Interaction between α-Synuclein and Bioactive Lipids: Neurodegeneration, Disease Biomarkers and Emerging Therapies
Abstract
1. Introduction
2. Genetics and Biochemistry of Synucleins at the Base of Their Preferential Interaction with Lipids in Physiological and Pathological Conditions
3. Alpha-Synuclein and Biological Membranes: The Importance of Protein-Lipids Interactions
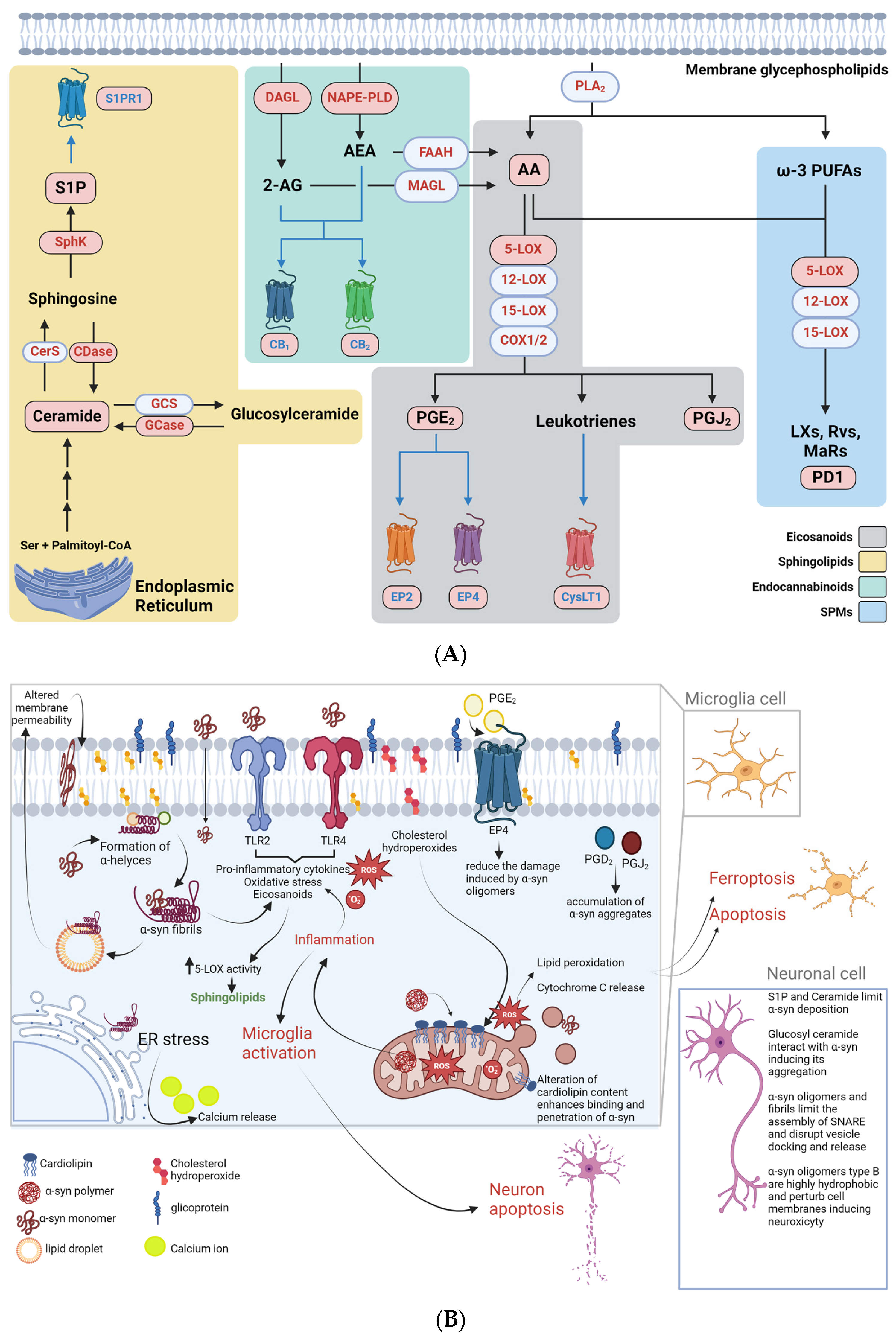
4. Endogenous Bioactive Lipids and Synucleinopathies
4.1. Eicosanoids
4.2. Sphingolipids
4.3. Endocannabinoids
4.4. Specialized Pro-Resolving Mediators
| Lipid Class | Molecular Target | Effect | Reference |
|---|---|---|---|
| Eicosanoids | Prostaglandins | Increased production in animal models of ischemia after α-Syn ablation | [98] |
| AA | Reduction in arachidonic acid incorporation and production in α-Syn knock out mice | [99] | |
| PGE2 | Enhanced PGE2-induced toxicity in α-syn-overexpressing neurons. | [104] | |
| Enhanced α-Syn clear out in EP2−/− microglia | [105] | ||
| EP4 agonism hinders α-Syn oligomer-induced damage in primary microglia | [107] | ||
| PGJ2 | Accumulation of α-Syn in rodent brain and SK-N-SH cells | [109] | |
| 5-LOX | Enhanced expression in DLB patients and mouse models | [112,113] | |
| CysLT1 | Montelukast ameliorates DLB clinical phenotype and α-Syn brain load in DLB mouse models. | ||
| Clinical trial EudraCT: 2020-000148 | [113] | ||
| Sphingolipids | GCase | It interacts with α-Syn and promotes its aggregation | [115,116,117] |
| Mutations are associated with PD | |||
| S1P | Production and aggregation of α-Syn | [119] | |
| Ceramide | |||
| SphK1 | SphK1 inhibition leads to neuronal death of dopaminergic neurons and to α-Syn overproduction | [120] | |
| Reduced activity in the MPTP-induced PD mouse model | [121] | ||
| S1PR1 | Fingolimod is beneficial in PD mouse models | [122] | |
| Acid Ceramidase | Inhibition leads to reduced α-Syn accumulation | [123] | |
| Polymorphisms of acid ceramidase genes (e.g., ASAH1) are associated with PD | [124] | ||
| SMPD1 | Control of sphingolipid metabolism and lysosomial burden that is linked with PD pathogenesis | [124,125] | |
| PSAP | |||
| Endocannabinoids | CB1 | Reduces bradikynesia in PD and enhances L-DOPA therapeutic effects | [127,128,129] |
| CB2 | Stimulation reduces loss of TH-positive neurons in pharmacological models of PD | [132,133] | |
| Upregulated in SNpc damage | [134] | ||
| AEA, 2-AG | Elevated levels following SNpc damage | ||
| MAGL | Inhibition protects DA neurons in MPTP/probenecid-based PD models | [139] | |
| FAAH | Inhibition is protective in rodent models | [140,141,142,143] | |
| SPMs | PD1 | Neuroprotective in PD rat models | [146] |
5. Targeting the Interaction of Alpha-Synuclein with Lipids for Discovering New Potential Biomarkers of Synucleinopathies
6. The Importance of α-Syn–Lipids Interactions along the Gut-to-Brain Axis
6.1. Role of Short Chain Fatty Acids
6.2. Interaction of α-Syn with Cholesterol and Lipoproteins—The Emerging Role of Hepatic Lipids Peroxidation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clayton, D.F.; George, J.M. The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998, 21, 249–254. [Google Scholar] [CrossRef]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef]
- Nakajo, S.; Omata, K.; Aiuchi, T.; Shibayama, T.; Okahashi, I.; Ochiai, H.; Nakai, Y.; Nakaya, K.; Nakamura, Y. Purification and characterisation of a novel brain-specific 14-kDa protein. J. Neurochem. 1990, 55, 2031–2038. [Google Scholar] [CrossRef]
- Ueda, K.; Fukushima, H.; Masliah, E.; Xia, Y.; Iwai, A.; Yoshimoto, M.; Otero, D.A.; Kondo, J.; Ihara, Y.; Saitoh, T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 11282–11286. [Google Scholar] [CrossRef] [PubMed]
- George, J.M.; Jin, H.; Woods, W.S.; Clayton, D.F. Characterisation of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 1995, 15, 361–372. [Google Scholar] [CrossRef]
- Jakes, R.; Spillantini, M.G.; Goedert, M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994, 345, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.N.; Wood, J.N. Peripheral nervous system-specific genes identified by subtractive cDNA cloning. J. Biol. Chem. 1995, 270, 21264–21270. [Google Scholar] [CrossRef]
- Ji, H.; Liu, Y.E.; Jia, T.; Wang, M.; Liu, J.; Xiao, G.; Joseph, B.K.; Rosen, C.; Shi, Y.E. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997, 57, 759–764. [Google Scholar] [PubMed]
- Campion, D.; Martin, C.; Heilig, R.; Charbonnier, F.; Moreau, V.; Flaman, J.M.; Petit, J.L.; Hannequin, D.; Brice, A.; Frebourg, T. The NACP/synuclein gene: Chromosomal assignment and screening for alterations in Alzheimer disease. Genomics 1995, 26, 254–257. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Baillie, D.A.; St Clair, D.; Brookes, A.J. High-resolution mapping of SNCA encoding α-synuclein, the non-A β component of Alzheimer’s disease amyloid precursor, to human chromosome 4q21.3→q22 by fluorescence in situ hybridization. Cytogenet. Cell Genet. 1995, 71, 54–55. [Google Scholar] [CrossRef]
- Lavedan, C.; Dehejia, A.; Pike, B.; Dutra, A.; Leroy, E.; Ide, S.E.; Root, H.; Rubenstein, J.; Boyer, R.L.; Chandrasekharappa, S.; et al. Contig map of the Parkinson’s disease region on 4q21-q23. DNA Res. 1998, 5, 19–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.; Kuhn, W.; Muller, T.; Woitalla, D.; Graeber, M.; Kosel, S.; Przuntek, H.; Epplen, J.T.; Schols, L.; Riess, O. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat. Genet. 1998, 18, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. α-Synuclein locus triplication causes Parkinson’s disease. Science 2003, 302, 841. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Pan, Y.; Price, A.C.; Sterling, W.; Copeland, N.G.; Jenkins, N.A.; Price, D.L.; Lee, M.K. Parkinson’s disease α-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 2006, 26, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A.; Durr, A.; Wood, N.W.; Parkinson, M.H.; Camuzat, A.; Hulot, J.S.; Morrison, K.E.; Renton, A.; Sussmuth, S.D.; Landwehrmeyer, B.G.; et al. Genetic variants of the α-synuclein gene SNCA are associated with multiple system atrophy. PLoS ONE 2009, 4, e7114. [Google Scholar] [CrossRef]
- Scholz, S.W.; Houlden, H.; Schulte, C.; Sharma, M.; Li, A.; Berg, D.; Melchers, A.; Paudel, R.; Gibbs, J.R.; Simon-Sanchez, J.; et al. SNCA variants are associated with increased risk for multiple system atrophy. Ann. Neurol. 2009, 65, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; de Silva, H.A.; Pettenati, M.J.; Rao, P.N.; St George-Hyslop, P.; Roses, A.D.; Xia, Y.; Horsburgh, K.; Ueda, K.; Saitoh, T. The human NACP/α-synuclein gene: Chromosome assignment to 4q21.3-q22 and TaqI RFLP analysis. Genomics 1995, 26, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Divane, A.; Goedert, M. Assignment of human α-synuclein (SNCA) and β-synuclein (SNCB) genes to chromosomes 4q21 and 5q35. Genomics 1995, 27, 379–381. [Google Scholar] [CrossRef]
- Xia, Y.; Rohan de Silva, H.A.; Rosi, B.L.; Yamaoka, L.H.; Rimmler, J.B.; Pericak-Vance, M.A.; Roses, A.D.; Chen, X.; Masliah, E.; DeTeresa, R.; et al. Genetic studies in Alzheimer’s disease with an NACP/α-synuclein polymorphism. Ann. Neurol. 1996, 40, 207–215. [Google Scholar] [CrossRef]
- Lavedan, C. The synuclein family. Genome Res. 1998, 8, 871–880. [Google Scholar] [CrossRef] [PubMed]
- George, J.M. The synucleins. Genome Biol. 2002, 3, 1–6. [Google Scholar] [CrossRef]
- Clayton, D.F.; George, J.M. Synucleins in synaptic plasticity and neurodegenerative disorders. J. Neurosci. Res. 1999, 58, 120–129. [Google Scholar] [CrossRef]
- Cheng, F.; Vivacqua, G.; Yu, S. The role of α-synuclein in neurotransmission and synaptic plasticity. J. Chem. Neuroanat. 2011, 42, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Segrest, J.P.; De Loof, H.; Dohlman, J.G.; Brouillette, C.G.; Anantharamaiah, G.M. Amphipathic helix motif: Classes and properties. Proteins 1990, 8, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Jonas, A.; Clayton, D.F.; George, J.M. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998, 273, 9443–9449. [Google Scholar] [CrossRef] [PubMed]
- Perrin, R.J.; Woods, W.S.; Clayton, D.F.; George, J.M. Interaction of human α-Synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J. Biol. Chem. 2000, 275, 34393–34398. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Sode, K.; Ochiai, S.; Kobayashi, N.; Usuzaka, E. Effect of reparation of repeat sequences in the human α-synuclein on fibrillation ability. Int. J. Biol. Sci. 2006, 3, 1–7. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Vorum, H.; Lindersson, E.; Jensen, P.H. Ca2+ binding to α-synuclein regulates ligand binding and oligomerization. J. Biol. Chem. 2001, 276, 22680–22684. [Google Scholar] [CrossRef]
- Crowther, R.A.; Jakes, R.; Spillantini, M.G.; Goedert, M. Synthetic filaments assembled from C-terminally truncated α-synuclein. FEBS Lett. 1998, 436, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Paik, S.R.; Yang, C.H.; Kim, J. Structural changes in α-synuclein affect its chaperone-like activity in vitro. Protein Sci. 2000, 9, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Paik, S.R.; Yang, C.H. Structural and functional implications of C-terminal regions of α-synuclein. Biochemistry 2002, 41, 13782–13790. [Google Scholar] [CrossRef]
- Kanda, S.; Bishop, J.F.; Eglitis, M.A.; Yang, Y.; Mouradian, M.M. Enhanced vulnerability to oxidative stress by α-synuclein mutations and C-terminal truncation. Neuroscience 2000, 97, 279–284. [Google Scholar] [CrossRef]
- Giasson, B.I.; Duda, J.E.; Murray, I.V.; Chen, Q.; Souza, J.M.; Hurtig, H.I.; Ischiropoulos, H.; Trojanowski, J.Q.; Lee, V.M. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 2000, 290, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Giasson, B.I.; Chen, Q.; Lee, V.M.; Ischiropoulos, H. Dityrosine cross-linking promotes formation of stable α-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000, 275, 18344–18349. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. A-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Cairns, N.J.; Lantos, P.L.; Goedert, M. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 1998, 251, 205–208. [Google Scholar] [CrossRef]
- Shahmoradian, S.H.; Lewis, A.J.; Genoud, C.; Hench, J.; Moors, T.E.; Navarro, P.P.; Castano-Diez, D.; Schweighauser, G.; Graff-Meyer, A.; Goldie, K.N.; et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef]
- Cremades, N.; Cohen, S.I.; Deas, E.; Abramov, A.Y.; Chen, A.Y.; Orte, A.; Sandal, M.; Clarke, R.W.; Dunne, P.; Aprile, F.A.; et al. Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell 2012, 149, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, L.; Sheynis, T.; Xue, W.F.; Orlova, E.V.; Hellewell, A.L.; Jelinek, R.; Hewitt, E.W.; Radford, S.E.; Saibil, H.R. Direct three-dimensional visualization of membrane disruption by amyloid fibrils. Proc. Natl. Acad. Sci. USA 2012, 109, 20455–20460. [Google Scholar] [CrossRef] [PubMed]
- Pieri, L.; Madiona, K.; Melki, R. Structural and functional properties of prefibrillar α-synuclein oligomers. Sci. Rep. 2016, 6, 24526. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, P.H.; Zhen, W.; Poon, A.W.; Conway, K.A.; Lansbury, P.T., Jr. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 1996, 35, 13709–13715. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; Choi, J.G.; Selkoe, D.J. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011, 477, 107–110. [Google Scholar] [CrossRef]
- Wang, W.; Perovic, I.; Chittuluru, J.; Kaganovich, A.; Nguyen, L.T.; Liao, J.; Auclair, J.R.; Johnson, D.; Landeru, A.; Simorellis, A.K.; et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc. Natl. Acad. Sci. USA 2011, 108, 17797–17802. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, U.; Newman, A.J.; Luth, E.S.; Bartels, T.; Selkoe, D. In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells. J. Biol. Chem. 2013, 288, 6371–6385. [Google Scholar] [CrossRef] [PubMed]
- Luth, E.S.; Bartels, T.; Dettmer, U.; Kim, N.C.; Selkoe, D.J. Purification of α-synuclein from human brain reveals an instability of endogenous multimers as the protein approaches purity. Biochemistry 2015, 54, 279–292. [Google Scholar] [CrossRef]
- Burre, J.; Sharma, M.; Sudhof, T.C. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. USA 2014, 111, E4274–E4283. [Google Scholar] [CrossRef]
- Burre, J.; Vivona, S.; Diao, J.; Sharma, M.; Brunger, A.T.; Sudhof, T.C. Properties of native brain α-synuclein. Nature 2013, 498, E4–E6; discussion E6–E7. [Google Scholar] [CrossRef]
- Sarchione, A.; Marchand, A.; Taymans, J.M.; Chartier-Harlin, M.C. A-Synuclein and Lipids: The Elephant in the Room? Cells 2021, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reitbock, P.; Anichtchik, O.; Bellucci, A.; Iovino, M.; Ballini, C.; Fineberg, E.; Ghetti, B.; Della Corte, L.; Spano, P.; Tofaris, G.K.; et al. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 2010, 133, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Chen, X.; Rizo, J.; Jahn, R.; Sudhof, T.C. A broken α-helix in folded α-Synuclein. J. Biol. Chem. 2003, 278, 15313–15318. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Carlus, D.; Yahi, N. The fusogenic tilted peptide (67-78) of α-synuclein is a cholesterol binding domain. Biochim. Biophys. Acta 2011, 1808, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Dervisoglu, R.; Antonschmidt, L.; Nimerovsky, E.; Sant, V.; Kim, M.; Ryazanov, S.; Leonov, A.; Fuentes-Monteverde, J.C.; Wegstroth, M.; Giller, K.; et al. Anle138b interaction in α-synuclein aggregates by dynamic nuclear polarization NMR. Methods 2023, 214, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynowicz, M.; Bar-On, D.; Calo, L.; Anichtchik, O.; Iovino, M.; Xia, J.; Ryazanov, S.; Leonov, A.; Giese, A.; Dalley, J.W.; et al. Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson’s disease model. Acta Neuropathol. 2019, 138, 575–595. [Google Scholar] [CrossRef] [PubMed]
- Antonschmidt, L.; Matthes, D.; Dervisoglu, R.; Frieg, B.; Dienemann, C.; Leonov, A.; Nimerovsky, E.; Sant, V.; Ryazanov, S.; Giese, A.; et al. The clinical drug candidate anle138b binds in a cavity of lipidic α-synuclein fibrils. Nat. Commun. 2022, 13, 5385. [Google Scholar] [CrossRef] [PubMed]
- Heras-Garvin, A.; Weckbecker, D.; Ryazanov, S.; Leonov, A.; Griesinger, C.; Giese, A.; Wenning, G.K.; Stefanova, N. Anle138b modulates α-synuclein oligomerization and prevents motor decline and neurodegeneration in a mouse model of multiple system atrophy. Mov. Disord. 2019, 34, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.; Bamm, V.V.; Stykel, M.G.; Coackley, C.L.; Humphries, K.M.; Jamieson-Williams, R.; Ambasudhan, R.; Mosser, D.D.; Lipton, S.A.; Harauz, G.; et al. Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat. Commun. 2018, 9, 817. [Google Scholar] [CrossRef]
- Ellis, C.E.; Murphy, E.J.; Mitchell, D.C.; Golovko, M.Y.; Scaglia, F.; Barcelo-Coblijn, G.C.; Nussbaum, R.L. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol. Cell Biol. 2005, 25, 10190–10201. [Google Scholar] [CrossRef]
- Luth, E.S.; Stavrovskaya, I.G.; Bartels, T.; Kristal, B.S.; Selkoe, D.J. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J. Biol. Chem. 2014, 289, 21490–21507. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Camilleri, A.; Caruana, M.; Ruf, V.C.; Schmidt, F.; Leonov, A.; Ryazanov, S.; Griesinger, C.; Cauchi, R.J.; Kamp, F.; et al. Cardiolipin Promotes Pore-Forming Activity of A-Synuclein Oligomers in Mitochondrial Membranes. ACS Chem. Neurosci. 2019, 10, 3815–3829. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, C.M.; Jiang, Z.; Lee, J.C. Biophysics of α-synuclein membrane interactions. Biochim. Biophys. Acta 2012, 1818, 162–171. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, B.D.; Claessens, M.M.; Subramaniam, V. Lipid bilayer disruption by oligomeric α-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim. Biophys. Acta 2009, 1788, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- van Maarschalkerweerd, A.; Vetri, V.; Vestergaard, B. Cholesterol facilitates interactions between α-synuclein oligomers and charge-neutral membranes. FEBS Lett. 2015, 589, 2661–2667. [Google Scholar] [CrossRef]
- Burre, J.; Sharma, M.; Sudhof, T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Fuller, N.; Rand, R.P.; St George-Hyslop, P.; Fraser, P.E. Defective membrane interactions of familial Parkinson’s disease mutant A30P α-synuclein. J. Mol. Biol. 2002, 315, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Bussell, R., Jr.; Eliezer, D. Effects of Parkinson’s disease-linked mutations on the structure of lipid-associated α-synuclein. Biochemistry 2004, 43, 4810–4818. [Google Scholar] [CrossRef]
- Kostka, M.; Hogen, T.; Danzer, K.M.; Levin, J.; Habeck, M.; Wirth, A.; Wagner, R.; Glabe, C.G.; Finger, S.; Heinzelmann, U.; et al. Single particle characterisation of iron-induced pore-forming α-synuclein oligomers. J. Biol. Chem. 2008, 283, 10992–11003. [Google Scholar] [CrossRef]
- Tofaris, G.K. Initiation and progression of α-synuclein pathology in Parkinson’s disease. Cell. Mol. Life Sci. 2022, 79, 210. [Google Scholar] [CrossRef]
- Musteikyte, G.; Jayaram, A.K.; Xu, C.K.; Vendruscolo, M.; Krainer, G.; Knowles, T.P.J. Interactions of α-synuclein oligomers with lipid membranes. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183536. [Google Scholar] [CrossRef]
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Volles, M.J.; Lansbury, P.T., Jr. Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 2002, 41, 4595–4602. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Hartley, D.; Petre, B.M.; Walz, T.; Lansbury, P.T., Jr. Neurodegenerative disease: Amyloid pores from pathogenic mutations. Nature 2002, 418, 291. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Petre, B.M.; Wall, J.; Simon, M.; Nowak, R.J.; Walz, T.; Lansbury, P.T., Jr. α-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J. Mol. Biol. 2002, 322, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Claessens, M. Disruptive membrane interactions of α-synuclein aggregates. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Okochi, M.; Walter, J.; Koyama, A.; Nakajo, S.; Baba, M.; Iwatsubo, T.; Meijer, L.; Kahle, P.J.; Haass, C. Constitutive phosphorylation of the Parkinson’s disease associated α-synuclein. J. Biol. Chem. 2000, 275, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Paleologou, K.E.; Oueslati, A.; Shakked, G.; Rospigliosi, C.C.; Kim, H.Y.; Lamberto, G.R.; Fernandez, C.O.; Schmid, A.; Chegini, F.; Gai, W.P.; et al. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits α-synuclein oligomerization, and influences synuclein-membrane interactions. J. Neurosci. 2010, 30, 3184–3198. [Google Scholar] [CrossRef]
- Bertoncini, C.W.; Jung, Y.S.; Fernandez, C.O.; Hoyer, W.; Griesinger, C.; Jovin, T.M.; Zweckstetter, M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 1430–1435. [Google Scholar] [CrossRef]
- Hejjaoui, M.; Butterfield, S.; Fauvet, B.; Vercruysse, F.; Cui, J.; Dikiy, I.; Prudent, M.; Olschewski, D.; Zhang, Y.; Eliezer, D.; et al. Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: A-synuclein phosphorylation at tyrosine 125. J. Am. Chem. Soc. 2012, 134, 5196–5210. [Google Scholar] [CrossRef] [PubMed]
- Dedmon, M.M.; Lindorff-Larsen, K.; Christodoulou, J.; Vendruscolo, M.; Dobson, C.M. Mapping long-range interactions in α-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J. Am. Chem. Soc. 2005, 127, 476–477. [Google Scholar] [CrossRef]
- Kumar, P.; Schilderink, N.; Subramaniam, V.; Huber, M. Membrane Binding of Parkinson’s Protein α-Synuclein: Effect of Phosphorylation at Positions 87 and 129 by the S to D Mutation Approach. Isr. J. Chem. 2017, 57, 762–770. [Google Scholar] [CrossRef]
- Uceda, A.B.; Frau, J.; Vilanova, B.; Adrover, M. On the effect of methionine oxidation on the interplay between α-synuclein and synaptic-like vesicles. Int. J. Biol. Macromol. 2023, 229, 92–104. [Google Scholar] [CrossRef]
- Burre, J.; Sharma, M.; Sudhof, T.C. Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities. J. Neurosci. 2012, 32, 15227–15242. [Google Scholar] [CrossRef] [PubMed]
- Lesage, S.; Anheim, M.; Letournel, F.; Bousset, L.; Honore, A.; Rozas, N.; Pieri, L.; Madiona, K.; Durr, A.; Melki, R.; et al. G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 2013, 73, 459–471. [Google Scholar] [CrossRef]
- Rauschenberger, L.; Behnke, J.; Grotemeyer, A.; Knorr, S.; Volkmann, J.; Ip, C.W. Age-dependent neurodegeneration and neuroinflammation in a genetic A30P/A53T double-mutated α-synuclein mouse model of Parkinson’s disease. Neurobiol. Dis. 2022, 171, 105798. [Google Scholar] [CrossRef]
- Ruf, W.P.; Meirelles, J.L.; Danzer, K.M. Spreading of α-synuclein between different cell types. Behav. Brain Res. 2023, 436, 114059. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef]
- Yap, T.L.; Velayati, A.; Sidransky, E.; Lee, J.C. Membrane-bound α-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol. Genet. Metab. 2013, 108, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.L.; Jiang, Z.; Heinrich, F.; Gruschus, J.M.; Pfefferkorn, C.M.; Barros, M.; Curtis, J.E.; Sidransky, E.; Lee, J.C. Structural features of membrane-bound glucocerebrosidase and α-synuclein probed by neutron reflectometry and fluorescence spectroscopy. J. Biol. Chem. 2015, 290, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Man, W.K.; Tahirbegi, B.; Vrettas, M.D.; Preet, S.; Ying, L.; Vendruscolo, M.; De Simone, A.; Fusco, G. The docking of synaptic vesicles on the presynaptic membrane induced by α-synuclein is modulated by lipid composition. Nat. Commun. 2021, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Bido, S.; Muggeo, S.; Massimino, L.; Marzi, M.J.; Giannelli, S.G.; Melacini, E.; Nannoni, M.; Gambare, D.; Bellini, E.; Ordazzo, G.; et al. Author Correction: Microglia-specific overexpression of α-synuclein leads to severe dopaminergic neurodegeneration by phagocytic exhaustion and oxidative toxicity. Nat. Commun. 2021, 12, 7359. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.K.; Tao, K.X.; Wang, X.B.; Yao, X.Y.; Pang, M.Z.; Liu, J.Y.; Wang, F.; Liu, C.F. Role of α-synuclein in microglia: Autophagy and phagocytosis balance neuroinflammation in Parkinson’s disease. Inflamm. Res. 2023, 72, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Khanapure, S.P.; Garvey, D.S.; Janero, D.R.; Letts, L.G. Eicosanoids in inflammation: Biosynthesis, pharmacology, and therapeutic frontiers. Curr. Top. Med. Chem. 2007, 7, 311–340. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Botta, E.; Holinstat, M. Eicosanoids in inflammation in the blood and the vessel. Front. Pharmacol. 2022, 13, 997403. [Google Scholar] [CrossRef] [PubMed]
- Golovko, M.Y.; Murphy, E.J. Brain prostaglandin formation is increased by α-synuclein gene-ablation during global ischemia. Neurosci. Lett. 2008, 432, 243–247. [Google Scholar] [CrossRef]
- Golovko, M.Y.; Rosenberger, T.A.; Faergeman, N.J.; Feddersen, S.; Cole, N.B.; Pribill, I.; Berger, J.; Nussbaum, R.L.; Murphy, E.J. Acyl-CoA synthetase activity links wild-type but not mutant α-synuclein to brain arachidonate metabolism. Biochemistry 2006, 45, 6956–6966. [Google Scholar] [CrossRef]
- Kim, C.; Ho, D.H.; Suk, J.E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.J.; et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013, 4, 1562. [Google Scholar] [CrossRef]
- Kim, C.; Lee, H.J.; Masliah, E.; Lee, S.J. Non-cell-autonomous Neurotoxicity of α-synuclein Through Microglial Toll-like Receptor 2. Exp. Neurobiol. 2016, 25, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia 2013, 61, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Maguire-Zeiss, K.A.; Giuliano, R.; Prifti, L.; Venkatesh, K.; Federoff, H.J. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging 2008, 29, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.E.; Kumar, B.; Andreatta, C.; Nahreini, P.; Hanson, A.J.; Yan, X.D.; Prasad, K.N. Overexpression of α-synuclein decreased viability and enhanced sensitivity to prostaglandin E2, hydrogen peroxide, and a nitric oxide donor in differentiated neuroblastoma cells. J. Neurosci. Res. 2004, 76, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shie, F.S.; Liu, J.; Wang, Y.; Davis, J.; Schantz, A.M.; Montine, K.S.; Montine, T.J.; Zhang, J. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated α-synuclein. J. Neuroinflamm. 2007, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Alhakbany, M.; Aldbass, A.; Qasem, H.; Al-Mazidi, S.; Bhat, R.S.; Al-Ayadhi, L. Correction to: A-Synuclein, cyclooxygenase-2 and prostaglandins-EP2 receptors as neuroinflammatory biomarkers of autism spectrum disorders: Use of combined ROC curves to increase their diagnostic values. Lipids Health Dis. 2021, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.S.; Salinas, K.; Garduno, A.C.; Johansson, J.U.; Wang, Q.; Manning-Bog, A.; Andreasson, K.I. Anti-Inflammatory and Neuroprotective Effects of PGE2 EP4 Signalling in Models of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2017, 12, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Pierre, S.R.; Lemmens, M.A.; Figueiredo-Pereira, M.E. Subchronic infusion of the product of inflammation prostaglandin J2 models sporadic Parkinson’s disease in mice. J. Neuroinflamm. 2009, 6, 18. [Google Scholar] [CrossRef]
- Ogburn, K.D.; Figueiredo-Pereira, M.E. Cytoskeleton/endoplasmic reticulum collapse induced by prostaglandin J2 parallels centrosomal deposition of ubiquitinated protein aggregates. J. Biol. Chem. 2006, 281, 23274–23284. [Google Scholar] [CrossRef]
- Gusach, A.; Luginina, A.; Marin, E.; Brouillette, R.L.; Besserer-Offroy, E.; Longpre, J.M.; Ishchenko, A.; Popov, P.; Patel, N.; Fujimoto, T.; et al. Structural basis of ligand selectivity and disease mutations in cysteinyl leukotriene receptors. Nat. Commun. 2019, 10, 5573. [Google Scholar] [CrossRef]
- Strempfl, K.; Unger, M.S.; Flunkert, S.; Trost, A.; Reitsamer, H.A.; Hutter-Paier, B.; Aigner, L. Leukotriene Signalling as a Target in α-Synucleinopathies. Biomolecules 2022, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Marschallinger, J.; Altendorfer, B.; Rockenstein, E.; Holztrattner, M.; Garnweidner-Raith, J.; Pillichshammer, N.; Leister, I.; Hutter-Paier, B.; Strempfl, K.; Unger, M.S.; et al. The Leukotriene Receptor Antagonist Montelukast Reduces A-Synuclein Load and Restores Memory in an Animal Model of Dementia with Lewy Bodies. Neurotherapeutics 2020, 17, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Gelosa, P.; Colazzo, F.; Tremoli, E.; Sironi, L.; Castiglioni, L. Cysteinyl Leukotrienes as Potential Pharmacological Targets for Cerebral Diseases. Mediat. Inflamm. 2017, 2017, 3454212. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Author Correction: Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, G.; Fratila, O.; Buhas, C.; Judea-Pusta, C.T.; Negrut, N.; Bustea, C.; Bungau, S. Cross-talks among GBA mutations, glucocerebrosidase, and α-synuclein in GBA-associated Parkinson’s disease and their targeted therapeutic approaches: A comprehensive review. Transl. Neurodegener. 2021, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.A. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet 2008, 372, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Lesage, S.; Condroyer, C.; Hecham, N.; Anheim, M.; Belarbi, S.; Lohman, E.; Viallet, F.; Pollak, P.; Abada, M.; Durr, A.; et al. Mutations in the glucocerebrosidase gene confer a risk for Parkinson disease in North Africa. Neurology 2011, 76, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Czubowicz, K.; Jesko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef]
- Pyszko, J.A.; Strosznajder, J.B. The key role of sphingosine kinases in the molecular mechanism of neuronal cell survival and death in an experimental model of Parkinson’s disease. Folia Neuropathol. 2014, 52, 260–269. [Google Scholar] [CrossRef]
- Motyl, J.; Przykaza, L.; Boguszewski, P.M.; Kosson, P.; Strosznajder, J.B. Pramipexole and Fingolimod exert neuroprotection in a mouse model of Parkinson’s disease by activation of sphingosine kinase 1 and Akt kinase. Neuropharmacology 2018, 135, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yang, X.; Yang, L.; Li, M.; Wood, K.; Liu, Q.; Zhu, X. Neuroprotective effects of fingolimod in mouse models of Parkinson’s disease. FASEB J. 2017, 31, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jeon, S.; Burbulla, L.F.; Krainc, D. Acid ceramidase inhibition ameliorates α-synuclein accumulation upon loss of GBA1 function. Hum. Mol. Genet. 2018, 27, 1972–1988. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Hatano, T.; Ueno, S.I.; Funayama, M.; Ishikawa, K.I.; Okuzumi, A.; Noda, S.; Sato, S.; Satake, W.; Toda, T.; et al. Variants in saposin D domain of prosaposin gene linked to Parkinson’s disease. Brain 2020, 143, 1190–1205. [Google Scholar] [CrossRef] [PubMed]
- Robak, L.A.; Jansen, I.E.; van Rooij, J.; Uitterlinden, A.G.; Kraaij, R.; Jankovic, J.; International Parkinson’s Disease Genomics, C.; Heutink, P.; Shulman, J.M. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 2017, 140, 3191–3203. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Fernandez-Espejo, E.; Caraballo, I.; de Fonseca, F.R.; El Banoua, F.; Ferrer, B.; Flores, J.A.; Galan-Rodriguez, B. Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with very severe nigral lesion in experimental parkinsonism. Neurobiol. Dis. 2005, 18, 591–601. [Google Scholar] [CrossRef]
- Gonzalez, S.; Scorticati, C.; Garcia-Arencibia, M.; de Miguel, R.; Ramos, J.A.; Fernandez-Ruiz, J. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson’s disease. Brain Res. 2006, 1073–1074, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, J.E.; Harris, O.; Cassin, J. The CB1 antagonist rimonabant is adjunctively therapeutic as well as monotherapeutic in an animal model of Parkinson’s disease. Behav. Brain Res. 2009, 203, 304–307. [Google Scholar] [CrossRef]
- Sam, A.H.; Salem, V.; Ghatei, M.A. Rimonabant: From RIO to Ban. J. Obes. 2011, 2011, 432607. [Google Scholar] [CrossRef]
- Bisogno, T.; Oddi, S.; Piccoli, A.; Fazio, D.; Maccarrone, M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol. Res. 2016, 111, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Price, D.A.; Martinez, A.A.; Seillier, A.; Koek, W.; Acosta, Y.; Fernandez, E.; Strong, R.; Lutz, B.; Marsicano, G.; Roberts, J.L.; et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur. J. Neurosci. 2009, 29, 2177–2186. [Google Scholar] [CrossRef]
- Garcia, C.; Palomo-Garo, C.; Garcia-Arencibia, M.; Ramos, J.; Pertwee, R.; Fernandez-Ruiz, J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson’s disease. Br. J. Pharmacol. 2011, 163, 1495–1506. [Google Scholar] [CrossRef]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Differential upregulation of the cannabinoid CB2 receptor in neurotoxic and inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2015, 269, 133–141. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, Y.K.; Ahn, S.; Hwang, T.Y.; Lee, H.; Park, H.J. A Comprehensive Phenotype of Non-motor Impairments and Distribution of A-Synuclein Deposition in Parkinsonism-Induced Mice by a Combination Injection of MPTP and Probenecid. Front. Aging Neurosci. 2020, 12, 599045. [Google Scholar] [CrossRef]
- Fornai, F.; Schluter, O.M.; Lenzi, P.; Gesi, M.; Ruffoli, R.; Ferrucci, M.; Lazzeri, G.; Busceti, C.L.; Pontarelli, F.; Battaglia, G.; et al. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and α-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 3413–3418. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Scipioni, L.; Arosio, B.; Mari, D.; Oddi, S.; Maccarrone, M. Anti-Inflammatory Effects of Fatty Acid Amide Hydrolase Inhibition in Monocytes/Macrophages from Alzheimer’s Disease Patients. Biomolecules 2021, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; De Caris, M.G.; Maggi, E.; Armeli, F.; Coccurello, R.; Bisogno, T.; D’Erme, M.; Maccarrone, M.; Mancini, P.; Businaro, R. Fatty Acid Amide Hydrolase (FAAH) Inhibition Modulates Amyloid-Beta-Induced Microglia Polarization. Int. J. Mol. Sci. 2021, 22, 7711. [Google Scholar] [CrossRef]
- Pasquarelli, N.; Porazik, C.; Bayer, H.; Buck, E.; Schildknecht, S.; Weydt, P.; Witting, A.; Ferger, B. Contrasting effects of selective MAGL and FAAH inhibition on dopamine depletion and GDNF expression in a chronic MPTP mouse model of Parkinson’s disease. Neurochem. Int. 2017, 110, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Viveros-Paredes, J.M.; Gonzalez-Castaneda, R.E.; Escalante-Castaneda, A.; Tejeda-Martinez, A.R.; Castaneda-Achutigui, F.; Flores-Soto, M.E. Effect of inhibition of fatty acid amide hydrolase on MPTP-induced dopaminergic neuronal damage. Neurologia 2019, 34, 143–152. [Google Scholar] [CrossRef]
- Escamilla-Ramirez, A.; Garcia, E.; Palencia-Hernandez, G.; Colin-Gonzalez, A.L.; Galvan-Arzate, S.; Tunez, I.; Sotelo, J.; Santamaria, A. URB597 and the Cannabinoid WIN55,212-2 Reduce Behavioral and Neurochemical Deficits Induced by MPTP in Mice: Possible Role of Redox Modulation and NMDA Receptors. Neurotox. Res. 2017, 31, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Celorrio, M.; Fernandez-Suarez, D.; Rojo-Bustamante, E.; Echeverry-Alzate, V.; Ramirez, M.J.; Hillard, C.J.; Lopez-Moreno, J.A.; Maldonado, R.; Oyarzabal, J.; Franco, R.; et al. Fatty acid amide hydrolase inhibition for the symptomatic relief of Parkinson’s disease. Brain Behav. Immun. 2016, 57, 94–105. [Google Scholar] [CrossRef]
- Johnston, T.H.; Huot, P.; Fox, S.H.; Wakefield, J.D.; Sykes, K.A.; Bartolini, W.P.; Milne, G.T.; Pearson, J.P.; Brotchie, J.M. Fatty acid amide hydrolase (FAAH) inhibition reduces L-3,4-dihydroxyphenylalanine-induced hyperactivity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned non-human primate model of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2011, 336, 423–430. [Google Scholar] [CrossRef]
- Serhan, C.N. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol. Aspects Med. 2017, 58, 1–11. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945. [Google Scholar] [CrossRef]
- Magalhaes, P.; Lashuel, H.A. Opportunities and challenges of α-synuclein as a potential biomarker for Parkinson’s disease and other synucleinopathies. NPJ Park. Dis. 2022, 8, 93. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Phan, K.; Bhatia, S.; Pickford, R.; Wu, P.; Dzamko, N.; Halliday, G.M.; Kim, W.S. Increased unsaturated lipids underlie lipid peroxidation in synucleinopathy brain. Acta Neuropathol. Commun. 2022, 10, 165. [Google Scholar] [CrossRef]
- Mackmull, M.T.; Nagel, L.; Sesterhenn, F.; Muntel, J.; Grossbach, J.; Stalder, P.; Bruderer, R.; Reiter, L.; van de Berg, W.D.J.; de Souza, N.; et al. Global, in situ analysis of the structural proteome in individuals with Parkinson’s disease to identify a new class of biomarker. Nat. Struct. Mol. Biol. 2022, 29, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Canerina-Amaro, A.; Pereda, D.; Diaz, M.; Rodriguez-Barreto, D.; Casanas-Sanchez, V.; Heffer, M.; Garcia-Esparcia, P.; Ferrer, I.; Puertas-Avendano, R.; Marin, R. Differential Aggregation and Phosphorylation of Alpha Synuclein in Membrane Compartments Associated With Parkinson Disease. Front. Neurosci. 2019, 13, 382. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, F.; Ricci, A.; Rota, L.; Colla, E. Subcellular localization of α-synuclein aggregates and their interaction with membranes. Neural Regen. Res. 2018, 13, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, V.; Gustafsson, G.; Sanders, L.H.; Howlett, E.H.; Sigvardson, J.; Kasrayan, A.; Ingelsson, M.; Bergstrom, J.; Erlandsson, A. Extensive uptake of α-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol. Cell. Neurosci. 2017, 82, 143–156. [Google Scholar] [CrossRef]
- Assayag, K.; Yakunin, E.; Loeb, V.; Selkoe, D.J.; Sharon, R. Polyunsaturated fatty acids induce α-synuclein-related pathogenic changes in neuronal cells. Am. J. Pathol. 2007, 171, 2000–2011. [Google Scholar] [CrossRef]
- Papay, R.; Zuscik, M.J.; Ross, S.A.; Yun, J.; McCune, D.F.; Gonzalez-Cabrera, P.; Gaivin, R.; Drazba, J.; Perez, D.M. Mice expressing the α1B-adrenergic receptor induces a synucleinopathy with excessive tyrosine nitration but decreased phosphorylation. J. Neurochem. 2002, 83, 623–634. [Google Scholar] [CrossRef]
- Min, J.O.; Ho, H.A.; Lee, W.; Jung, B.C.; Park, S.J.; Kim, S.; Lee, S.J. Statins suppress cell-to-cell propagation of α-synuclein by lowering cholesterol. Cell Death Dis. 2023, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, G.; Paciotti, S.; Concha-Marambio, L.; Rizzo, D.; Wojdaƚa, A.L.; Chiasserini, D.; Gatticchi, L.; Cerofolini, L.; Giuntini, S.; De Luca, C.M.G.; et al. Cerebrospinal fluid lipoproteins inhibit α-synuclein aggregation by interacting with oligomeric species in seed amplification assays. Mol. Neurodegener. 2023, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Allsop, D. α-Synuclein Interacts with Lipoproteins in Plasma. J. Mol. Neurosci. 2017, 63, 165–172. [Google Scholar] [CrossRef]
- Paslawski, W.; Zareba-Paslawska, J.; Zhang, X.; Holzl, K.; Wadensten, H.; Shariatgorji, M.; Janelidze, S.; Hansson, O.; Forsgren, L.; Andren, P.E.; et al. α-synuclein-lipoprotein interactions and elevated ApoE level in cerebrospinal fluid from Parkinson’s disease patients. Proc. Natl. Acad. Sci. USA 2019, 116, 15226–15235. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.R.; Li, K.; Unger, T.L.; Gallagher, M.D.; Van Deerlin, V.M.; Agarwal, P.; Leverenz, J.; Roberts, J.; Samii, A.; Gross, R.G.; et al. Lower plasma apolipoprotein A1 levels are found in Parkinson’s disease and associate with apolipoprotein A1 genotype. Mov. Disord. 2015, 30, 805–812. [Google Scholar] [CrossRef]
- Fyfe, I. APOE*ε4 promotes synucleinopathy. Nat. Rev. Neurol. 2020, 16, 185. [Google Scholar] [CrossRef]
- Real, R.; Martinez-Carrasco, A.; Reynolds, R.H.; Lawton, M.A.; Tan, M.M.X.; Shoai, M.; Corvol, J.C.; Ryten, M.; Bresner, C.; Hubbard, L.; et al. Association between the LRP1B and APOE loci and the development of Parkinson’s disease dementia. Brain 2023, 146, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjaerg-Hansen, A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017, 357, j1648. [Google Scholar] [CrossRef]
- Fang, F.; Zhan, Y.; Hammar, N.; Shen, X.; Wirdefeldt, K.; Walldius, G.; Mariosa, D. Lipids, Apolipoproteins, and the Risk of Parkinson Disease. Circ. Res. 2019, 125, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Chase, T.N.; Bennett, M.C. Muscarinic receptor stimulation induces translocation of an α-synuclein oligomer from plasma membrane to a light vesicle fraction in cytoplasm. J. Biol. Chem. 2001, 276, 28212–28218. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D.L.; Troyer, M.D.; Nakamura, K.; Kubo, S.; Anthony, M.D.; Edwards, R.H. Lipid rafts mediate the synaptic localization of α-synuclein. J. Neurosci. 2004, 24, 6715–6723. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.B.; Murphy, D.D.; Grider, T.; Rueter, S.; Brasaemle, D.; Nussbaum, R.L. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein α-synuclein. J. Biol. Chem. 2002, 277, 6344–6352. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.; Vendruscolo, M. Modulation of the Interactions Between α-Synuclein and Lipid Membranes by Post-translational Modifications. Front. Neurol. 2021, 12, 661117. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Duran, R.; Lopez, G.; Kurzawa-Akanbi, M.; McKeith, I.G.; Chinnery, P.F.; Morris, C.M.; Theuns, J.; Crosiers, D.; Cras, P.; et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013, 70, 727–735. [Google Scholar] [CrossRef]
- Kurzawa-Akanbi, M.; Hanson, P.S.; Blain, P.G.; Lett, D.J.; McKeith, I.G.; Chinnery, P.F.; Morris, C.M. Glucocerebrosidase mutations alter the endoplasmic reticulum and lysosomes in Lewy body disease. J. Neurochem. 2012, 123, 298–309. [Google Scholar] [CrossRef]
- Lin, G.; Wang, L.; Marcogliese, P.C.; Bellen, H.J. Sphingolipids in the Pathogenesis of Parkinson’s Disease and Parkinsonism. Trends Endocrinol. Metab. 2019, 30, 106–117. [Google Scholar] [CrossRef]
- Williams, E.T.; Chen, X.; Moore, D.J. VPS35, the Retromer Complex and Parkinson’s Disease. J. Park. Dis. 2017, 7, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Miura, E.; Hasegawa, T.; Konno, M.; Suzuki, M.; Sugeno, N.; Fujikake, N.; Geisler, S.; Tabuchi, M.; Oshima, R.; Kikuchi, A.; et al. VPS35 dysfunction impairs lysosomal degradation of α-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiol. Dis. 2014, 71, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dahabiyeh, L.A.; Nimer, R.M.; Rashed, M.; Wells, J.D.; Fiehn, O. Serum-Based Lipid Panels for Diagnosis of Idiopathic Parkinson’s Disease. Metabolites 2023, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- den Heijer, J.M.; Cullen, V.C.; Pereira, D.R.; Yavuz, Y.; de Kam, M.L.; Grievink, H.W.; Moerland, M.; Leymarie, N.; Khatri, K.; Sollomoni, I.; et al. A Biomarker Study in Patients with GBA1-Parkinson’s Disease and Healthy Controls. Mov. Disord. 2023, 38, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Lerche, S.; Schulte, C.; Wurster, I.; Machetanz, G.; Roeben, B.; Zimmermann, M.; Deuschle, C.; Hauser, A.K.; Bohringer, J.; Krageloh-Mann, I.; et al. The Mutation Matters: CSF Profiles of GCase, Sphingolipids, α-Synuclein in PDGBA. Mov. Disord. 2021, 36, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.E.; Park, H.; Chiang, M.S.R.; Tuncali, I.; Liu, G.; Locascio, J.J.; Shirvan, J.; Hutten, S.J.; Rotunno, M.S.; Viel, C.; et al. Glucosylceramide in cerebrospinal fluid of patients with GBA-associated and idiopathic Parkinson’s disease enrolled in PPMI. NPJ Park. Dis. 2021, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Kurzawa-Akanbi, M.; Tammireddy, S.; Fabrik, I.; Gliaudelyte, L.; Doherty, M.K.; Heap, R.; Matecko-Burmann, I.; Burmann, B.M.; Trost, M.; Lucocq, J.M.; et al. Altered ceramide metabolism is a feature in the extracellular vesicle-mediated spread of α-synuclein in Lewy body disorders. Acta Neuropathol. 2021, 142, 961–984. [Google Scholar] [CrossRef]
- Xing, Y.; Tang, Y.; Zhao, L.; Wang, Q.; Qin, W.; Ji, X.; Zhang, J.; Jia, J. Associations between plasma ceramides and cognitive and neuropsychiatric manifestations in Parkinson’s disease dementia. J. Neurol. Sci. 2016, 370, 82–87. [Google Scholar] [CrossRef]
- Mielke, M.M.; Maetzler, W.; Haughey, N.J.; Bandaru, V.V.; Savica, R.; Deuschle, C.; Gasser, T.; Hauser, A.K.; Graber-Sultan, S.; Schleicher, E.; et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: A pilot study. PLoS ONE 2013, 8, e73094. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Mirick, G.E.; Serhan, C.N.; Selkoe, D.J. Altered fatty acid composition of dopaminergic neurons expressing α-synuclein and human brains with α-synucleinopathies. J. Biol. Chem. 2003, 278, 49874–49881. [Google Scholar] [CrossRef]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Hattori, N.; Uchida, K.; Tanaka, M.; Stadtman, E.R.; Mizuno, Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. USA 1996, 93, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, N.; Antonecchia, E.; Angiolillo, A.; Bender, V.; Camerlenghi, M.; Xie, Q.; Di Costanzo, A. Metabolomics of blood reveals age-dependent pathways in Parkinson’s Disease. Cell Biosci. 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nong, Y.; Zhang, X.; Mai, T.; Cai, J.; Liu, J.; Lai, K.P.; Zhang, Z. Comparative plasma metabolomic analysis to identify biomarkers for lead-induced cognitive impairment. Chem. Biol. Interact. 2022, 366, 110143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, S.; Kang, W.; Chen, P.; Liu, J. Associations between polyunsaturated fatty acid concentrations and Parkinson’s disease: A two-sample Mendelian randomization study. Front. Aging Neurosci. 2023, 15, 1123239. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Haque, A.; Imberdis, T.; Baru, V.; Barrasa, M.I.; Nuber, S.; Termine, D.; Ramalingam, N.; Ho, G.P.H.; Noble, T.; et al. Lipidomic Analysis of α-Synuclein Neurotoxicity Identifies Stearoyl CoA Desaturase as a Target for Parkinson Treatment. Mol. Cell 2019, 73, 1001–1014.e8. [Google Scholar] [CrossRef]
- Imberdis, T.; Negri, J.; Ramalingam, N.; Terry-Kantor, E.; Ho, G.P.H.; Fanning, S.; Stirtz, G.; Kim, T.E.; Levy, O.A.; Young-Pearse, T.L.; et al. Cell models of lipid-rich α-synuclein aggregation validate known modifiers of α-synuclein biology and identify stearoyl-CoA desaturase. Proc. Natl. Acad. Sci. USA 2019, 116, 20760–20769. [Google Scholar] [CrossRef] [PubMed]
- Nuber, S.; Nam, A.Y.; Rajsombath, M.M.; Cirka, H.; Hronowski, X.; Wang, J.; Hodgetts, K.; Kalinichenko, L.S.; Muller, C.P.; Lambrecht, V.; et al. A Stearoyl-Coenzyme A Desaturase Inhibitor Prevents Multiple Parkinson Disease Phenotypes in α-Synuclein Mice. Ann. Neurol. 2021, 89, 74–90. [Google Scholar] [CrossRef]
- Nuber, S.; Chung, C.Y.; Tardiff, D.F.; Bechade, P.A.; McCaffery, T.D.; Shimanaka, K.; Choi, J.; Chang, B.; Raja, W.; Neves, E.; et al. A Brain-Penetrant Stearoyl-CoA Desaturase Inhibitor Reverses α-Synuclein Toxicity. Neurotherapeutics 2022, 19, 1018–1036. [Google Scholar] [CrossRef]
- de Bus, I.; Witkamp, R.; Zuilhof, H.; Albada, B.; Balvers, M. The role of n-3 PUFA-derived fatty acid derivatives and their oxygenated metabolites in the modulation of inflammation. Prostaglandins Other Lipid Mediat. 2019, 144, 106351. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; Isaac, A.R.; Malcher-Lopes, R.; Diaz, B.L.; Trevenzoli, I.H.; De Melo Reis, R.A. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2018, 21, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Inceoglu, B.; Zolkowska, D.; Yoo, H.J.; Wagner, K.M.; Yang, J.; Hackett, E.; Hwang, S.H.; Lee, K.S.; Rogawski, M.A.; Morisseau, C.; et al. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS ONE 2013, 8, e80922. [Google Scholar] [CrossRef]
- Sarparast, M.; Dattmore, D.; Alan, J.; Lee, K.S.S. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients 2020, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Sura, P.; Sura, R.; Enayetallah, A.E.; Grant, D.F. Distribution and expression of soluble epoxide hydrolase in human brain. J. Histochem. Cytochem. 2008, 56, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Atone, J.; Wagner, K.; Hashimoto, K.; Hammock, B.D. Cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases. Prostaglandins Other Lipid Mediat. 2020, 147, 106385. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.; Vito, S.; Inceoglu, B.; Hammock, B.D. The role of long chain fatty acids and their epoxide metabolites in nociceptive signalling. Prostaglandins Other Lipid Mediat. 2014, 113–115, 2–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, G.; Lee, K.S.; Hammock, B.D.; Gebremedhin, D.; Harder, D.R.; Koehler, R.C.; Sapirstein, A. Inhibition of soluble epoxide hydrolase augments astrocyte release of vascular endothelial growth factor and neuronal recovery after oxygen-glucose deprivation. J. Neurochem. 2017, 140, 814–825. [Google Scholar] [CrossRef]
- Wagner, K.M.; McReynolds, C.B.; Schmidt, W.K.; Hammock, B.D. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol. Ther. 2017, 180, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, F.; Vivacqua, G.; Lazzeri, G.; Ferese, R.; Iannacone, S.; Onori, P.; Morini, S.; D’Este, L.; Fornai, F. Chronic MPTP in Mice Damage-specific Neuronal Phenotypes within Dorsal Laminae of the Spinal Cord. Neurotox. Res. 2021, 39, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, G.; Biagioni, F.; Busceti, C.L.; Ferrucci, M.; Madonna, M.; Ryskalin, L.; Yu, S.; D’Este, L.; Fornai, F. Motor Neurons Pathology After Chronic Exposure to MPTP in Mice. Neurotox. Res. 2020, 37, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, G.; Biagioni, F.; Yu, S.; Casini, A.; Bucci, D.; D’Este, L.; Fornai, F. Loss of spinal motor neurons and alteration of α-synuclein immunostaining in MPTP induced Parkinsonism in mice. J. Chem. Neuroanat. 2012, 44, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fischer, D.; Guerreiro, S.; Hunot, S.; Saurini, F.; Marien, M.; Sokoloff, P.; Hirsch, E.C.; Hartmann, A.; Michel, P.P. Modelling Parkinson-like neurodegeneration via osmotic minipump delivery of MPTP and probenecid. J. Neurochem. 2008, 107, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wu, Q.; Lin, L.; Sun, A.; Liu, S.; Li, X.; Cao, X.; Gao, T.; Luo, P.; Zhu, X.; et al. Soluble Epoxide Hydrolase Deficiency or Inhibition Attenuates MPTP-Induced Parkinsonism. Mol. Neurobiol. 2015, 52, 187–195. [Google Scholar] [CrossRef]
- Ren, Q.; Ma, M.; Yang, J.; Nonaka, R.; Yamaguchi, A.; Ishikawa, K.I.; Kobayashi, K.; Murayama, S.; Hwang, S.H.; Saiki, S.; et al. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E5815–E5823. [Google Scholar] [CrossRef]
- Pallas, M.; Vazquez, S.; Sanfeliu, C.; Galdeano, C.; Grinan-Ferre, C. Soluble Epoxide Hydrolase Inhibition to Face Neuroinflammation in Parkinson’s Disease: A New Therapeutic Strategy. Biomolecules 2020, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, X.; Huang, X.; Qin, C.; Fang, Y.; Liu, Y.; Zhang, G.; Pan, D.; Wang, W.; Xie, M. Soluble epoxide hydrolase inhibition provides multi-target therapeutic effects in rats after spinal cord injury. Mol. Neurobiol. 2016, 53, 1565–1578. [Google Scholar] [CrossRef]
- Hashimoto, K. Role of Soluble Epoxide Hydrolase in Metabolism of PUFAs in Psychiatric and Neurological Disorders. Front. Pharmacol. 2019, 10, 36. [Google Scholar] [CrossRef]
- Haeggstrom, J.Z.; Funk, C.D. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.X.; Wang, F.; Liu, J.Y.; Liu, C.F. Relationship Between Short-chain Fatty Acids and Parkinson’s Disease: A Review from Pathology to Clinic. Neurosci. Bull. 2024, 40, 500–516. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004, 447, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, P.S.; Dallas, S.; Wilson, B.; Block, M.L.; Wang, C.C.; Kinyamu, H.; Lu, N.; Gao, X.; Leng, Y.; et al. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int. J. Neuropsychopharmacol. 2008, 11, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, W.W.; Lu, C.Z. Histone decacetylase inhibitors prevent mitochondrial fragmentation and elicit early neuroprotection against MPP+. CNS Neurosci. Ther. 2014, 20, 308–316. [Google Scholar] [CrossRef]
- Chuang, D.M.; Leng, Y.; Marinova, Z.; Kim, H.J.; Chiu, C.T. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009, 32, 591–601. [Google Scholar] [CrossRef]
- Paiva, I.; Pinho, R.; Pavlou, M.A.; Hennion, M.; Wales, P.; Schutz, A.L.; Rajput, A.; Szego, E.M.; Kerimoglu, C.; Gerhardt, E.; et al. Sodium butyrate rescues dopaminergic cells from α-synuclein-induced transcriptional deregulation and DNA damage. Hum. Mol. Genet. 2017, 26, 2231–2246. [Google Scholar] [CrossRef]
- Kontopoulos, E.; Parvin, J.D.; Feany, M.B. A-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 2006, 15, 3012–3023. [Google Scholar] [CrossRef]
- Singh, Y.; Trautwein, C.; Romani, J.; Salker, M.S.; Neckel, P.H.; Fraccaroli, I.; Abeditashi, M.; Woerner, N.; Admard, J.; Dhariwal, A.; et al. Overexpression of human α-Synuclein leads to dysregulated microbiome/metabolites with ageing in a rat model of Parkinson disease. Mol. Neurodegener. 2023, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.N.; Pahan, K. HATs and HDACs in neurodegeneration: A tale of disconcerted acetylation homeostasis. Cell Death Differ. 2006, 13, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Taliyan, R. Targeting histone deacetylases: A novel approach in Parkinson’s disease. Park. Dis. 2015, 2015, 303294. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Wang, C.C.; Bortner, C.D.; Peng, G.S.; Wu, X.; Pang, H.; Lu, R.B.; Gean, P.W.; Chuang, D.M.; Hong, J.S. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 2007, 149, 203–212. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Gonzalez-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signalling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Liu, C.; Zhang, M.; Zhang, X.; Ge, S.; Zhao, L. Neuroprotective effects of short-chain fatty acids in MPTP induced mice model of Parkinson’s disease. Exp. Gerontol. 2021, 150, 111376. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterisation of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Getachew, B.; Csoka, A.B.; Bhatti, A.; Copeland, R.L.; Tizabi, Y. Butyrate Protects Against Salsolinol-Induced Toxicity in SH-SY5Y Cells: Implication for Parkinson’s Disease. Neurotox. Res. 2020, 38, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F.; Liu, S.; Du, J.; Hu, X.; Xiong, J.; Fang, R.; Chen, W.; Sun, J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017, 381, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.F.; Shan, C.; Zhuang, S.Y.; Zhuang, Q.Q.; Ghosh, A.; Zhu, K.C.; Kong, X.K.; Wang, S.M.; Gong, Y.L.; Yang, Y.Y.; et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luo, Y.; Ray Chaudhuri, K.; Reynolds, R.; Tan, E.K.; Pettersson, S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain 2021, 144, 2571–2593. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.J.; Menozzi, E.; Koletsi, S.; Bates, R.; Gegg, M.E.; Chau, K.Y.; Blottiere, H.M.; Macnaughtan, J.; Schapira, A.H.V. α-Synuclein expression in response to bacterial ligands and metabolites in gut enteroendocrine cells: An in vitro proof of concept study. Brain Commun. 2023, 5, fcad285. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. Gut microbiome, short-chain fatty acids, α-synuclein, neuroinflammation, and ROS/RNS: Relevance to Parkinson’s disease and therapeutic implications. Redox Biol. 2024, 71, 103092. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Stevens, M.V.; Akter, M.H.; Rusk, S.E.; Huang, R.J.; Cohen, A.; Noguchi, A.; Springer, D.; Bocharov, A.V.; Eggerman, T.L.; et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Investig. 2011, 121, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Gellermann, G.P.; Appel, T.R.; Tannert, A.; Radestock, A.; Hortschansky, P.; Schroeckh, V.; Leisner, C.; Lutkepohl, T.; Shtrasburg, S.; Rocken, C.; et al. Raft lipids as common components of human extracellular amyloid fibrils. Proc. Natl. Acad. Sci. USA 2005, 102, 6297–6302. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Nosi, D.; Forzan, M.; Russo, E.; Calamai, M.; Pieri, L.; Formigli, L.; Quercioli, F.; Soria, S.; Pavone, F.; et al. Toxic effects of amyloid fibrils on cell membranes: The importance of ganglioside GM1. FASEB J. 2012, 26, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Ashley, R.H.; Harroun, T.A.; Hauss, T.; Breen, K.C.; Bradshaw, J.P. Autoinsertion of soluble oligomers of Alzheimer’s Aβ(1-42) peptide into cholesterol-containing membranes is accompanied by relocation of the sterol towards the bilayer surface. BMC Struct. Biol. 2006, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Tang, Y.; Zhou, S.; Toh, B.H.; McLean, C.; Li, H. Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol. Cell Neurosci. 2010, 43, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Yahi, N. The driving force of α-synuclein insertion and amyloid channel formation in the plasma membrane of neural cells: Key role of ganglioside- and cholesterol-binding domains. Adv. Exp. Med. Biol. 2013, 991, 15–26. [Google Scholar] [CrossRef]
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Park. Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.; Gegg, M.E.; Migdalska-Richards, A.; Doherty, M.K.; Whitfield, P.D.; Schapira, A.H. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: Relevance to Parkinson disease. Hum. Mol. Genet. 2016, 25, 3432–3445. [Google Scholar] [CrossRef]
- Cleeter, M.W.; Chau, K.Y.; Gluck, C.; Mehta, A.; Hughes, D.A.; Duchen, M.; Wood, N.W.; Hardy, J.; Mark Cooper, J.; Schapira, A.H. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem. Int. 2013, 62, 1–7. [Google Scholar] [CrossRef]
- Yang, S.Y.; Beavan, M.; Chau, K.Y.; Taanman, J.W.; Schapira, A.H.V. A Human Neural Crest Stem Cell-Derived Dopaminergic Neuronal Model Recapitulates Biochemical Abnormalities in GBA1 Mutation Carriers. Stem Cell Rep. 2017, 8, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Sillence, D.J.; Puri, V.; Marks, D.L.; Butters, T.D.; Dwek, R.A.; Pagano, R.E.; Platt, F.M. Glucosylceramide modulates membrane traffic along the endocytic pathway. J. Lipid Res. 2002, 43, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.A.; Fowler, D.M.; Zhang, Q.; Nieva, J.; Powers, E.T.; Wentworth, P., Jr.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate α-synuclein fibrilization. Nat. Chem. Biol. 2006, 2, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Marwarha, G.; Ghribi, O. Does the oxysterol 27-hydroxycholesterol underlie Alzheimer’s disease-Parkinson’s disease overlap? Exp. Gerontol. 2015, 68, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Lutjohann, D.; Breuer, O.; Ahlborg, G.; Nennesmo, I.; Siden, A.; Diczfalusy, U.; Bjorkhem, I. Cholesterol homeostasis in human brain: Evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA 1996, 93, 9799–9804. [Google Scholar] [CrossRef] [PubMed]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Marwarha, G.; Rhen, T.; Schommer, T.; Ghribi, O. The oxysterol 27-hydroxycholesterol regulates α-synuclein and tyrosine hydroxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors–relevance to Parkinson’s disease. J. Neurochem. 2011, 119, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Kim, W.S.; Garner, B. Regulation of α-synuclein expression by liver X receptor ligands in vitro. Neuroreport 2008, 19, 1685–1689. [Google Scholar] [CrossRef]
- Schommer, J.; Marwarha, G.; Schommer, T.; Flick, T.; Lund, J.; Ghribi, O. 27-Hydroxycholesterol increases α-synuclein protein levels through proteasomal inhibition in human dopaminergic neurons. BMC Neurosci. 2018, 19, 17. [Google Scholar] [CrossRef]
- Sharon, R.; Goldberg, M.S.; Bar-Josef, I.; Betensky, R.A.; Shen, J.; Selkoe, D.J. α-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 9110–9115. [Google Scholar] [CrossRef]
- Merched, A.; Xia, Y.; Visvikis, S.; Serot, J.M.; Siest, G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol. Aging 2000, 21, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.R.; Berlyand, Y.; Xie, S.X.; Alcalay, R.N.; Chahine, L.M.; Chen-Plotkin, A.S. Plasma apolipoprotein A1 associates with age at onset and motor severity in early Parkinson’s disease patients. Mov. Disord. 2015, 30, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and cholesterol handling in the brain. Cardiovasc. Res. 2014, 103, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eckel, R.H. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 2014, 25, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.T.; Bullock, K.M.; Erickson, M.A.; Zhang, J.; Banks, W.A. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides 2014, 62, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.F.; Ekmark-Lewen, S.; Perdiki, M.; Klingstedt, T.; Hoffmann, A.; Wiechec, E.; Nilsson, P.; Nilsson, K.P.R.; Alafuzoff, I.; Ingelsson, M.; et al. Accumulation of α-synuclein within the liver, potential role in the clearance of brain pathology associated with Parkinson’s disease. Acta Neuropathol. Commun. 2021, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Vivacqua, G.; Vaccaro, R.; Renzi, A.; Leone, S.; Pannarale, L.; Franchitto, A.; Onori, P.; Mancinelli, R.; Gaudio, E. Expression and role of cocaine-amphetamine regulated transcript (CART) in the proliferation of biliary epithelium. Eur. J. Histochem. 2023, 67, 21. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, G.; Renzi, A.; Carpino, G.; Franchitto, A.; Gaudio, E. Expression of brain derivated neurotrophic factor and of its receptors: TrKB and p75NT in normal and bile duct ligated rat liver. Ital. J. Anat. Embryol. 2014, 119, 111–129. [Google Scholar] [PubMed]
- Yang, H.M.; Cheng, Y.Z.; Hou, T.Z.; Fan, J.K.; Gu, L.; Zhang, J.N.; Zhang, H. Upregulation of Parkinson’s disease-associated protein α-synuclein suppresses tumorigenesis via interaction with mGluR5 and gamma-synuclein in liver cancer. Arch. Biochem. Biophys. 2023, 744, 109698. [Google Scholar] [CrossRef]
- Kakimoto, T.; Hosokawa, M.; Ichimura-Shimizu, M.; Ogawa, H.; Miyakami, Y.; Sumida, S.; Tsuneyama, K. Accumulation of α-synuclein in hepatocytes in nonalcoholic steatohepatitis and its usefulness in pathological diagnosis. Pathol. Res. Pract. 2023, 247, 154525. [Google Scholar] [CrossRef]
- Padilla-Godinez, F.J.; Ramos-Acevedo, R.; Martinez-Becerril, H.A.; Bernal-Conde, L.D.; Garrido-Figueroa, J.F.; Hiriart, M.; Hernandez-Lopez, A.; Arguero-Sanchez, R.; Callea, F.; Guerra-Crespo, M. Protein Misfolding and Aggregation: The Relatedness between Parkinson’s Disease and Hepatic Endoplasmic Reticulum Storage Disorders. Int. J. Mol. Sci. 2021, 22, 12467. [Google Scholar] [CrossRef]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjaerbaek, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-first versus body-first Parkinson’s disease: A multimodal imaging case-control study. Brain 2020, 143, 3077–3088. [Google Scholar] [CrossRef]
- Horsager, J.; Knudsen, K.; Sommerauer, M. Clinical and imaging evidence of brain-first and body-first Parkinson’s disease. Neurobiol. Dis. 2022, 164, 105626. [Google Scholar] [CrossRef]
- Borghammer, P. The brain-first vs. body-first model of Parkinson’s disease with comparison to alternative models. J. Neural Transm. 2023, 130, 737–753. [Google Scholar] [CrossRef]
- Binder, C.J. Lipid modification and lipid peroxidation products in innate immunity and inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 369–370. [Google Scholar] [CrossRef]
- Friedli, O.; Freigang, S. Cyclopentenone-containing oxidized phospholipids and their isoprostanes as pro-resolving mediators of inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 382–392. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guo, Y.; Yin, X. Lipid Peroxidation in Ferroptosis and Association with Nonalcoholic Fatty Liver Disease. Front. Biosci. 2023, 28, 332. [Google Scholar] [CrossRef] [PubMed]
- Girard, V.; Jollivet, F.; Knittelfelder, O.; Celle, M.; Arsac, J.N.; Chatelain, G.; Van den Brink, D.M.; Baron, T.; Shevchenko, A.; Kuhnlein, R.P.; et al. Abnormal accumulation of lipid droplets in neurons induces the conversion of α-Synuclein to proteolytic resistant forms in a Drosophila model of Parkinson’s disease. PLoS Genet. 2021, 17, e1009921. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanluca, C.; Spagnolo, P.; Mancinelli, R.; De Bartolo, M.I.; Fava, M.; Maccarrone, M.; Carotti, S.; Gaudio, E.; Leuti, A.; Vivacqua, G. Interaction between α-Synuclein and Bioactive Lipids: Neurodegeneration, Disease Biomarkers and Emerging Therapies. Metabolites 2024, 14, 352. https://doi.org/10.3390/metabo14070352
Sanluca C, Spagnolo P, Mancinelli R, De Bartolo MI, Fava M, Maccarrone M, Carotti S, Gaudio E, Leuti A, Vivacqua G. Interaction between α-Synuclein and Bioactive Lipids: Neurodegeneration, Disease Biomarkers and Emerging Therapies. Metabolites. 2024; 14(7):352. https://doi.org/10.3390/metabo14070352
Chicago/Turabian StyleSanluca, Chiara, Paolo Spagnolo, Romina Mancinelli, Maria Ilenia De Bartolo, Marina Fava, Mauro Maccarrone, Simone Carotti, Eugenio Gaudio, Alessandro Leuti, and Giorgio Vivacqua. 2024. "Interaction between α-Synuclein and Bioactive Lipids: Neurodegeneration, Disease Biomarkers and Emerging Therapies" Metabolites 14, no. 7: 352. https://doi.org/10.3390/metabo14070352
APA StyleSanluca, C., Spagnolo, P., Mancinelli, R., De Bartolo, M. I., Fava, M., Maccarrone, M., Carotti, S., Gaudio, E., Leuti, A., & Vivacqua, G. (2024). Interaction between α-Synuclein and Bioactive Lipids: Neurodegeneration, Disease Biomarkers and Emerging Therapies. Metabolites, 14(7), 352. https://doi.org/10.3390/metabo14070352











