Effect of Bile on Hemodynamics and Blood Micro-Rheological Parameters in Experimental Models of Bilhemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sampling Protocol
2.1.1. Experimental Protocol in Rats
2.1.2. Experimental Protocol in Pigs
2.1.3. Blood Sampling Protocol
2.2. Hematological Parameters
2.3. Red Blood Cell Conventional and Osmotic Gradient Deformability Measurements
2.4. Determination of Red Blood Cell Aggregation
2.5. Statistical Analysis
3. Results
3.1. Hemodynamic Variables
3.2. Hematological Parameters
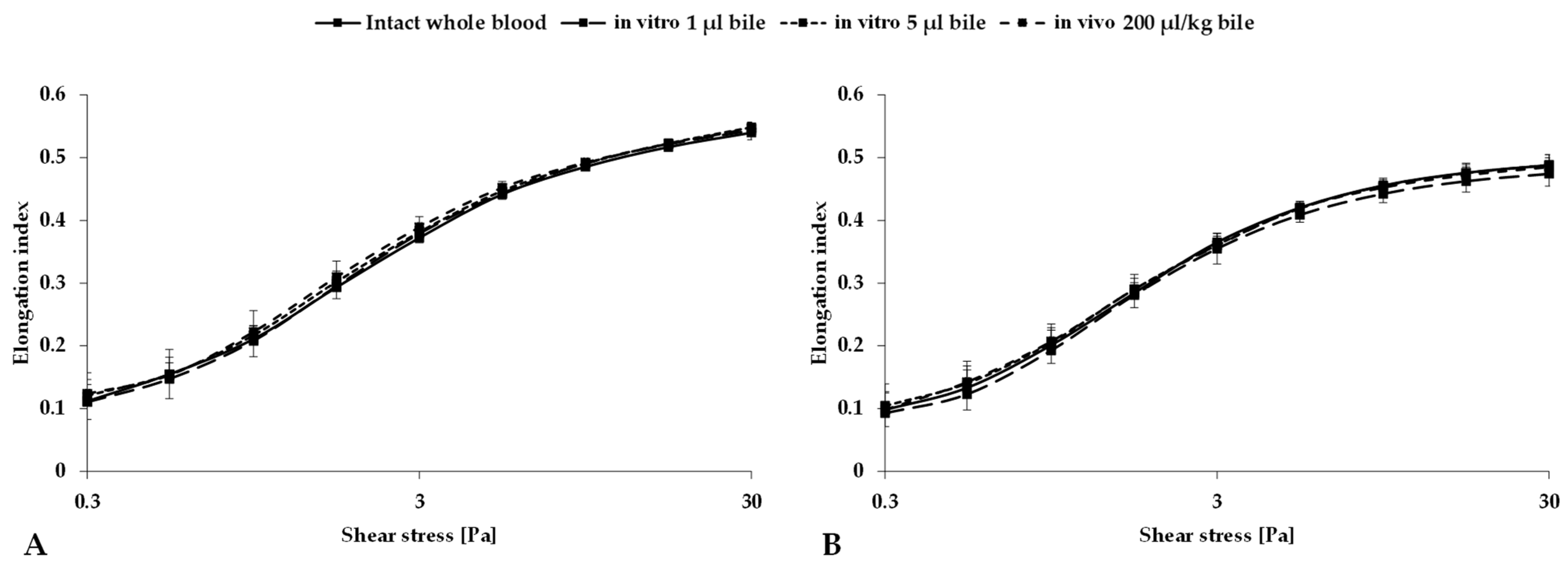
3.3. Red Blood Cell Deformability
3.4. Red Blood Cell Aggregation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haberlik, A.; Cendron, M.; Sauer, H. Biliovenous fistula in children after blunt liver trauma: Proposal for a simple surgical treatment. J. Pediatr. Surg. 1992, 27, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, V.P.; Laupland, K.B.; Colwell, C.B.; Soriya, G.; Magder, S.; Ball, J.; DiCocco, J.M.; Fabian, T.C.; Moran, G.J.; Easton-Carr, R.; et al. Biliovenous fistula. In Encyclopedia of Intensive Care Medicine; Vincent, J.L., Hall, J.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 303–305. [Google Scholar]
- Antebi, E.; Adar, R.; Zweig, A.; Barzilay, J.; Mozes, M. Bilemia: An unusual complication of bile ducts stones. Ann. Surg. 1973, 177, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Yopp, A. Hemobilia and bilhemia. In Blumgart’s Surgery of the Liver, Biliary Tract and Pancreas, 2-Volume Set; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1915–1927. [Google Scholar]
- Navuluri, R. Hemobilia. Semin. Intervent. Radiol. 2016, 33, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Han, J.; Girotra, M.; Tabibian, J.H. Hemobilia: Perspective and role of the advanced endoscopist. Gastroenterol. Res. Pract. 2018, 2018, 3670739. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gupta, S.; Soin, A.; Nundy, S. Management of massive haemobilia in an indian hospital. Indian J. Surg. 2008, 70, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Gable, D.R.; Allen, J.W.; Harrell, D.J.; Carrillo, E.H. Endoscopic treatment of posttraumatic “bilhemia”: Case report. J. Trauma 1997, 43, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.; Wetscher, G.; Pointner, R.; Schwab, G.; Tschmelitsch, J.; Gadenstätter, M.; Bodner, E. Traumatic bilhemia. Surgery 1994, 116, 24–27. [Google Scholar] [PubMed]
- Sandblom, P.; Jakobsson, B.; Lindgren, H.; Lunderquist, A. Fatal bilhemia. Surgery 2000, 127, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Turk, E.; Temir, Z.G.; Karkiner, A.; Memis, A.; Topalak, O.; Evciler, H.; Ucan, B.; Karaca, I. Bilhemia, an unusual complication after blunt liver trauma in a child: Case report and review of the literature. Eur. J. Pediatr. Surg. 2010, 20, 212–214. [Google Scholar] [CrossRef]
- Çakır, M.S.; Guzelbey, T.; Kınacı, E.; Sevinc, M.M.; Kilickesmez, O. Delayed bilhemia complicating percutaneous transhepatic biliary drainage: Successful treatment with primary coil embolization. Radiol. Case Rep. 2019, 14, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Maxeiner, H. Iatrogene biliovenöse fistel. Z. Rechtsmed. 1984, 91, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, R.; Jamindar, P.; Williams, R. Venobiliary fistula with haemobilia: A rare complication of percutaneous liver biopsy. BMJ Case Rep. 2017, 2017, bcr2016218930. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Walter, R.; Contesse, J.; Reinhart, W.H. Impairment of blood rheology by cholestatic jaundice in human beings. J. Lab. Clin. Med. 2003, 142, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, B. Red blood cell’s deformability in obstructive jaundice. Wiadomości Lek. 2001, 54, 262–267. [Google Scholar]
- Huang, L.; Yu, Q.; Peng, H. Hemorheological alteration in patients with cirrhosis clinically diagnosed with portal vein system thrombosis after splenectomy. Med. Sci. Monit. 2021, 27, e931157. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Therapeutic targets for cholestatic liver injury. Expert Opin. Ther. Targets 2016, 20, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liang, Z. Clinical significance of serum total bile acid and hemorheology indexes in intrahepatic cholestasis of pregnancy. J. Clin. Med. Prac. 2014, 23, 70–72. [Google Scholar] [CrossRef]
- Nemeth, N.; Peto, K.; Magyar, Z.; Klarik, Z.; Varga, G.; Oltean, M.; Mantas, A.; Czigany, Z.; Tolba, R.H. Hemorheological and microcirculatory factors in liver ischemia-reperfusion injury—An update on pathophysiology, molecular mechanisms and protective strategies. Int. J. Mol. Sci. 2021, 22, 1864. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.F.; Varlet-Marie, E.; Myzia, J.; Raynaud de Mauverger, E.; Pretorius, E. Metabolic influences modulating erythrocyte deformability and eryptosis. Metabolites 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Szanto, S.; Mody, T.; Gyurcsik, Z.; Babjak, L.B.; Somogyi, V.; Barath, B.; Varga, A.; Matrai, A.A.; Nemeth, N. Alterations of selected hemorheological and metabolic parameters induced by physical activity in untrained men and sportsmen. Metabolites 2021, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumaravel, M. Influence of jaundice on aggregation process and deformability of erythrocytes. Clin. Hemorheol. Microcirc. 1995, 15, 273–290. [Google Scholar] [CrossRef]
- Baillie, J. Hemobilia. Gastroenterol. Hepatol. 2012, 8, 270–272. [Google Scholar]
- Wong, Y.-C.; Wang, L.-J.; Wu, C.-H.; Chen, H.-W.; Fu, C.-J.; Yuan, K.-C.; Lin, B.-C.; Hsu, Y.-P.; Kang, S.-C. Detection and characterization of traumatic bile leaks using Gd-EOB-DTPA enhanced magnetic resonance cholangiography. Sci. Rep. 2018, 8, 14612. [Google Scholar] [CrossRef] [PubMed]
- Spinn, M.P.; Patel, M.K.; Cotton, B.A.; Lukens, F.J. Successful endoscopic therapy of traumatic bile leaks. Case Rep. Gastroenterol. 2013, 7, 56–62. [Google Scholar] [CrossRef]
- De Nisco, G.; Chiastra, C.; Hartman, E.M.J.; Hoogendoorn, A.; Daemen, J.; Calò, K.; Gallo, D.; Morbiducci, U.; Wentzel, J.J. Comparison of swine and human computational hemodynamics models for the study of coronary atherosclerosis. Front. Bioeng. Biotechnol. 2021, 9, 731924. [Google Scholar] [CrossRef] [PubMed]
- Längin, M.; Konrad, M.; Reichart, B.; Mayr, T.; Vandewiele, S.; Postrach, J.; Mokelke, M.; Radan, J.; Brenner, P.; Bauer, A.; et al. Hemodynamic evaluation of anesthetized baboons and piglets by transpulmonary thermodilution: Normal values and interspecies differences with respect to xenotransplantation. Xenotransplantation 2020, 27, e12576. [Google Scholar] [CrossRef] [PubMed]
- Si, G.L.R.; Yao, P.; Shi, L. Rapid determination of bile acids in bile from various mammals by reversed-phase ultra-fast liquid chromatography. J. Chromatogr. Sci. 2015, 53, 1060–1065. [Google Scholar] [CrossRef][Green Version]
- Thakare, R.; Alamoudi, J.A.; Gautam, N.; Rodrigues, A.D.; Alnouti, Y. Species differences in bile acids I. plasma and urine bile acid composition. J. Appl. Toxicol. 2018, 38, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Iqbal, S.; Godfrey, P.P.; Billington, D. Membranes and bile formation. composition of several mammalian biles and their membrane-damaging properties. Biochem. J. 1979, 178, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Windberger, U.; Baskurt, O.K. Comparative hemorheology. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 267–285. [Google Scholar]
- Nemeth, N.; Kiss, F.; Miszti-Blasius, K. Interpretation of osmotic gradient ektacytometry (osmoscan) data: A comparative study for methodological standards. Scand. J. Clin. Lab. Investig. 2015, 75, 213–222. [Google Scholar] [CrossRef]
- Gholampour, S.; Mehrjoo, S. Effect of bifurcation in the hemodynamic changes and rupture risk of small intracranial aneurysm. Neurosurg. Rev. 2021, 44, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Li, J.; Chen, Z.; Wang, R.; Shen, Y.; Zhang, R.; Zhou, F.; Zhang, Y. Pathological hemodynamic changes and leukocyte transmigration disrupt the blood–spinal cord barrier after spinal cord injury. J. Neuro. 2023, 20, 118. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Long, W.; Wu, W.; Jiang, C.; Liu, X.; Li, L. The changes of hemodynamic parameters, pathology and c-kit mRNA expression in myocardium after acute myocardial infarction in rats. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2009, 26, 540–544. [Google Scholar] [PubMed]
- Moon, C.M.; Kim, S.K.; Heo, S.H.; Shin, S.S. Hemodynamic changes in the portal vein with age: Evaluation using four-dimensional flow MRI. Sci. Rep. 2023, 13, 7397. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Escobar, C.; Zamora, C.; Rejas, C.; Varas, J.; Párraga, M.; San Martin, S.; Montedónico, S. Bile duct ligature in young rats: A revisited animal model for biliary atresia. Eur. J. Histochem. 2017, 61, 2803. [Google Scholar] [CrossRef] [PubMed]
- Rolf, L.L.; Bartels, K.E.; Nelson, E.C.; Berlin, K.D. Chronic bile duct cannulation in laboratory rats. Lab. Anim. Sci. 1991, 41, 486–492. [Google Scholar]
- Hardeman, M.; Goedhart, P.; Shin, S. Methods in hemorheology. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 242–266. [Google Scholar]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of red blood cell elongation index—Shear stress curves obtained by ektacytometry. Scand. J. Clin. Lab. Investig. 2009, 69, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Data reduction methods for ektacytometry in clinical hemorheology. Clin. Hemorheol. Microcirc. 2013, 54, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Alexy, T.; Shin, S.; Hardeman, M.R.; Meiselman, H.J. Comparison of three instruments for measuring red blood cell aggregation. Clin. Hemorheol. Microcirc. 2009, 43, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Vayá, A.; Martinez Triguero, M.; Ricart, A.; Plumé, G.; Solves, P.; Corella, D.; Romagnoli, M. Erythrocyte aggregability and AB0 blood groups. Clin. Hemorheol. Microcirc. 2009, 41, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Neu, B.; Meiselman, H.J. Measurement of red blood cell aggregation. In Red Blood Cell Aggregation; Baskurt, O.K., Neu, B., Meiselman, H.J., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 63–132. [Google Scholar]
- Baskurt, O.K.; Neu, B.; Meiselman, H.J. Alterations in red blood cell aggregation. In Red Blood Cell Aggregation; Baskurt, O.K., Neu, B., Meiselman, H.J., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 223–267. [Google Scholar]
- Schmid-Schonbein, H.; Malotta, H.; Striesow, F. Erythrocyte aggregation: Causes, consequences and methods of assessment. Tijdschr NvKC 1990, 15, 88–97. [Google Scholar]
- Lee, B.K.; Alexy, T.; Wenby, R.B.; Meiselman, H.J. Red blood cell aggregation quantitated via Myrenne aggregometer and yield shear stress. Biorheology 2007, 44, 29–35. [Google Scholar] [PubMed]
- Wolfensohn, S.; Lloyd, M. (Eds.) Handbook of Laboratory Animal Management and Welfare, 4th ed.; Willey Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Song, E.; Segal, I.; Hodkinson, J.; Kew, M.C. Sinus bradycardia in obstructive jaundice--correlation with total serum bile acid concentrations. S. Afr. Med. J. 1983, 64, 548–551. [Google Scholar] [PubMed]
- Zhang, B.C.; Chen, J.H.; Xiang, C.H.; Su, M.Y.; Zhang, X.S.; Ma, Y.F. Increased serum bile acid level is associated with high-risk coronary artery plaques in an asymptomatic population detected by coronary computed tomography angiography. J. Thorac. Dis. 2019, 11, 5063–5070. [Google Scholar] [CrossRef] [PubMed]
- Chong Nguyen, C.; Duboc, D.; Rainteau, D.; Sokol, H.; Humbert, L.; Seksik, P.; Bellino, A.; Abdoul, H.; Bouazza, N.; Treluyer, J.M.; et al. Circulating bile acids concentration is predictive of coronary artery disease in human. Sci. Rep. 2021, 11, 22661. [Google Scholar] [CrossRef]
- Simko, V.; Kelley, R.E. Effect of physical exercise on bile and red blood cell lipids in humans. Atherosclerosis 1979, 32, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Maenz, D.D.; Forsyth, G.W. Calcium ionophore activity of intestinal secretory compounds. Digestion 1984, 30, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, W.Q.; Fu, M.J.; Li, J.; Hu, C.H.; Chen, Y.; Zhou, M.M.; Gao, Z.J.; He, Y.L. Overview of bile acid signaling in the cardiovascular system. World J. Clin. Cases 2021, 9, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Leng, A.; Zhang, G.; Qu, J. Cardiac complications caused by biliary diseases: A review of clinical manifestations, pathogenesis and treatment strategies of cholecardia syndrome. Pharmacol. Res. 2024, 199, 107006. [Google Scholar] [CrossRef]
- Desai, M.S.; Penny, D.J. Bile acids induce arrhythmias: Old metabolite, new tricks. Heart 2013, 99, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Vasavan, T.; Ferraro, E.; Ibrahim, E.; Dixon, P.; Gorelik, J.; Williamson, C. Heart and bile acids—Clinical consequences of altered bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Voiosu, A.; Wiese, S.; Voiosu, T.; Bendtsen, F.; Møller, S. Bile acids and cardiovascular function in cirrhosis. Liver Int. 2017, 37, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Iqbal, M.J.; Farooq, H. Jaundice and sinus bradycardia 100 years of an unsolved mystery. Pak. Heart J. 2012, 43, 17–19. [Google Scholar]
- Joubert, P. An in vivo investigation of the negative chronotropic effect of cholic acid in the rat. Clin. Exp. Pharmacol. Physiol. 1978, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Binah, O.; Rubinstein, I.; Bomzon, A.; Better, O.S. Effects of bile acids on ventricular muscle contraction and electrophysiological properties: Studies in rat papillary muscle and isolated ventricular myocytes. Naunyn Schmiedebergs Arch. Pharmacol. 1987, 335, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Vacca, G.; Battaglia, A.; Grossini, E.; Mary, D.A.; Molinari, C. Reflex coronary vasoconstriction caused by gallbladder distension in anesthetized pigs. Circulation 1996, 94, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Pozdeev, V.I.; Gatidis, S.; Qadri, S.M.; Häussinger, D.; Kubitz, R.; Herebian, D.; Mayatepek, E.; Lang, F.; Lang, K.S.; et al. Bile acid-induced suicidal erythrocyte death. Cell Physiol. Biochem. 2016, 38, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Oelberg, D.G.; Dubinsky, W.P.; Sackman, J.W.; Wang, L.B.; Adcock, E.W.; Lester, R. Bile salts induce calcium uptake in vitro by human erythrocytes. Hepatology 1987, 7, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Samartsev, V.N.; Khoroshavina, E.I.; Pavlova, E.K.; Dubinin, M.V.; Semenova, A.A. Bile acids as inducers of protonophore and ionophore permeability of biological and artificial membranes. Membranes 2023, 13, 472. [Google Scholar] [CrossRef]
- Vural Yilmaz, Z.; Gencosmanoglu Turkmen, G.; Daglar, K.; Yılmaz, E.; Kara, O.; Uygur, D. Elevated red blood cell distribution width is associated with intrahepatic cholestasis of pregnancy. Ginekol. Pol. 2017, 88, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Child, P.; Rafter, J. Calcium enhances the hemolytic action of bile salts. Biochim. Biophys. Acta 1986, 855, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Rodrigues, C.M.; Clerici, C.; Solinas, A.; Morelli, A.; Gartung, C.; Boyer, J. Bile acid concentrations in human and rat liver tissue and in hepatocyte nuclei. Gastroenterology 1997, 112, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dawson, P.A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Thakare, R.; Alamoudi, J.A.; Gautam, N.; Rodrigues, A.D.; Alnouti, Y. Species differences in bile acids II. Bile acid metabolism. J. Appl. Toxicol. 2018, 38, 1336–1352. [Google Scholar] [CrossRef] [PubMed]


| Species | Rat | Pig | |||
|---|---|---|---|---|---|
| Parameter | MAP | HR | MAP | HR | |
| rate of decrease (%) | 15.2 ± 6.9 | 4.6 ± 2.1 | 32.1 ± 14 * | 25.2 ± 11.6 * | |
| duration of decrease (s) | 10.6 ± 2.6 | 48.3 ± 18.9 * | |||
| time required for normalization (s) | 50% | 24.4 ± 7 | 83.3 ± 25.2 * | ||
| 80% | 34.8 ± 8.4 | 110.3 ± 22 * | |||
| 100% | 45 ± 9.5 | 130 ± 20 * | |||
| Intact Blood | In Vitro 1 µL Bile | In Vitro 5 µL Bile | In Vivo (Bilhemia) | |||||
|---|---|---|---|---|---|---|---|---|
| Rat | Pig | Rat | Pig | Rat | Pig | Rat | Pig | |
| RBC [1012/L] | 8.25 ± 0.33 | 6.95 ± 0.64 | 8.76 ± 0.58 | 6.42 ± 0.71 * | 8.6 ± 0.43 | 6.52 ± 0.65 | 8.68 ± 0.5 | 7.01 ± 1.18 |
| WBC [109/L] | 7.08 ± 1.31 | 24.78 ± 2.74 | 6.98 ± 2.48 | 25.01 ± 2.96 | 6.63 ± 1.93 | 24.57 ± 2.75 | 6.71 ± 1.92 | 21.78 ± 4.3 |
| Hgb [g/L] | 147.7 ± 5.5 | 123.8 ± 14.9 | 159 ± 8.5 * | 115.1 ± 13.4 | 156.4 ± 7.2 * | 116.9 ± 12.6 | 160.6 ± 7.8 * | 123.9 ± 19.9 |
| Hct [%] | 44.35 ± 1.33 | 41.09 ± 4.67 | 48.34 ± 2.61 * | 38.09 ± 4.22 | 47.57 ± 2.19 * | 38.76 ± 3.95 | 48.64 ± 2.65 * | 40.86 ± 6.91 |
| MCV [fL] | 53.82 ± 1.33 | 59.04 ± 2.26 | 55.24 ± 1.76 | 59.33 ± 1.92 | 55.36 ± 1.67 | 59.48 ± 2.01 | 56.03 ± 1.74 * | 59.07 ± 2.06 |
| MCH [pg] | 17.31 ± 1.9 | 17.79 ± 0.78 | 18.19 ± 1.01 | 17.93 ± 0.92 | 18.21 ± 0.71 | 17.93 ± 0.93 | 18.51 ± 0.69 | 17.88 ± 0.93 |
| MCHC [g/L] | 321.5 ± 32.8 | 301.2 ± 4.9 | 329 ± 10.5 | 302 ± 7 | 328.8 ± 3.7 | 301.4 ± 6.8 | 330.2 ± 4.4 | 302.9 ± 8.1 |
| Plt [109/L] | 744.6 ± 175.1 | 409.9 ± 94.3 | 808.5 ± 114.9 | 441.9 ± 78.8 | 785.8 ± 110.5 | 427.6 ± 66.5 | 862.7 ± 148.9 | 385.6 ± 110.7 |
| Intact Blood | In Vitro 1 µL Bile | In Vitro 5 µL Bile | In Vivo (Bilhemia) | |||||
|---|---|---|---|---|---|---|---|---|
| Rat | Pig | Rat | Pig | Rat | Pig | Rat | Pig | |
| EI at 3 Pa | 0.372 ± 0.01 | 0.365 ± 0.01 | 0.379 ± 0.02 | 0.355 ± 0.02 | 0.381 ± 0.01 | 0.361 ± 0.02 | 0.389 ± 0.02 * | 0.36 ± 0.02 |
| EImax | 0.568 ± 0.01 | 0.521 ± 0.02 | 0.574 ± 0.01 | 0.503 ± 0.02 | 0.573 ± 0.02 | 0.517 ± 0.03 | 0.571 ± 0.02 | 0.512 ± 0.02 |
| SS1/2 [Pa] | 1.611 ± 0.21 | 1.403 ± 0.27 | 1.609 ± 0.23 | 1.347 ± 0.26 | 1.544 ± 0.24 | 1.355 ± 0.29 | 1.463 ± 0.34 | 1.31 ± 0.23 |
| EImax/SS1/2 [Pa−1] | 0.358 ± 0.04 | 0.383 ± 0.07 | 0.362 ± 0.05 | 0.385 ± 0.07 | 0.381 ± 0.05 | 0.383 ± 0.08 | 0.404 ± 0.07 | 0.402 ± 0.06 |
| Intact Blood | In Vitro 1 µL Bile | In Vitro 5 µL Bile | In Vivo (Bilhemia) | |||||
|---|---|---|---|---|---|---|---|---|
| Rat | Pig | Rat | Pig | Rat | Pig | Rat | Pig | |
| EI min | 0.156 ± 0.012 | 0.147 ± 0.013 | 0.154 ± 0.007 | 0.142 ± 0.014 | 0.159 ± 0.016 | 0.139 ± 0.006 | 0.158 ± 0.014 | 0.145 ± 0.008 |
| EI max | 0.525 ± 0.023 | 0.528 ± 0.005 | 0.532 ± 0.024 | 0.528 ± 0.004 | 0.528 ± 0.019 | 0.528 ± 0.004 | 0.532 ± 0.03 | 0.521 ± 0.008 |
| EI hyper | 0.263 ± 0.012 | 0.264 ± 0.003 | 0.266 ± 0.012 | 0.264 ± 0.002 | 0.264 ± 0.01 | 0.264 ± 0.002 | 0.266 ± 0.015 | 0.261 ± 0.004 |
| O min [mOsm/kg] | 136 ± 6.03 | 182 ± 7.86 | 137.17 ± 7.17 | 182 ± 5.76 | 139.33 ± 6.92 | 179 ± 4.69 | 142.67 ± 5.68 | 183 ± 8.16 |
| O (EI max) [mOsm/kg] | 277 ± 17.4 | 357.88 ± 10.09 | 281.67 ± 21.84 | 354.13 ± 6.56 | 280.33 ± 26.11 | 355.43 ± 9.69 | 288.17 ± 16.14 | 352.25 ± 10.86 |
| O hyper [mOsm/kg] | 428.33 ± 3.14 | 492.83 ± 9.95 | 431 ± 4.16 | 487 ± 6.16 | 435.25 ± 6.24 | 489.8 ± 8.26 | 439.5 ± 3.7 | 491 ± 8.17 |
| Area | 141.85 ± 9.56 | 135.03 ± 3.71 | 143.17 ± 7.33 | 134.33 ± 1.82 | 139.9 ± 7.71 | 135.1 ± 3.13 | 141.92 ± 14.12 | 132.84 ± 4.01 |
| ΔEI | 0.369 ± 0.017 | 0.381 ± 0.016 | 0.379 ± 0.021 | 0.386 ± 0.015 | 0.369 ± 0.009 | 0.388 ± 0.009 | 0.374 ± 0.019 | 0.377 ± 0.015 |
| ΔO | 141 ± 12.92 | 175.88 ± 10.29 | 144.5 ± 17.76 | 172.13 ± 5 | 141 ± 20.83 | 176.43 ± 6.5 | 145.5 ± 14.15 | 169.25 ± 7.2 |
| rEI | 3.38 ± 0.18 | 3.62 ± 0.35 | 3.46 ± 0.16 | 3.75 ± 0.37 | 3.34 ± 0.24 | 3.79 ± 0.19 | 3.38 ± 0.17 | 3.62 ± 0.25 |
| rO | 2.04 ± 0.07 | 1.97 ± 0.08 | 2.05 ± 0.11 | 1.95 ± 0.04 | 2.01 ± 0.12 | 1.99 ± 0.03 | 2.02 ± 0.1 | 1.93 ± 0.06 |
| ΔEI/ΔO | 2.6 × 10−3 ± 3 × 10−4 | 2.2 × 10−3 ± 2 × 10−4 | 2.7 × 10−3 ± 4 × 10−4 | 2.2 × 10−3 ± 1 × 10−4 | 2.7 × 10−3 ± 4 × 10−4 | 2.2 × 10−3 ± 9 × 10−5 | 2.6 × 10−3 ± 3 × 10−4 | 2.2 × 10−3 ± 1 × 10−4 |
| rEI/rO | 1.66 ± 0.11 | 1.84 ± 0.19 | 1.69 ± 0.14 | 1.93 ± 0.19 | 1.66 ± 0.06 | 1.91 ± 0.1 | 1.68 ± 0.14 | 1.88 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matrai, A.A.; Varga, A.; Fazekas, L.A.; Bedocs-Barath, B.; Nellamkuzhi, N.J.; Nghi, T.B.; Nemeth, N.; Deak, A. Effect of Bile on Hemodynamics and Blood Micro-Rheological Parameters in Experimental Models of Bilhemia. Metabolites 2024, 14, 211. https://doi.org/10.3390/metabo14040211
Matrai AA, Varga A, Fazekas LA, Bedocs-Barath B, Nellamkuzhi NJ, Nghi TB, Nemeth N, Deak A. Effect of Bile on Hemodynamics and Blood Micro-Rheological Parameters in Experimental Models of Bilhemia. Metabolites. 2024; 14(4):211. https://doi.org/10.3390/metabo14040211
Chicago/Turabian StyleMatrai, Adam Attila, Adam Varga, Laszlo Adam Fazekas, Barbara Bedocs-Barath, Noel Johny Nellamkuzhi, Tran Bao Nghi, Norbert Nemeth, and Adam Deak. 2024. "Effect of Bile on Hemodynamics and Blood Micro-Rheological Parameters in Experimental Models of Bilhemia" Metabolites 14, no. 4: 211. https://doi.org/10.3390/metabo14040211
APA StyleMatrai, A. A., Varga, A., Fazekas, L. A., Bedocs-Barath, B., Nellamkuzhi, N. J., Nghi, T. B., Nemeth, N., & Deak, A. (2024). Effect of Bile on Hemodynamics and Blood Micro-Rheological Parameters in Experimental Models of Bilhemia. Metabolites, 14(4), 211. https://doi.org/10.3390/metabo14040211







