Metabolomic Profiling and In Vivo Antiepileptic Effect of Zygophyllum album Aerial Parts and Roots Crude Extracts against Pentylenetetrazole-Induced Kindling in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Metabolomic Profiling with LC/MS/MS
2.3. In Vivo Study of Z. album
2.3.1. Drugs and Chemicals
2.3.2. Experimental Animals and Study Protocol
2.3.3. Assessment of Seizure Activity in PTZ-Kindled Mice
2.3.4. Tissue Sampling
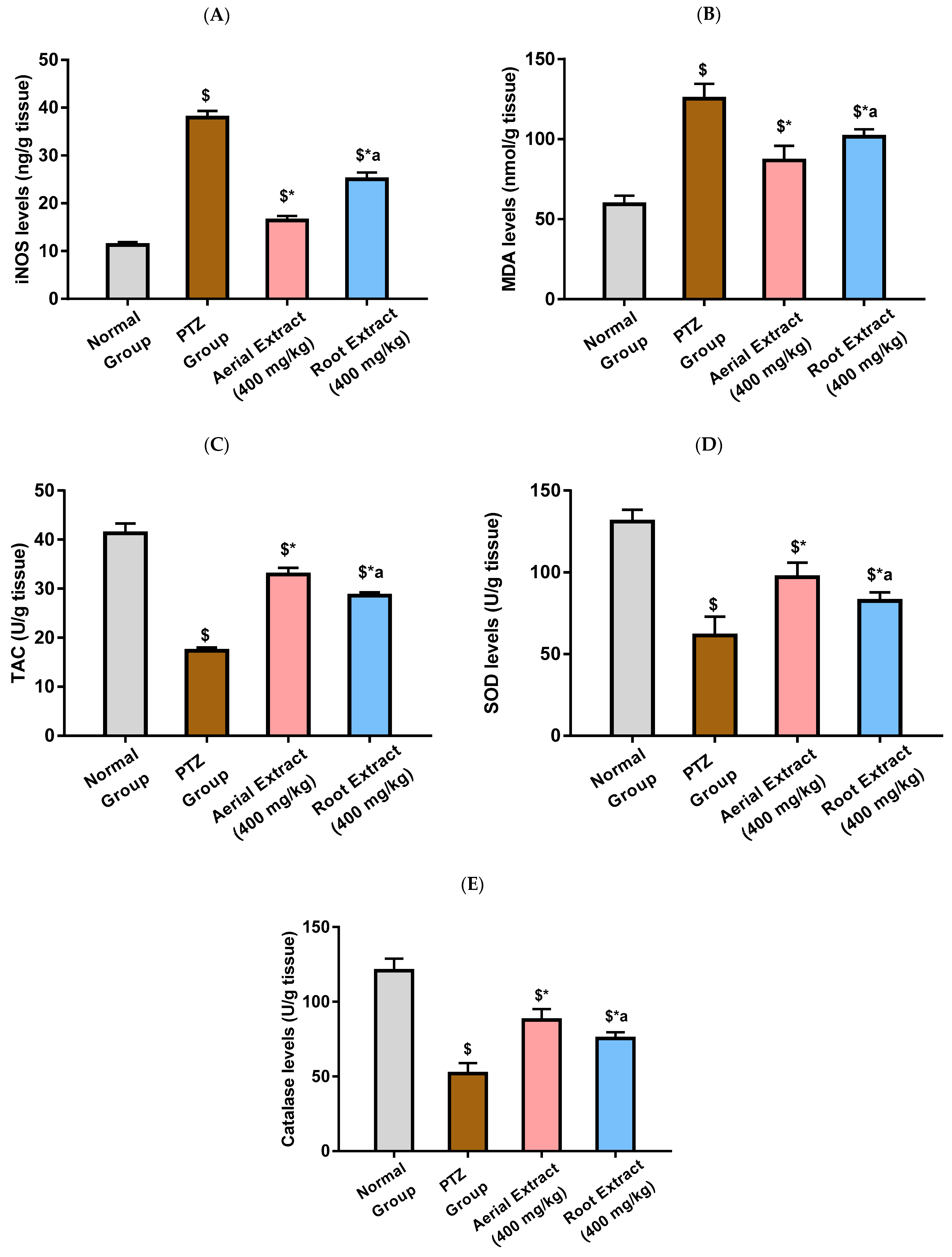
2.3.5. Determination of Oxidative Stress Markers (iNOS, TAC, MDA, SOD and Catalase)
2.3.6. Determination of the Inflammatory Markers (IL-1β and IL-18)
2.3.7. Quantitative RT-PCR Analysis for Determination of Inflammatory Biomarkers
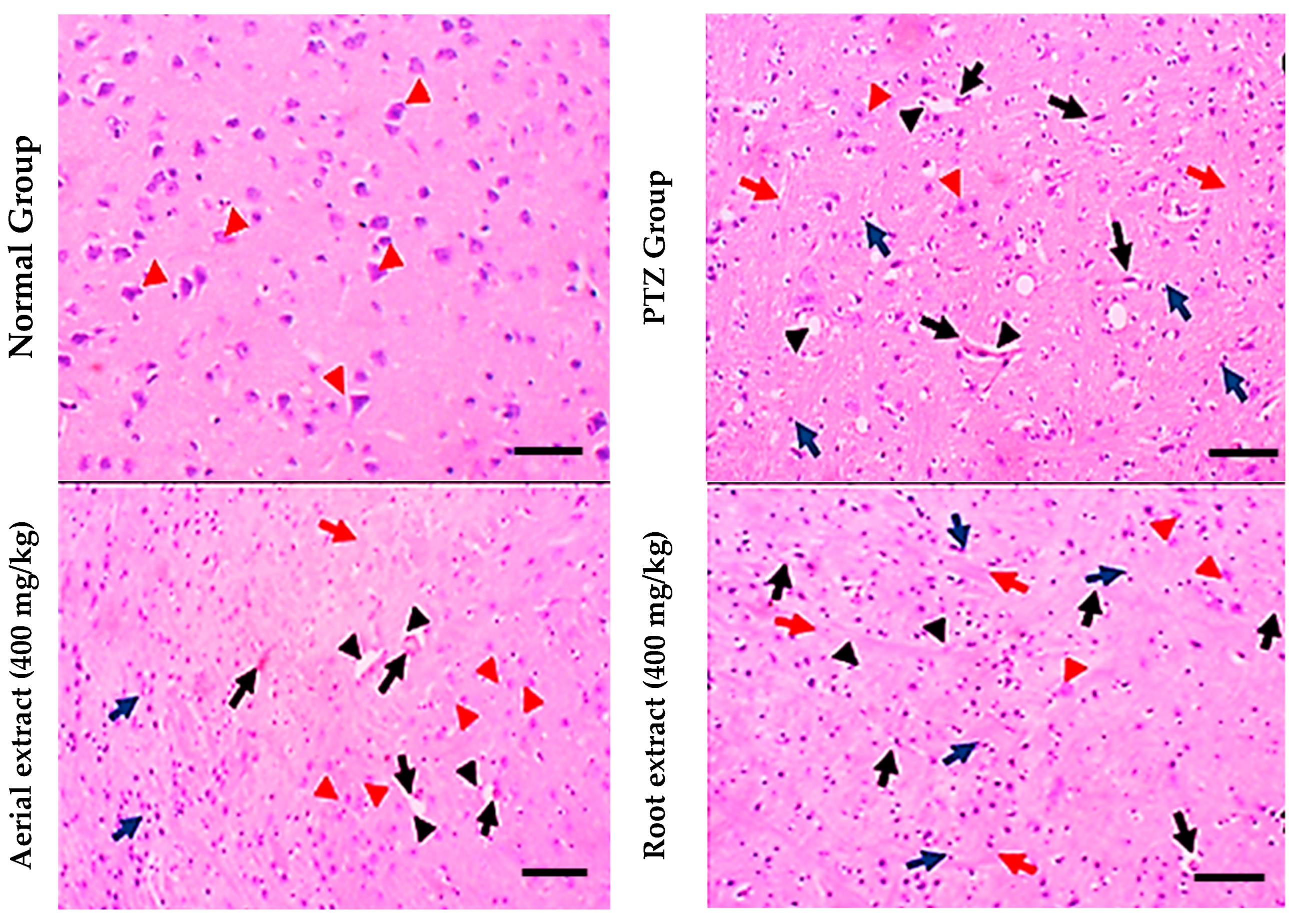
2.3.8. Histopathological Study of Brain Tissue Sections in Different Groups
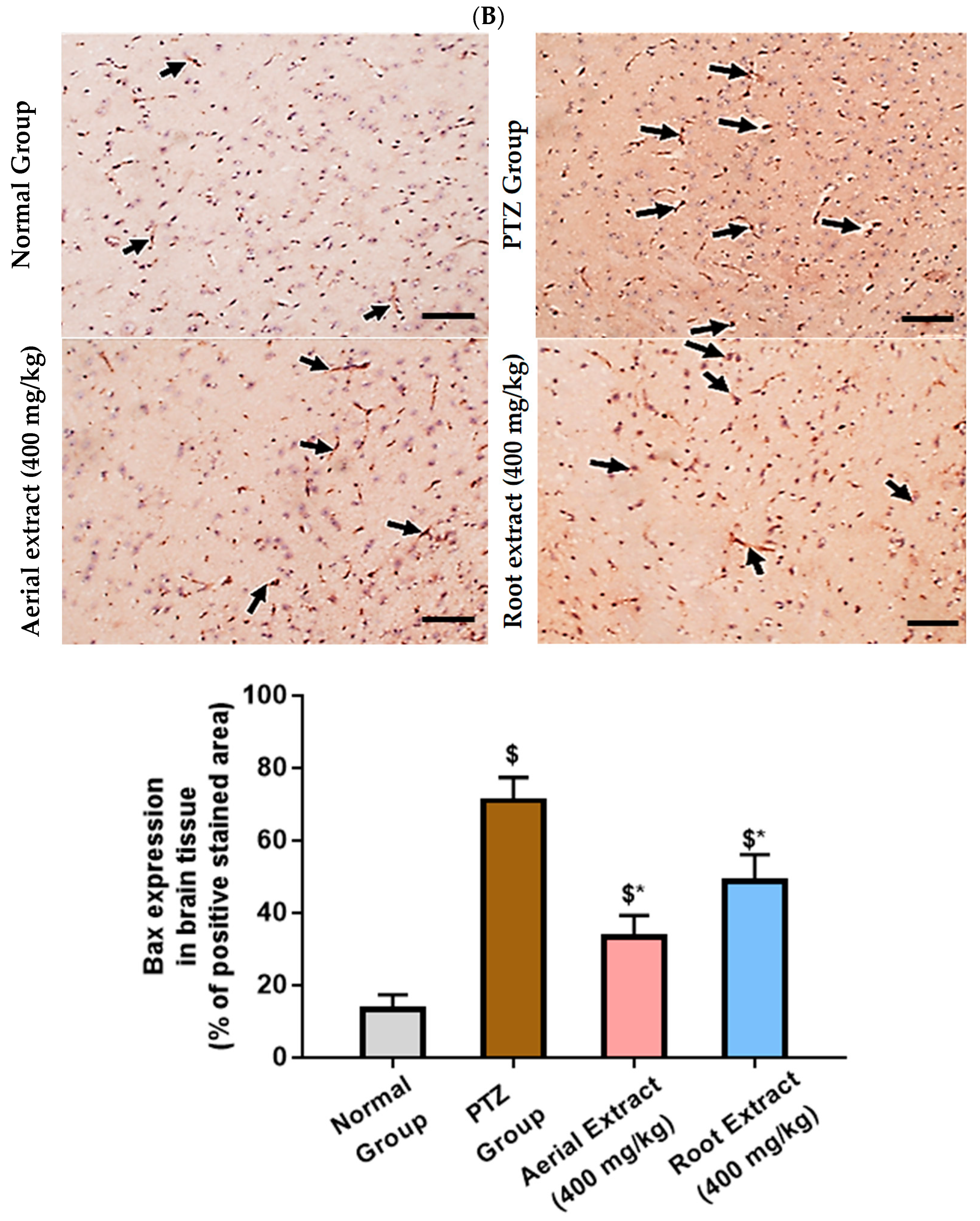
2.3.9. Immunohistochemical Staining and Determination of Apoptotic Markers Immunoexpression (Bcl-2 and Bax)
2.3.10. Statistical Analysis
3. Results and Discussion
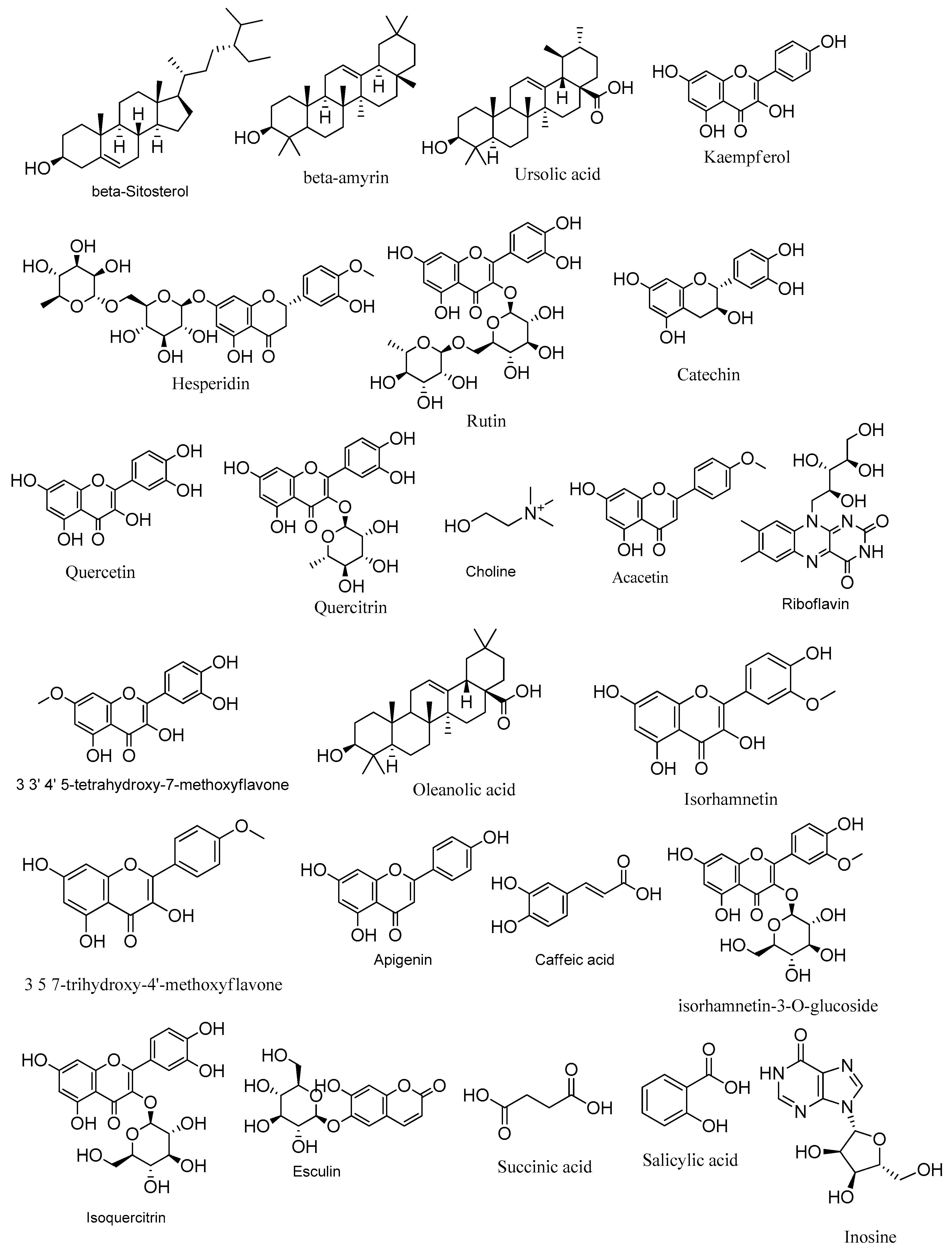
3.1. LC-ESI-TOF-MS/MS Analysis of Zygophyllum album
| No. | Ret. Time (min) | Measured m/z | Calculated m/z | Mass Error (ppm) | Adduct | Molecular Formula | MS/MS Spectrum | Deduced Name | Ref. | Plant Part |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26.52 | 415.3951 | 415.3940 | 2.65 | [M + H]+ | C29H50O | 255, 147 | β-Sitosterol | [34,35] | Roots |
| 415.3935 | −1.20 | Aerial | ||||||||
| 2 | 22.88 | 427.3952 | 427.3940 | 2.81 | [M + H]+ | C30H50O | 177, 259, 299 | β-amyrin | [36] | Roots |
| 427.3928 | −2.81 | Aerial | ||||||||
| 3 | 22.58 | 457.3687 | 457.3682 | 1.09 | [M + H]+ | C30H48O3 | 203, 161, 95 | Ursolic acid | [37,38] | Roots |
| 457.3674 | −1.75 | Aerial | ||||||||
| 4 | 9.99 | 287.0562 | 287.0556 | 2.09 | [M + H]+ | C15H10O6 | 241, 223 | Kaempferol | [39,40] | Roots |
| 287.0543 | −4.53 | Aerial | ||||||||
| 5 | 6.41 | 611.1993 | 611.1976 | 2.78 | [M + H]+ | C28H34O15 | 611 | Hesperidin | [41] | Aerial only |
| 6 | 6.39 | 611.1582 | 611.1612 | −4.91 | [M + H]+ | C27H30O16 | 303, 609 | Rutin | [42,43] | Roots |
| 611.1588 | −3.93 | Aerial | ||||||||
| 7 | 4.62 | 291.0857 | 291.0869 | −4.12 | [M + H]+ | C15H14O6 | 205, 179 | Catechin | [44] | Roots |
| 291.0863 | −2.06 | Aerial | ||||||||
| 8 | 9.51 | 303.0520 | 303.0505 | 4.95 | [M + H]+ | C15H9O7 | 68, 121 | Quercetin | [45] | Roots |
| 303.0491 | −4.62 | Aerial | ||||||||
| 9 | 7.23 | 449.1064 | 449.1084 | −4.45 | [M + H]+ | C21H20O11 | 254, 346 | Quercitrin | [46] | Aerial only |
| 10 | 1.19 | 104.1071 | 104.1070 | 0.96 | [M + H]+ | C5H14NO | 104, 60 | Choline | [47] | Roots |
| 104.1066 | −3.84 | Aerial | ||||||||
| 11 | 14.16 | 285.0762 | 285.0763 | −0.35 | [M + H]+ | C16H12O5 | 285, 193, 153 | Acacetin | [48] | Aerial only |
| 12 | 14.38 | 377.1446 | 377.1461 | −3.98 | [M + H]+ | C17H20N4O6 | 359, 341 | (-)-Riboflavin | [49] | Aerial only |
| 13 | 8.71 | 317.0657 | 317.0661 | −1.26 | [M + H]+ | C16H12O7 | 302, 224 | 3,3′,4′,5-tetrahydroxy-7-methoxy flavone(Rhamnetin) | [50] | Roots |
| 317.0648 | −4.10 | Aerial | ||||||||
| 14 | 22.53 | 457.3659 | 457.3682 | −5.03 | [M + H]+ | C30H48O3 | 457, 393 | Oleanolic acid | [51] | Roots |
| 457.3674 | −1.75 | Aerial | ||||||||
| 15 | 7.48 | 317.0647 | 317.0661 | −4.42 | [M + H]+ | C16H12O7 | 300, 151 | Isorhamnetin | [52,53] | Roots |
| 317.0643 | −5.67 | Aerial | ||||||||
| 16 | 9.59 | 301.0701 | 301.0712 | −3.65 | [M + H]+ | C16H12O6 | 269, 349 | 3,5,7-trihydroxy-4′-methoxyfla-vone (Kaempferide) | [54] | Aerialonly |
| No. | Ret. Time (min) | Measured m/z | Calculated m/z | Mass Error (ppm) | Adduct | Molecular Formula | MS/MS Spectrum | Deduced Name | Ref. | Plant Part |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.32 | 269.0439 | 269.0450 | −4.09 | [M − H]− | C15H10O5 | 269 | Apigenin | [43] | Roots |
| 269.0459 | 3.35 | Aerial | ||||||||
| 2 | 1.33 | 179.0343 | 179.0344 | −0.56 | [M − H]− | C9H8O4 | 180, 161 | Caffeic acid | [43] | Aerial only |
| 3 | 7.27 | 477.1031 | 477.1033 | −0.42 | [M − H]− | C22H22O12 | 477, 314, 285, 271, 243 | isorhamnetin-3-O-glucoside | [55,56,57] | Roots |
| 477.1032 | −0.21 | Aerial | ||||||||
| 4 | 6.22 | 463.0879 | 463.0877 | 0.43 | [M − H]− | C21H20O12 | 343, 303 | Isoquercitrin | [58] | Roots |
| 463.0879 | 0.43 | Aerial | ||||||||
| 5 | 7.15 | 339.0699 | 339.0716 | −5.01 | [M − H]− | C15H16O9 | 133, 148 | Esculin | [59] | Aerial only |
| 6 | 1.08 | 117.0192 | 117.0188 | 3.42 | [M − H]− | C4H6O4 | 99 | Succinic acid | [60] | Aerial only |
| 7 | 1.31 | 137.0242 | 137.0239 | 2.19 | [M − H]− | C7H6O3 | 75, 93 | Salicylic acid | [61] | Roots |
| 137.0241 | 1.46 | Aerial | ||||||||
| 8 | 1.13 | 267.0734 | 267.0729 | 1.87 | [M − H]− | C10H12N4O5 | 267, 113, 92, 89, 71, 59 | Inosine | [62] | Roots |
| 267.0716 | 4.87 | Aerial |
3.2. The Antiepileptic Effect of Z. album Aerial Parts and Roots Crude Extracts against Pentylenetetrazole (PTZ)-Induced Kindling in Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on nature: The role of nanomedicine in the development of clinical natural drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.E.; Nafie, M.S.; Hal, D.M.; Abdel-Kader, M.S.; Abu-Elsaoud, A.M.; Ahmed, S.A.; Ibrahim, A.K.; Badr, J.M.; Abdelhameed, R.F.A. A New Saponin (Zygo-albuside D) from Zygophyllum album Roots Triggers Apoptosis in Non-Small Cell Lung Car-cinoma (A549 Cells) through CDK-2 Inhibition. ACS Omega 2023, 8, 30630–30639. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.R.; Kawashty, S.A.; Tantawy, M.E.; Saleh, N.A.M. Chemosystematic studies of Nitraria retusa and selected taxa of Zygophyllaceae in Egypt. Plant Syst. Evol. 2009, 277, 251–264. [Google Scholar] [CrossRef]
- Shawky, E.; Gabr, N.; El-gindi, M.; Mekky, R. A Comprehensive Review on Genus Zygophyllum. J. Adv. Pharm. Res. 2019, 3, 1–16. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.; Fattah, S.A.; Mehanna, E.T.; Hal, D.M.; Mosaad, S.M.; Abdel-Kader, M.S.; Ibrahim, A.K.; Ahmed, S.A.; Badr, J.M.; Eltamany, E.E. Zygo-Albuside A: New Saponin from Zygophyllum album L. with Significant Antioxidant, Anti-Inflammatory and Antiapoptotic Effects against Methotrexate-Induced Testicular Damage. Int. J. Mol. Sci. 2022, 23, 10799. [Google Scholar] [CrossRef] [PubMed]
- Feriani, A.; Tir, M.; Gómez-Caravaca, A.M.; Del Mar Contreras, M.; Talhaoui, N.; Taamalli, A.; Segura-Carretero, A.; Ghazouani, L.; Mufti, A.; Tlili, N.; et al. HPLC-DAD-ESI-QTOF- MS/MS profiling of Zygophyllum album roots extract and assessment of its cardioprotective effect against deltamethrin-induced myocardial injuries in rat, by suppression of oxidative stress-related inflammation and apoptosis via NF-κB signaling pathway. J. Ethnopharmacol. 2020, 247, 112266. [Google Scholar] [CrossRef] [PubMed]
- Mnafgui, K.; Kchaou, M.; Ben-Salah, H.; Hajji, R.; Khabbabi, G.; Elfeki, A.; Allouche, N.; Gharsallah, N. Essential oil of Zygophyllum album inhibits key-digestive enzymes related to diabetes and hypertension and attenuates symptoms of diarrhea in alloxan-induced diabetic rats. Pharm. Biol. 2016, 54, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Megdiche, W.; Ksouri, R. The halophytic genus Zygophyllum and Nitraria from North Africa: A phytochemical and pharmacological overview. In Medicinal and Aromatic Plants of the World—Africa; Neffati, M., Najjaa, H., Máthé, Á., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 3, pp. 345–356. [Google Scholar]
- Rong, S.; Wan, D.; Fan, Y.; Liu, S.; Sun, K.; Huo, J.; Zhang, P.; Li, X.; Xie, X.; Wang, F.; et al. Amentoflavone affects epileptogenesis and exerts neuroprotective effects by inhibiting NLRP3 inflammasome. Front. Pharmacol. 2019, 10, 856. [Google Scholar] [CrossRef]
- El-Sayed, R.M.; Fawzy, M.N.; Zaki, H.F.; Abd El-Haleim, E.A. Neuroprotection impact of biochanin A against pentylenetetrazol-kindled mice: Targeting NLRP3 inflammasome/TXNIP pathway and autophagy modulation. Int. Immunopharmacol. 2023, 115, 109711. [Google Scholar] [CrossRef]
- Shen, K.; Jiang, W.; Zhang, C.; Cai, L.; Wang, Q.; Yu, H.; Tang, Z.; Gu, Z.; Chen, B. Molecular mechanism of a specific NLRP3 inhibitor to alleviate seizure severity induced by pentylenetetrazole. Curr. Mol. Pharmacol. 2021, 14, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, J.; Zhu, C.; Guo, B.; Yang, W.; He, W.; Li, X.; Wang, Y.; Li, W.; Wang, F.; et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole-kindled mice. Int. J. Mol. Med. 2021, 48, 219. [Google Scholar] [CrossRef] [PubMed]
- Próchnicki, T.; Latz, E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab. 2017, 26, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Güveli, B.T.; Rosti, R.Ö.; Güzeltaş, A.; Tuna, E.B.; Ataklı, D.; Sencer, S.; Yekeler, E.; Kayserili, H.; Dirican, A.; Bebek, N.; et al. Teratogenicity of Antiepileptic Drugs. Clin. Psychopharmacol. Neurosci. 2017, 15, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-C.; Lee, H.-S.; Chang, K.-P.; Lee, Y.-Y.; Lai, H.-C.; Hung, P.-L.; Lee, H.-F.; Chi, C.-S. The impact of anti-epileptic drugs on growth and bone metabolism. Int. J. Mol. Sci. 2016, 17, 1242. [Google Scholar] [CrossRef]
- Abdel-Hamed, A.R.; Mehanna, E.T.; Hazem, R.M.; Badr, J.M.; Abo-Elmatty, D.M.; Abdel-Kader, M.S.; Goda, M.S. Plicosepalus acacia Extract and Its Major Constituents, Methyl Gallate and Quercetin, Potentiate Thera-peutic Angiogenesis in Diabetic Hind Limb Ischemia: HPTLC Quantification and LC-MS/MS Metabolic Pro-filing. Antioxidants 2021, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Edition, E. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Sefil, F.; Bagirici, F.; Acar, M.; Marangoz, C. Influence of carbenoxolone on the anticonvulsant efficacy of phenytoin in pentylenetetrazole kindled rats. Acta Neurobiol. Exp. (Wars) 2012, 72, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Mnafgui, K.; Hamden, K.; Ben Salah, H.; Kchaou, M.; Nasri, M.; Slama, S.; Derbali, F.; Allouche, N.; Elfeki, A. Inhibitory activities of Zygophyllum album: A natural weight-lowering plant on key enzymes in high-fat diet-fed rats. Evid. Based Complement. Altern. Med. 2012, 2012, 620384. [Google Scholar] [CrossRef]
- El-Megiri, N.; Mostafa, Y.M.; Ahmed, A.; Mehanna, E.T.; El-Azab, M.F.; Alshehri, F.; Alahdal, H.; El-Sayed, N.M. Pioglitazone ameliorates hippocampal neurodegeneration, disturbances in glucose metabolism and AKT/mTOR signaling pathways in pentyelenetetrazole-kindled mice. Pharmaceuticals 2022, 15, 1113. [Google Scholar] [CrossRef]
- Liu, X.-J.; Wang, Y.-Q.; Shang, S.-Q.; Xu, S.; Guo, M. TMT induces apoptosis and necroptosis in mouse kidneys through oxidative stress-induced activation of the NLRP3 inflammasome. Ecotoxicol. Environ. Saf. 2022, 230, 113167. [Google Scholar] [CrossRef]
- Elhady, S.S.; Abdelhameed, R.F.; Mehanna, E.T.; Wahba, A.S.; Elfaky, M.A.; Koshak, A.E.; Noor, A.O.; Bogari, H.A.; Malatani, R.T.; Goda, M.S. Metabolic profiling, chemical composition, antioxidant capacity, and in vivo hepato-and nephroprotective effects of Sonchus cornutus in mice exposed to cisplatin. Antioxidants 2022, 11, 819. [Google Scholar] [CrossRef]
- Fujimura, K.; Karasawa, T.; Komada, T.; Yamada, N.; Mizushina, Y.; Baatarjav, C.; Matsumura, T.; Otsu, K.; Takeda, N.; Mizukami, H.; et al. NLRP3 inflammasome-driven IL-1β and IL-18 contribute to lipopolysaccharide-induced septic cardiomyopathy. J. Mol. Cell Cardiol. 2023, 180, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, J.; Kim, J.-S.; Shin, J.; Lee, S.K.; Park, H.-W. Targeting TBK1 attenuates LPS-induced NLRP3 inflammasome activation by regulating of mTORC1 pathways in trophoblasts. Front. Immunol. 2021, 12, 743700. [Google Scholar] [CrossRef]
- Altun, S.; Budak, H. The protective effect of the cardiac thioredoxin system on the heart in the case of iron overload in mice. J. Trace Elem. Med. Biol. 2021, 64, 126704. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, Y.; Cao, J.; Lu, Y.; Sun, G.; Yang, J. Role of ASM/Cer/TXNIP signaling module in the NLRP3 inflammasome activation. Lipids Health Dis. 2021, 20, 19. [Google Scholar] [CrossRef]
- Ye, X.; Shao, S.; Wang, Y.; Su, W. Ginsenoside Rg2 alleviates neurovascular damage in 3xTg-AD mice with Alzheimer’s disease through the MAPK-ERK pathway. J. Chem. Neuroanat. 2023, 133, 102346. [Google Scholar] [CrossRef]
- Jokinen, M.P.; Lieuallen, W.G.; Boyle, M.C.; Johnson, C.L.; Malarkey, D.E.; Nyska, A. Morphologic aspects of rodent cardiotoxicity in a retrospective evaluation of National Toxicology Program studies. Toxicol. Pathol. 2011, 39, 850–860. [Google Scholar] [CrossRef]
- Ilić, I.R.; Stojanović, N.M.; Radulović, N.S.; Živković, V.V.; Randjelović, P.J.; Petrović, A.S.; Božić, M.; Ilić, R.S. The quantitative ER immunohistochemical analysis in breast cancer: Detecting the 3+ 0, 4+ 0, and 5+ 0 allred score cases. Medicina 2019, 55, 461. [Google Scholar] [CrossRef]
- Goda, M.S.; El-Kattan, N.; Abdel-Azeem, M.A.; Allam, K.A.; Badr, J.M.; Nassar, N.A.; Almalki, A.J.; Alharbi, M.; Elhady, S.S.; Eltamany, E.E. Antimicrobial Potential of Different Isolates of Chaetomium globosum Combined with Liquid Chromatography Tandem Mass Spectrometry Chemical Profiling. Biomolecules 2023, 13, 1683. [Google Scholar] [CrossRef]
- Hal, D.M.; Eltamany, E.; Abdelhameed, R.F.; Ibrahim, A.K.; Badr, J. Chemical Review on Zygophyllum genus. Rec. Pharm. Biomed. Sci. 2022, 6, 105–129. [Google Scholar] [CrossRef]
- Mo, S.; Dong, L.; Hurst, W.J.; Van Breemen, R.B. Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography–tandem mass spectrometry. Lipids 2013, 48, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Münger, L.H.; Boulos, S.; Nyström, L. UPLC-MS/MS based identification of dietary steryl glucosides by investigation of corresponding free sterols. Front. Chem. 2018, 6, 342. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Katsube, Y.; Otsuka, M.; Zhang, H.; Tansakul, P.; Xiang, T.; Ebizuka, Y. Identification of a product specific β-amyrin synthase from Arabidopsis thaliana. Plant Physiol. Biochem. 2009, 47, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.; Abdel-Hamid, M.E.; Hamza, H.; Masterova, I.; Grancai, D. Development of LC–MS method for determination of ursolic acid: Application to the analysis of ursolic acid in Staphylea holocarpa Hemsl. J. Pharm. Biomed. Anal. 2003, 31, 961–968. [Google Scholar] [CrossRef]
- Falev, D.I.; Ul’yanovskii, N.V.; Ovchinnikov, D.V.; Faleva, A.V.; Kosyakov, D.S. Screening and semi-quantitative determination of pentacyclic triterpenoids in plants by liquid chromatography–tandem mass spectrometry in precursor ion scan mode. Phytochem. Anal. 2021, 32, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Munyao Mutie, F.; Xu, Y.-B.; Saleri, F.D.; Hu, G.-W.; Guo, M.-Q. Antioxidant, anti-inflammatory activities and polyphenol profile of Rhamnus prinoides. Pharmaceuticals 2020, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of Portuguese propolis by LC—MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhou, D.; Gao, J.; Zhu, Y.; Sun, H.; Bi, K. Simultaneous determination of naringin, hesperidin, neohesperidin, naringenin and hesperetin of Fractus aurantii extract in rat plasma by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 58, 58–64. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Quantification of rutin in rat’s brain by UHPLC/ESI-Q-TOF-MS/MS after intranasal administration of rutin loaded chitosan nanoparticles. EXCLI J. 2016, 15, 518–531. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, W.; Huang, M.; Xu, W.; Li, H.; Ye, M.; Zhang, X.; Chu, K. Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in Yinhua Kanggan tablet by UPLC-QqQ-MS/MS. Molecules 2015, 20, 12209–12228. [Google Scholar] [CrossRef] [PubMed]
- Stöggl, W.; Huck, C.; Bonn, G.K. Structural elucidation of catechin and epicatechin in sorrel leaf extracts using liquid-chromatography coupled to diode array-, fluorescence-, and mass spectrometric detection. J. Sep. Sci. 2004, 27, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.-Y.; He, Y.-J.; Yang, Y.-H.; Yan, X.-P.; Li, Z.-B.; Yang, H. Systematic Identification of Bioactive Compositions in Leaves of Morus Cultivars Using UHPLC-ESI-QTOF-MS/MS and Comprehensive Screening of High-Quality Resources. Separations 2022, 9, 76. [Google Scholar] [CrossRef]
- Santos, A.L.; Soares, M.G.; de Medeiros, L.S.; Ferreira, M.J.; Sartorelli, P. Identification of flavonoid-3-O-glycosides from leaves of Casearia arborea (Salicaceae) by UHPLC-DAD-ESI-HRMS/MS combined with molecular networking and NMR. Phytochem. Anal. 2021, 32, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.J.; Guy, P.A.; Rezzi, S.; Ross, A.B. Quantitative measurement of betaine and free choline in plasma, cereals and cereal products by isotope dilution LC-MS/MS. J. Agric. Food Chem. 2010, 58, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-G.; Cha, E.; Joo, J.; Hwang, J.S.; Kim, S.; Park, T.; Jeong, Y.-S.; Maeng, H.-J.; Kim, S.-B.; Yoon, I.-S. Investigation of the factors responsible for the poor oral bioavailability of acacetin in rats: Physicochemical and biopharmaceutical aspects. Pharmaceutics 2021, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Zhong, X.; Li, J.; Wang, X.; Ji, L.; Shang, X. Rapid characterization of chemical components in edible mushroom Sparassis crispa by UPLC-orbitrap MS analysis and potential inhibitory effects on allergic rhinitis. Molecules 2019, 24, 3014. [Google Scholar] [CrossRef] [PubMed]
- Mahrous, F.S.M.; Mohammed, H.; Sabour, R.; Ismail, L.d. LC-ESI-QTOF-MS/MS of Holoptelea integrifolia (Roxb.) Planch. leaves and In silico study of phenolic compounds’ antiviral activity against the HSV1 virus. AIJPMS 2021, 1, 91–101. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Zhang, W.; Chen, Z. Identification and quantification of oleanolic acid and ursolic acid in Chinese herbs by liquid chromatography–ion trap mass spectrometry. Biomed. Chromatogr. 2011, 25, 1381–1388. [Google Scholar] [CrossRef]
- Shahat, A.A.; Abdelshafeek, K.A.; Husseiny, H.A. Isolation and identification of a new flavonoid glycoside from Carrichtera annua L. seeds. Pharmacogn. Res. 2011, 3, 151–154. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in pollen typhae for transformation rule exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, L.; Xi, H.; Guan, L.; Fang, J.; Liu, Y.; Wu, B.; Li, S. Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Anal. Chim. Acta 2012, 724, 127–135. [Google Scholar] [CrossRef]
- Cantos, E.; Espin, J.C.; Tomás-Barberán, F.A. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC− DAD− MS− MS. J. Agric. Food Chem. 2002, 50, 5691–5696. [Google Scholar] [CrossRef]
- Gad El-Hak, H.N.; Mahmoud, H.S.; Ahmed, E.A.; Elnegris, H.M.; Aldayel, T.S.; Abdelrazek, H.M.; Soliman, M.T.; El-Menyawy, M.A.I. Methanolic Phoenix dactylifera L. extract ameliorates cisplatin-induced hepatic injury in male rats. Nutrients 2022, 14, 1025. [Google Scholar] [CrossRef]
- MassBank of North America (MoNA). Available online: https://mona.fiehnlab.ucdavis.edu/ (accessed on 12 May 2022).
- Hu, H.; Tekin, V.; Hu, B.; Yaghoobi, M.; Khan, A.; Ghosh, A.K.; Panda, S.K.; Huang, H.; Luyten, W. Metabolic profiling of Chimonanthus grammatus via UHPLC-HRMS-MS with computer-assisted structure elucidation and its antimicrobial activity. Front. Plant Sci. 2023, 14, 1138913. [Google Scholar] [CrossRef]
- Li, Y.-y.; Song, Y.-y.; Liu, C.-h.; Huang, X.-t.; Zheng, X.; Li, N.; Xu, M.-l.; Mi, S.-q.; Wang, N.-s. Simultaneous determination of esculin and its metabolite esculetin in rat plasma by LC–ESI-MS/MS and its application in pharmacokinetic study. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2012, 907, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Al Kadhi, O.; Melchini, A.; Mithen, R.; Saha, S. Development of a LC-MS/MS method for the simultaneous detection of tricarboxylic acid cycle intermediates in a range of biological matrices. J. Anal. Methods Chem. 2017, 2017, 5391832. [Google Scholar] [CrossRef]
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem. 2017, 215, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, J.; Kawagishi, H.; Jiang, C.; Zhou, Q.; Tong, Z.; Tong, Y.; Wang, P. Study on Secondary Metabolites of Endophytic Fungus, Aspergillus fumigatus, from Crocus sativus L. Guided byUHPLC-HRMS/MS-Based Molecular Network. Int. J. Anal. Chem. 2022, 2022, 7067665. [Google Scholar] [CrossRef]
- He, K.; Zhu, X.; Liu, Y.; Miao, C.; Wang, T.; Li, P.; Zhao, L.; Chen, Y.; Gong, J.; Cai, C.; et al. Inhibition of NLRP3 inflammasome by thioredoxin-interacting protein in mouse Kupffer cells as a regulatory mechanism for non-alcoholic fatty liver disease development. Oncotarget 2017, 8, 37657–37672. [Google Scholar] [CrossRef]
- Kulsoom, B.; Shamsi, T.S.; Afsar, N.A.; Memon, Z.; Ahmed, N.; Hasnain, S.N. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: Are we ready for Bcl-2-directed therapy? Cancer Manag. Res. 2018, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Tambe, R.; Jain, P.; Patil, S.; Ghumatkar, P.; Sathaye, S. Protective effects of diosgenin in pentylenetetrazole induced kindling model of epilepsy in mice. Neurochem. Neuropharm. 2015, 1, 106. [Google Scholar] [CrossRef]
- Alvi, A.M.; Al Kury, L.T.; Alattar, A.; Ullah, I.; Muhammad, A.J.; Alshaman, R.; Shah, F.A.; Khan, A.U.; Feng, J.; Li, S. Carveol attenuates seizure severity and neuroinflammation in pentylenetetrazole-kindled epileptic rats by regulating the Nrf2 signaling pathway. Oxid. Med. Cell Longev. 2021, 2021, 9966663. [Google Scholar] [CrossRef]
- Muke, S.; Kaikini, A.; Peshattiwar, V.; Bagle, S.; Dighe, V.; Sathaye, S. Neuroprotective effect of coumarin nasal formulation: Kindling model assessment of epilepsy. Front. Pharmacol. 2018, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.M.; Abdelfattah, M.S.; El-khadragy, M.; Al-Megrin, W.A.; Fehaid, A.; Kassab, R.B.; Abdel Moneim, A.E. Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice. Green. Process Synth. 2023, 12, 20230010. [Google Scholar] [CrossRef]
- Palumbo, L.; Carinci, M.; Guarino, A.; Asth, L.; Zucchini, S.; Missiroli, S.; Rimessi, A.; Pinton, P.; Giorgi, C. The NLRP3 Inflammasome in Neurodegenerative Disorders: Insights from Epileptic Models. Biomedicines 2023, 11, 2825. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Rabidas, S.S.; Prakash, C.; Tyagi, J.; Suryavanshi, J.; Kumar, P.; Bhattacharya, J.; Sharma, D. A comprehensive review on anti-inflammatory response of flavonoids in experimentally-induced epileptic seizures. Brain Sci. 2023, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Qin, T.; Qu, R.; Ma, S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav. Brain Res. 2016, 296, 318–325. [Google Scholar] [CrossRef]
- Bellavite, P. Neuroprotective potentials of flavonoids: Experimental studies and mechanisms of action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, F.; Xing, Z.; Chen, J.; Li, D. Beneficial effects of natural flavonoids on neuroinflammation. Front. Immunol. 2022, 13, 1006434. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Li, Z.; Hua, Q.; Song, P.; Gao, L.; Zhou, L.; Cai, Q. Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway. Int. J. Mol. Sci. 2023, 24, 14771. [Google Scholar] [CrossRef] [PubMed]
- Feriani, A.; Tir, M.; Gomez-Caravaca, A.M.; del Mar Contreras, M.; Taamalli, A.; Segura-Carretero, A.; Ghazouani, L.; Mufti, A.; Tlili, N.; El Feki, A. Zygophyllum album leaves extract prevented hepatic fibrosis in rats, by reducing liver injury and suppressing oxidative stress, inflammation, apoptosis and the TGF-β1/Smads signaling pathways. Exploring of bioactive compounds using HPLC–DAD–ESI–QTOF-MS/MS. Inflammopharmacology 2020, 28, 1735–1750. [Google Scholar] [CrossRef] [PubMed]



| GenBank Accession No. | Gene | Primers | Annealing Temperature | Reference |
|---|---|---|---|---|
| NM_145827.4 | NLRP3 | Forward: 5′-AGCCTTCCAGGATCCTCTTC-3′ | 52 °C | [26] |
| Reverse: 5′-CTTGGGCAGCAGTTTCTTTC-3′ | ||||
| NM_001009935.2 | TXNIP | Forward: 5′-GATACCCCAGAAGCTCCTCC-3′ | 54 °C | [27] |
| Reverse: 5′-ACCTCAGTGTAAGTGGGTGG-3′ | ||||
| NM_009807.2 | Caspase-1 | Forward: 5′-TGGCAGGAATTCTGGAGCTT-3′ | 53 °C | [28] |
| Reverse: 5′-CTTGAGGGTCCCAGTCAGTC-3′ | ||||
| NM_001289726.2 | GAPDH | Forward: 5′-ATGACTCTACCCACGGCAAG-3′ | 55 °C | [29] |
| Reverse: 5′-GATCTCGCTCCTGGAAGATG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Hamed, A.R.; Wahba, A.S.; Khodeer, D.M.; Abdel-Kader, M.S.; Badr, J.M.; Mahgoub, S.; Hal, D.M. Metabolomic Profiling and In Vivo Antiepileptic Effect of Zygophyllum album Aerial Parts and Roots Crude Extracts against Pentylenetetrazole-Induced Kindling in Mice. Metabolites 2024, 14, 316. https://doi.org/10.3390/metabo14060316
Abdel-Hamed AR, Wahba AS, Khodeer DM, Abdel-Kader MS, Badr JM, Mahgoub S, Hal DM. Metabolomic Profiling and In Vivo Antiepileptic Effect of Zygophyllum album Aerial Parts and Roots Crude Extracts against Pentylenetetrazole-Induced Kindling in Mice. Metabolites. 2024; 14(6):316. https://doi.org/10.3390/metabo14060316
Chicago/Turabian StyleAbdel-Hamed, Asmaa R., Alaa S. Wahba, Dina M. Khodeer, Maged S. Abdel-Kader, Jihan M. Badr, Sebaey Mahgoub, and Dina M. Hal. 2024. "Metabolomic Profiling and In Vivo Antiepileptic Effect of Zygophyllum album Aerial Parts and Roots Crude Extracts against Pentylenetetrazole-Induced Kindling in Mice" Metabolites 14, no. 6: 316. https://doi.org/10.3390/metabo14060316
APA StyleAbdel-Hamed, A. R., Wahba, A. S., Khodeer, D. M., Abdel-Kader, M. S., Badr, J. M., Mahgoub, S., & Hal, D. M. (2024). Metabolomic Profiling and In Vivo Antiepileptic Effect of Zygophyllum album Aerial Parts and Roots Crude Extracts against Pentylenetetrazole-Induced Kindling in Mice. Metabolites, 14(6), 316. https://doi.org/10.3390/metabo14060316





