Integrated Microbiome-Metabolomics Analysis Reveals the Potential Mechanism of Dandelion Root Polysaccharides to Ameliorate Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Dandelion Root Polysaccharides
2.2. Animal Experiment Design
2.3. Collection of Blood and Feces Samples
2.4. Fecal Bacteria 16S rRNA Sequencing Analysis
2.5. Extraction and Detection of Serum Metabolites
2.6. Correlation Analysis between Metabolites and Intestinal Flora
2.7. Data Analysis
3. Results and Discussion
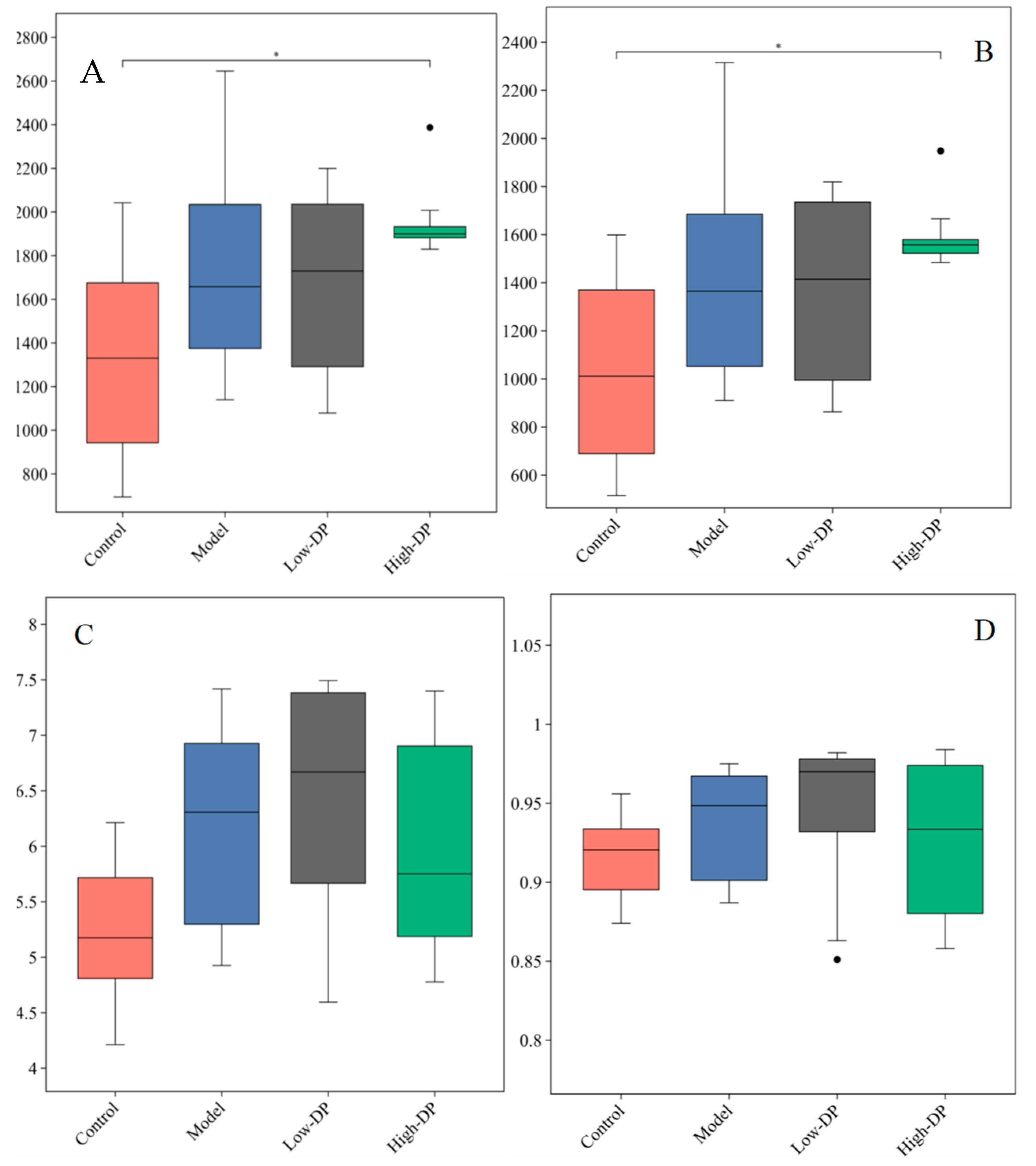
3.1. The Effects of DPs on α Diversity of Intestinal Flora in Mice
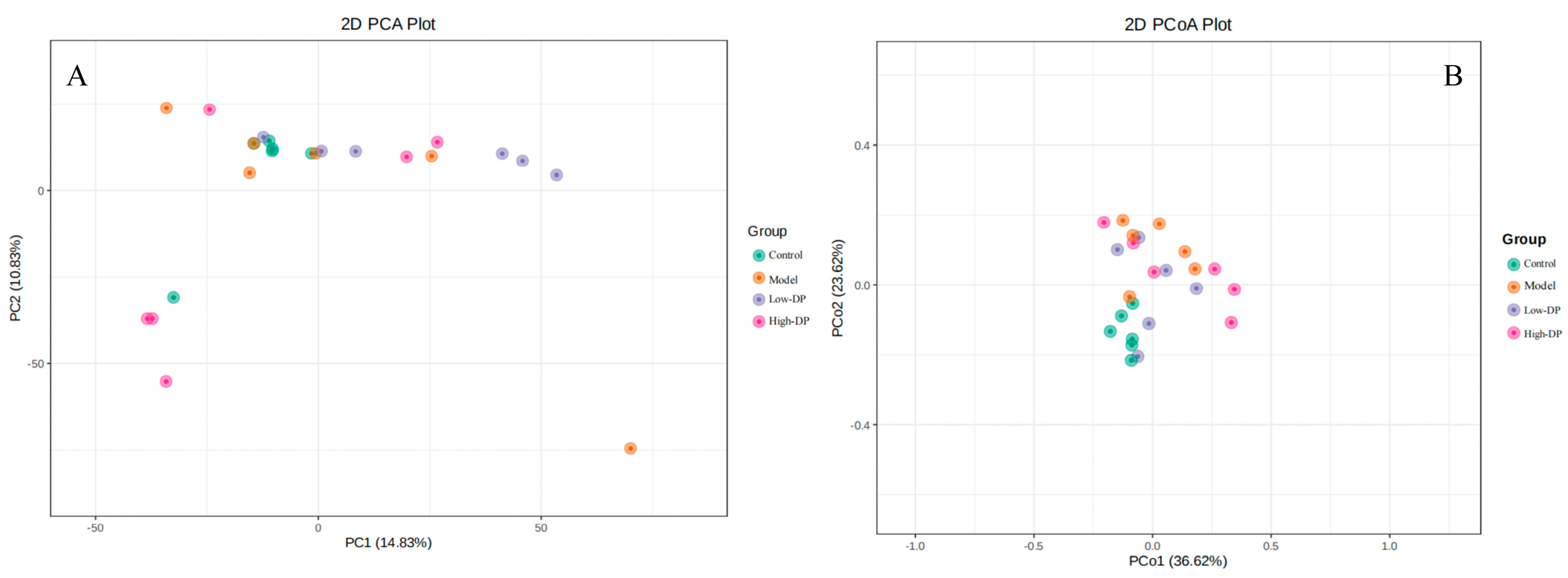
3.2. The Effects of AAP on the β Diversity of Intestinal Flora in Mice
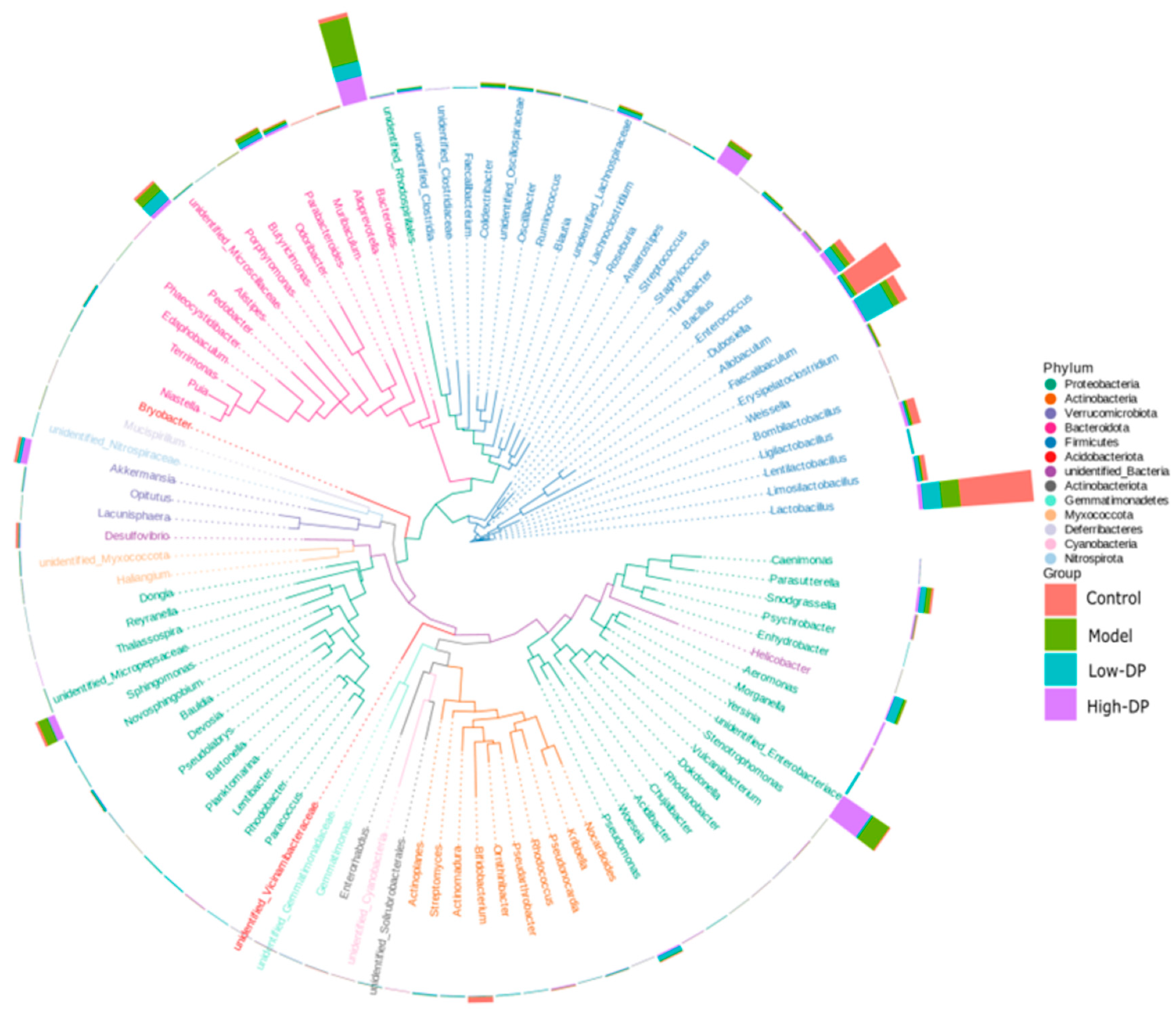
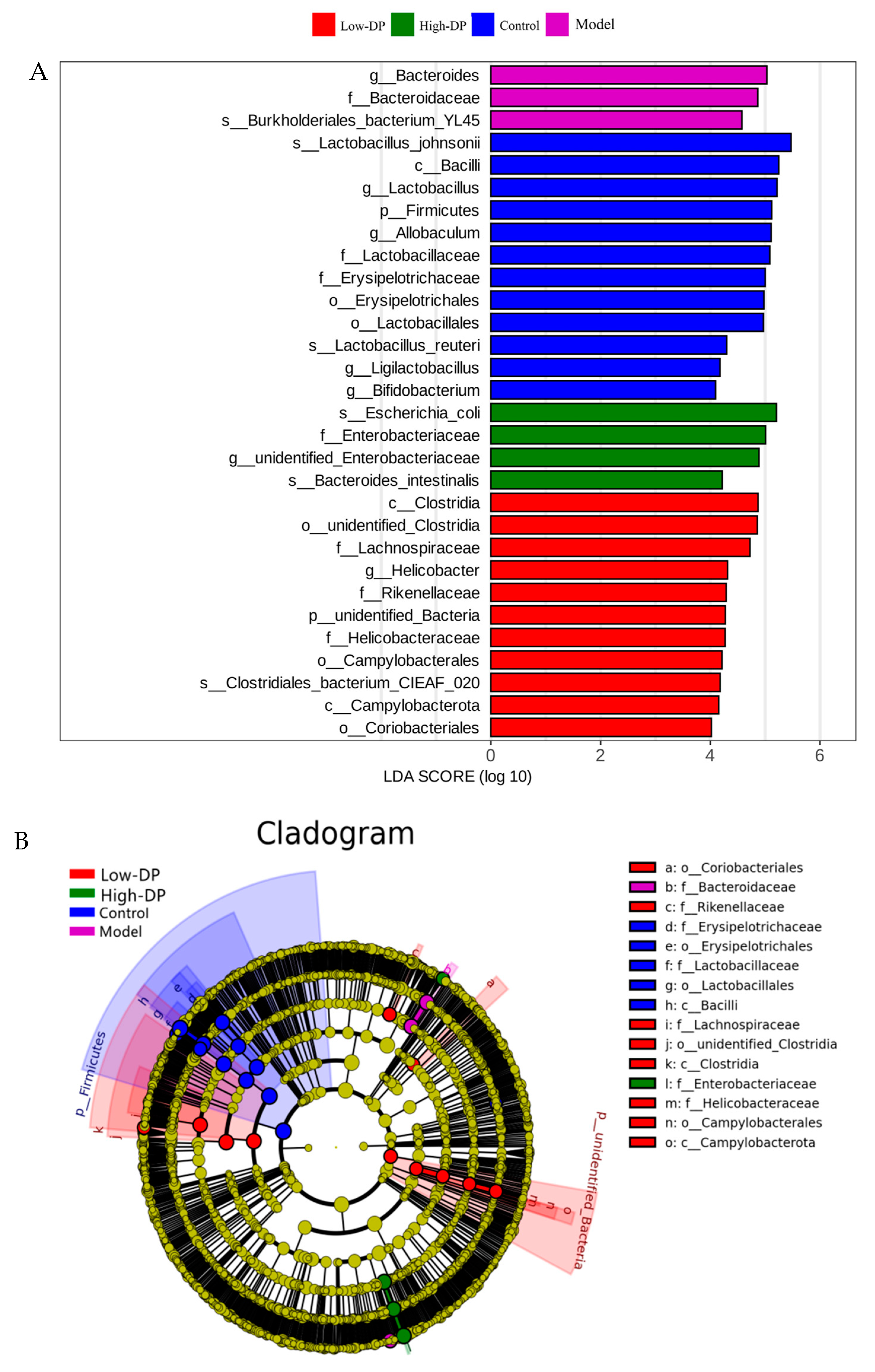
3.3. Effect of Polysaccharides from Dandelion Root on the Composition of Intestinal Flora in Mice
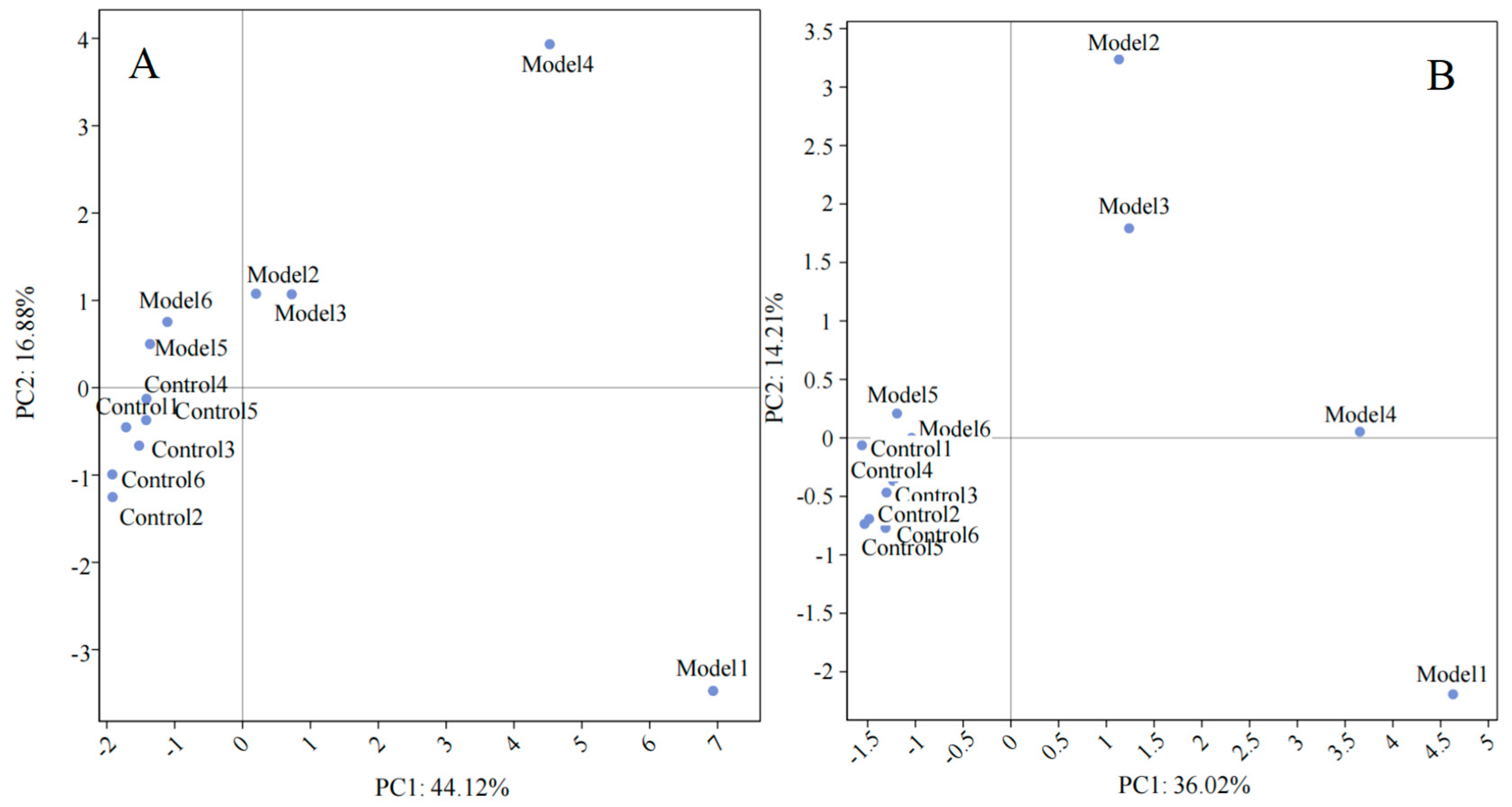
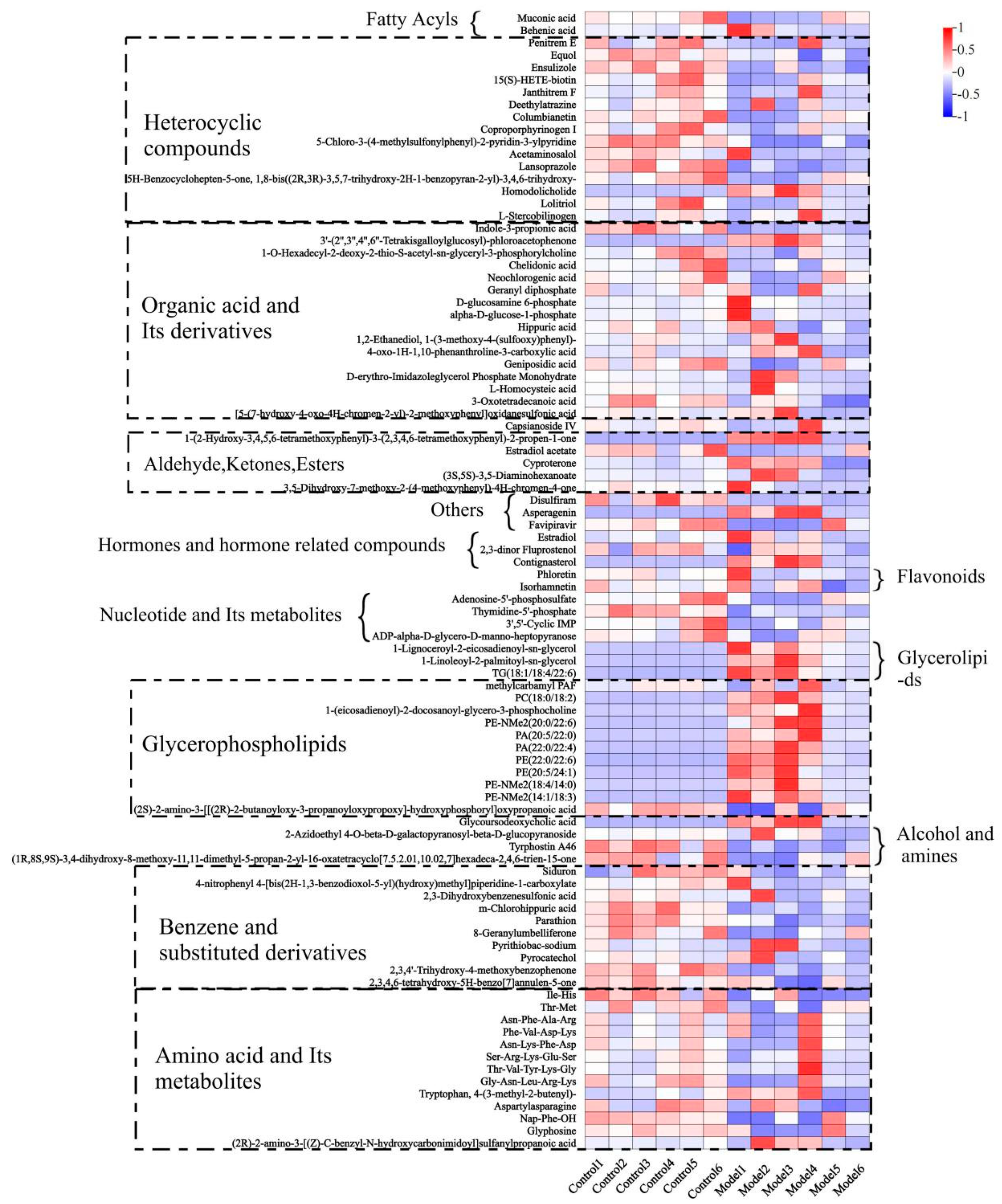
3.4. Screening of Mouse Serum Biomarkers after DP Consumption
- (1)
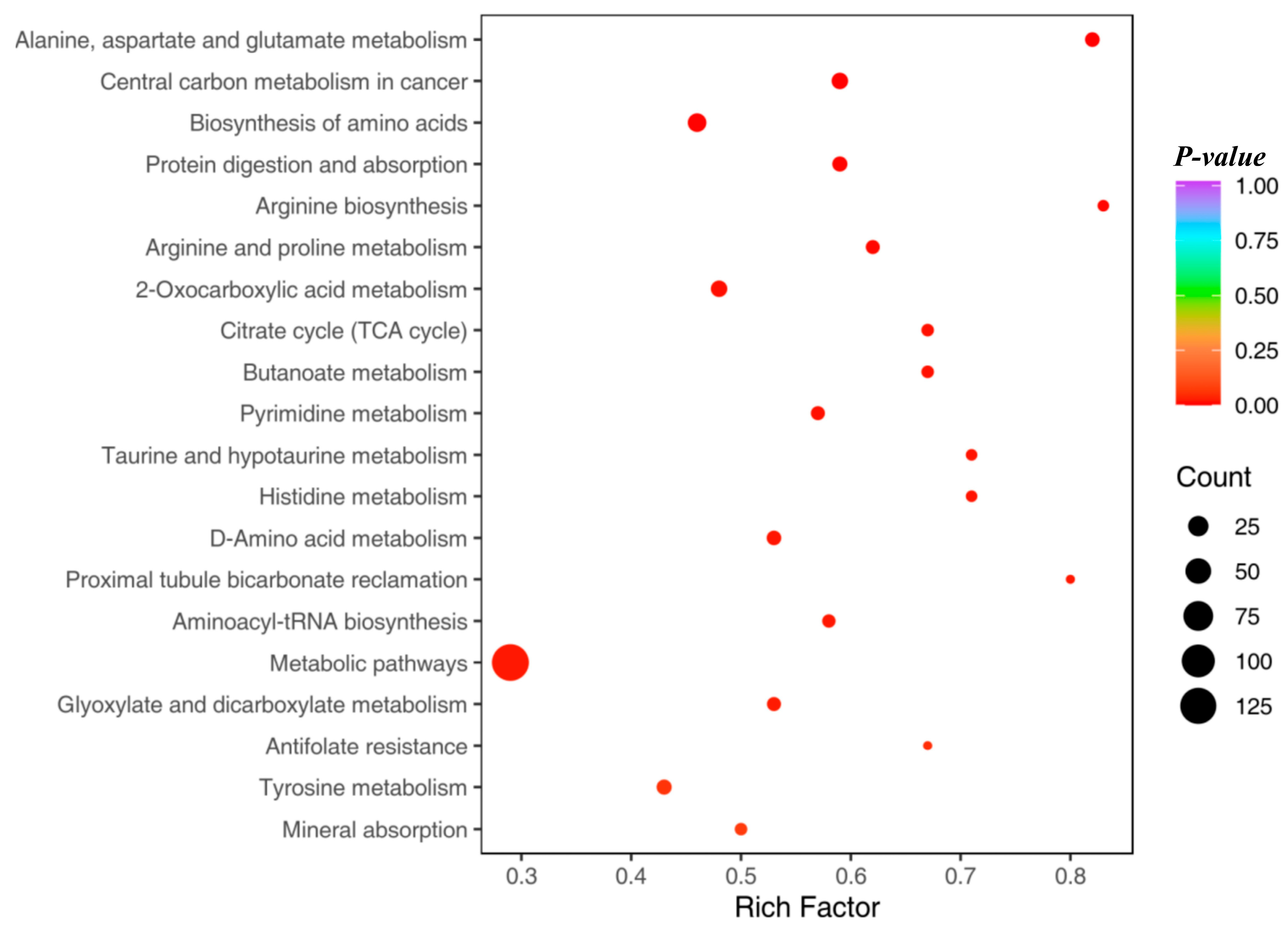
- UC biomarker identification and metabolic pathway analysis
3.5. Changes in Serum Biomarkers in Mice after Dandelion Polysaccharide Intake
- (1)
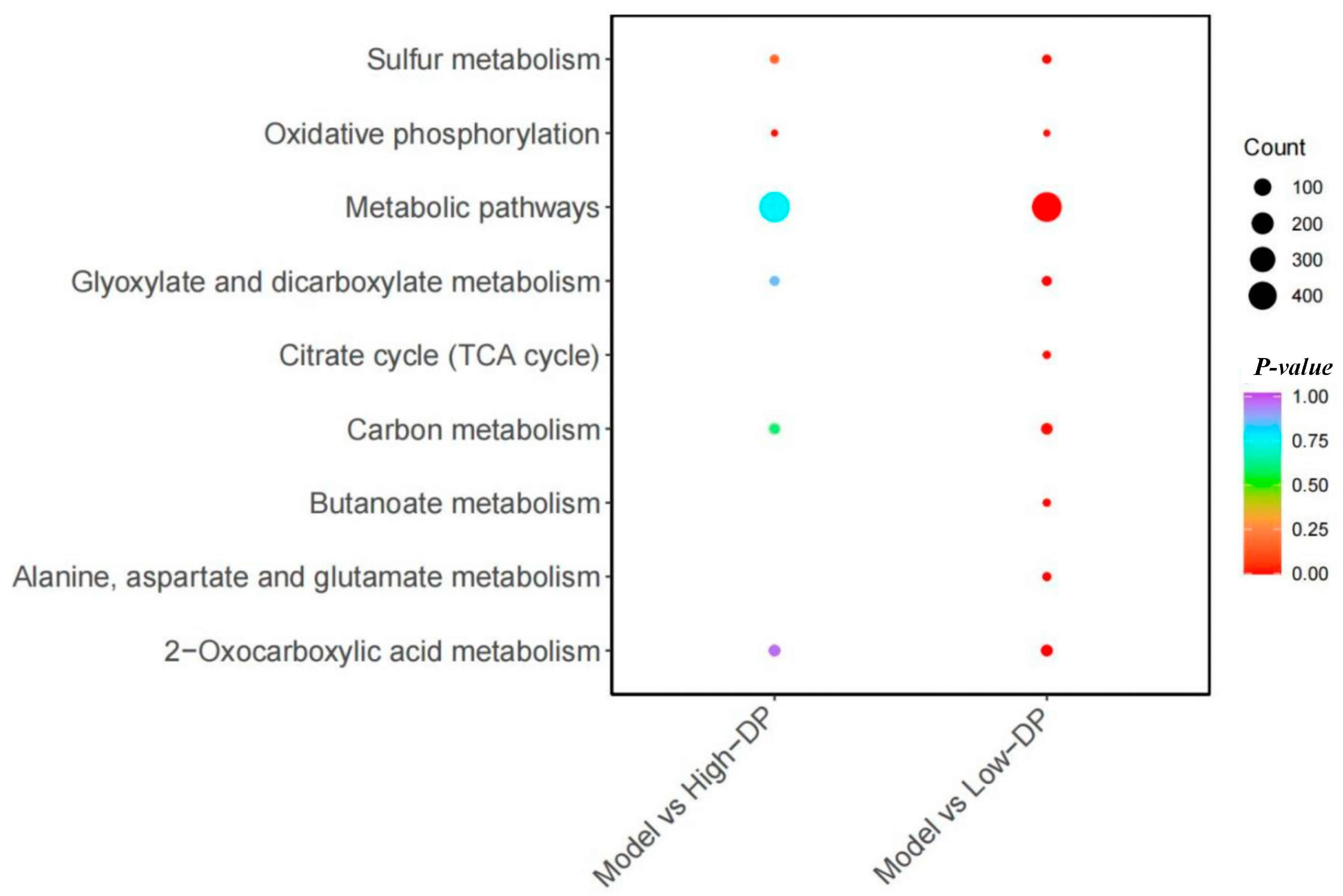
- Effects of DPs on key differential metabolites and metabolic pathway analysis
3.6. Correlation Analysis of Intestinal Flora and Metabolites in Mice after Intake of Auricularia Auricula Polysaccharides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Tian, Y.; Zhao, C.; Li, S.; Wang, T.; Qiao, B.; Fu, Y. Application of fingerprint combined with quantitative analysis and multivariate chemometric methods in quality evaluation of dandelion (Taraxacum mongolicum). R. Soc. Open Sci. 2021, 8, 210614. [Google Scholar] [CrossRef]
- Yuan-Yuan, J.; Rong-Fa, G.; Yi-Hang, W.; Xiao-Ping, Y.; Wen-Yan, L.; Yong-Yong, Z.; Tao, L.; Jun, Z.; Shu-Yun, S.; Yu, Z. Taraxacum mongolicum extract exhibits a protective effect on hepatocytes and an antiviral effect against hepatitis B virus in animal and human cells. Mol. Med. Rep. 2014, 9, 1381–1387. [Google Scholar]
- Wei, L.; Changyeol, L.; Young, H.K.; Jin, Y.M.; Sang, H.S. Chemical constituents of the aerial part of Taraxacum mongolicum and their chemotaxonomic significance Natural Product Research. Nat. Prod. Res. 2017, 28, 2303–2307. [Google Scholar]
- Hua, T.; Yujuan, H. Effects of dandelion polysaccharide on the inflammatory response and MAPK/ERK pathway of gastric mucosa in rats with Helicobacter pylori-associated gastritis. Mod. J. Integr. Chin. West. Med. 2021, 281, 114519. [Google Scholar]
- Natchanok, T.; Subramanian, P.; ChangSheng, L.; Nan, M.; Marimuthu, N.P. Polysaccharide extracted from Taraxacum platycarpum root exerts immunomodulatory activity via MAPK and NF-kB pathways in RAW264.7 cells. J. Ethnopharmacol. 2021, 281, 114519. [Google Scholar]
- Le, N. Extraction, Isolation and Purification of Polysaccharides from Leaves of Northeast Dandelion and Their Application in Beverages. Master’s Thesis, Changchun University, Changchun, China, 2022. [Google Scholar]
- Bo, H. Biological functions of dandelion extract and its application in livestock production. New Agric. 2023, 21, 56–57. [Google Scholar]
- Yanni, Z.; Yaodong, G.; Chenfei, L.; Fangjuan, L. Study on the regulation of intestinal flora and anti-inflammatory effects of dandelion polysaccharide in mice with ulcerative colitis combined with dysbiosis. Biomed. Eng. Clin. 2022, 26, 414–419. [Google Scholar]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of disease: Inflammatory bowel diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Israr, K.; Naeem, U.; Lajia, Z.; Yanrui, B.; Ashiq, K.; Tang, Z.; Tuanjie, C.; Chunjiang, Z. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Sokal, A.; Filip, R. What was first, obesity or inflammatory bowel disease? What does the gut microbiota have to do with it. Nutrients 2020, 12, 3073. [Google Scholar] [CrossRef] [PubMed]
- Zecai, Z.; Hongyang, C.; Peng, S.; Jiuxi, L.; Yongguo, C.; Naisheng, Z. Ping weisan alleviates chronic colitis in mice by regulating intestinal microbiota composition. J. Ethnopharmacol. 2020, 255, 112715. [Google Scholar]
- Joshua, A.O.; Bejan, J.S.; Crystal, R.N.; Sarah, H.-C.; Maria, E.B.; Richard, U.E.; Lauren, A.; Trevor, M.D.; Brian, S.R.; Rheinallt, M.J.P. Lactobacillus rhamnosus GG orchestrates an antitumor immune response. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1311–1327. [Google Scholar]
- Eric, A.F.; Alexandra, S.-M.; Julian, A.-P.; Nadine, F.; Henry, J.H.; Stefan, R.; Tommi, V.; Brantley, H.; Himel, M.; Lauren, J.M.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar]
- Sitkin, S.; Tkachenko, E.; Vakhitov, T.; Oreshko, L.; Zhigalova, T. Oral butyrate plus inulin improve serum metabolomic profile and gut microbiota composition in ulcerative colitis and celiac disease. J. Crohns Colitis 2014, 8 (Suppl. S1), S232. [Google Scholar] [CrossRef][Green Version]
- Min, L.; Baohong, W.; Menghui, Z.; Liping, Z. Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. USA 2008, 105, 2117–2122. [Google Scholar]
- Steinmeyer, S.; Lee, K.; Jayaraman, A.; Alaniz, R.C. Microbiota metabolite regulation of host immune homeostasis: A mechanistic missing link. Curr. Allergy Asthma Rep. 2015, 15, 24. [Google Scholar] [CrossRef]
- Yulan, W.; Baohong, W.; Junfang, W.; Xiangyang, J.; Huiru, T.; Ole, H.N. Modulation of gut microbiota in pathological states. Engineering 2017, 3, 83–89. [Google Scholar]
- Zedan, Z.; Fengyun, Z.; Jiaqi, Z.; Lin, X.; Xudong, T. Treatment of ulcerative colitis based on intestinal flora. Chin. J. Tradit. Chin. Med. 2021, 36, 3468–3471. [Google Scholar]
- Tian, H.; Lam, S.M.; Shui, G. Metabolomics, a powerfultool for agricultural research. Int. J. Mol. Sci. 2016, 17, 1871. [Google Scholar] [CrossRef] [PubMed]
- Diab, J.; Hansen, T.; Goll, R.; Hans, S.; Einar, J. Mucosal metabolo-mic profiling and pathway analysis reveal the metabolicsignature of ulcerative colitis. Metabolites 2019, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.; Mills, P.S.; Dulai, Y.V.-B.; Consuelo, S.; Noëmie, D.; Romana, R.G.; Lakshmi, E.B.; Mario, M.; Qiyun, Z.; Kelly, W.; et al. Multiomics analyses of the ulcerative colitis gut microbi-ome link Bacteroides vulgatus proteases with disease severity. Nat. Rev. Microbiol. 2022, 7, 262–276. [Google Scholar]
- Shenkun, Y.; Lijun, Y.; Rong, D. Inhibition of IEC-6 Cell Proliferation and the Mechanism of Ulcerative Colitis in C57BL/6 Mice by Dandelion Root Polysaccharides. Foods 2023, 12, 3800. [Google Scholar] [CrossRef] [PubMed]
- Mashael, R.; Aljumaah, M.M.; Alkhulaifi, A.M.; Abudabos, R.S.; Aljumaah, A.N.; Stanley, D. Impact of challenge on C. perfringens impact on Alpha diversity, lower Richness in association with challenge (P = 0.047) (left) marginally influenced Chao1 index (P = 0.089) (right), NC: Non-challenged. PLoS ONE 2020, 18, e0232781. [Google Scholar]
- Sashuang, D.; Benhua, Z.; Ling, H.; Yuling, Z.; Jiaqi, X.; Jing, D.; Liyan, H.; Zhenlin, L.; Jie, W.; Hong, W.; et al. Effect of a Humanized Diet Profile on Colonization Efficiency and Gut Microbial Diversity in Human Flora-Associated Mice Frontiers in Nutrition. Front. Nutr. 2021, 23, 8. [Google Scholar]
- Nikhra, V. The Nutritional Links for Gut Microbial Dysbiosis and its Metabolic and Endocrinal. Fallouts Gastroenterol. Hepatol. Int. Journa 2022, 7, 1–19. [Google Scholar]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kenji, K.; Naoki, T.; Takanobu, T.; Masayuki, I. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-inducedMCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Fangmei, Z.; Yue, L.; Senmiao, C.; Xiaodan, B.; Siyu, F.; Yishan, L.; Mingyuan, Z.; Yuchi, C.; Bingqi, Z.; Chao-Dong, Q.; et al. Ameliorating role of Tetrastigma hemsleyanum polysaccharides in antibiotic-induced intestinal mucosal barrier dysfunction in mice based on microbiome and metabolome analyses. Int. J. Biol. Macromol. 2023, 241, 124419. [Google Scholar]
- Yue, L.; Yishan, L.; Zian, M.; Xingcan, C.; Yuchi, C.; Bingqi, Z.; Ying, Y.; Zhongxiang, D.; Fangmei, Z. Polysaccharides from Tetrastigma Hemsleyanum Diels et Gilg ameliorated inflammatory bowel disease by rebuilding the intestinal mucosal barrier and inhibiting inflammation through the SCFA-GPR41/43 signaling pathway. Int. J. Biol. Macromol. 2023, 250, 126167. [Google Scholar]
- Ziwen, H.; Jingyan, G.; Huiwen, Z.; Jingquan, Y.; Yiqing, Z.; Yajun, W.; Ting, L.; Ming, Y.; Bo, L.; Ann, C.; et al. Atractylodes macrocephala Koidz polysaccharide improves glycolipid metabolism disorders through activation of aryl hydrocarbon receptor by gut flora-produced tryptophan metabolites. Int. J. Biol. Macromol. 2023, 1, 126987. [Google Scholar]
- Sumio, O.; Toshiki, K. S4.1—Impact of intestinal flora on host metabolism of drug, sugar and lipid. Drug Metab. Pharmacokinet. 2020, 1, S9. [Google Scholar]
- Agnieszka, Ł.-N.; Marta, O.; Natalia, N.-J.; Aleksandra, M.; Konrad, K.; Maciej, M.; Marcin, M.; Sebastian, K. Crude Polysaccharide Fraction from Rosa rugosa Thunb. Root-Chemical Characterisation, Enzyme Inhibitory, Antioxidant and Antiproliferative Activity. Appl. Sci. 2022, 12, 10126. [Google Scholar] [CrossRef]
- Naghma, K.; Vijayan, N.; Ravinder, K.; Neha, G. Overview on L-asparagine monohydrate single crystal: A non-essential amino acid. J. Nonlinear Opt. Phys. Mater. 2022, 32, 2330001. [Google Scholar]
- Ling, W.; Ke-Chun, Y.; Yunqing, H.; Ming, G.; Fan, Y.; Zhenxia, C. Gut microbiome in tumorigenesis and therapy of colorectal cancer. J. Cell. Physiol. 2022, 238, 94–108. [Google Scholar]
- Yaxin, Z.; Yin, G.; Jing, J.; Xiao-Bing, C.; Shi, C.; Linling, L.; Zhiyong, H.; Rongxin, L.; Peng, Z.; Jieying, Y.; et al. Stigmasterol attenuates hepatic steatosis in rats by strengthening the intestinal barrier and improving bile acid metabolism. Sci. Food 2022, 6, 38. [Google Scholar]
- Marta, A.; Fik-Jaskółka, A.F.; Mkrtchyan, A.S.; Saghyan, R.P.; Agnieszka, B.; Liana, A.H.; Hayarpi, S.; Valentina, R.; Giovanni, N.R. Biological macromolecule binding and anticancer activity of synthetic alkyne-containing l-phenylalanine derivatives. Amino Acids 2020, 52, 755–769. [Google Scholar]
- Xianglin, P.; Haoyu, W.; Ziming, Z.; Xiao, L.H.; Linlin, Y.; Junxi, L.; Kaiping, W.; Yu, Z. Pectic polysaccharide from Smilax china L. ameliorated ulcerative colitis by inhibiting the galectin-3/NLRP3 inflammasome pathway. Carbohydr. Polym. 2022, 277, 118864. [Google Scholar]
- Tingting, Z.; Haoran, W.; Zhenjiang, L.; Yang, L.; De, J.; Bin, L.; Xiaodan, H. Recent Perspective of Lactobacillus in Reducing Oxidative Stress to Prevent Disease Antioxidants. Antioxidants 2023, 21, 769. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Dong, R. Integrated Microbiome-Metabolomics Analysis Reveals the Potential Mechanism of Dandelion Root Polysaccharides to Ameliorate Ulcerative Colitis. Metabolites 2024, 14, 351. https://doi.org/10.3390/metabo14070351
Yan S, Dong R. Integrated Microbiome-Metabolomics Analysis Reveals the Potential Mechanism of Dandelion Root Polysaccharides to Ameliorate Ulcerative Colitis. Metabolites. 2024; 14(7):351. https://doi.org/10.3390/metabo14070351
Chicago/Turabian StyleYan, Shengkun, and Rong Dong. 2024. "Integrated Microbiome-Metabolomics Analysis Reveals the Potential Mechanism of Dandelion Root Polysaccharides to Ameliorate Ulcerative Colitis" Metabolites 14, no. 7: 351. https://doi.org/10.3390/metabo14070351
APA StyleYan, S., & Dong, R. (2024). Integrated Microbiome-Metabolomics Analysis Reveals the Potential Mechanism of Dandelion Root Polysaccharides to Ameliorate Ulcerative Colitis. Metabolites, 14(7), 351. https://doi.org/10.3390/metabo14070351




