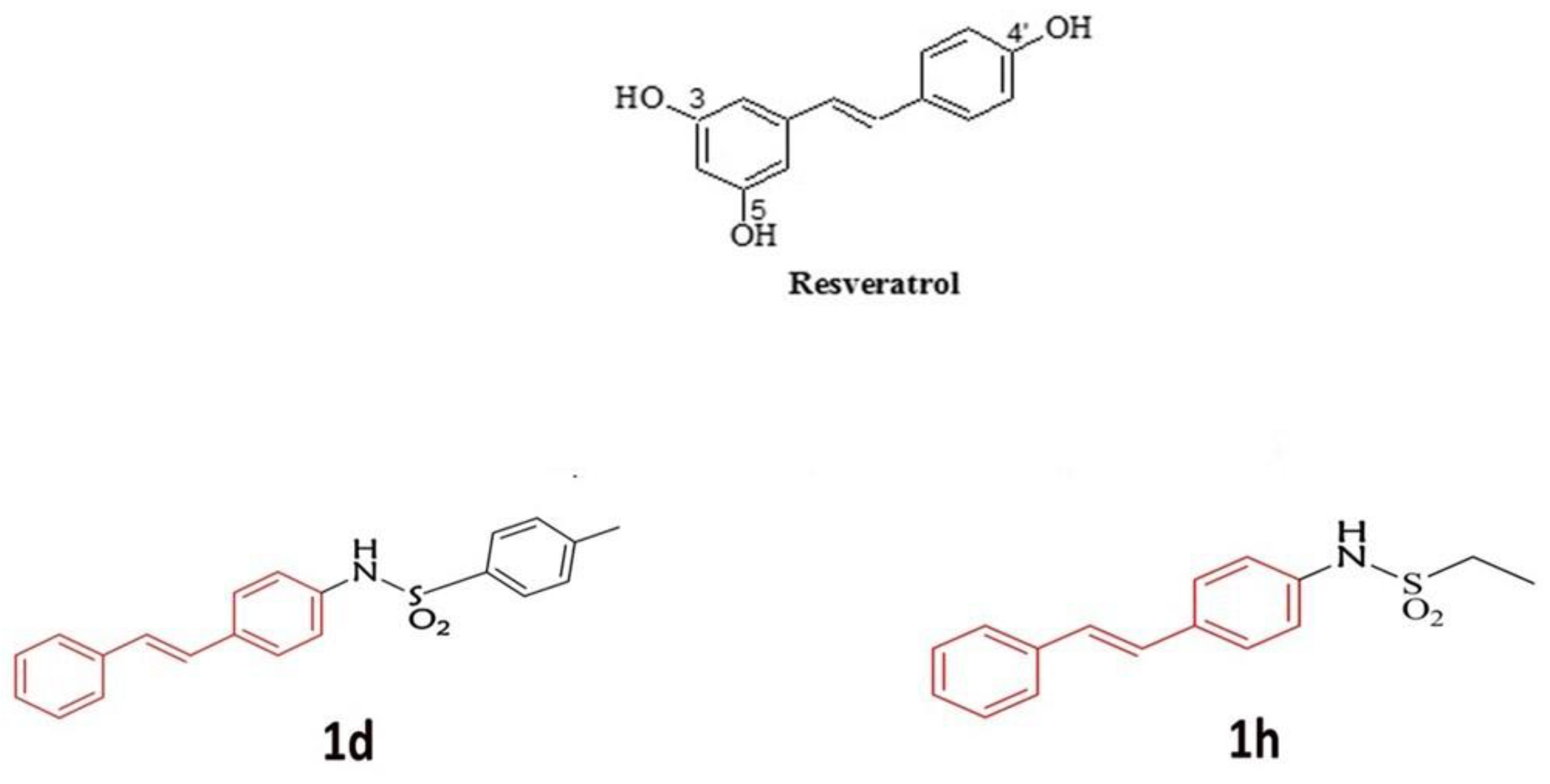
2.1. Description of Derivatives of RSV
2.2. Cell Culture and Treatments
- -
- (CTRL) cells were treated with 0.1% of DMSO;
- -
- (RSV) cells were treated with 5 µM RSV;
- -
- (1d) cells were treated with 5 µM 1d derivatives;
- -
- (1h) cells were treated with 5 µM 1h derivatives;
- -
- (DEX) cells were treated with 200 µM of dexamethasone (DEX; Sigma Aldrich, St. Louis, MO, USA) for 30 min to induce oxidative stress. This dose was chosen among different concentrations based on their potential to induce the highest level of ROS (Figure S1 in the Supplementary Materials);
- -
- (RSV+DEX) cells were treated with 200 µM of DEX. After treatment with DEX, the medium containing DEX was removed, and cells were treated with 5 µM RSV;
- -
- (1d+DEX) cells were treated with 200 µM of DEX. After treatment with DEX, the medium containing DEX was removed, and cells were treated with 5 µM 1d;
- -
- (1h+DEX) cells were treated with 200 µM of DEX. After treatment with DEX, the medium containing DEX was removed, and cells were treated with 5 µM 1h.
2.3. Cell Viability
2.4. CSLM
2.5. ROS Levels
2.6. SOD Activity
2.7. Gene Expression
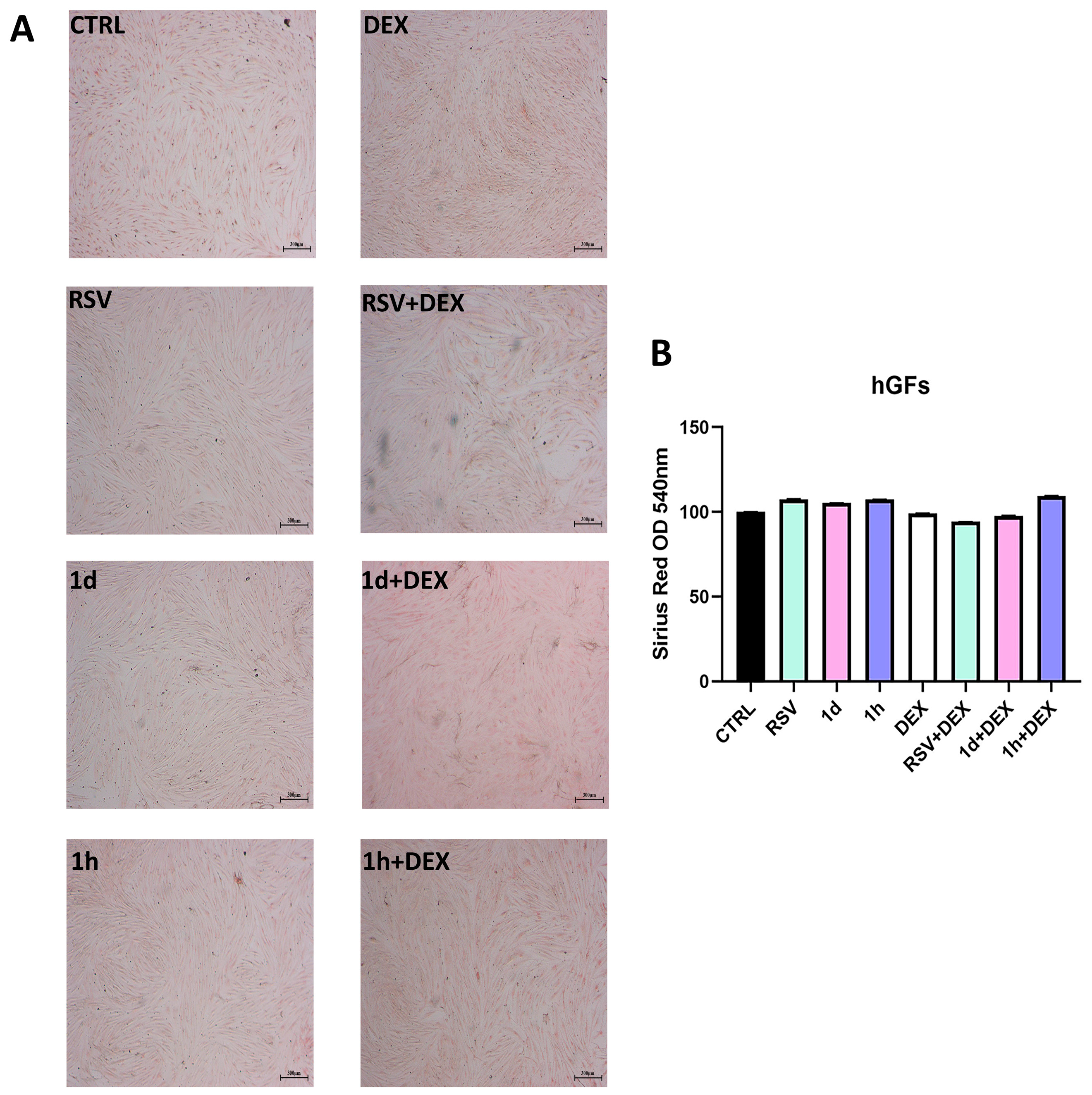
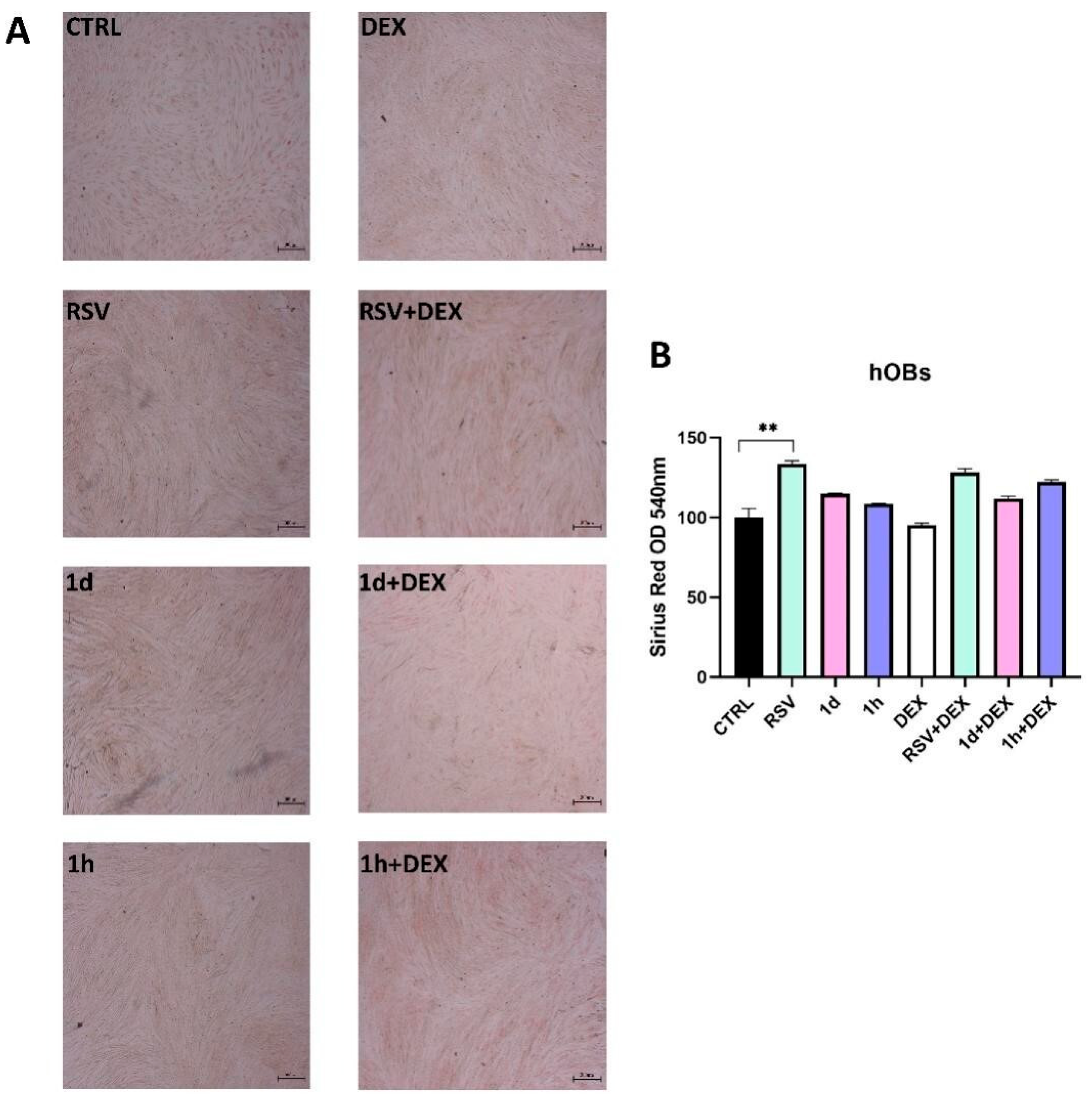
2.8. Picro-Sirius Red Staining and Spectrophotometric Analysis
2.9. Statistical Analysis
3. Results
3.1. Influence of 1d and 1h in Combination with Dexamethasone on Cell Viability
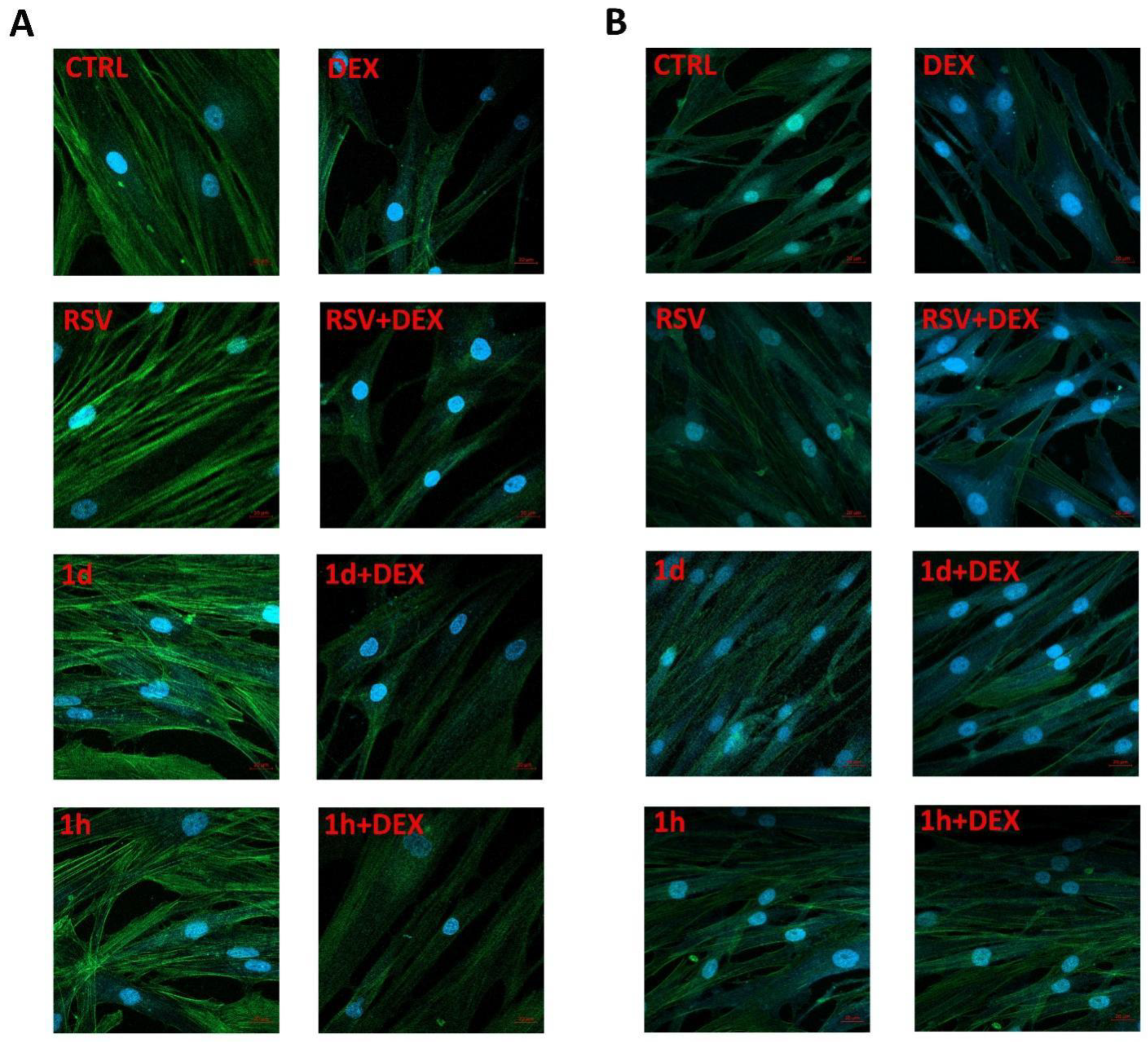
3.2. Influence of 1d and 1h in Combination with Dexamethasone on Morphology
3.3. Influence of 1d and 1h in Combination with Dexamethasone on ROS Levels
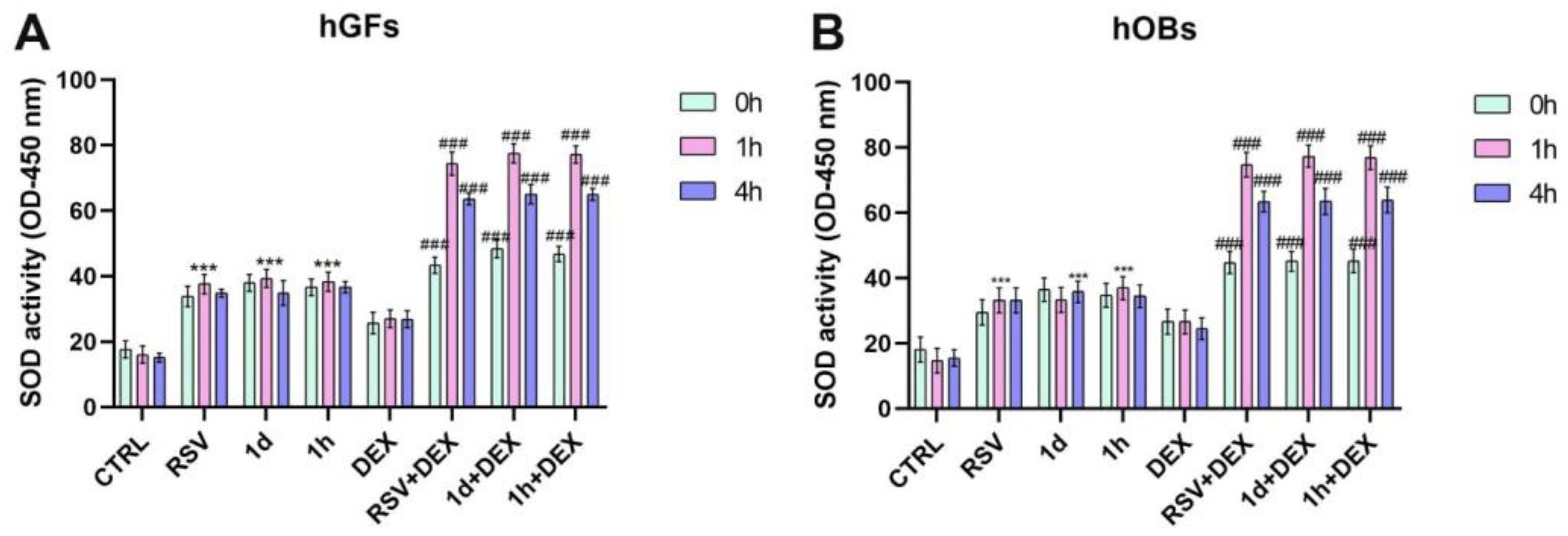
3.4. Influence of 1d and 1h in Combination with Dexamethasone on SOD Activity
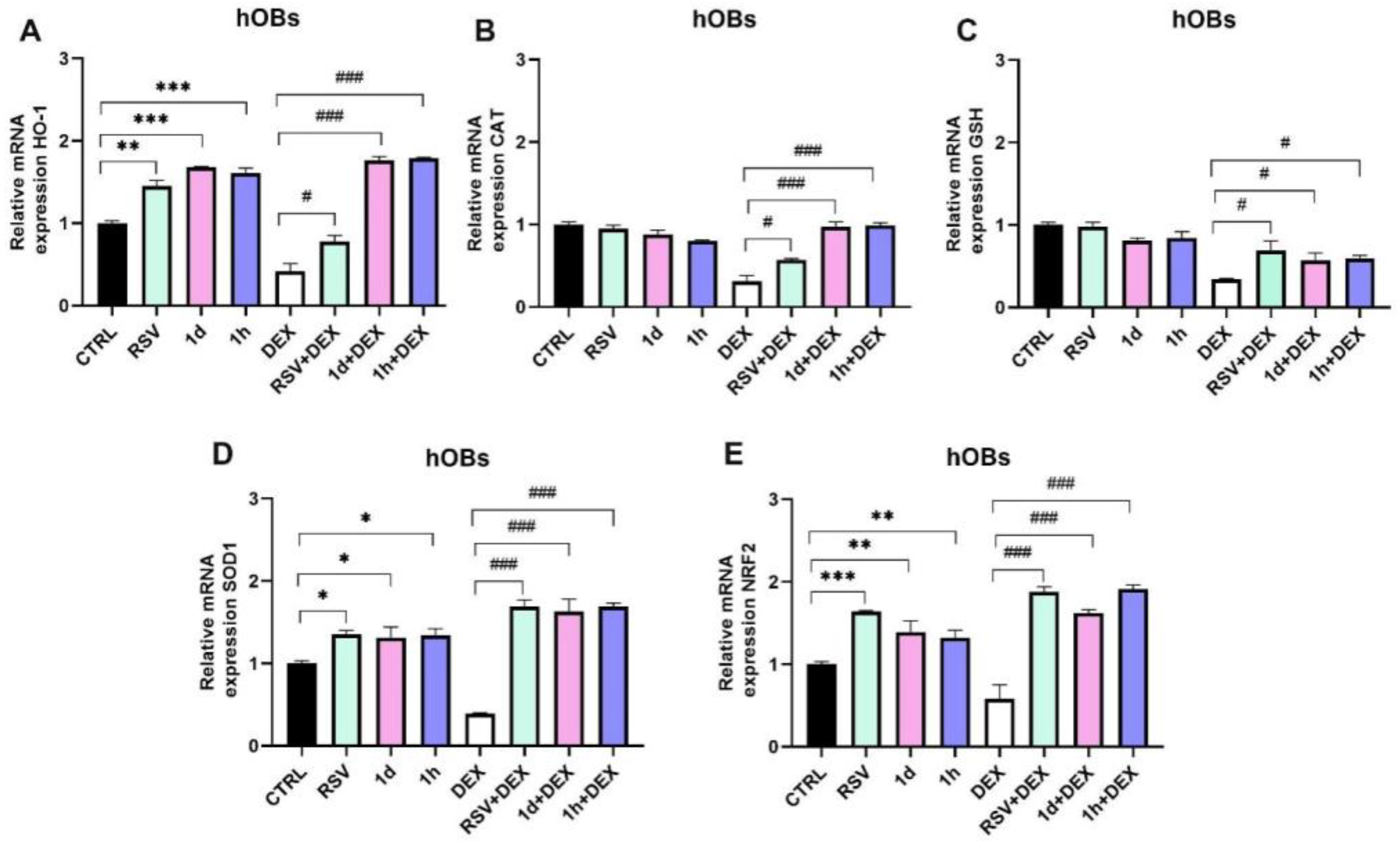
3.5. Influence of 1d and 1h in Combination with Dexamethasone on Antioxidant Gene Expression
3.6. Influence of 1d and 1h in Combination with Dexamethasone on Collagen Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Ayroldi, E.; Ricci, E.; Gentili, M.; Migliorati, G.; Riccardi, C. A Glance at the Use of Glucocorticoids in Rare Inflammatory and Autoimmune Diseases: Still an Indispensable Pharmacological Tool? Front. Immunol. 2021, 11, 613435. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments. Curr. Osteoporos. Rep. 2019, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, R.; Mago, J. Corticosteroids in dentistry. Indian J. Dent. Sci. 2016, 8, 252. [Google Scholar] [CrossRef]
- Nils, H.J.; Recatala, C.A.; Castano, A.; Ribas, D.; Flores-Fraile, J. Efficacy/Safety of the Use of Glucocorticoids in Oral and Maxillofacial Surgery. Dent. J. 2023, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M.; Mumby, S. Glucocorticoids. Handb. Exp. Pharmacol. 2016, 237, 171–196. [Google Scholar]
- Bjelaković, G.; Beninati, S.; Pavlović, D.; Kocić, G.; Jevtović, T.; Kamenov, L.; Šaranac, L.; Bjelaković, B.; Stojanović, I.; Bašić, J. Glucocorticoids and oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2007, 18, 115–128. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.G.; Kovoor, C.; Brown, K. Direct molecular targets of resveratrol: Identifying key interactions to unlock complex mechanisms. Ann. N. Y. Acad. Sci. 2015, 1348, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2018, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Trilli, I.; Del Vecchio, G.; Palmieri, G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G. Oxidative Stress and Natural Products in Orthodontic Treatment: A Systematic Review. Nutrients 2023, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.Q.; Moraes, W.M., Jr.; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Gerszon, J.; Rodacka, A.; Puchała, M. Antioxidant Properties of Resveratrol and its Protective Effects in Neurodegenerative Diseases. Adv. Cell Biol. 2014, 4, 97–117. [Google Scholar] [CrossRef]
- Constantinescu, T.; Mihis, A.G. Resveratrol as a privileged molecule with antioxidant activity. Food Chem. Adv. 2023, 3, 100539. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef]
- de Vries, K.; Strydom, M.; Steenkamp, V. A Brief Updated Review of Advances to Enhance Resveratrol’s Bioavailability. Molecules 2021, 26, 4367. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Fantacuzzi, M.; Gallorini, M.; Gambacorta, N.; Ammazzalorso, A.; Aturki, Z.; Balaha, M.; Carradori, S.; Giampietro, L.; Maccallini, C.; Cataldi, A.; et al. Design, Synthesis and Biological Evaluation of Aromatase Inhibitors Based on Sulfonates and Sulfonamides of Resveratrol. Pharmaceuticals 2021, 14, 984. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Amoroso, R.; Giampietro, L. Stilbene derivatives as new perspective in antifungal medicinal chemistry. Drug Dev. Res. 2019, 80, 285–293. [Google Scholar] [CrossRef]
- Di Fermo, P.; Di Lodovico, S.; Amoroso, R.; De Filippis, B.; D’Ercole, S.; Di Campli, E.; Cellini, L.; Di Giulio, M. Searching for New Tools to Counteract the Helicobacter pylori Resistance: The Positive Action of Resveratrol Derivatives. Antibiotics 2020, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Florio, R.; De Filippis, B.; Veschi, S.; di Giacomo, V.; Lanuti, P.; Catitti, G.; Brocco, D.; di Rienzo, A.; Cataldi, A.; Cacciatore, I.; et al. Resveratrol Derivative Exhibits Marked Antiproliferative Actions, Affecting Stemness in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2023, 24, 1977. [Google Scholar] [CrossRef]
- Di Filippo, E.S.; Giampietro, L.; De Filippis, B.; Balaha, M.; Ferrone, V.; Locatelli, M.; Pietrangelo, T.; Tartaglia, A.; Amoroso, R.; Fulle, S. Synthesis and Biological Evaluation of Halogenated E-Stilbenols as Promising Antiaging Agents. Molecules 2020, 25, 5770. [Google Scholar] [CrossRef]
- Apaydın, S.; Török, M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg. Med. Chem. Lett. 2019, 29, 2042–2050. [Google Scholar] [CrossRef]
- D’amico, E.; Pierfelice, T.V.; Amoroso, R.; Cacciatore, I.; D’arcangelo, C.; Lepore, S.; D’ercole, S.; Di Pietro, N.; Di Rienzo, A.; Petrini, M.; et al. Emerging Effects of Resveratrol Derivatives in Cells Involved in Oral Wound Healing: A Preliminary Study. Int. J. Mol. Sci. 2023, 24, 3276. [Google Scholar] [CrossRef]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Jt. Surg. 2021, 103, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, P.; Ballerini, P.; Buccella, S.; Ciccarelli, R.; Rathbone, M.P.; Romano, S.; D’Alimonte, I.; Caciagli, F.; Di Iorio, P.; Pokorski, M. Guanosine Protects Glial Cells Against 6-Hydroxydopamine Toxicity. In Neurotransmitter Interactions and Cognitive Function; Springer Nature: Berlin/Heidelberg, Germany, 2014; pp. 23–33. [Google Scholar]
- Bouvard, B.; Gallois, Y.; Legrand, E.; Audran, M.; Chappard, D. Glucocorticoids reduce alveolar and trabecular bone in mice. Jt. Bone Spine 2012, 80, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-L.; Tang, X.-L. Effect of glucocorticoid-induced oxidative stress on the expression of Cbfa1. Chem. Interactions 2014, 207, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shen, S.; Zhang, S.; Huang, M.; Zhang, L.; Chen, X. Autophagy in Bone Remodeling: A Regulator of Oxidative Stress. Front. Endocrinol. 2022, 13, 898634. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-L.; Li, T.-T.; Yang, F.; Tzeng, C.-M. Baicalin Ameliorates Dexamethasone-Induced Osteoporosis by Regulation of the RANK/RANKL/OPG Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 195–206. [Google Scholar] [CrossRef]
- Wang, L.; Heckmann, B.L.; Yang, X.; Long, H. Osteoblast autophagy in glucocorticoid-induced osteoporosis. J. Cell. Physiol. 2019, 234, 3207–3215. [Google Scholar] [CrossRef]
- Liu, S.; Fang, T.; Yang, L.; Chen, Z.; Mu, S.; Fu, Q. Gastrodin protects MC3T3-E1 osteoblasts from dexamethasone-induced cellular dysfunction and promotes bone formation via induction of the NRF2 signaling pathway. Int. J. Mol. Med. 2018, 41, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Habeos, I.G.; Samuelson, A.V.; Bohmann, D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 41–48. [Google Scholar] [CrossRef]
- Alavi, M.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell Int. 2021, 21, 579. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Souza, I.C.; Martins, L.A.M.; Coelho, B.P.; Grivicich, I.; Guaragna, R.M.; Gottfried, C.; Borojevic, R.; Guma, F.C.R. Resveratrol inhibits cell growth by inducing cell cycle arrest in activated hepatic stellate cells. Mol. Cell. Biochem. 2008, 315, 1–7. [Google Scholar] [CrossRef]
- Joe, A.K.; Liu, H.; Suzui, M.; E Vural, M.; Xiao, D.; Weinstein, I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002, 8, 893–903. [Google Scholar]
- Berardi, V.; Ricci, F.; Castelli, M.; Galati, G.; Risuleo, G. Resveratrol exhibits a strong cytotoxic activity in cultured cells and has an antiviral action against polyomavirus: Potential clinical use. J. Exp. Clin. Cancer Res. 2009, 28, 96–97. [Google Scholar] [CrossRef]
- Pal, K.; Raghuram, G.V.; Dsouza, J.; Shinde, S.; Jadhav, V.; Shaikh, A.; Rane, B.; Tandel, H.; Kondhalkar, D.; Chaudhary, S.; et al. A pro-oxidant combination of resveratrol and copper down-regulates multiple biological hallmarks of ageing and neurodegeneration in mice. Sci. Rep. 2022, 12, 17209. [Google Scholar] [CrossRef]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Resveratrol Induces Apoptosis through ROS-Dependent Mitochondria Pathway in HT-29 Human Colorectal Carcinoma Cells. J. Agric. Food Chem. 2008, 56, 4813–4818. [Google Scholar] [CrossRef]
- Martins, L.A.M.; Coelho, B.P.; Behr, G.; Pettenuzzo, L.F.; Souza, I.C.C.; Moreira, J.C.F.; Borojevic, R.; Gottfried, C.; Guma, F.C.R. Resveratrol Induces Pro-oxidant Effects and Time-Dependent Resistance to Cytotoxicity in Activated Hepatic Stellate Cells. Cell Biochem. Biophys. 2014, 68, 247–257. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Bonazza, V.; Buffoli, B.; Nocini, P.F.; Albanese, M.; Zotti, F.; Inchingolo, F.; Rezzani, R.; Rodella, L.F. Beneficial Effects of Concentrated Growth Factors and Resveratrol on Human Osteoblasts In Vitro Treated with Bisphosphonates. BioMed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.-M.; Guo, C.; Han, J.-F. Resveratrol promotes osteoblastic differentiation in a rat model of postmenopausal osteoporosis by regulating autophagy. Nutr. Metab. 2020, 17, 29. [Google Scholar] [CrossRef]
- Testa, B.; Crivori, P.; Reist, M.; Carrupt, P.-A. The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Perspect. Drug Discov. Des. 2000, 19, 179–211. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, E.; Cinquini, C.; Petrini, M.; Barone, A.; Iezzi, G.; D’Ercole, S.; De Filippis, B.; Pierfelice, T.V. The Application of Resveratrol Derivatives in Oral Cells Reduces the Oxidative Stress Induced by Glucocorticoids. Metabolites 2024, 14, 350. https://doi.org/10.3390/metabo14070350
D’Amico E, Cinquini C, Petrini M, Barone A, Iezzi G, D’Ercole S, De Filippis B, Pierfelice TV. The Application of Resveratrol Derivatives in Oral Cells Reduces the Oxidative Stress Induced by Glucocorticoids. Metabolites. 2024; 14(7):350. https://doi.org/10.3390/metabo14070350
Chicago/Turabian StyleD’Amico, Emira, Chiara Cinquini, Morena Petrini, Antonio Barone, Giovanna Iezzi, Simonetta D’Ercole, Barbara De Filippis, and Tania Vanessa Pierfelice. 2024. "The Application of Resveratrol Derivatives in Oral Cells Reduces the Oxidative Stress Induced by Glucocorticoids" Metabolites 14, no. 7: 350. https://doi.org/10.3390/metabo14070350
APA StyleD’Amico, E., Cinquini, C., Petrini, M., Barone, A., Iezzi, G., D’Ercole, S., De Filippis, B., & Pierfelice, T. V. (2024). The Application of Resveratrol Derivatives in Oral Cells Reduces the Oxidative Stress Induced by Glucocorticoids. Metabolites, 14(7), 350. https://doi.org/10.3390/metabo14070350











