Metabolic Traits and Risk of Ischemic Stroke in Japanese and European Populations: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
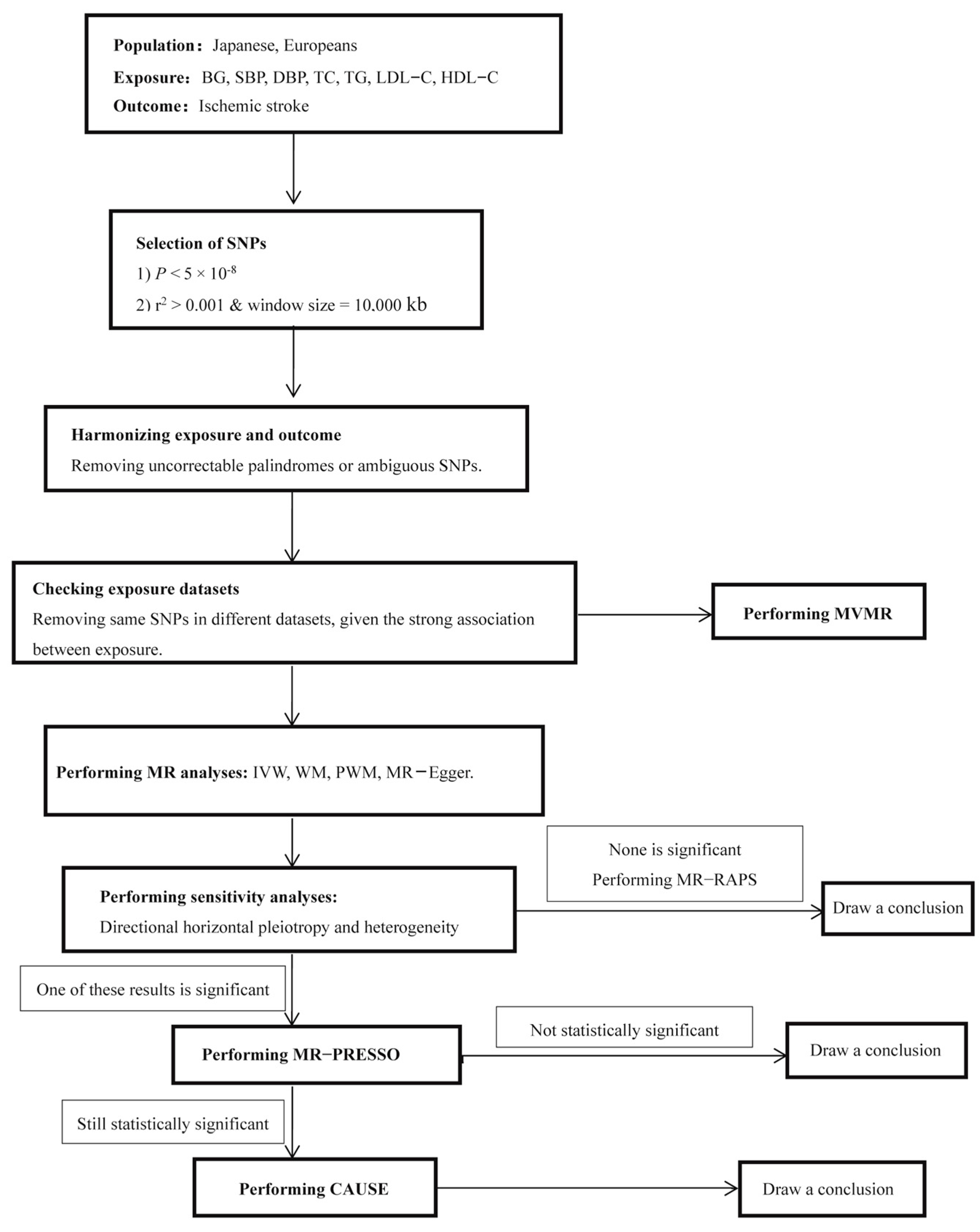
2. Materials and Methods
2.1. Data Source
2.1.1. Japanese Population
2.1.2. European Population
2.2. SNP Selection
2.3. MR Analyses
3. Results
3.1. Results Description
3.1.1. Japanese Population
3.1.2. European Population
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nguyen, G.; Cercy, K.; Johnson, C.O.; Alam, T.; Parmar, P.G.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abejie, A.N.; et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar]
- Diener, H.-C.; Hankey, G.J. Primary and Secondary Prevention of Ischemic Stroke and Cerebral Hemorrhage: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1804–1818. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Filipp, S.L.; Sims, M.; Musani, S.K.; Gurka, M.J. Risk of Ischemic Stroke Increases Over the Spectrum of Metabolic Syndrome Severity. Stroke 2020, 51, 2548–2552. [Google Scholar] [CrossRef]
- Horn, J.W.; Feng, T.; Mørkedal, B.; Strand, L.B.; Horn, J.; Mukamal, K.; Janszky, I. Obesity and Risk for First Ischemic Stroke Depends on Metabolic Syndrome: The HUNT Study. Stroke 2021, 52, 3555–3561. [Google Scholar] [CrossRef]
- Decker, J.J.; Norby, F.L.; Rooney, M.R.; Soliman, E.Z.; Lutsey, P.L.; Pankow, J.S.; Alonso, A.; Chen, L.Y. Metabolic Syndrome and Risk of Ischemic Stroke in Atrial Fibrillation: ARIC Study. Stroke 2019, 50, 3045–3050. [Google Scholar] [CrossRef]
- Fatumo, S.; Karhunen, V.; Chikowore, T.; Sounkou, T.; Udosen, B.; Ezenwa, C.; Nakabuye, M.; Soremekun, O.; Daghlas, I.; Ryan, D.K.; et al. Metabolic Traits and Stroke Risk in Individuals of African Ancestry: Mendelian Randomization Analysis. Stroke 2021, 52, 2680–2684. [Google Scholar] [CrossRef]
- He, Q.; Wang, W.; Li, H.; Xiong, Y.; Tao, C.; Ma, L.; You, C. Genetic insights into the risk of metabolic syndrome and its components on stroke and its subtypes: Bidirectional Mendelian randomization. J. Cereb. Blood Flow Metab. 2023, 43, 126–137. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Boehm, F.J.; Zhou, X. Statistical methods for Mendelian randomization in genome-wide association studies: A review. Comput. Struct. Biotechnol. J. 2022, 20, 2338–2351. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Cinelli, C.; LaPierre, N.; Hill, B.L.; Sankararaman, S.; Eskin, E. Robust Mendelian randomization in the presence of residual population stratification, batch effects and horizontal pleiotropy. Nat. Commun. 2022, 13, 1093. [Google Scholar] [CrossRef]
- Morrison, J.; Knoblauch, N.; Marcus, J.H.; Stephens, M.; He, X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat. Genet. 2020, 52, 740–747. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Zhu, L.; Jiao, S.; Chen, Y.; Sun, Y. Metabolic disorders and risk of cardiovascular diseases: A two-sample mendelian randomization study. BMC Cardiovasc. Disord 2023, 23, 529. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Gill, D.; Webb, A.J.S.; Evangelou, E.; Elliott, P.; Sudlow, C.L.M.; Dehghan, A.; Malik, R.; Tzoulaki, I.; Dichgans, M. Genetically determined blood pressure, antihypertensive drug classes, and risk of stroke subtypes. Neurology 2020, 95, e353–e361. [Google Scholar] [CrossRef]
- Hindy, G.; Engström, G.; Larsson, S.C.; Traylor, M.; Markus, H.S.; Melander, O.; Orho-Melander, M. Role of Blood Lipids in the Development of Ischemic Stroke and its Subtypes: A Mendelian Randomization Study. Stroke 2018, 49, 820–827. [Google Scholar] [CrossRef]
- Yuan, S.; Tang, B.; Zheng, J.; Larsson, S.C. Circulating Lipoprotein Lipids, Apolipoproteins and Ischemic Stroke. Ann. Neurol. 2020, 88, 1229–1236. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, W.J.; Khera, A.V.; Kim, J.Y.; Yon, D.K.; Lee, S.W.; Shin, J.I.; Won, H.-H. Association between adiposity and cardiovascular outcomes: An umbrella review and meta-analysis of observational and Mendelian randomization studies. Eur. Heart J. 2021, 42, 3388–3403. [Google Scholar] [CrossRef]
- Marini, S.; Merino, J.; Montgomery, B.E.; Malik, R.; Sudlow, C.L.; Dichgans, M.; Florez, J.C.; Rosand, J.; Gill, D.; Anderson, C.D. Mendelian Randomization Study of Obesity and Cerebrovascular Disease. Ann. Neurol. 2020, 87, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Tewara, M.A.; Li, Y.; Li, W.; Chen, X.; Yuan, T.; Liu, C.; Li, J.; Wang, B.; Li, H.; et al. Causal Pathways from Body Components and Regional Fat to Extensive Metabolic Phenotypes: A Mendelian Randomization Study. Obesity 2020, 28, 1536–1549. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hou, L.; Shi, X.; Sun, X.; Liu, X.; Yu, Y.; Yuan, Z.; Li, H.; Xue, F. Impact of nonrandom selection mechanisms on the causal effect estimation for two-sample Mendelian randomization methods. PLoS Genet. 2022, 18, e1010107. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.Y.F.; Fung, W.T.; Schooling, C.M.; Au Yeung, S.L.; Kwok, M.K.; Yu, E.Y.T.; Wang, Y.; Chan, E.W.Y.; Wong, I.C.K.; Lam, C.L.K. Blood Pressure and Risk of Cardiovascular Disease in UK Biobank: A Mendelian Randomization Study. Hypertension 2021, 77, 367–375. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Harshfield, E.L.; Malik, R.; Franceschini, N.; Langenberg, C.; Wareham, N.J.; Markus, H.S.; Dichgans, M. Diabetes Mellitus, Glycemic Traits, and Cerebrovascular Disease: A Mendelian Randomization Study. Neurology 2021, 96, e1732–e1742. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, K.; Akiyama, M.; Kanai, M.; Takahashi, A.; Kawakami, E.; Sugishita, H.; Sakaue, S.; Matoba, N.; Low, S.-K.; Okada, Y.; et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat. Genet. 2020, 52, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Akiyama, M.; Takahashi, A.; Matoba, N.; Momozawa, Y.; Ikeda, M.; Iwata, N.; Ikegawa, S.; Hirata, M.; Matsuda, K.; et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 2018, 50, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.-K.; van der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018, 50, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Lagou, V.; Mägi, R.; Hottenga, J.-J.; Grallert, H.; Perry, J.R.B.; Bouatia-Naji, N.; Marullo, L.; Rybin, D.; Jansen, R.; Min, J.L.; et al. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat. Commun. 2021, 12, 24. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar]
- Tin, A.; Köttgen, A. Mendelian Randomization Analysis as a Tool to Gain Insights into Causes of Diseases: A Primer. J. Am. Soc. Nephrol. JASN 2021, 32, 2400–2407. [Google Scholar] [CrossRef]
- Brown, B.C.; Knowles, D.A. Welch-weighted Egger regression reduces false positives due to correlated pleiotropy in Mendelian randomization. Am. J. Hum. Genet. 2021, 108, 2319–2335. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, Y.; Wang, J.; Small, D.S. Powerful three-sample genome-wide design and robust statistical inference in summary-data Mendelian randomization. Int. J. Epidemiol. 2019, 48, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; He, L.; Wang, H.; Rong, X.; Chen, M.; Shen, Q.; Li, X.; Li, M.; Peng, Y. Genetic liability for prescription opioid use and risk of cardiovascular diseases: A multivariable Mendelian randomization study. Addiction 2022, 117, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.-J.A.; Shakhbazov, K.; Visscher, P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013, 42, 1497–1501. [Google Scholar] [CrossRef]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef]
- Yang, P.; Song, L.; Zhang, Y.; Zhang, X.; Chen, X.; Li, Y.; Sun, L.; Wan, Y.; Billot, L.; Li, Q.; et al. Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): A multicentre, open-label, blinded-endpoint, randomised controlled trial. Lancet 2022, 400, 1585–1596. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Heshmatollah, A.; Ma, Y.; Fani, L.; Koudstaal, P.J.; Ikram, M.A.; Ikram, M.K. Visit-to-visit blood pressure variability and the risk of stroke in the Netherlands: A population-based cohort study. PLoS Med. 2022, 19, e1003942. [Google Scholar] [CrossRef]
- Flint, A.C.; Conell, C.; Ren, X.; Banki, N.M.; Chan, S.L.; Rao, V.A.; Melles, R.B.; Bhatt, D.L. Effect of Systolic and Diastolic Blood Pressure on Cardiovascular Outcomes. N. Engl. J. Med. 2019, 381, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Psaty, B.M.; Lumley, T.; Furberg, C.D.; Schellenbaum, G.; Pahor, M.; Alderman, M.H.; Weiss, N.S. Health outcomes associated with various antihypertensive therapies used as first-line agents: A network meta-analysis. JAMA 2003, 289, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiao, Y.; Zhang, H.; Zhang, Y.; Hua, J.; Jin, S.; Liu, G. Circulating Vitamin D Levels and Alzheimer’s Disease: A Mendelian Randomization Study in the IGAP and UK Biobank. J. Alzheimer’s Dis. JAD 2020, 73, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.D.; Tyrrell, J.; Borges, M.-C.; Beaumont, R.N.; Knight, B.A.; Wood, A.R.; Ring, S.M.; Hattersley, A.T.; Freathy, R.M.; Lawlor, D.A. Association of maternal circulating 25(OH)D and calcium with birth weight: A mendelian randomisation analysis. PLoS Med. 2019, 16, e1002828. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Bowden, J.; Davey Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 2018, 27, R195–R208. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Millwood, I.Y.; Kartsonaki, C.; Hill, M.R.; Bennett, D.A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R.; et al. Lipids, Lipoproteins, and Metabolites and Risk of Myocardial Infarction and Stroke. J. Am. Coll. Cardiol. 2018, 71, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Pikula, A.; Beiser, A.S.; Wang, J.; Himali, J.J.; Kelly-Hayes, M.; Kase, C.S.; Yang, Q.; Seshadri, S.; Wolf, P.A. Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham Study. Neurology 2015, 84, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, Z.; Fang, W.; Wang, X.; Cai, Z.; Chen, G.; Wu, W.; Chen, Z.; Wu, S.; Chen, Y. Remnant Cholesterol Variability and Incident Ischemic Stroke in the General Population. Stroke 2022, 53, 1934–1941. [Google Scholar] [CrossRef]
- Sun, L.; Clarke, R.; Bennett, D.; Guo, Y.; Walters, R.G.; Hill, M.; Parish, S.; Millwood, I.Y.; Bian, Z.; Chen, Y.; et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat. Med. 2019, 25, 569–574. [Google Scholar] [CrossRef]
- Boden-Albala, B.; Cammack, S.; Chong, J.; Wang, C.; Wright, C.; Rundek, T.; Elkind, M.S.V.; Paik, M.C.; Sacco, R.L. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: Findings from the Northern Manhattan Study (NOMAS). Diabetes Care 2008, 31, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.A.; Reaven, P.D.; Wiitala, W.L.; Bahn, G.D.; Reda, D.J.; Ge, L.; McCarren, M.; Duckworth, W.C.; Emanuele, N.V. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 372, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; IsmaiL-Beigi, F.; Grimm, R.H.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [PubMed]
- Johnston, K.C.; Bruno, A.; Pauls, Q.; Hall, C.E.; Barrett, K.M.; Barsan, W.; Fansler, A.; Van de Bruinhorst, K.; Janis, S.; Durkalski-Mauldin, V.L. Intensive vs Standard Treatment of Hyperglycemia and Functional Outcome in Patients With Acute Ischemic Stroke: The SHINE Randomized Clinical Trial. JAMA 2019, 322, 326–335. [Google Scholar] [CrossRef]
- Bains, N.K.; Huang, W.; French, B.R.; Siddiq, F.; Gomez, C.R.; Qureshi, A.I. Hyperglycemic control in acute ischemic stroke patients undergoing endovascular treatment: Post hoc analysis of the Stroke Hyperglycemia Insulin Network Effort trial. J. Neurointerv. Surg. 2023, 15, 370–374. [Google Scholar] [CrossRef]
| Exposure | SNP (N) | OR (95% CI) | Beta (SE) | p |
|---|---|---|---|---|
| BG | 11 | 1.036 (0.848–1.265) | 0.035 (0.102) | 0.731 |
| SBP | 12 | 1.870 (1.122–3.116) | 0.626 (0.261) | 0.016 * |
| DBP | 7 | 1.966 (0.829–4.663) | 0.676 (0.441) | 0.125 |
| TC | 30 | 1.044 (0.914–1.193) | 0.043 (0.068) | 0.523 |
| TG | 18 | 0.992 (0.915–1.074) | 0.008 (0.041) | 0.840 |
| LDL–C | 18 | 1.038 (0.907–1.189) | 0.038 (0.069) | 0.585 |
| HDL–C | 37 | 0.961 (0.893–1.034) | 0.040 (0.038) | 0.285 |
| Exposure | Method | SNP (N) | OR (95% CI) | Beta (SE) | p |
|---|---|---|---|---|---|
| BG | WM | 11 | 1.029 (0.815–1.300) | 0.029 (0.117) | 0.806 |
| PWM | 11 | 1.026 (0.815–1.293) | 0.026 (0.118) | 0.825 | |
| MR–Egger | 11 | 0.667 (0.252–1.763) | −0.404 (0.496) | 0.436 | |
| SBP | WM | 12 | 1.871 (1.234–2.836) | 0.626 (0.212) | 3.187 × 10−3 * |
| PWM | 12 | 2.446 (1.575–3.799) | 0.894 (0.225) | 6.854 × 10−5 * | |
| MR–Egger | 12 | 0.732 (0.050–10.661) | −0.311 (1.366) | 0.824 | |
| DBP | WM | 7 | 2.182 (1.139–4.178) | 0.780 (0.332) | 0.019 * |
| PWM | 7 | 3.597 (1.832–7.062) | 1.280 (0.344) | 2.000 × 10−4 * | |
| MR–Egger | 7 | 12.610 (0.506–313.959) | 2.534 (1.640) | 0.183 | |
| TC | WM | 30 | 1.006 (0.835–1.213) | 0.006 (0.095) | 0.947 |
| PWM | 30 | 1.005 (0.832–1.214) | 0.005 (0.096) | 0.961 | |
| MR–Egger | 30 | 1.109 (0.763–1.611) | 0.104 (0.191) | 0.591 | |
| TG | WM | 18 | 0.969 (0.878–1.070) | −0.031 (0.050) | 0.534 |
| PWM | 18 | 0.969 (0.877–1.071) | −0.031 (0.051) | 0.542 | |
| MR–Egger | 18 | 0.934 (0.828–1.049) | −0.070 (0.060) | 0.263 | |
| LDL−C | WM | 18 | 1.019 (0.858–1.210) | 0.0189 (0.088) | 0.829 |
| PWM | 18 | 1.019 (0.848–1.225) | 0.0189 (0.094) | 0.840 | |
| MR–Egger | 18 | 1.036 (0.730–1.471) | 0.035 (0.179) | 0.846 | |
| HDL−C | WM | 37 | 0.973 (0.882–1.074) | −0.027 (0.050) | 0.586 |
| PWM | 37 | 0.976 (0.883–1.077) | −0.024 (0.051) | 0.625 | |
| MR–Egger | 37 | 1.026 (0.895–1.175) | 0.026 (0.069) | 0.715 |
| Exposure | MR–Analysis | SNP (N) | OR (95% CI) | Beta (SE) | Pa | Pb | Pc |
|---|---|---|---|---|---|---|---|
| SBP | Outlier-corrected | 10 | 2.168 (1.470–3.198) | 0.774 (0.198) | <0.001 * | 0.004 * | 0.503 |
| DBP | Outlier-corrected | 4 | 1.963 (0.771–4.994) | 0.674 (0.477) | <0.001 * | 0.230 | 0.956 |
| Exposure | SNP (N) | Model 1 | Model 2 | ∆ELPD | SE of ∆ELPD | Z | p |
|---|---|---|---|---|---|---|---|
| SBP | 1076 | Null | Sharing | −5.854 | 2.772 | −2.111 | 0.017 * |
| 1076 | Null | Causal | −8.805 | 4.311 | −2.042 | 0.021 * | |
| 1076 | Sharing | Causal | −2.950 | 1.708 | −1.726 | 0.042 * | |
| DBP | 977 | Null | Sharing | −8.479 | 3.631 | −2.335 | 0.001 * |
| 977 | Null | Causal | −10.830 | 4.894 | −2.213 | 0.013 * | |
| 977 | Sharing | Causal | −2.351 | 1.605 | −1.465 | 0.071 |
| Exposure | SNP (N) | OR (95% CI) | Beta (SE) | p |
|---|---|---|---|---|
| BG | 38 | 1.077 (0.957–1.213) | 0.075 (0.060) | 0.217 |
| SBP | 324 | 1.032 (1.026–1.038) | 0.032 (0.003) | 1.748 × 10−27 * |
| DBP | 319 | 1.044 (1.033–1.054) | 0.043 (0.005) | 2.623 × 10−17 * |
| TC | 34 | 1.045 (0.917–1.192) | 0.045 (0.067) | 0.506 |
| TG | 45 | 1.015 (0.943–1.093) | 0.015 (0.038) | 0.687 |
| LDL–C | 33 | 1.070 (0.937–1.222) | 0.068 (0.069) | 0.319 |
| HDL–C | 68 | 0.880 (0.798–0.971) | −0.127 (0.050) | 0.011 * |
| Exposure | Method | SNP (N) | OR (95% CI) | Beta (SE) | p |
|---|---|---|---|---|---|
| BG | WM | 38 | 0.966 (0.866–1.077) | −0.035 (0.055) | 0.533 |
| PWM | 38 | 0.966 (0.863–1.081) | −0.035 (0.058) | 0.548 | |
| MR–Egger | 38 | 0.925 (0.801–1.068) | −0.078 (0.073) | 0.295 | |
| SBP | WM | 324 | 1.033 (1.025–1.040) | 0.032 (0.004) | 1.141 × 10−17 * |
| PWM | 324 | 1.033 (1.025–1.041) | 0.033 (0.004) | 9.354 × 10−16 * | |
| MR–Egger | 324 | 1.043 (1.026–1.060) | 0.042 (0.008) | 8.903 × 10−7 * | |
| DBP | WM | 319 | 1.046 (1.033–1.060) | 0.045 (0.007) | 1.331 × 10−11 * |
| PWM | 319 | 1.046 (1.032–1.061) | 0.045 (0.007) | 8.600 × 10−11 * | |
| MR–Egger | 319 | 1.062 (1.034–1.091) | 0.060 (0.014) | 1.837 × 10−5 * | |
| TC | WM | 34 | 1.215 (1.025–1.441) | 0.195 (0.087) | 0.025 * |
| PWM | 34 | 1.220 (1.017–1.463) | 0.199 (0.093) | 0.032 * | |
| MR–Egger | 34 | 1.102 (0.799–1.520) | 0.097 (0.164) | 0.559 | |
| TG | WM | 45 | 1.008 (0.914–1.111) | 0.007 (0.050) | 0.880 |
| PWM | 45 | 1.009 (0.916–1.110) | 0.009 (0.049) | 0.861 | |
| MR–Egger | 45 | 0.951 (0.844–1.071) | −0.051 (0.061) | 0.411 | |
| LDL−C | WM | 33 | 0.980 (0.837–1.146) | −0.021 (0.080) | 0.796 |
| PWM | 33 | 0.980 (0.830–1.156) | −0.021 (0.084) | 0.804 | |
| MR–Egger | 33 | 1.094 (0.822–1.456) | 0.090 (0.146) | 0.543 | |
| HDL−C | WM | 68 | 0.893 (0.794–1.004) | −0.113 (0.060) | 0.059 |
| PWM | 68 | 0.890 (0.789–1.003) | −0.116 (0.061) | 0.058 | |
| MR–Egger | 68 | 1.145 (0.916–1.430) | 0.135 (0.113) | 0.238 |
| Exposure | MR–Analysis | SNP (N) | OR (95% CI) | Beta (SE) | Pa | Pb | Pc |
|---|---|---|---|---|---|---|---|
| SBP | Outlier-corrected | 318 | 1.033 (1.026–1.039) | 0.032 (0.003) | <0.001 * | 2.558 × 10−26 * | 0.977 |
| DBP | Outlier-corrected | 314 | 1.044 (1.034–1.054) | 0.043 (0.005) | <0.001 * | 2.167 × 10−17 * | 0.986 |
| HDL–C | Outlier-corrected | 67 | 0.899 (0.833–0.971) | −0.106 (0.039) | 0.001 * | 0.008 | 0.517 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lu, H.; Cao, M.; Zhang, J.; Liu, D.; Meng, X.; Zheng, D.; Wu, L.; Liu, X.; Wang, Y. Metabolic Traits and Risk of Ischemic Stroke in Japanese and European Populations: A Two-Sample Mendelian Randomization Study. Metabolites 2024, 14, 255. https://doi.org/10.3390/metabo14050255
Zhang J, Lu H, Cao M, Zhang J, Liu D, Meng X, Zheng D, Wu L, Liu X, Wang Y. Metabolic Traits and Risk of Ischemic Stroke in Japanese and European Populations: A Two-Sample Mendelian Randomization Study. Metabolites. 2024; 14(5):255. https://doi.org/10.3390/metabo14050255
Chicago/Turabian StyleZhang, Jinxia, Huimin Lu, Mingyang Cao, Jie Zhang, Di Liu, Xiaoni Meng, Deqiang Zheng, Lijuan Wu, Xiangdong Liu, and Youxin Wang. 2024. "Metabolic Traits and Risk of Ischemic Stroke in Japanese and European Populations: A Two-Sample Mendelian Randomization Study" Metabolites 14, no. 5: 255. https://doi.org/10.3390/metabo14050255
APA StyleZhang, J., Lu, H., Cao, M., Zhang, J., Liu, D., Meng, X., Zheng, D., Wu, L., Liu, X., & Wang, Y. (2024). Metabolic Traits and Risk of Ischemic Stroke in Japanese and European Populations: A Two-Sample Mendelian Randomization Study. Metabolites, 14(5), 255. https://doi.org/10.3390/metabo14050255







