Comparison of the Effect of Two Different Handling Conditions at Slaughter in Saliva Analytes in Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedure
- (A)
- Group A (improved handling group, 12 males, 12 females). The animals were loaded into the truck and transported for 15 km to the slaughterhouse in groups of 10 animals (1.25 m2 per animal), unloaded on arrival at the slaughterhouse and placed in a lairage area with free access to water. In order to minimize stress, those animals were the last to be loaded onto the truck, the first to be unloaded once at the slaughterhouse, and they were not mixed with unfamiliar ones in order to avoid the establishment of new hierarchical relationships with 1.25 m2 per animal.
- (B)
- Group B (stressful handling group, 12 males, 12 females). Animals from this group were loaded into the same truck and transported for the same length, but they were mixed with other animals at 0.55 m2 per animal. In order to increase stress level, they were the first animals to be loaded onto the truck and the last to be unloaded from it, thus the processing time was increased. After arrival at the slaughterhouse, the animals were mixed with unfamiliar animals using higher density (0.38 m2 per animal) with free access to water.
2.2. Sampling Procedure
2.3. Salivary Biomarkers Measurement
2.3.1. Stress Biomarkers
2.3.2. Immune System and Muscle Biomarkers
2.4. Statistical Analysis
3. Results
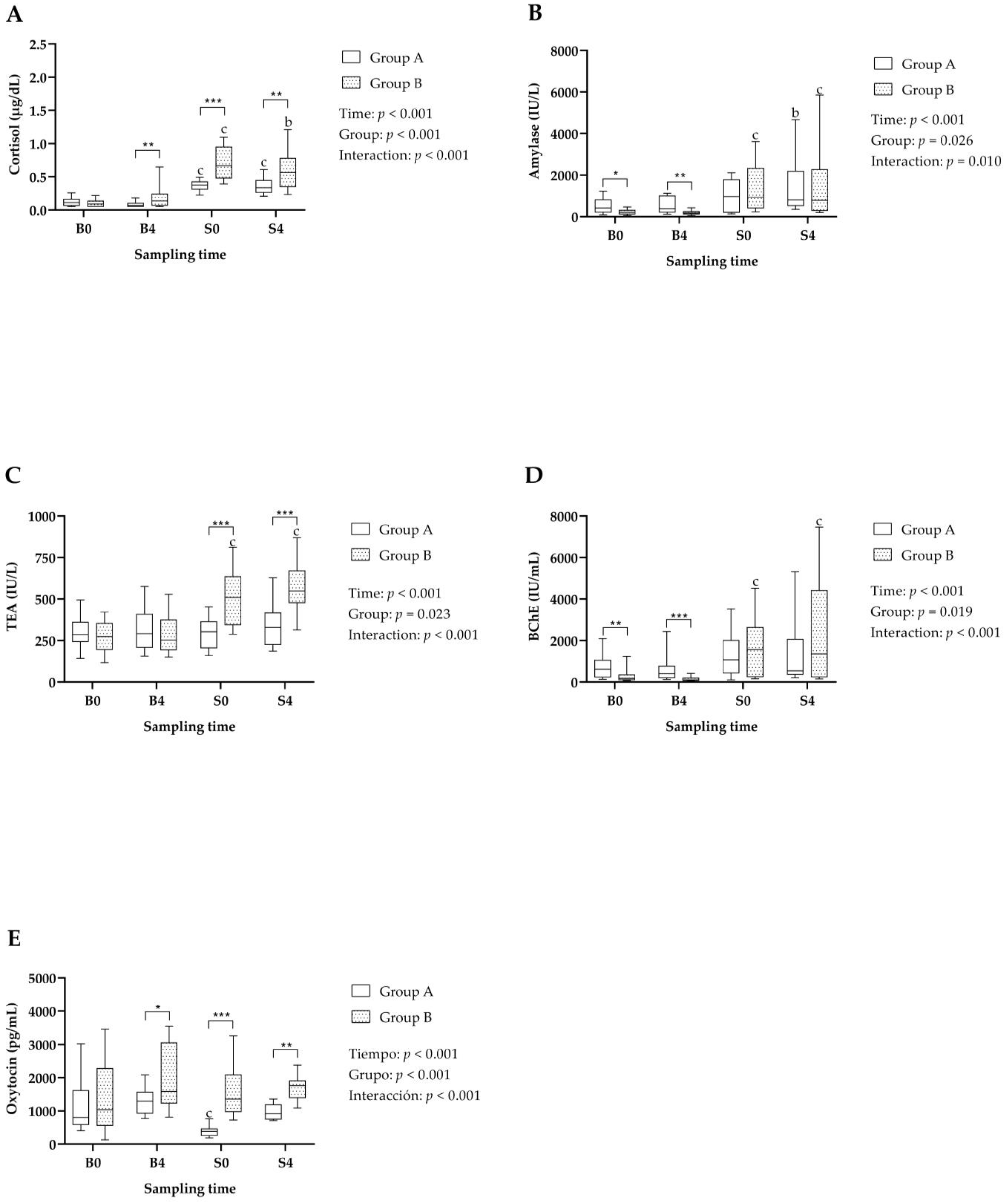
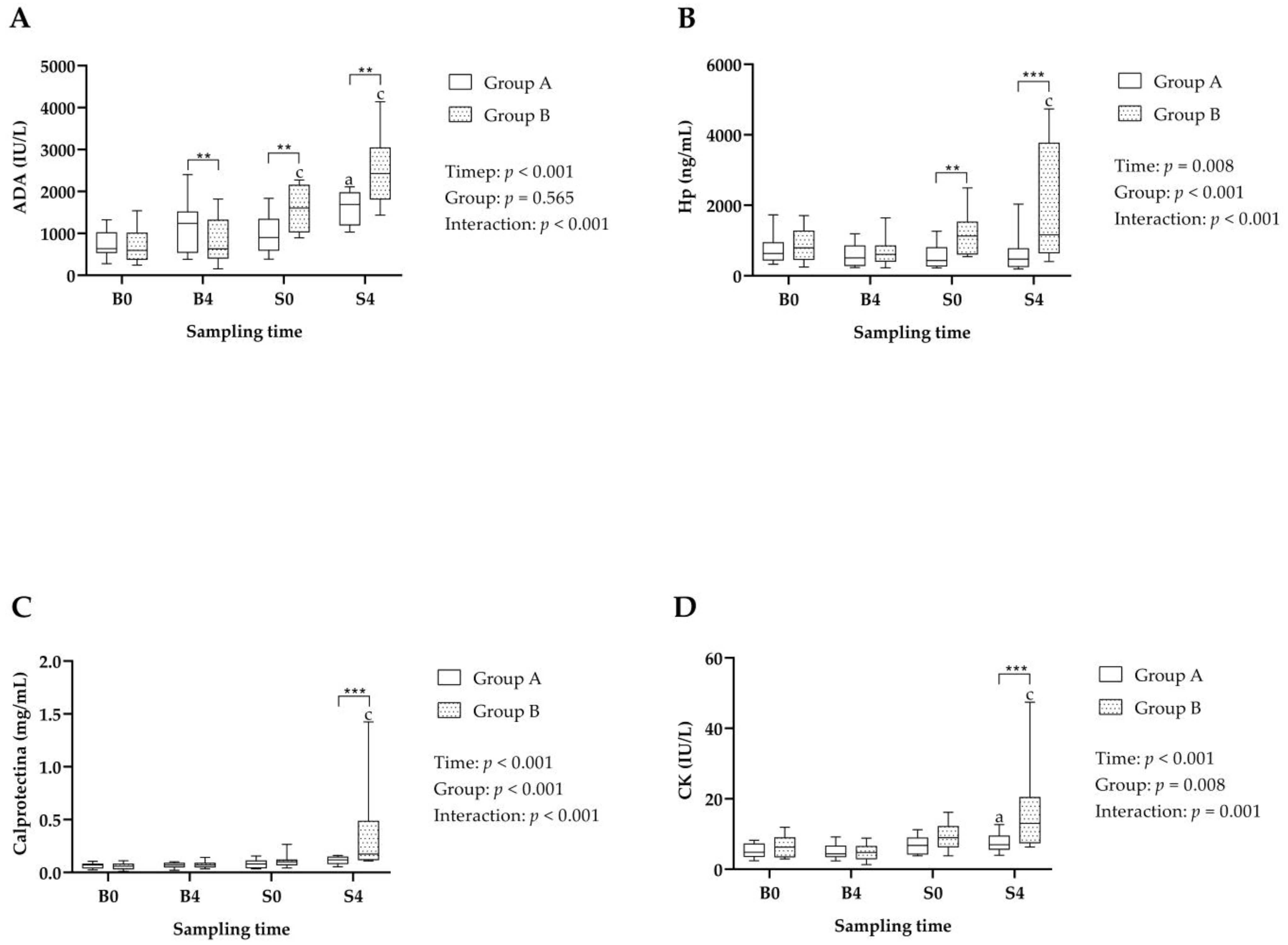
3.1. Stress Biomarkers
3.2. Immune System and Muscle Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Álvarez, D.; Garrido, M.D.; Bañón, S. Influence of Pre-Slaughter Process on Pork Quality: An Overview. Food Rev. Int. 2009, 25, 233–250. [Google Scholar] [CrossRef]
- Lammens, V.; Peeters, E.; De Maere, H.; De Mey, E.; Paelinck, H.; Leyten, J.; Geers, R. A Survey of Pork Quality in Relation to Pre-Slaughter Conditions, Slaughterhouse Facilities, and Quality Assurance. Meat Sci. 2007, 75, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Barton Gade, P. Pre-Slaughter Handling. In Encyclopedia of Meat Sciences; Klinth Jensen, W., Ed.; Elsevier: Oxford, UK, 2004; pp. 1012–1020. [Google Scholar]
- dalla Costa, O.A.; Faucitano, L.; Coldebella, A.; Ludke, J.V.; Peloso, J.V.; dalla Roza, D.; Paranhos da Costa, M.J.R. Effects of the Season of the Year, Truck Type and Location on Truck on Skin Bruises and Meat Quality in Pigs. Livest. Sci. 2007, 107, 29–36. [Google Scholar] [CrossRef]
- Ritter, M.J.; Ellis, M.; Bertelsen, C.R.; Bowman, R.; Brinkmann, J.; Dedecker, J.M.; Keffaber, K.K.; Murphy, C.M.; Peterson, B.A.; Schlipf, J.M.; et al. Effects of Distance Moved during Loading and Floor Space on the Trailer during Transport on Losses of Market Weight Pigs on Arrival at the Packing Plant. J. Anim. Sci. 2007, 85, 3454–3461. [Google Scholar] [CrossRef]
- Ferlazzo, A. Large Animals Transportation Procedures in Europe: Present and Future. Vet. Res. Commun. 2003, 27 (Suppl. S1), 513–514. [Google Scholar] [CrossRef]
- Temple, D.; Manteca, X.; Velarde, A.; Dalmau, A. Assessment of Animal Welfare through Behavioural Parameters in Iberian Pigs in Intensive and Extensive Conditions. Appl. Anim. Behav. Sci. 2011, 131, 29–39. [Google Scholar] [CrossRef]
- Dalmau, A.; Temple, D.; Rodríguez, P.; Llonch, P.; Velarde, A. Application of the Welfare Quality® Protocol at Pig Slaughterhouses. Anim. Welf. 2009, 18, 497–505. [Google Scholar] [CrossRef]
- Śmiecińska, K.; Denaburski, J.; Sobotka, W. Slaughter Value, Meat Quality, Creatine Kinase Activity and Cortisol Levels in the Blood Serum of Growing-Finishing Pigs Slaughtered Immediately after Transport and after a Rest Period. Pol. J. Vet. Sci. 2011, 14, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Averos, X.; Herranz, A.; Sanchez, R.; Comella, J.X.; Gosalvez, L.F. Serum Stress Parameters in Pigs Transported to Slaughter under Commercial Conditions in Different Seasons. Vet. Med. 2007, 52, 333–342. [Google Scholar] [CrossRef]
- Nannoni, E.; Widowski, T.; Torrey, S.; Fox, J.; Rocha, L.M.; Gonyou, H.; Weschenfelder, A.V.; Crowe, T.; Martelli, G.; Faucitano, L. Water Sprinkling Market Pigs in a Stationary Trailer. 2. Effects on Selected Exsanguination Blood Parameters and Carcass and Meat Quality Variation. Livest. Sci. 2014, 160, 124–131. [Google Scholar] [CrossRef]
- Lamy, E.; Mau, M. Saliva Proteomics as an Emerging, Non-Invasive Tool to Study Livestock Physiology, Nutrition and Diseases. J. Proteom. 2012, 75, 4251–4258. [Google Scholar] [CrossRef]
- Rey-Salgueiro, L.; Martinez-Carballo, E.; Fajardo, P.; Chapela, M.; Espiñeira, M.; Simal-Gandara, J. Meat Quality in Relation to Swine Well-Being after Transport and during Lairage at the Slaughterhouse. Meat Sci. 2018, 142, 38–43. [Google Scholar] [CrossRef]
- Jama, N.; Maphosa, V.; Hoffman, L.; Muchenje, V. Effect of Sex and Time to Slaughter (Transportation and Lairage Duration) on the Levels of Cortisol, Creatine Kinase and Subsequent Relationship with Pork Quality. Meat Sci. 2016, 116, 43–49. [Google Scholar] [CrossRef]
- López-Arjona, M.; Escribano, D.; Mateo, S.; Contreras-Aguilar, M.; Rubio, C.; Tecles, F.; Cerón, J.; Martínez-Subiela, S. Changes in Oxytocin Concentrations in Saliva of Pigs after a Transport and during Lairage at Slaughterhouse. Res. Vet. Sci. 2020, 133, 26–30. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; López-Arjona, M.; Martínez-Miró, S.; Escribano, D.; Hernández-Ruipérez, F.; Cerón, J.J.; Tecles, F. Changes in Saliva Analytes during Pregnancy, Farrowing and Lactation in Sows: A Sialochemistry Approach. Vet. J. 2021, 273, 105679. [Google Scholar] [CrossRef]
- Botía, M.; Ortín-Bustillo, A.; López-Martínez, M.J.; Fuentes, P.; Escribano, D.; González-Bulnes, A.; Manzanilla, E.G.; Martínez-Subiela, S.; Tvarijonaviciute, A.; López-Arjona, M.; et al. Gaining Knowledge about Biomarkers of the Immune System and Inflammation in the Saliva of Pigs: The Case of Myeloperoxidase, S100A12, and ITIH4. Res. Vet. Sci. 2023, 164, 104997. [Google Scholar] [CrossRef]
- Simmons, N.J.; Young, O.A.; Dobbie, P.M.; Singh, K.; Thompson, B.C.; Speck, P.A. Post-Mortem Calpain-System Kinetics in Lamb: Effects of Clenbuterol and Preslaughter Exercise. Meat Sci. 1997, 47, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Gajana, C.S.; Nkukwana, T.T.; Marume, U.; Muchenje, V. Effects of Transportation Time, Distance, Stocking Density, Temperature and Lairage Time on Incidences of Pale Soft Exudative (PSE) and the Physico-Chemical Characteristics of Pork. Meat Sci. 2013, 95, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Guàrdia, M.D.; Estany, J.; Balasch, S.; Oliver, M.A.; Gispert, M.; Diestre, A. Risk Assessment of DFD Meat Due to Pre-Slaughter Conditions in Pigs. Meat Sci. 2010, 70, 709–716. [Google Scholar] [CrossRef]
- Barton Gade, P. Effect of Rearing System and Mixing at Loading on Transport and Lairage Behaviour and Meat Quality: Comparison of Outdoor and Conventionally Raised Pigs. Animal 2008, 2, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Jung, K.C.; Choe, J.H.; Kim, B.C. Effects of Muscle Cortisol Concentration on Muscle Fiber Characteristics, Pork Quality, and Sensory Quality of Cooked Pork. Meat Sci. 2012, 91, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Dallman, M.; Hellhammer, D. Regulation of the Hypothalamo-Pituitary-Adrenal Axis, Chronic Stress, and Energy: The Role of the Brain and Networks. In The Handbook of Stress Science: Biology, Psychology, and Health; Contrada, R., Baum, A., Eds.; Springer Publishing Company: New York, NY, USA, 2011; pp. 11–36. [Google Scholar]
- Bottoms, G.D.; Roesel, O.F.; Rausch, F.D.; Akins, E.L. Circadian Variation in Plasma Cortisol and Corticosterone in Pigs and Mares. Am. J. Vet. Res. 1972, 33, 785–790. [Google Scholar] [PubMed]
- Merlot, E.; Mounier, A.M.; Prunier, A. Endocrine Response of Gilts to Various Common Stressors: A Comparison of Indicators and Methods of Analysis. Physiol. Behav. 2011, 102, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Tecles, F.; Contreras-Aguilar, M.D.; Martínez-Miró, S.; Tvarijonaviciute, A.; Martínez-Subiela, S.; Escribano, D.; Cerón, J.J. Total Esterase Measurement in Saliva of Pigs: Validation of an Automated Assay, Characterization and Changes in Stress and Disease Conditions. Res. Vet. Sci. 2017, 114, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, S.; Yamamoto, M.; Nagasawa, M.; Mogi, K.; Kikusui, T.; Ohtani, N.; Ohta, M. Urinary Oxytocin as a Noninvasive Biomarker of Positive Emotion in Dogs. Horm. Behav. 2011, 60, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Lürzel, S.; Bückendorf, L.; Waiblinger, S.; Rault, J. Salivary Oxytocin in Pigs, Cattle, and Goats during Positive Human-Animal Interactions. Psychoneuroendocrinology 2020, 115, 104636. [Google Scholar] [CrossRef] [PubMed]
- López-Arjona, M.; Padilla, L.; Roca, J.; Cerón, J.; Martínez-Subiela, S. Ejaculate Collection Influences the Salivary Oxytocin Concentrations in Breeding Male Pigs. Animals 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Valros, A.; Lopez-Martinez, M.J.; Munsterhjelm, C.; Lopez-Arjona, M.; Ceron, J.J. Novel Saliva Biomarkers for Stress and Infection in Pigs: Changes in Oxytocin and Procalcitonin in Pigs with Tail-Biting Lesions. Res. Vet. Sci. 2022, 153, 49–56. [Google Scholar] [CrossRef]
- Nishioka, T.; Anselmo-Franci, J.A.; Li, P.; Callahan, M.F.; Morris, M. Stress Increases Oxytocin Release within the Hypothalamic Paraventricular Nucleus. Brain Res. 1998, 781, 57–61. [Google Scholar] [CrossRef]
- Tabak, B.A.; Rosenfield, D.; Sunahara, C.S.; Alvi, T.; Szeto, A.; Mendez, A.J. Social Anxiety Is Associated with Greater Peripheral Oxytocin Reactivity to Psychosocial Stress. Psychoneuroendocrinology 2022, 140, 105712. [Google Scholar] [CrossRef]
- Taylor, S.E.; Gonzaga, G.C.; Klein, L.C.; Hu, P.; Greendale, G.A.; Seeman, T.E. Relation of Oxytocin to Psychological Stress Responses and Hypothalamic-Pituitary-Adrenocortical Axis Activity in Older Women. Psychosom. Med. 2006, 68, 238–245. [Google Scholar] [CrossRef]
- Grippo, A.J.; Gerena, D.; Huang, J.; Kumar, N.; Shah, M.; Ughreja, R.; Sue Carter, C. Social Isolation Induces Behavioral and Neuroendocrine Disturbances Relevant to Depression in Female and Male Prairie Voles. Psychoneuroendocrinology 2007, 32, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Grippo, A.J.; Trahanas, D.M.; Zimmerman, R.R.; Porges, S.W.; Carter, C.S. Oxytocin Protects against Negative Behavioral and Autonomic Consequences of Long-Term Social Isolation. Psychoneuroendocrinology 2009, 34, 1542–1553. [Google Scholar] [CrossRef]
- Tops, M.; Van Peer, J.M.; Korf, J.; Wijers, A.A.; Tucker, D.M. Anxiety, Cortisol, and Attachment Predict Plasma Oxytocin. Psychophysiology 2007, 44, 444–449. [Google Scholar] [CrossRef]
- Murata, H. Stress and Acute Phase Protein Response: An Inconspicuous but Essential Linkage. Vet. J. 2007, 173, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.; Escribano, D.; Martínez-Miró, S.; López-Arjona, L.; Rubio, C.; Martínez-Subiela, S.; Cerón, J.; Tecles, F. Application of a Score for Evaluation of Pain, Distress and Discomfort in Pigs with Lameness and Prolapses: Correlation with Saliva Biomarkers and Severity of the Disease. Res. Vet. Sci. 2019, 126, 155–163. [Google Scholar] [CrossRef] [PubMed]
- García-Celdrán, M.; Ramis, G.; Quereda, J.; Armero, E. Reduction of Transport-Induced Stress on Finishing Pigs by Increasing Lairage Time at the Slaughter House. J. Swine Health Prod. 2012, 20, 118–122. [Google Scholar]
- Soler, L.; Gutiérrez, A.; Escribano, D.; Fuentes, M.; Cerón, J.J. Response of Salivary Haptoglobin and Serum Amyloid A to Social Isolation and Short Road Transport Stress in Pigs. Res. Vet. Sci. 2013, 95, 298–302. [Google Scholar] [CrossRef]
- Hirten, R.P.; Danieletto, M.; Scheel, R.; Shervey, M.; Ji, J.; Hu, L.; Sauk, J.; Chang, L.; Arnrich, B.; Böttinger, E.; et al. Longitudinal Autonomic Nervous System Measures Correlate With Stress and Ulcerative Colitis Disease Activity and Predict Flare. Inflamm. Bowel Dis. 2021, 27, 1576–1584. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Zhao, W.; Chen, J.; Maas, K.; Hussain, N.; Henderson, W.A.; Cong, X. Trends of Fecal Calprotectin Levels and Associations with Early Life Experience in Preterm Infants. Interdiscip. Nurs. Res. 2022, 1, 36–42. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Becerril-Herrera, M.; Trujillo-Ortega, M.; Alonso-Spilsbury, M.; Flores-Peinado, S.; Guerrero-Legarreta, I. Effects of Pre-Slaughter Transport, Lairage and Sex on Pig Chemical Serologic Profiles. J. Anim. Vet. Adv. 2009, 8, 246–250. [Google Scholar]
- McLellan, C.P.; Lovell, D.I.; Gass, G.C. Creatine Kinase and Endocrine Responses of Elite Players Pre, During, and Post Rugby League Match Play. J. Strength Cond. Res. 2010, 24, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Muchenje, V.; Ndou, S. How Pig Pre-Slaughter Welfare Affects Pork Quality and the Pig Industry. Porcus 2011, 29, 38–39. [Google Scholar]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the Hypothalamic–Pituitary–Adrenal Function as a Tool to Evaluate Animal Welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Ruis, M.; Te Brake, J.; Engel, B.; Ekkel, E.; Buist, W.; Blokhuis, H.; Koolhaas, J. The Circadian Rhythm of Salivary Cortisol in Growing Pigs: Effects of Age, Gender, and Stress. Physiol. Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botía, M.; Escribano, D.; Ortín-Bustillo, A.; López-Martínez, M.J.; Fuentes, P.; Jiménez-Caparrós, F.J.; Hernández-Gómez, J.L.; Avellaneda, A.; Cerón, J.J.; Rubio, C.P.; et al. Comparison of the Effect of Two Different Handling Conditions at Slaughter in Saliva Analytes in Pigs. Metabolites 2024, 14, 234. https://doi.org/10.3390/metabo14040234
Botía M, Escribano D, Ortín-Bustillo A, López-Martínez MJ, Fuentes P, Jiménez-Caparrós FJ, Hernández-Gómez JL, Avellaneda A, Cerón JJ, Rubio CP, et al. Comparison of the Effect of Two Different Handling Conditions at Slaughter in Saliva Analytes in Pigs. Metabolites. 2024; 14(4):234. https://doi.org/10.3390/metabo14040234
Chicago/Turabian StyleBotía, María, Damián Escribano, Alba Ortín-Bustillo, María J. López-Martínez, Pablo Fuentes, Francisco J. Jiménez-Caparrós, Juan L. Hernández-Gómez, Antonio Avellaneda, José J. Cerón, Camila P. Rubio, and et al. 2024. "Comparison of the Effect of Two Different Handling Conditions at Slaughter in Saliva Analytes in Pigs" Metabolites 14, no. 4: 234. https://doi.org/10.3390/metabo14040234
APA StyleBotía, M., Escribano, D., Ortín-Bustillo, A., López-Martínez, M. J., Fuentes, P., Jiménez-Caparrós, F. J., Hernández-Gómez, J. L., Avellaneda, A., Cerón, J. J., Rubio, C. P., Tvarijonaviciute, A., Martínez-Subiela, S., López-Arjona, M., & Tecles, F. (2024). Comparison of the Effect of Two Different Handling Conditions at Slaughter in Saliva Analytes in Pigs. Metabolites, 14(4), 234. https://doi.org/10.3390/metabo14040234







