Exploring Possible Links: Thigh Muscle Mass, Apolipoproteins, and Glucose Metabolism in Peripheral Artery Disease—Insights from a Pilot Sub-Study following Endovascular Treatment
Abstract
1. Introduction
2. Materials and Methods
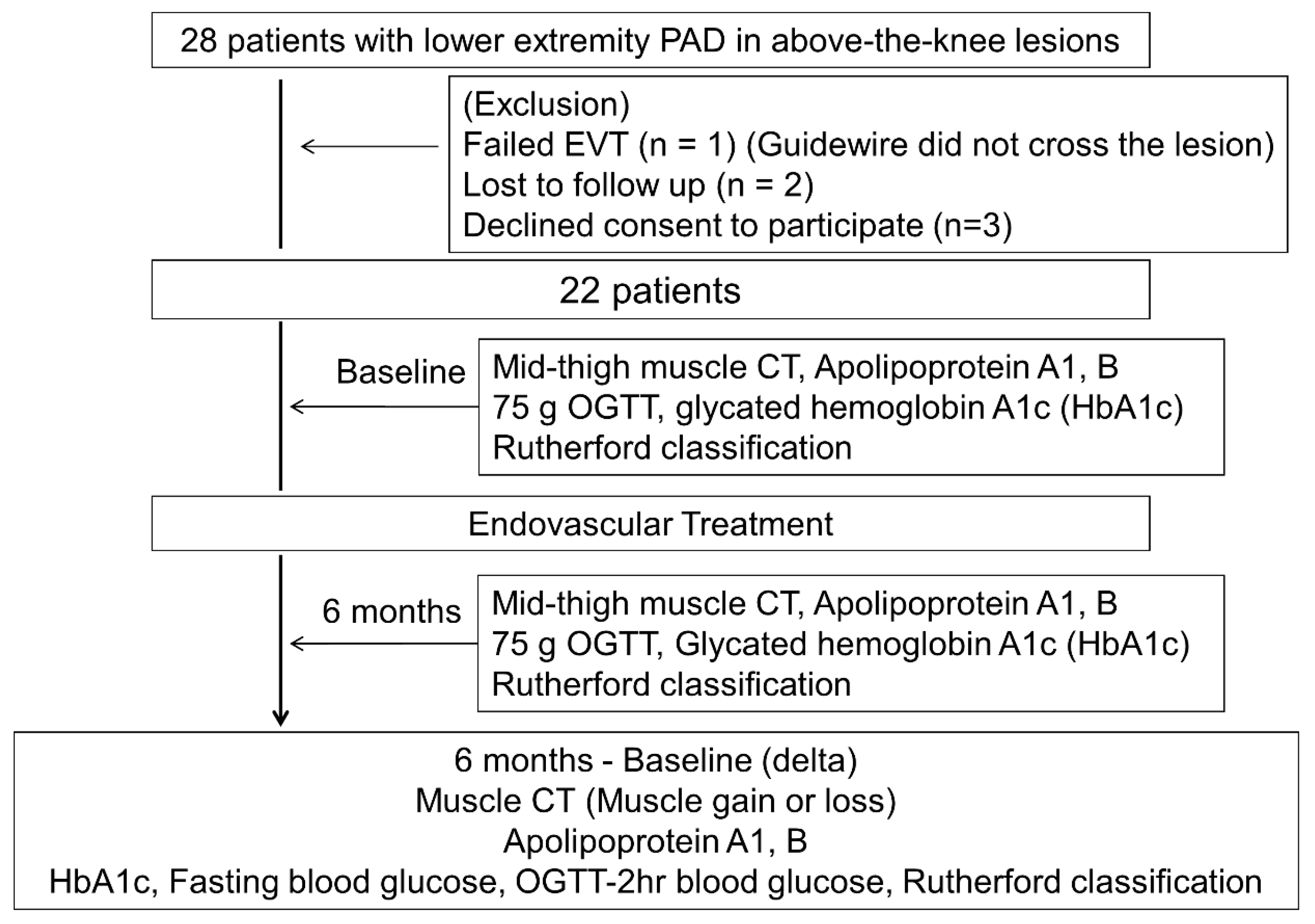
2.1. Study Design
2.2. Ethics
2.3. Endovascular Treatment
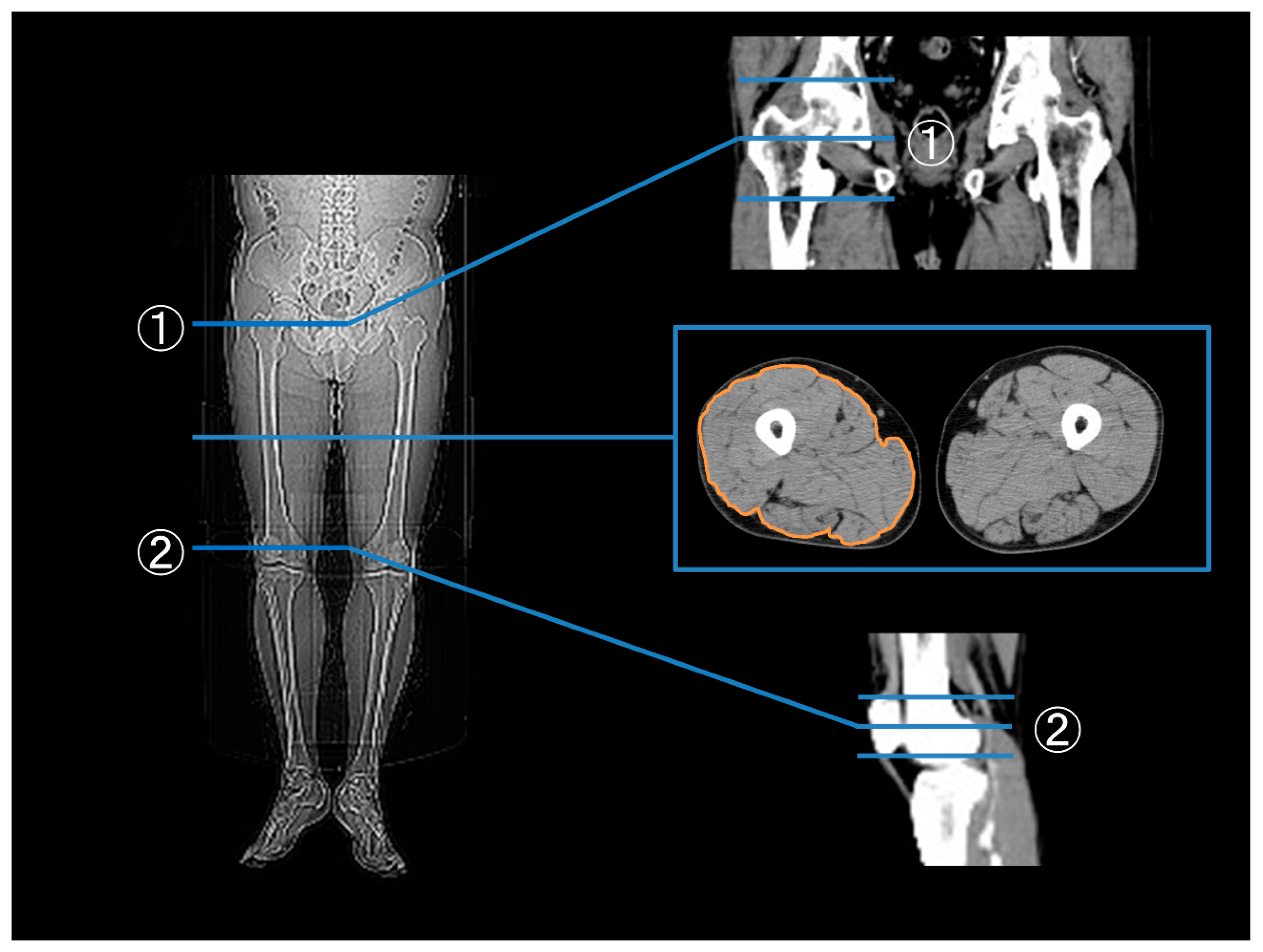
2.4. Measurements of the Thigh Muscle Area, Apo A1, B, OGTT, and Glycated HbA1c
2.5. ABI Measurement
2.6. Statistical Analyses
3. Results
3.1. Thigh Muscle Area before and after EVT
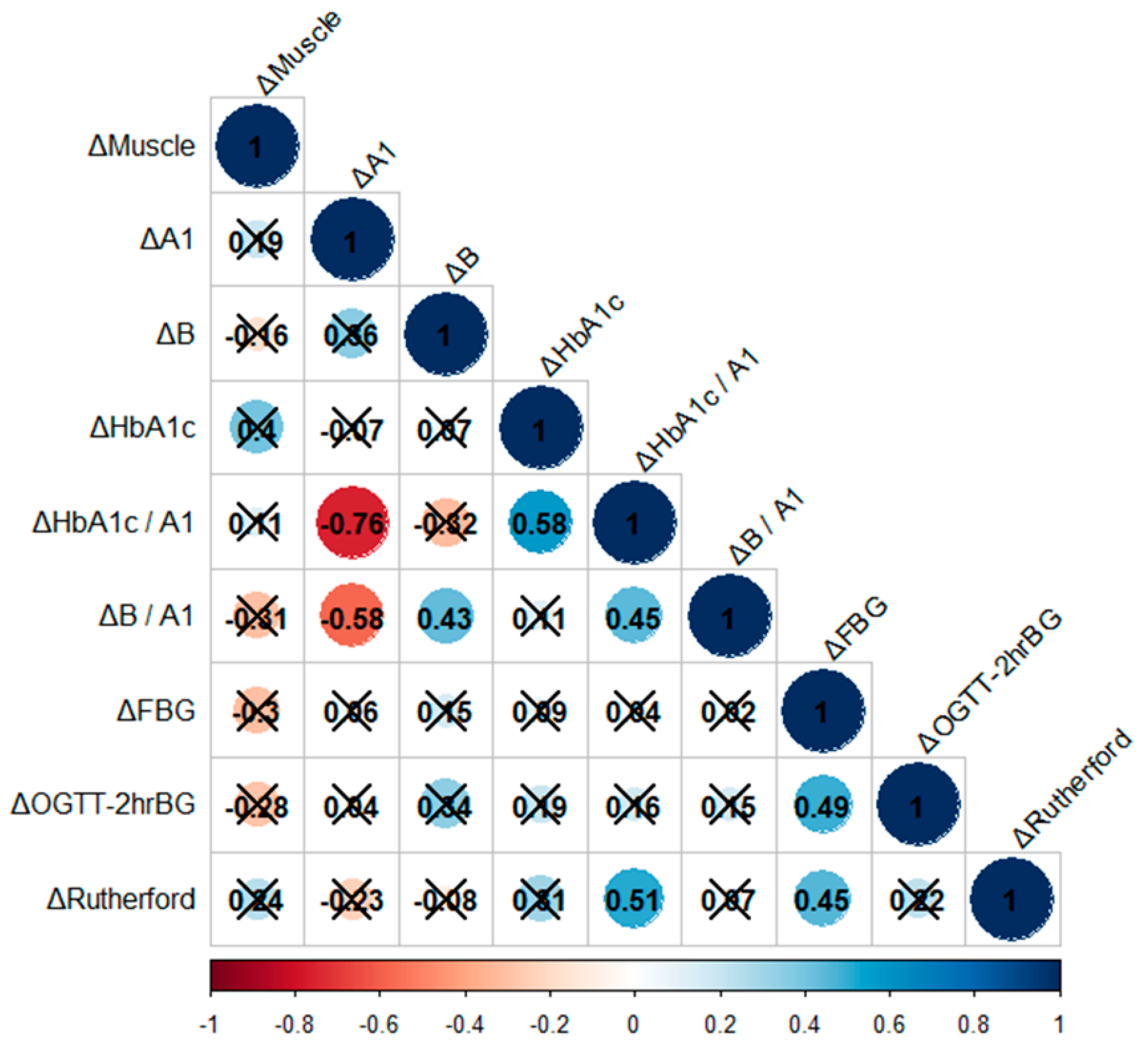
3.2. Correlation Analysis among the Changes in Apo A1, B, Glucose Metabolism, Intermittent Claudication Symptom, and Skeletal Muscle Mass
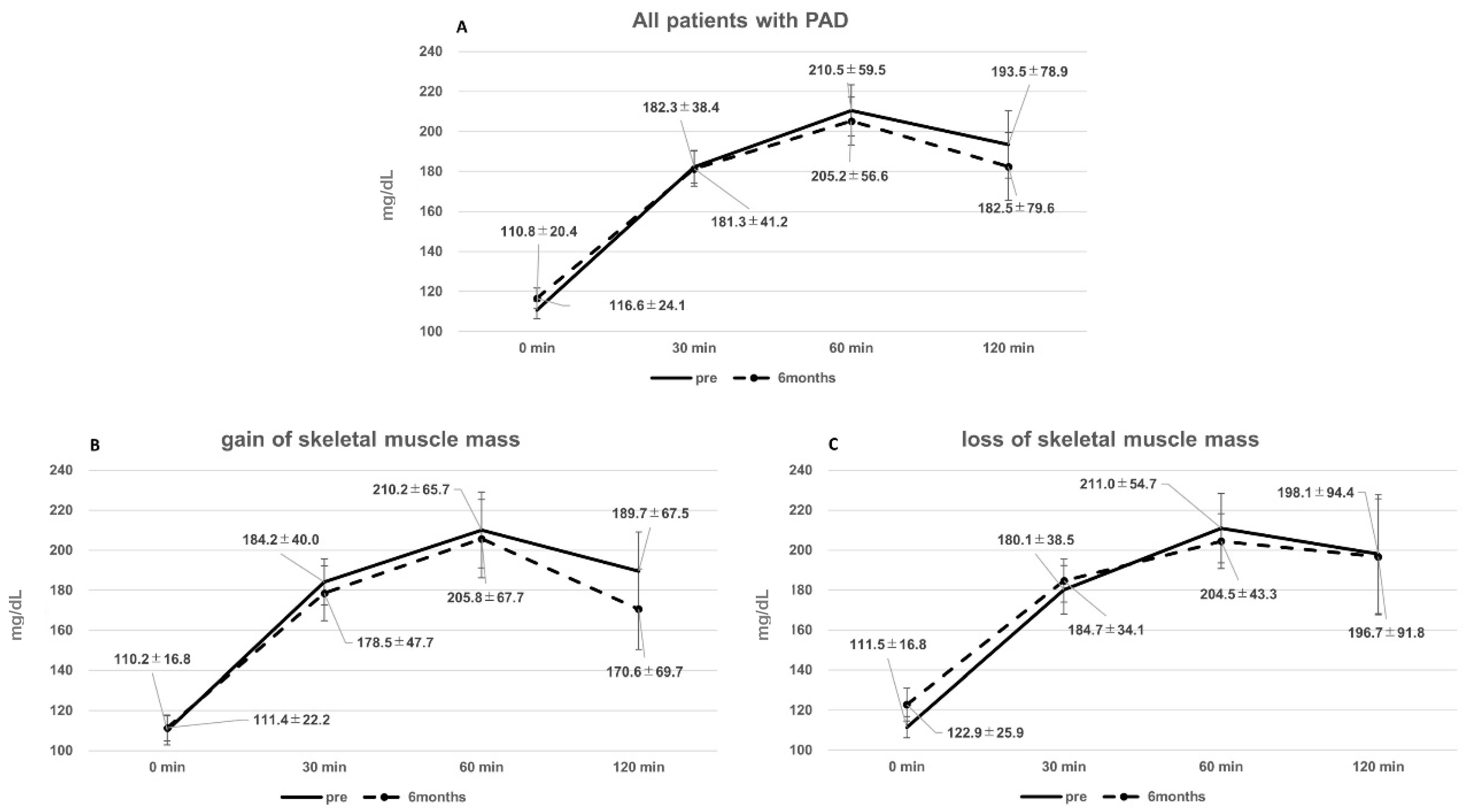
3.3. Glucose Tolerance and Control before and after EVT
3.4. Changes in Apo A1 and B before and after EVT
3.5. Atherosclerotic Metrics of Apo B/Apo A1 and HbA1c/Apo A1
4. Discussion
- There was no significant correlation between changes in muscle area and levels of Apo A1, Apo B, glucose metabolism, and Rutherford classification.
- Apo A1 significantly increased in patients with skeletal muscle gain after EVT, while Apo B did not change in either group.
- Patients with muscle gain showed improved glucose tolerance, whereas patients with muscle loss had increased fasting glucose levels.
4.1. Changes in Apo A1 and B, Glucose Metabolism, Intermittent Claudication Symptom, and Skeletal Muscle Mass
4.2. Atherosclerosis Indicators: HbA1c/Apo A1 and Apo B/Apo A1
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Landolfo, C.; Niebauer, J.; Ozemek, C.; Arena, R.; Lavie, C.J. Promoting physical activity and exercise: JACC health promotion series. J. Am. Coll. Cardiol. 2018, 72, 1622–1639. [Google Scholar] [CrossRef]
- Sniderman, A.; Furberg, C.; Keech, A.; van Lennep, J.R.; Frohlich, J.; Jungner, I.; Walldius, G. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment. Lancet 2003, 361, 777–780. [Google Scholar] [CrossRef]
- Song, F.; Zhou, Y.; Zhang, K.; Liang, Y.-F.; He, X.; Li, L. The role of the plasma glycosylated hemoglobin A1c/Apolipoprotein Al ratio in predicting cardiovascular outcomes in acute coronary syndrome. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 570–578. [Google Scholar] [CrossRef]
- Bodde, M.C.; Hermans, M.P.J.; Jukema, J.W.; Schalij, M.J.; Lijfering, W.M.; Rosendaal, F.R.; Romijn, F.P.H.T.M.; Ruhaak, L.R.; van der Laarse, A.; Cobbaert, C.M. Apolipoproteins A1, B, and apoB/apoA1 ratio are associated with first ST-segment elevation myocardial infarction but not with recurrent events during long-term follow-up. Clin. Res. Cardiol. 2019, 108, 520–538. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ 2020, 370, m2297. [Google Scholar] [CrossRef]
- Temelkova-Kurktschiev, T.S.; Koehler, C.; Henkel, E.; Leonhardt, W.; Fuecker, K.; Hanefeld, M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000, 23, 1830–1834. [Google Scholar] [CrossRef]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef]
- Tang, S.; Tabet, F.; Cochran, B.J.; Torres, L.F.C.; Wu, B.J.; Barter, P.J.; Rye, K.-A. Apolipoprotein AI enhances insulin-dependent and insulin-independent glucose uptake by skeletal muscle. Sci. Rep. 2019, 9, 1350. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Domingo-Espín, J.; Lundsgaard, A.-M.; Kleinert, M.; Israelsen, I.; Carl, C.S.; Nicolaisen, T.S.; Kjøbsted, R.; Jeppesen, J.F.; Wojtaszewski, J.F.; et al. ApoA-1 improves glucose tolerance by increasing glucose uptake into heart and skeletal muscle independently of AMPKα2. Mol. Metab. 2020, 35, 100949. [Google Scholar] [CrossRef]
- Christoffersen, C.; Nielsen, L.B.; Axler, O.; Andersson, A.; Johnsen, A.H.; Dahlbaäck, B. Isolation and characterization of human apolipoprotein M-containing lipoproteins. J. Lipid Res. 2006, 47, 1833–1843. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, B.; Luo, G.; Nilsson-Ehle, P.; Xu, N. Hyperglycemia down-regulates apolipoprotein M expression in vivo and in vitro. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 879–882. [Google Scholar] [CrossRef]
- Xepapadaki, E.; Nikdima, I.; Sagiadinou, E.C.; Zvintzou, E.; Kypreos, K.E. HDL and type 2 diabetes: The chicken or the egg? Diabetologia 2021, 64, 1917–1926. [Google Scholar] [CrossRef]
- Miyakuni, T.; Komiyama, H.; Takano, M.; Ikeda, T.; Matsushita, M.; Kobayashi, N.; Otsuka, T.; Miyauchi, Y.; Asai, K.; Seino, Y.; et al. A preliminary pilot study investigating the impact of endovascular treatment on leg muscle volume in peripheral artery disease and its relation to baseline glycemic control. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 269–276. [Google Scholar] [CrossRef]
- MacDonald, T.L.; Pattamaprapanont, P.; Pathak, P.; Fernandez, N.; Freitas, E.C.; Hafida, S.; Mitri, J.; Britton, S.L.; Koch, L.G.; Lessard, S.J. Hyperglycaemia is associated with impaired muscle signalling and aerobic adaptation to exercise. Nat. Metab. 2020, 2, 902–917. [Google Scholar] [CrossRef]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 2019, 4, e124952. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Marsigliante, S. The effects of exercise training on lipid metabolism and coronary heart disease. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H76–H88. [Google Scholar] [CrossRef]
- Hardman, R.L.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. Overview of classification systems in peripheral artery disease. Semin. Interv. Radiol. 2014, 31, 378–388. [Google Scholar] [CrossRef]
- Kittiskulnam, P.; Carrero, J.J.; Chertow, G.M.; Kaysen, G.A.; Delgado, C.; Johansen, K.L. Sarcopenia among patients receiving hemodialysis: Weighing the evidence. J. Cachexia Sarcopenia Muscle 2017, 8, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, M.; Meyer, A.; Charles, A.L.; Giannini, M.; Chakfé, N.; Lejay, A.; Geny, B. Sarcopenia and peripheral arterial disease: A systematic review. J. Cachexia Sarcopenia Muscle 2020, 11, 866–886. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.F.; Berends, B.; Vedder, I.R.; Levolger, S.; Gupta, M.; Schuurmann, R.C.; de Vries, J.-P.P.M.; Bokkers, R.P. Quantification of muscle mass in the legs of patients with peripheral arterial occlusive disease: Associations between volumetric and cross-sectional single-slice measurements for identification of atrophy and focal sarcopenia. J. Cardiovasc. Surg. 2019, 60, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; LeBLANC, P.; Whittom, F.; Carrier, G.; Jobin, J.; Belleau, R.; Maltais, F. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Abrams, J.; Aurigemma, G.P.; Bond, M.G.; Clark, L.T.; Criqui, M.H.; Crouse, J.R., 3rd; Friedman, L.; Fuster, V.; Herrington, D.M.; et al. Prevention Conference V: Beyond secondary prevention: Identifying the high-risk patient for primary prevention: Noninvasive tests of atherosclerotic burden: Writing Group III. Circulation 2000, 101, E16–E22. [Google Scholar] [CrossRef] [PubMed]
- Champely, S.; Ekstrom, C.; Dalgaard, P.; Gill, J.; Weibelzahl, S.; Anandkumar, A. Package ‘pwr’. R Package Version. Package ‘pwr’ R Package Version. 2018, 1(2). Available online: https://cran.r-project.org/web/packages/pwr/ (accessed on 11 March 2024).
- Schwartz, R.S. Effects of exercise training on high density lipoproteins and apolipoprotein AI in old and young men. Metab.-Clin. Exp. 1988, 37, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Balducci, S.; Guidetti, L.; Mazzuca, P.; Rossi, E.; Schena, F. Walking for subjects with type 2 diabetes: A systematic review and joint AMD/SID/SISMES evidence-based practical guideline. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1882–1898. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; Macfadyen, J.G.; et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 2009, 373, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Vanags, L.Z.; Wong, N.K.; Nicholls, S.J.; Bursill, C.A. High-Density Lipoproteins and Apolipoprotein A-I Improve Stent Biocompatibility. Arter. Thromb. Vasc. Biol. 2018, 38, 1691–1701. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Arsenault, B.J.; Hovingh, G.K.; Mora, S.; Pedersen, T.R.; Larosa, J.C.; Welch, K.M.; Amarenco, P.; Demicco, D.A.; Tonkin, A.M.; et al. Levels and changes of HDL cholesterol and apolipoprotein AI in relation to risk of cardiovascular events among statin-treated patients: A meta-analysis. Circulation 2013, 128, 1504–1512. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef]
- Care, D. 6. Glycemic targets: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42 (Suppl. 1), S61–S70. [Google Scholar]
- O’Connor, S.; Turcotte, A.-F.; Gagnon, C.; Rudkowska, I. Increased dairy product intake modifies plasma glucose concentrations and glycated hemoglobin: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2019, 10, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.P.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Fiatarone, M.A.; Fielding, R.A.; Kahn, B.B.; Ferrara, C.M.; Shepherd, P.; Fisher, E.C.; Wolfe, R.R.; Elahi, D.; Evans, W.J. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am. J. Physiol. 1993, 264, E855–E862. [Google Scholar] [CrossRef] [PubMed]
- Liem, A.H.; van de Woestijne, A.P.; van Lennep, H.W.O.R.; Zwinderman, A.H.; van der Steeg, W.A.; Jukema, J.W. ApoB/A1 and LDL-C/HDL-C and the prediction of cardiovascular risk in statin-treated patients. Curr. Med. Res. Opin. 2008, 24, 359–364. [Google Scholar] [CrossRef]
- Takahara, M.; the J-EVT and J-PCI investigators; Iida, O.; Kohsaka, S.; Soga, Y.; Fujihara, M.; Shinke, T.; Amano, T.; Ikari, Y. Diabetes mellitus and other cardiovascular risk factors in lower-extremity peripheral artery disease versus coronary artery disease: An analysis of 1,121,359 cases from the nationwide databases. Cardiovasc. Diabetol. 2019, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Armstrong, D.G.; Albert, S.F.; Frykberg, R.G.; Hellman, R.; Kirkman, M.S.; Lavery, L.A.; LeMaster, J.W.; Mills, J.L.; Mueller, M.J.; et al. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008, 31, 1679–1685. [Google Scholar] [CrossRef]
- Volpato, S.; Bianchi, L.; Lauretani, F.; Lauretani, F.; Bandinelli, S.; Guralnik, J.M.; Zuliani, G.; Ferrucci, L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care 2012, 35, 1672–1679. [Google Scholar] [CrossRef]
- Grøvle, L.; Haugen, A.J.; Natvig, B.; Brox, J.I.; Grotle, M. The prognosis of self-reported paresthesia and weakness in disc-related sciatica. Eur. Spine J. 2013, 22, 2488–2495. [Google Scholar] [CrossRef]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Spertus, J.A.; Winder, J.A.; Dewhurst, T.A.; Deyo, R.A.; Prodzinski, J.; McDonnell, M.; Fihn, S.D. Development and evaluation of the Seattle Angina Questionnaire: A new functional status measure for coronary artery disease. J. Am. Coll. Cardiol. 1995, 25, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, T.S.; McDermott, M.M. Lower Extremity Peripheral Artery Disease Without Chronic Limb-Threatening Ischemia: A Review. JAMA 2021, 325, 2188–2198. [Google Scholar] [CrossRef]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; the Trend Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef]
| All | Gain of Skeletal Muscle | Loss of Skeletal Muscle | p-Value | |
|---|---|---|---|---|
| Number of patients | 22 | 12 | 10 | |
| Age (years) | 72.4 ± 7.4 | 69.7 ± 7.5 | 75.6 ± 6.2 | 0.06 |
| Male | 20 (91) | 12 (100) | 8 (80) | N.S |
| Female | 2 (9) | 0 (0) | 2 (20) | |
| Hypertension | 18 (82) | 10 (83) | 8 (80) | N.S |
| Dyslipidemia | 19 (86) | 10 (83) | 8 (80) | N.S |
| Diabetes | 10 (45) | 6 (50) | 4 (40) | N.S |
| HbA1c (%) | 6.1 ± 0.7 | 5.8 ± 0.4 * | 6.5 ± 0.8 * | 0.0175 * |
| BMI | 24.4 ± 3.5 | 25.1 ± 2.6 | 23.5 ± 4.4 | N.S |
| Past smoker | 10 (45) | 6 (50) | 4 (40) | N.S |
| Rutherford classification | 1.5 ± 0.6 | 1.6 ± 0.5 | 1.5 ± 0.7 | N.S |
| Medications | ||||
| Dual antiplatelet therapy | 22 (100) | 12 (100) | 10 (100) | N.S |
| Aspirin | 19 (86) | 10 (83) | 9 (90) | N.S |
| Clopidogrel | 9 (41) | 5 (42) | 4 (40) | N.S |
| Cilostazol | 6 (27) | 2 (8) | 4 (40) | N.S |
| Prasugrel | 6 (27) | 4 (33) | 2 (20) | N.S |
| Statin | 21 (95) | 11 (92) | 10 (100) | N.S |
| ACE-I/ARB | 22 (100) | 12 (100) | 10 (100) | N.S |
| β-blocker | 15 (68) | 8 (67) | 7 (70) | N.S |
| Anti-diabetic drugs | 9 (41) | 5 (42) | 4 (40) | N.S |
| Voglibose | 2 (9) | 1 (8) | 1 (10) | N.S |
| Metformin | 3 (14) | 2 (17) | 1 (10) | N.S |
| Glimepride | 1 (5) | 0 (0) | 1 (10) | N.S |
| DPP-4 inhibitor | 8 (36) | 5 (42) | 3 (30) | N.S |
| SGLT-2 inhibitor | 1 (5) | 0 (0) | 1 (10) | N.S |
| Thiazolidine | 1 (5) | 0 (0) | 1 (10) | N.S |
| Glinide | 2 (9) | 1 (8) | 1 (8) | N.S |
| Variables | All | Gain of Skeletal Muscle | Loss of Skeletal Muscle | p-Value |
|---|---|---|---|---|
| Location of lesions | N = 30 | N = 17 | N = 13 | |
| Unilateral lesion Bilateral lesions | 14 (64) 8 (36) | 7 (58) 5 (42) | 7 (70) 3 (30) | N.S |
| Iliac artery | 16 (53) | 10 (59) | 6 (46) | N.S |
| Superficial femoral artery | 9 (30) | 6 (35) | 5 (38) | N.S |
| Common femoral artery | 1 (3) | 0 (0) | 1 (8) | N.S |
| Popliteal artery | 3 (13) | 1 (6) | 0 (0) | N.S |
| Superficial + popliteal artery | 1 (5) | 0 (0) | 1 (8) | N.S |
| TASC II classification | ||||
| Type A | 17 (58) | 10 (59) | 7 (54) | N.S |
| Type B | 6 (20) | 3 (18) | 3 (23) | N.S |
| Type C | 2 (6) | 0 (0) | 2 (15) | N.S |
| Type D | 5 (16) | 4 (23) | 1 (8) | N.S |
| Stent implantation | 24 (80) | 15 (88) | 9 (70) | N.S |
| All (n = 22) | Before | After 6 Months | p-Value | Delta Value |
|---|---|---|---|---|
| Muscle area (cm2) | 239.8 ± 34.8 | 243.1 ± 40.8 | 0.21 | 2.5 ± 8.1 |
| Apo A1 (mg/dL) | 119.0 ± 17.4 ** | 129.6 ± 19.6 ** | 0.0027 ** | 10.7 ± 14.7 |
| Apo B (mg/dL) | 76.3 ± 17.3 * | 81.3 ± 17.7 * | 0.0404 * | 5.0 ± 9.4 |
| HbA1c (%) | 6.1 ± 0.7 | 6.2 ± 0.8 | 0.74 | 0.073 ± 0.517 |
| Apo B/Apo A1 | 0.65 ± 0.16 | 0.64 ± 0.16 | 0.11 | −0.012 ± 0.081 |
| HbA1c/Apo A1 | 0.053 ± 0.011 * | 0.049 ± 0.011 * | 0.0489 * | 0.073 ± 0.517 |
| Fasting blood glucose (mg/dL) | 110.8 ± 20.4 | 116.6 ± 24.1 | 0.24 | 5.9 ± 22.5 |
| 2 h -OGTT blood glucose (mg/dL) | 193.5 ± 78.9 | 182.5 ± 79.6 | 0.29 | −11.0 ± 48.0 |
| Rutherford classification | 1.5 ± 0.6 *** | 0.2 ± 0.4 *** | p *** < 0.001 | −1.4 ± 0.8 |
| Gain of Skeletal Muscle n = 12 | Loss of Skeletal Muscle n = 10 | |||||
|---|---|---|---|---|---|---|
| Before | 6 Months | p-Value | Before | 6 Months | p-Value | |
| Delta muscle area (cm2) | NA | 8.41 ± 5.93 | NA | NA | −4.67 ± 2.41 | NA |
| Apo A1 (mg/dL) | 121.8 ± 15.1 *** | 136.5 ± 19.5 *** | p *** < 0.001 | 115.6 ± 20.1 | 121.4 ± 17.1 | 0.32 |
| Apo B (mg/dL) | 76.4 ± 19.2 | 80.5 ± 17.0 | 0.10 | 78.6 ± 19.8 | 82.3 ± 19.3 | 0.24 |
| HbA1c (%) | 5.8 ± 0.4 | 6.0 ± 0.4 | 0.97 | 6.5 ± 0.8 | 6.5 ± 1.1 | 0.50 |
| Apo B/Apo A1 | 0.64 ± 0.17 | 0.60 ± 0.17 | 0.069 | 0.69 ± 0.19 | 0.68 ± 0.16 | 0.66 |
| HbA1c/Apo A1 | 0.049 ± 0.007 ** | 0.045 ± 0.008 ** | 0.007 ** | 0.058 ± 0.004 | 0.054 ± 0.004 | 0.37 |
| Fasting blood glucose (mg/dL) | 110.2 ± 16.8 | 111.4 ± 22.2 | 0.83 | 111.5 ± 16.8 | 122.9 ± 25.9 | 0.094 |
| 2 h-OGTT blood glucose (mg/dL) | 189.7 ± 67.5 | 170.6 ± 69.7 | 0.075 | 198.1 ± 94.4 | 196.7 ± 91.8 | 0.93 |
| Rutherford classification | 1.6 ± 0.5 *** | 0.3 ± 0.5 *** | p *** < 0.001 | 1.5 ± 0.7 *** | 0.1 ± 0.3 *** | p *** < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, T.; Komiyama, H.; Miyakuni, T.; Takano, M.; Asai, K. Exploring Possible Links: Thigh Muscle Mass, Apolipoproteins, and Glucose Metabolism in Peripheral Artery Disease—Insights from a Pilot Sub-Study following Endovascular Treatment. Metabolites 2024, 14, 192. https://doi.org/10.3390/metabo14040192
Ikeda T, Komiyama H, Miyakuni T, Takano M, Asai K. Exploring Possible Links: Thigh Muscle Mass, Apolipoproteins, and Glucose Metabolism in Peripheral Artery Disease—Insights from a Pilot Sub-Study following Endovascular Treatment. Metabolites. 2024; 14(4):192. https://doi.org/10.3390/metabo14040192
Chicago/Turabian StyleIkeda, Takeshi, Hidenori Komiyama, Tomoyo Miyakuni, Masamichi Takano, and Kuniya Asai. 2024. "Exploring Possible Links: Thigh Muscle Mass, Apolipoproteins, and Glucose Metabolism in Peripheral Artery Disease—Insights from a Pilot Sub-Study following Endovascular Treatment" Metabolites 14, no. 4: 192. https://doi.org/10.3390/metabo14040192
APA StyleIkeda, T., Komiyama, H., Miyakuni, T., Takano, M., & Asai, K. (2024). Exploring Possible Links: Thigh Muscle Mass, Apolipoproteins, and Glucose Metabolism in Peripheral Artery Disease—Insights from a Pilot Sub-Study following Endovascular Treatment. Metabolites, 14(4), 192. https://doi.org/10.3390/metabo14040192








