Molecular Networking-Based Metabolome, In Vitro Antidiabetic and Anti-Inflammatory Effects of Breonadia salicina (Vahl) Hepper & J.R.I. Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedure
2.2. Sampling and Extraction
2.3. UPLC-QTOF-MS Analysis
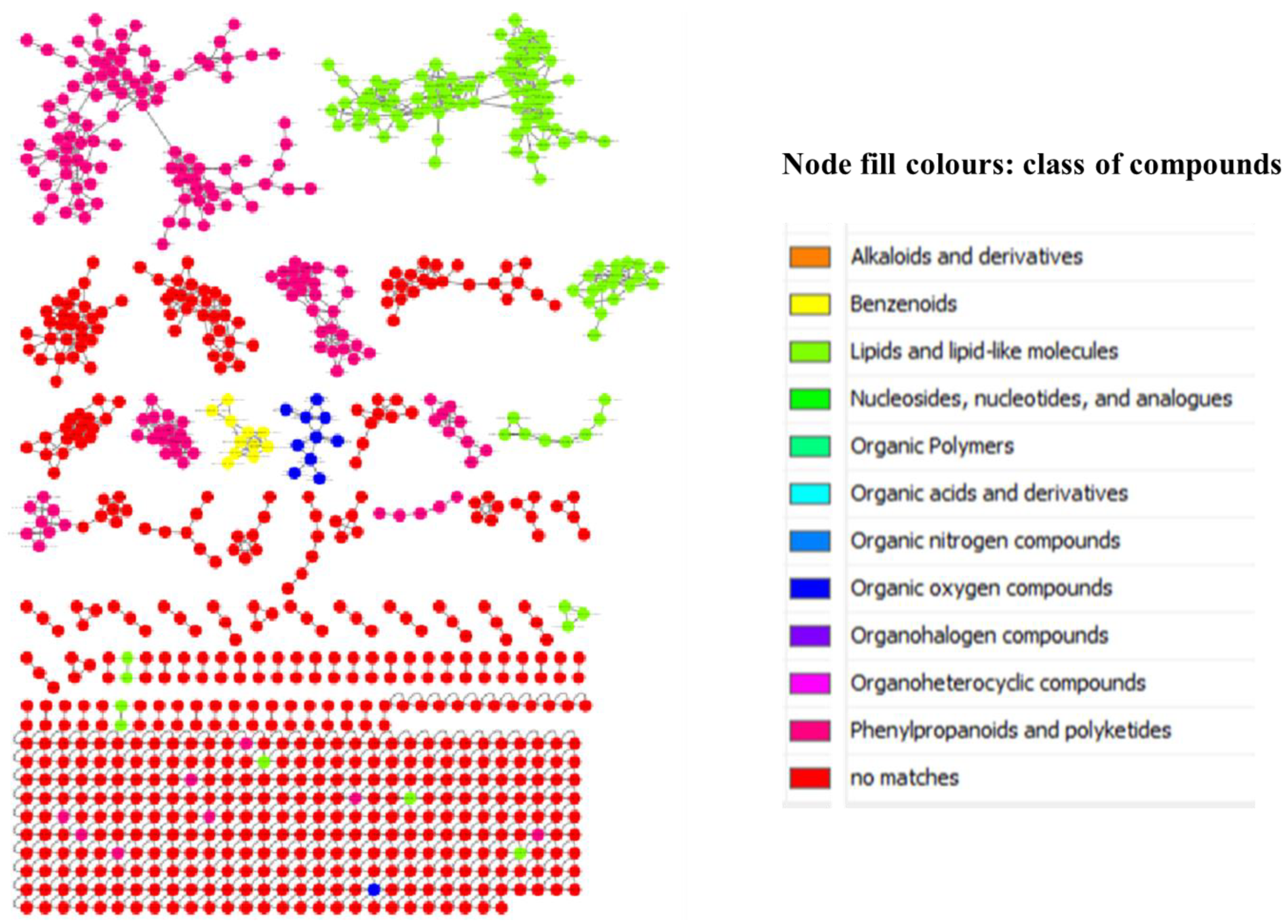
2.4. Global Natural Product Social Molecular Networking (GNPS) and Metabolite Annotation
2.5. Antidiabetic Activity
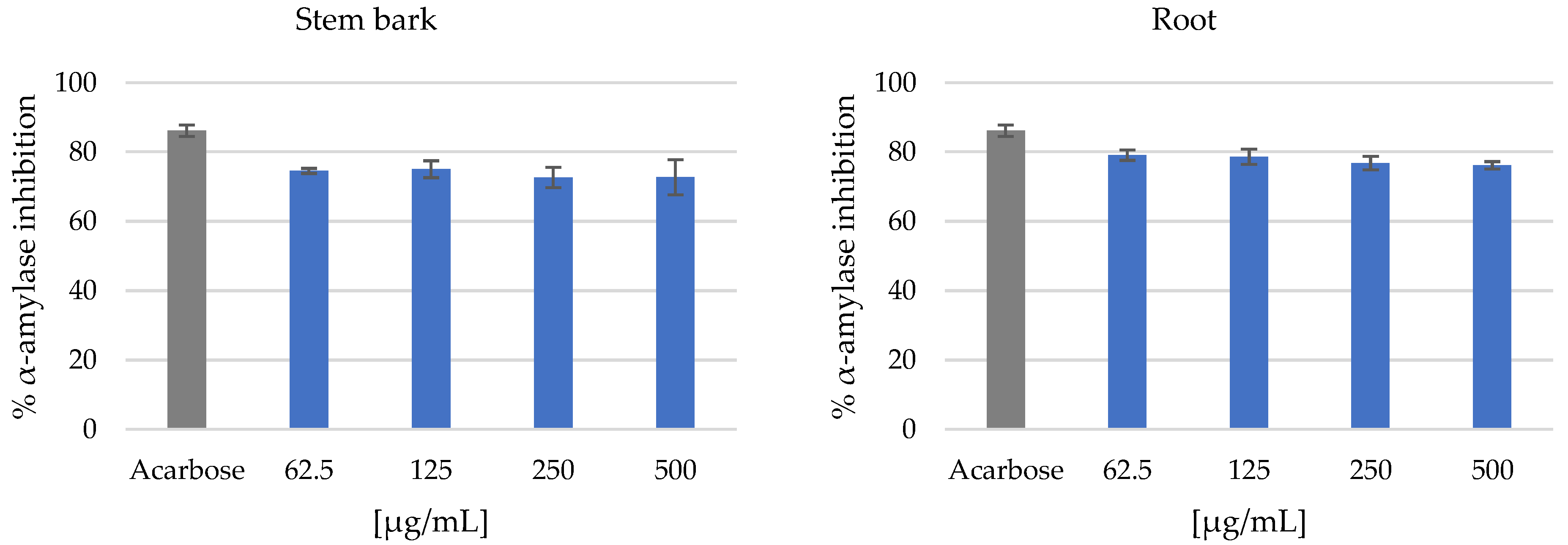
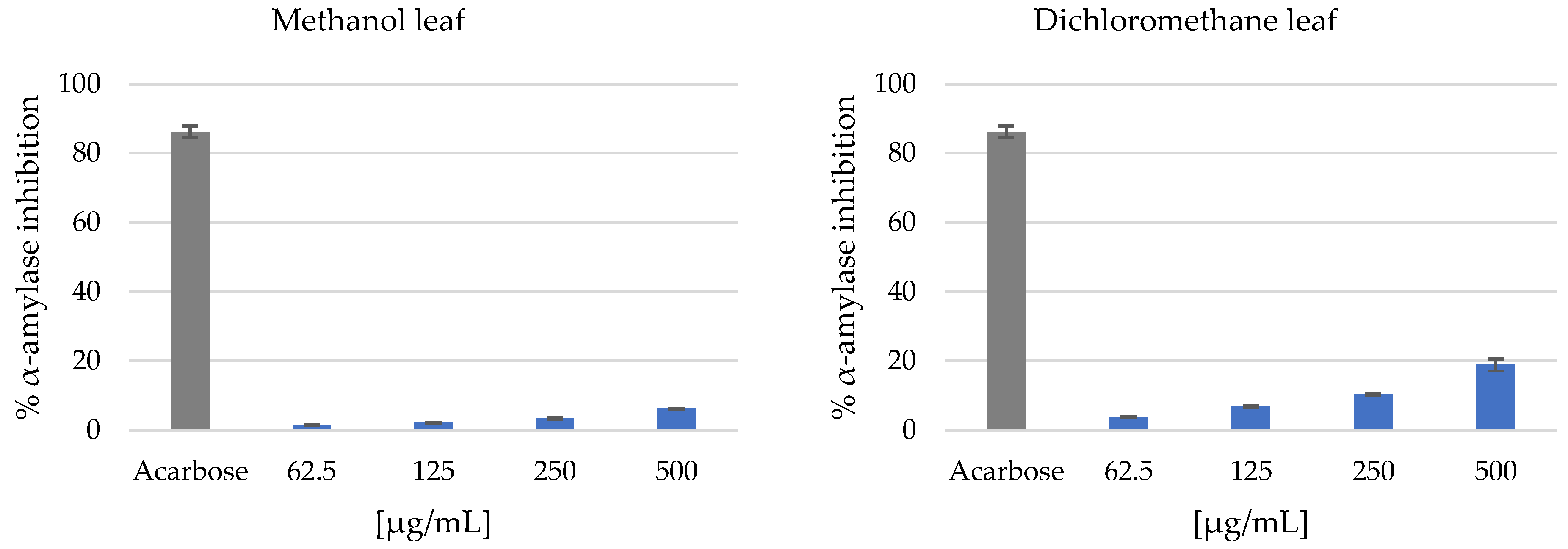
2.5.1. α-Amylase Inhibition Assay
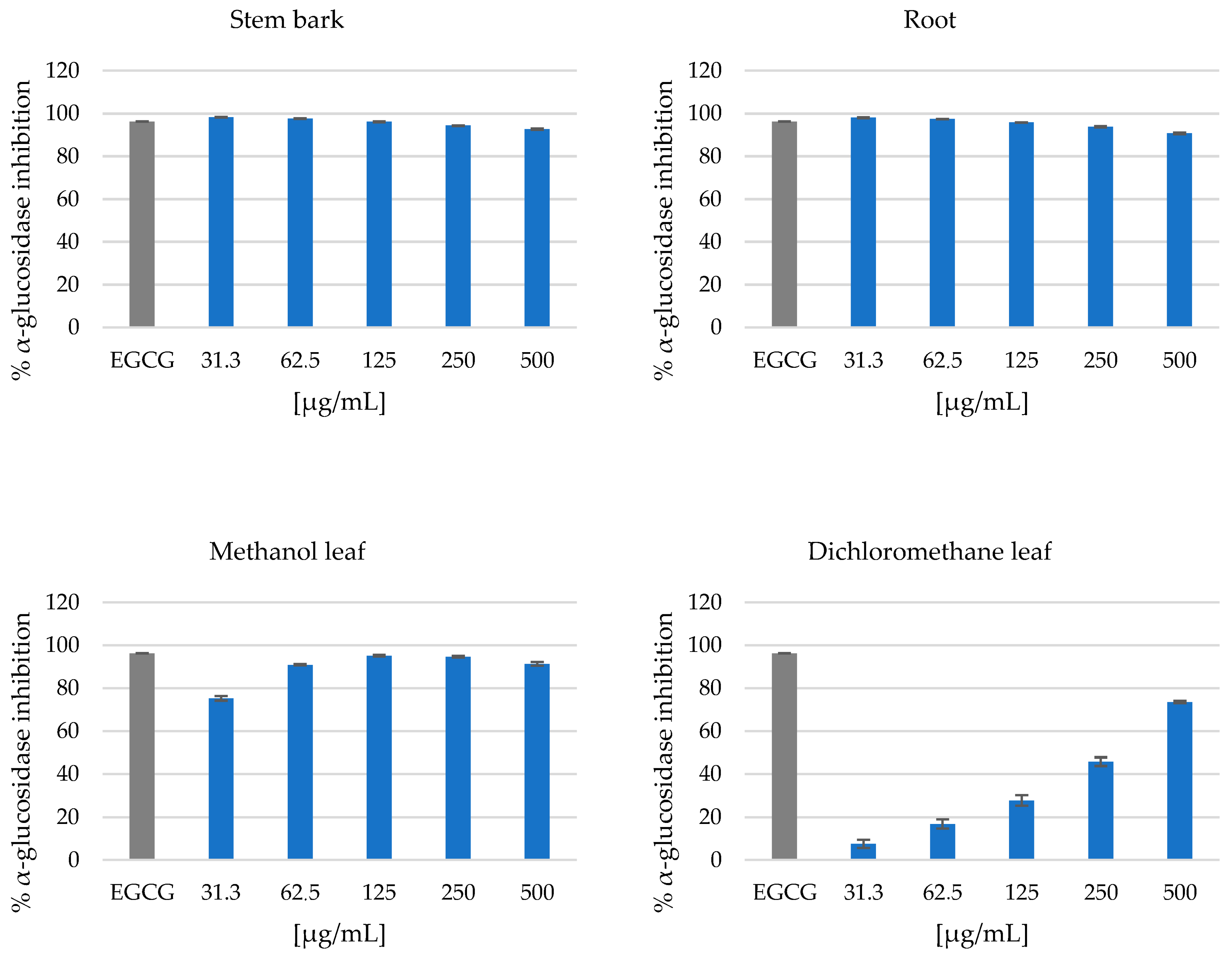
2.5.2. α-Glucosidase Inhibition Assay
2.6. Anti-Inflammatory Activity
2.7. Antiproliferation Activity
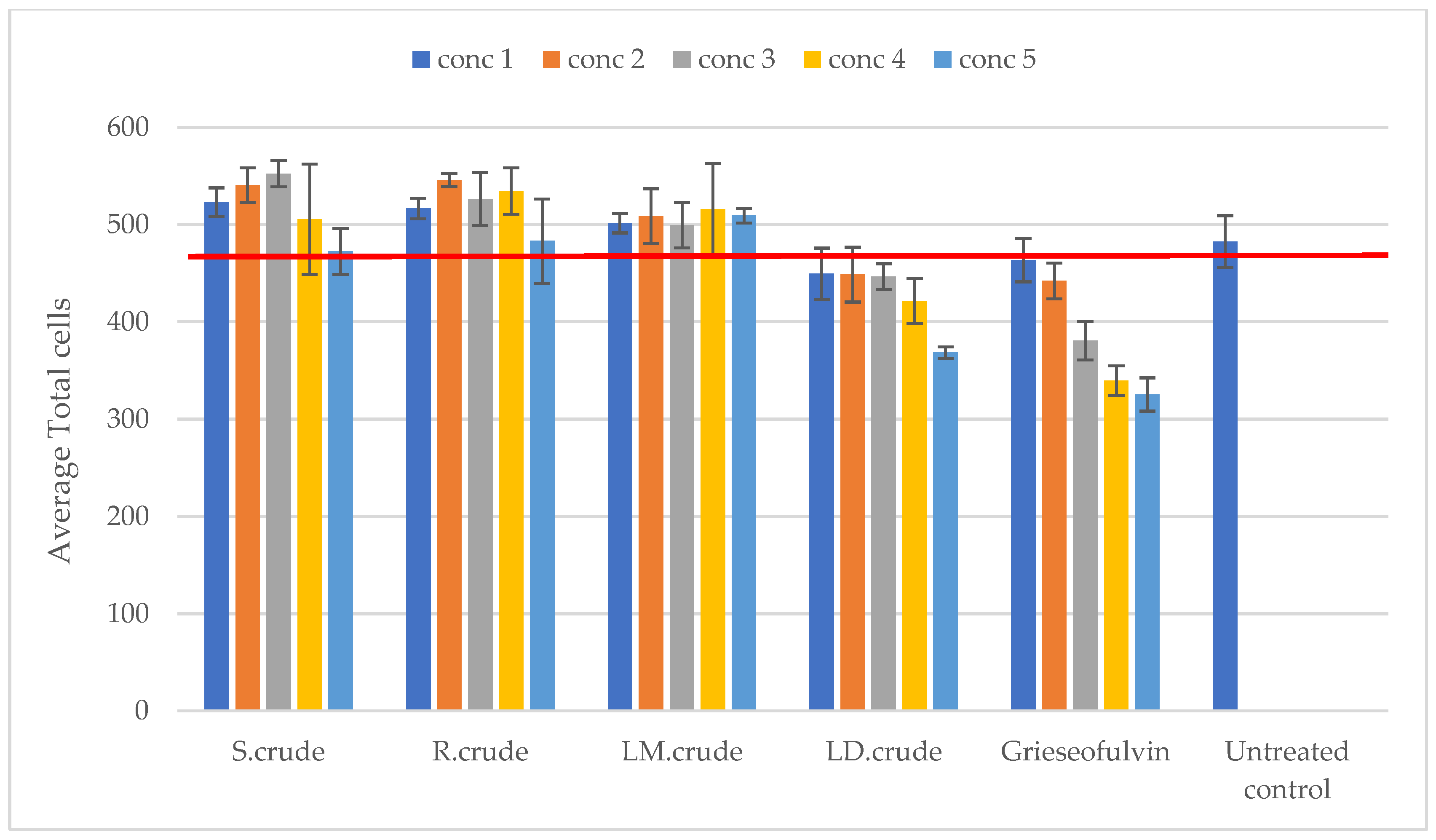
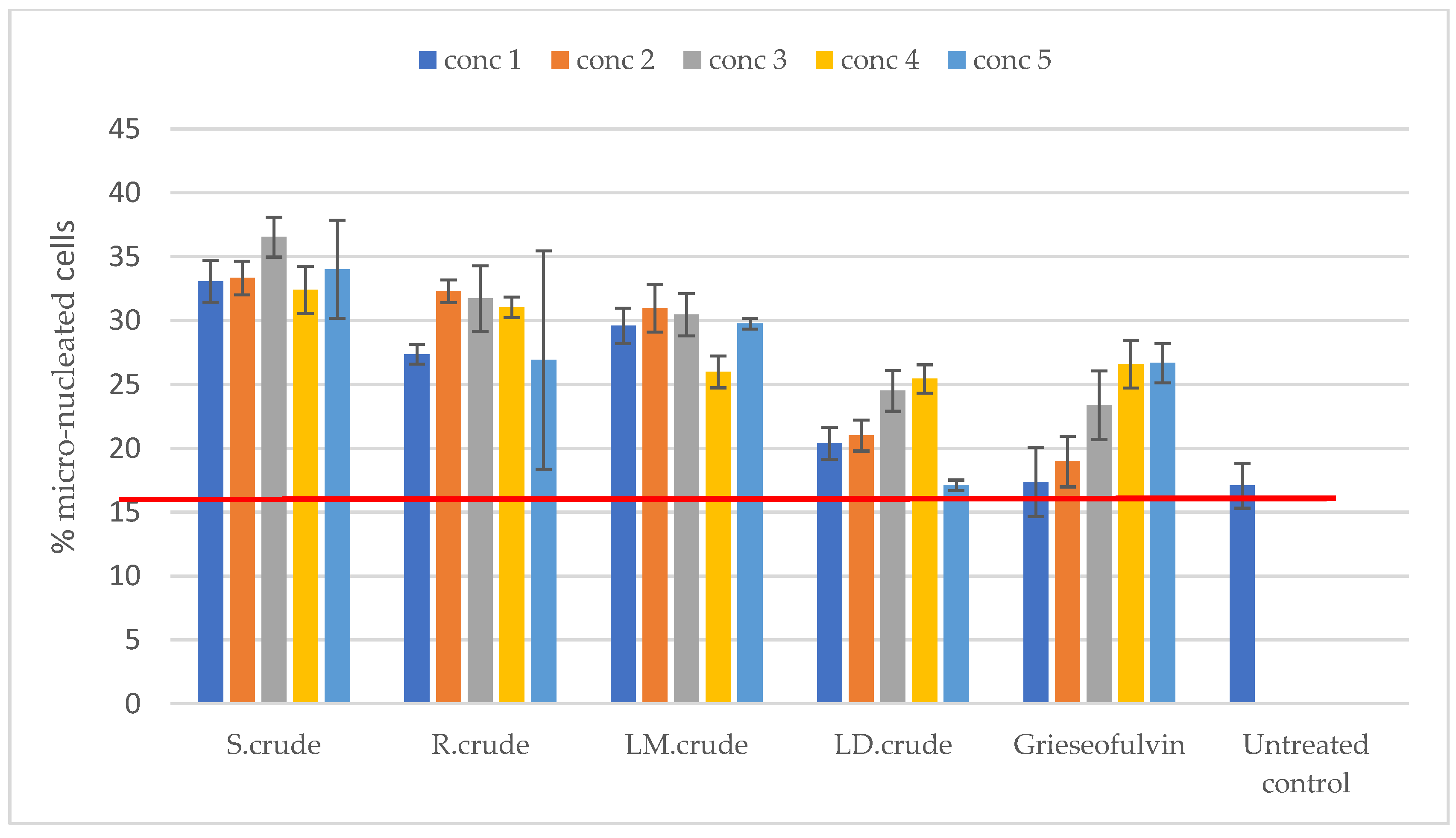
2.8. Genotoxicity
2.9. Cytotoxicity
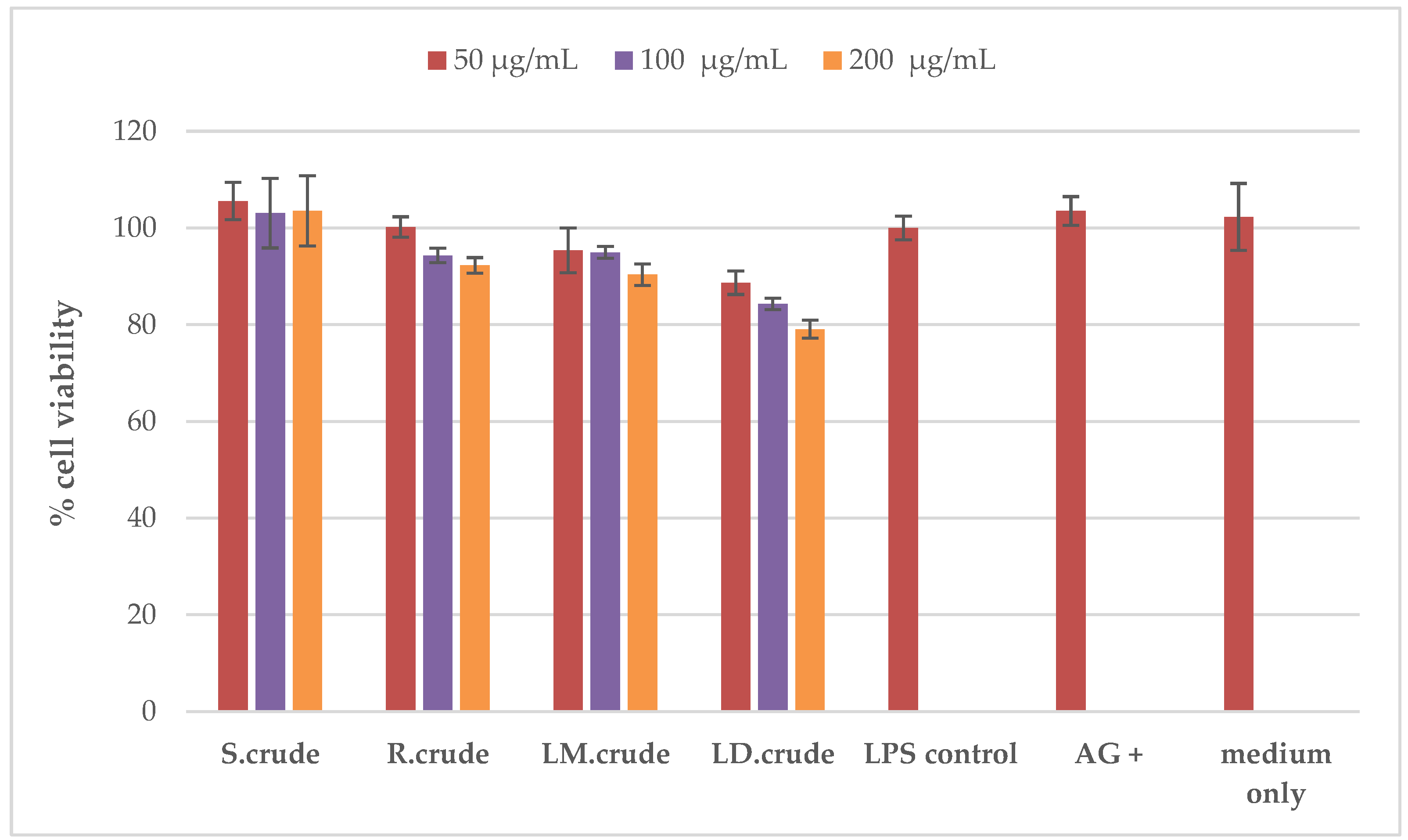
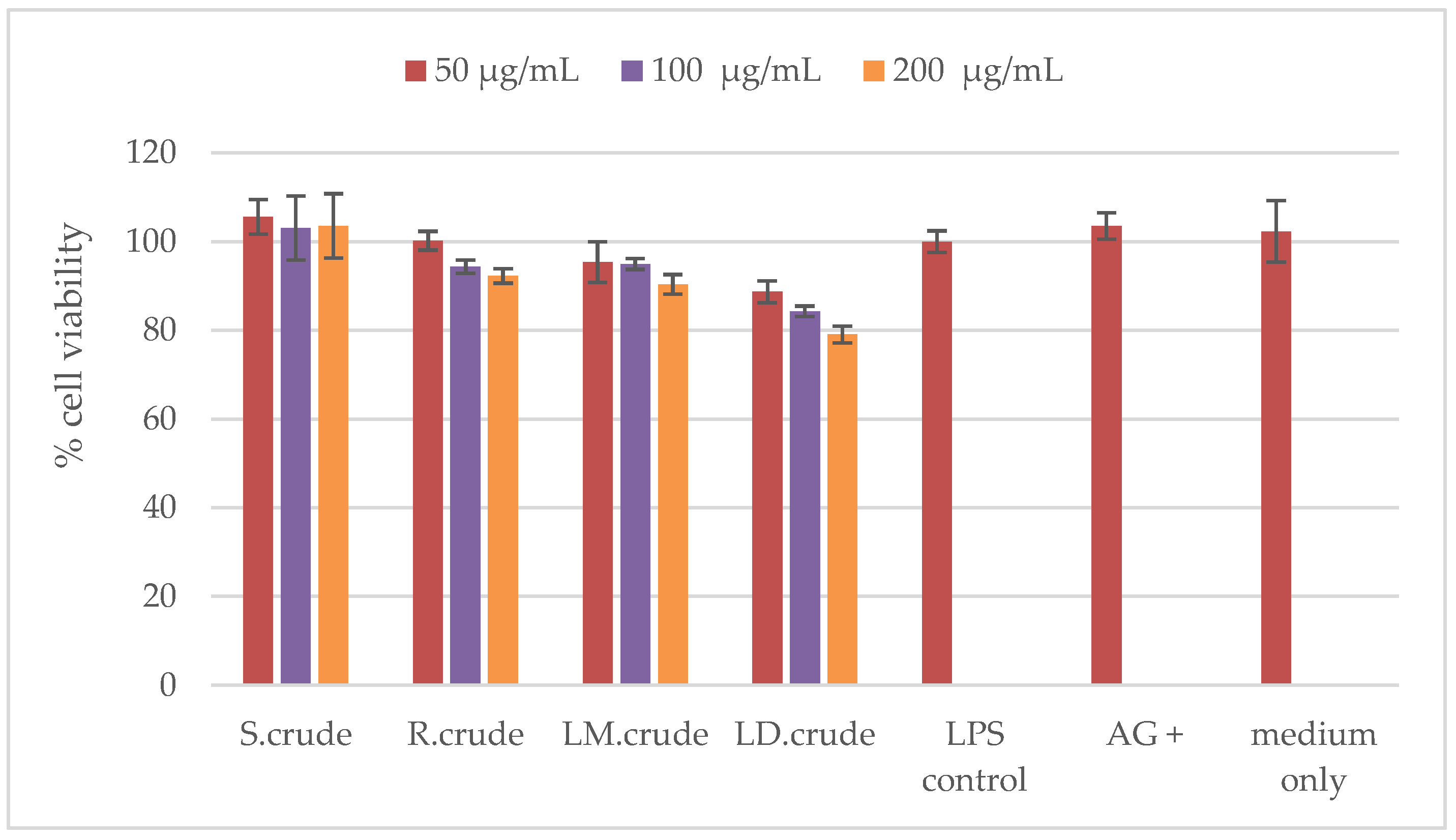
2.9.1. MTT Assay
2.9.2. Hoechst 33342/Propidium Iodide (PI) Dual Staining Method
2.10. Statistical Analysis
3. Results and Discussion
3.1. Molecular Networking of Breonadia Metabolites
3.2. Antidiabetic Activity
3.3. Anti-Inflammatory Activity
3.4. Antiproliferation Activity
3.5. Genotoxicity
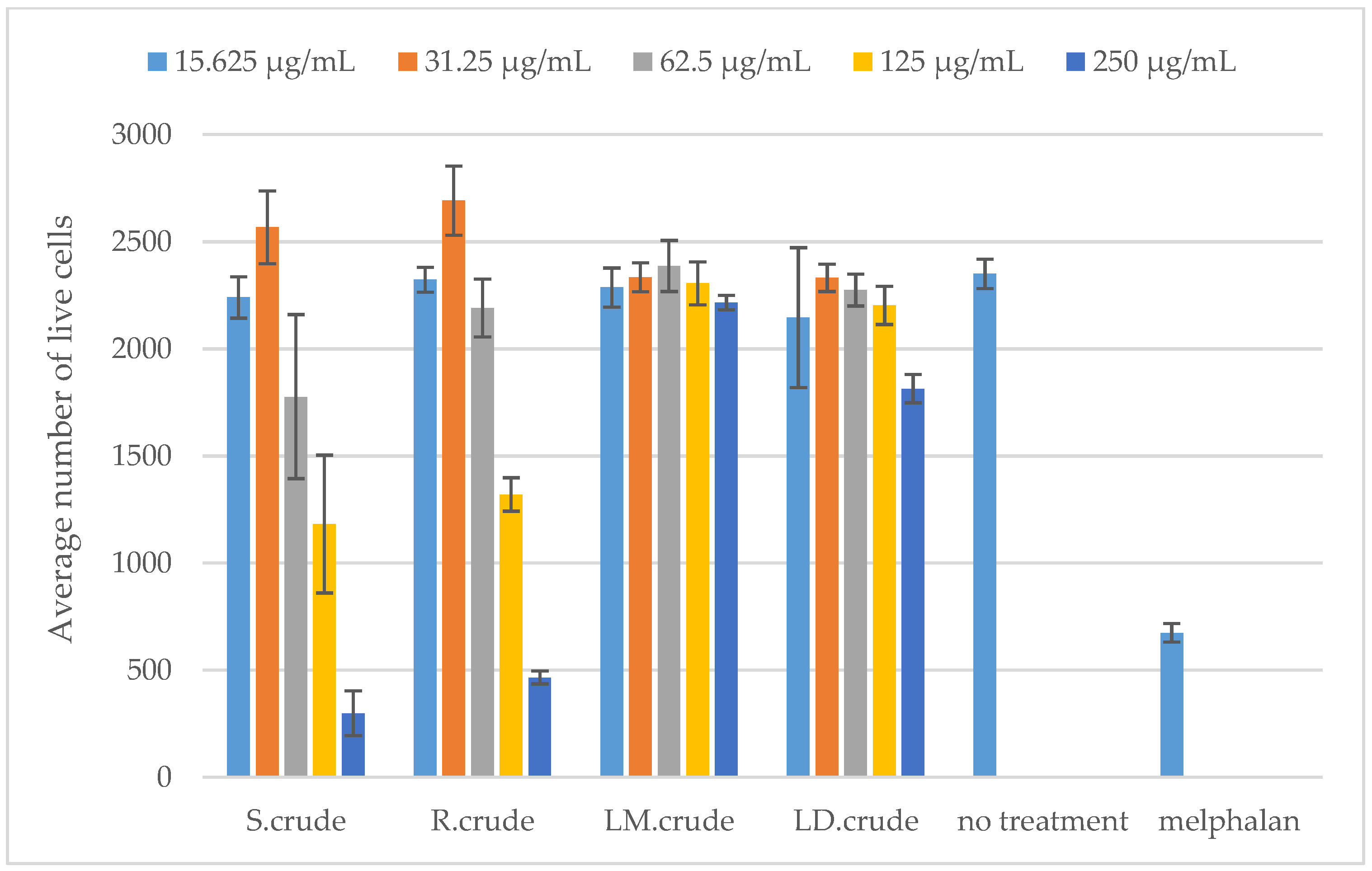
3.6. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaafar, A.R.Z.; Al-Qurainy, F.; Khan, S. Assessment of genetic diversity in the endangered populations of Breonadia salicina (Rubiaceae) growing in The Kingdom of Saudi Arabia using inter-simple sequence repeat markers. BMC Genet. 2014, 15, 109. [Google Scholar] [CrossRef]
- Ridsdale, C.E. A revision of the tribe Cephalantheae (Rubiaceae). Blumea Biodivers. Evol. Biogeogr. Plants 1976, 23, 177–188. [Google Scholar]
- Mahlo, S.M.; McGaw, L.J.; Eloff, J.N. Antifungal activity and cytotoxicity of isolated compounds from leaves of Breonadia salicina. J. Ethnopharmcol. 2013, 148, 909–913. [Google Scholar] [CrossRef]
- Sibandze, G.F.; van Zyl, R.L.; van Vuuren, S.F. The anti-diarrhoeal properties of Breonadia salicina, Syzygium cordatum and Ozoroa sphaerocarpa when used in combination in Swazi traditional medicine. J. Ethnopharm. 2010, 132, 506–511. [Google Scholar] [CrossRef]
- Ali, S.; Umar, A.Z.; Asmau, M.; Deepa, S.; Milli, J.; Fatima, H. In vitro antitrypanosomal activity of Breonadia salicina on Trypanosoma brucei brucei. Int. J. Pharma Sci. Res. 2018, 9, 103–107. [Google Scholar]
- Nvau, B.J.; Sami, B.; Ajibade, O.S.; Gray, I.A.; Igoli, J.O. Adicardin and Other Coumarins from Breonadia salicina (Vahl) Hepper. Trop. J. Nat. Prod. Res. 2019, 3, 298–301. [Google Scholar] [CrossRef]
- Al-Qurainy, F.; Gaafar, A.Z.; Khan, S.; Nadeem, M.; Tarroum, M.; Alaklabi, A.; Thomas, J. Antibacterial activity of leaf extract of Breonadia salicina (Rubiaceae), an endangered medicinal plant of Saudi Arabia. Gen. Mol. Res. 2013, 12, 3212–3219. [Google Scholar] [CrossRef]
- Ayo, S.G.; Habila, J.D.; Achika, J.I.; Akinwande, O.O. Isolation and characterization of 2,4-dihydroxycinnamic acid from the stem bark of Adina microcephala Delile. Chem. Soc. Nig. 1930, 47BCC70. Available online: http://www.proceeding.academicjournals.org/ (accessed on 1 May 2024).
- Tlhapi, B.D.; Ramaite, I.D.; Anokwuru, C.P. Metabolomic profiling and antioxidant activities of Breonadia salicina using 1H-NMR and UPLC-QTOF-MS analysis. Molecules 2021, 26, 6707. [Google Scholar] [CrossRef]
- Shiratsuchi, H.; Chang, S.; Wei, A.; El-Ghorab, A.H.; Shibamoto, T. Biological activities of low-molecular weight compounds found in foods and plants. J. Food Drug Anal. 2012, 20, 65. [Google Scholar] [CrossRef]
- Rosli, M.A.F.; Mediani, A.; Azizan, K.A.; Baharum, S.N.; Goh, H.H. UPLC-TOF-MS/MS-Based Metabolomics Analysis Reveals Species-Specific Metabolite Compositions in Pitchers of Nepenthes ampullaria, Nepenthes rafflesiana, and Their Hybrid Nepenthes × hookeriana. Front. Plant Sci. 2010, 2, 655004. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Suriyanarayanan, T.; Widyarman, A.S.; Lee, L.S.; Lau, M.; Ching, J.; Delaney, C.; Ramage, G. Multi-omics tools for studying microbial biofilms: Current perspectives and future directions. Crit. Rev. Microbiol. 2020, 46, 759–778. [Google Scholar] [CrossRef]
- Liang, Y.; Ke, X.; Xiao, Z.; Zhang, Y.; Chen, Y.; Li, Y.; Wang, Z.; Lin, L.; Yao, P.; Lu, J. Untargeted Metabolomic Profiling Using UHPLC-QTOF/MS Reveals Metabolic Alterations Associated with Autism. BioMed Res. Int. 2020, 2020, 6105608. [Google Scholar] [CrossRef]
- Martens, J.; Berden, G.; van Outersterp, R.E.; Kluijtmans, L.A.; Engelke, U.F.; van Karnebeek, C.D.; Wevers, R.A.; Oomens, J. Molecular identification in metabolomics using infrared ion spectroscopy. Sci. Rep. 2017, 7, 3363. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Ye, L.; Kang, Z.; Jia, D.; Yang, L.; Zhang, B. LC-MS-based metabolomic approach revealed the significantly different metabolic profiles of five commercial truffle species. Front. Microbiol. 2019, 10, 2227. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Chang, D.; Guo, F.; Pan, H.; Yang, Y. Metabolomics approach by 1H-NMR spectroscopy of serum reveals progression axes for asymptomatic hyperuricemia and gout. Arthritis Res. Ther. 2018, 20, 111. [Google Scholar] [CrossRef]
- Nalbantoglu, S. Metabolomics: Basic principles and strategies. Mol. Med. 2019, 10, 1–13. Available online: https://www.intechopen.com/chapters/68486 (accessed on 1 May 2024).
- Wang, Y.; Xu, L.; Shen, H.; Wang, J.; Liu, W.; Zhu, X.; Wang, R.; Sun, X.; Liu, L. Metabolomic analysis with GC-MS to reveal potential metabolites and biological pathways involved in Pb and Cd stress response of radish roots. Sci. Rep. 2015, 5, 18296. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by gas chromatography–mass spectrometry: Combined targeted and untargeted profiling. Cur. Prot. Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, T.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Lee, S.Y.; Abas, F.; Khatib, A.; Ismail, I.S.; Shaari, K.; Zawawi, N. Metabolite profiling of Neptunia oleracea and correlation with antioxidant and α-glucosidase inhibitory activities using 1H-NMR-based metabolomics. Phytochem. Lett. 2016, 16, 23–33. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Xu, Y.; Long, H.; Deng, Y.; Li, S.; Xi, Y.; Li, W.; Cai, H.; Zhang, B.; et al. Emerging LC-MS/MS-based molecular networking strategy facilitates foodomics to assess the function, safety, and quality of foods: Recent trends and future perspectives. Trends Food Sci. Technol. 2023, 139, 104114. [Google Scholar] [CrossRef]
- Clements, T.; Rautenbach, M.; Ndlovu, T.; Khan, S.; Khan, W.A. Metabolomics and molecular networking approach to elucidate the structures of secondary metabolites produced by Serratia marcescens strains. Front. Chem. 2021, 16, 633870. [Google Scholar] [CrossRef] [PubMed]
- Tlhapi, D.; Ramaite, I.D.I.; Anokwuru, C.; van Ree, T.; Madala, N.; Hoppe, H. Effects of seasonal variation on phytochemicals contributing to the antimalarial and antitrypanosomal activities of Breonadia salicina using a metabolomic approach. Heliyon 2024, 10, e24068. [Google Scholar] [CrossRef] [PubMed]
- Spohr, P.; Dinkla, K.; Klau, G.W.; El-Kebir, M. eXamine: Visualizing annotated networks in Cytoscape. F1000Research 2018, 7, 519. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Roskar, I.; Molek, P.; Vodnik, M.; Stempelj, M.; Strukelj, B.; Lunder, M. Peptide modulators of alpha-glucosidase. J. Diabetes Investig. 2015, 6, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.R.; Chaudet, M.M.; Jones, K. Structural studies of the intestinal α-glucosidases, maltase-glucoamylase and sucrase-isomaltase. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 11–13. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Dzoyem, J.P.; Shai, L.J.; Eloff, J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement. Altern. Med. 2015, 15, 159. [Google Scholar] [CrossRef]
- Stander, A.; Marais, S.; Stivaktas, V.; Vorster, V.; Albrecht, C.; Lottering, M.-L.; Joubert, A.M. In vitro effects of Sutherlandia frutescens water extracts on cell numbers, morphology, cell cycle progression and cell death in a tumorigenic and a non-tumorigenic epithelial breast cell line. J. Ethnopharm. 2009, 124, 45–60. [Google Scholar] [CrossRef]
- Gertsch, J. How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. J. Ethnopharm. 2009, 122, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.H.; Arlt, V.M. Genotoxicity: Damage to DNA and its consequences. In Molecular, Clinical and Environmental Toxicology; Birkhäuser Verlag: Basel, Switzerland, 2019; pp. 87–110. [Google Scholar] [CrossRef]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General cytotoxicity assessment by means of the MTT assay. In Protocols in In Vitro Hepatocyte Research; Humana Press: New York, NY, USA, 2015; pp. 333–348. [Google Scholar] [CrossRef]
- Carolina, L.E.M.A.; Varela-Ramirez, A.; Aguilera, R.J. Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr. Cell. Biochem. 2011, 1, 1–14. [Google Scholar]
- Przeor, M. Some common medicinal plants with antidiabetic activity, known and available in Europe (A Mini-Review). Pharma 2022, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.D.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as sources of anti-inflammatory agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef] [PubMed]
- Nkobole, N.; Houghton, P.J.; Hussein, A.; Lall, N. Antidiabetic activity of Terminalia sericea constituents. Nat. Prod. Chem. 2011, 6, 1934578X1100601106. [Google Scholar] [CrossRef]
- San, H.T.; Boonsnongchee, P.; Putalun, W.; Sritularak, B.; Likhitwitayawuid, K. Bergenin from Cissus javana DC.(Vitaceae) root extract enhances glucose uptake by rat L6 myotubes. Trop. J. Pharm. Res. 2020, 9, 1081–1086. [Google Scholar] [CrossRef]
- Astiti, M.A.; Jittmittraphap, A.; Leaungwutiwong, P.; Chutiwitoonchai, N.; Pripdeevech, P.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. LC-QTOF-MS/MS based molecular networking approach for the isolation of α-glucosidase inhibitors and virucidal agents from Coccinia grandis (L.) Voigt. Foods 2021, 10, 3041. [Google Scholar] [CrossRef]
- Sibandze, G.F. Pharmacological Properties of Swazi Medicinal Plants. Master’s Thesis, University of Witwatersrand, Johannesburg, South Africa, 2009. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tlhapi, D.; Ramaite, I.; Anokwuru, C.; van Ree, T. Molecular Networking-Based Metabolome, In Vitro Antidiabetic and Anti-Inflammatory Effects of Breonadia salicina (Vahl) Hepper & J.R.I. Wood. Metabolites 2024, 14, 291. https://doi.org/10.3390/metabo14060291
Tlhapi D, Ramaite I, Anokwuru C, van Ree T. Molecular Networking-Based Metabolome, In Vitro Antidiabetic and Anti-Inflammatory Effects of Breonadia salicina (Vahl) Hepper & J.R.I. Wood. Metabolites. 2024; 14(6):291. https://doi.org/10.3390/metabo14060291
Chicago/Turabian StyleTlhapi, Dorcas, Isaiah Ramaite, Chinedu Anokwuru, and Teunis van Ree. 2024. "Molecular Networking-Based Metabolome, In Vitro Antidiabetic and Anti-Inflammatory Effects of Breonadia salicina (Vahl) Hepper & J.R.I. Wood" Metabolites 14, no. 6: 291. https://doi.org/10.3390/metabo14060291
APA StyleTlhapi, D., Ramaite, I., Anokwuru, C., & van Ree, T. (2024). Molecular Networking-Based Metabolome, In Vitro Antidiabetic and Anti-Inflammatory Effects of Breonadia salicina (Vahl) Hepper & J.R.I. Wood. Metabolites, 14(6), 291. https://doi.org/10.3390/metabo14060291






