Plant-Derived Senotherapeutics for the Prevention and Treatment of Intervertebral Disc Degeneration and Aging
Abstract
1. Introduction
2. Intervertebral Disc—Intervertebral Disc Degeneration
3. Cellular Senescence Is a Main Etiologic Factor and/or Contributor in IDD Pathogenesis
4. Senotherapeutics
5. Plant-Derived Compounds with a Reported Senotherapeutic Activity in the IVD
5.1. Apigenin
5.2. Butein
5.3. p-Coumaric Acid
5.4. Curcumin
5.5. Dehydrocostus Lactone
5.6. 20-Deoxyingenol
5.7. Eupatilin
5.8. Evodiamine
5.9. Fisetin
5.10. Honokiol
5.11. Kaempferol
5.12. Kinsenoside
5.13. Luteolin
5.14. Morroniside
5.15. Myricetin
5.16. Polydatin
5.17. Proanthocyanidins
5.18. Quercetin
5.19. Resveratrol
5.20. o-Vanillin
6. Plant-Derived Metabolites with a Potential Senotherapeutic Role against IDD
7. Perspectives, Challenges and Practical Concerns
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, S.; Lam, E.W.; Tchkonia, T.; Kirkland, J.L.; Sun, Y. Senescent Cells: Emerging Targets for Human Aging and Age-Related Diseases. Trends Biochem. Sci. 2020, 45, 578–592. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Mannarino, M.; Wu-Martinez, O.; Sheng, K.; Li, L.; Navarro-Ramirez, R.; Jarzem, P.; Ouellet, J.A.; Cherif, H.; Haglund, L. Senolytic Combination Treatment Is More Potent Than Single Drugs in Reducing Inflammatory and Senescence Burden in Cells from Painful Degenerating IVDs. Biomolecules 2023, 13, 1257. [Google Scholar] [CrossRef]
- The Lancet Rheumatology. The global epidemic of low back pain. Lancet Rheumatol. 2023, 5, e305. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shao, T.; Lou, J.; Zhang, J.; Xia, C. Aging, cell senescence, the pathogenesis and targeted therapies of intervertebral disc degeneration. Front. Pharmacol. 2023, 14, 1172920. [Google Scholar] [CrossRef]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.R.; Sakai, D.; Nakai, T.; Toyama, K.; Mochida, J.; Alini, M.; Grad, S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur. Spine J. 2007, 16, 2174–2185. [Google Scholar] [CrossRef]
- Urban, J.P. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002, 30, 858–864. [Google Scholar] [CrossRef]
- Kouroumalis, A.; Mavrogonatou, E.; Savvidou, O.D.; Papagelopoulos, P.J.; Pratsinis, H.; Kletsas, D. Major traits of the senescent phenotype of nucleus pulposus intervertebral disc cells persist under the specific microenvironmental conditions of the tissue. Mech. Ageing Dev. 2019, 177, 118–127. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Kletsas, D. High osmolality activates the G1 and G2 cell cycle checkpoints and affects the DNA integrity of nucleus pulposus intervertebral disc cells triggering an enhanced DNA repair response. DNA Repair 2009, 8, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Dimozi, A.; Mavrogonatou, E.; Sklirou, A.; Kletsas, D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cells Mater. 2015, 30, 89–102; discussion 103. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Zhang, G.Z.; Liu, M.Q.; Zhang, L.J.; Kang, J.H.; Wang, Z.H.; Liu, W.Z.; Lin, A.X.; Kang, X.W. Natural Products of Pharmacology and Mechanisms in Nucleus Pulposus Cells and Intervertebral Disc Degeneration. Evid.-Based Complement. Altern. Med. 2021, 2021, 9963677. [Google Scholar] [CrossRef] [PubMed]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef]
- Perera, R.S.; Dissanayake, P.H.; Senarath, U.; Wijayaratne, L.S.; Karunanayake, A.L.; Dissanayake, V.H.W. Associations between disc space narrowing, anterior osteophytes and disability in chronic mechanical low back pain: A cross sectional study. BMC Musculoskelet. Disord. 2017, 18, 193. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Nakamae, T.; Nakanishi, K.; Tamura, T.; Tsuchikawa, Y.; Morisako, T.; Harada, T.; Maruyama, T.; Adachi, N. Evaluation of intervertebral disc degeneration using T2 signal ratio on magnetic resonance imaging. Eur. J. Radiol. 2022, 152, 110358. [Google Scholar] [CrossRef] [PubMed]
- Bouhsina, N.; Decante, C.; Hardel, J.B.; Rouleau, D.; Abadie, J.; Hamel, A.; Le Visage, C.; Lesoeur, J.; Guicheux, J.; Clouet, J.; et al. Comparison of MRI T1, T2, and T2* mapping with histology for assessment of intervertebral disc degeneration in an ovine model. Sci. Rep. 2022, 12, 5398. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Pratsinis, H.; Papadopoulou, A.; Karamanos, N.K.; Kletsas, D. Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol. J. Int. Soc. Matrix Biol. 2019, 75–76, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Papadopoulou, A.; Pratsinis, H.; Kletsas, D. Senescence-associated alterations in the extracellular matrix: Deciphering their role in the regulation of cellular function. Am. J. Physiol. Cell Physiol. 2023, 325, C633–C647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, B.; Wang, J.; Cheng, F.; Shi, K.; Ying, L.; Wang, C.; Xia, K.; Huang, X.; Gong, Z.; et al. Cell Senescence: A Nonnegligible Cell State under Survival Stress in Pathology of Intervertebral Disc Degeneration. Oxidative Med. Cell. Longev. 2020, 2020, 9503562. [Google Scholar] [CrossRef]
- Tominaga, K. The emerging role of senescent cells in tissue homeostasis and pathophysiology. Pathobiol. Aging Age Relat. Dis. 2015, 5, 27743. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Evans, E.H.; Kletsas, D.; Jaffray, D.C.; Eisenstein, S.M. Senescence in human intervertebral discs. Eur. Spine J. 2006, 15 (Suppl. S3), S312–S316. [Google Scholar] [CrossRef]
- Veroutis, D.; Kouroumalis, A.; Lagopati, N.; Polyzou, A.; Chamilos, C.; Papadodima, S.; Evangelou, K.; Gorgoulis, V.G.; Kletsas, D. Evaluation of senescent cells in intervertebral discs by lipofuscin staining. Mech. Ageing Dev. 2021, 199, 111564. [Google Scholar] [CrossRef] [PubMed]
- Kletsas, D. Senescent cells in the intervertebral disc: Numbers and mechanisms. Spine J. 2009, 9, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013, 13, 331–341. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Kouroumalis, A.; Papadopoulou, A.; Pratsinis, H.; Kletsas, D. Cell-based therapies for the regeneration of the intervertebral disc: Promises and challenges. Acta Orthop. Traumatol. Hell. 2021, 72, 21–29. [Google Scholar]
- Tosteson, A.N.; Tosteson, T.D.; Lurie, J.D.; Abdu, W.; Herkowitz, H.; Andersson, G.; Albert, T.; Bridwell, K.; Zhao, W.; Grove, M.R.; et al. Comparative effectiveness evidence from the spine patient outcomes research trial: Surgical versus nonoperative care for spinal stenosis, degenerative spondylolisthesis, and intervertebral disc herniation. Spine 2011, 36, 2061–2068. [Google Scholar] [CrossRef]
- Patil, P.; Dong, Q.; Wang, D.; Chang, J.; Wiley, C.; Demaria, M.; Lee, J.; Kang, J.; Niedernhofer, L.J.; Robbins, P.D.; et al. Systemic clearance of p16(INK4a)-positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell 2019, 18, e12927. [Google Scholar] [CrossRef]
- Che, H.; Li, J.; Li, Y.; Ma, C.; Liu, H.; Qin, J.; Dong, J.; Zhang, Z.; Xian, C.J.; Miao, D.; et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. eLife 2020, 9, e52570. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Xu, M.; Bradley, E.W.; Weivoda, M.M.; Hwang, S.M.; Pirtskhalava, T.; Decklever, T.; Curran, G.L.; Ogrodnik, M.; Jurk, D.; Johnson, K.O.; et al. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 780–785. [Google Scholar] [CrossRef]
- Li, W.; Qin, L.; Feng, R.; Hu, G.; Sun, H.; He, Y.; Zhang, R. Emerging senolytic agents derived from natural products. Mech. Ageing Dev. 2019, 181, 1–6. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Wu, C.Y.; Peng, H.H.; Voisin, L.; Perfettini, J.L.; Ko, Y.F.; Young, J.D. Emerging use of senolytics and senomorphics against aging and chronic diseases. Med. Res. Rev. 2020, 40, 2114–2131. [Google Scholar] [CrossRef] [PubMed]
- Mbara, K.C.; Devnarain, N.; Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharm. Med. 2022, 36, 331–352. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Deng, Y.; Chen, Y.; Wu, C.; Zhao, X.; Chen, X.; Wang, X.; Zhou, Y.; Zhang, X.; et al. Fisetin suppresses ferroptosis through Nrf2 and attenuates intervertebral disc degeneration in rats. Eur. J. Pharmacol. 2023, 964, 176298. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Roy, N.K.; Bordoloi, D.; Arfuso, F.; Mishra, S.; Sethi, G.; Bishayee, A.; Kunnumakkara, A.B. Butein in health and disease: A comprehensive review. Phytomedicine 2017, 25, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Shi, Y.; Chen, Z.; Zhou, X.; Luo, P.; Hong, C.; Tian, N.; Wu, Y.; Zhou, Y.; Lin, Y.; et al. Apigenin Alleviates Intervertebral Disc Degeneration via Restoring Autophagy Flux in Nucleus Pulposus Cells. Front. Cell Dev. Biol. 2021, 9, 787278. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, J.; Nisar, M.; Chen, T.; Xu, T.; Zheng, G.; Wang, C.; Jin, H.; Chen, J.; Gao, W.; et al. The Sirt1/P53 Axis in Diabetic Intervertebral Disc Degeneration Pathogenesis and Therapeutics. Oxidative Med. Cell. Longev. 2019, 2019, 7959573. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Li, Y.; Wang, Z.; Hang, K.; Ye, Z. p-Coumaric acid suppresses reactive oxygen species-induced senescence in nucleus pulposus cells. Exp. Ther. Med. 2022, 23, 183. [Google Scholar] [CrossRef]
- Kang, L.; Xiang, Q.; Zhan, S.; Song, Y.; Wang, K.; Zhao, K.; Li, S.; Shao, Z.; Yang, C.; Zhang, Y. Restoration of Autophagic Flux Rescues Oxidative Damage and Mitochondrial Dysfunction to Protect against Intervertebral Disc Degeneration. Oxidative Med. Cell. Longev. 2019, 2019, 7810320. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, Y.; Yurube, T.; Kakutani, K.; Takada, T.; Ito, M.; Takeoka, Y.; Kanda, Y.; Miyazaki, S.; Kuroda, R.; Nishida, K. Pharmacological inhibition of mTORC1 but not mTORC2 protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy induction. Osteoarthr. Cartil. 2019, 27, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Cherif, H.; Bisson, D.G.; Jarzem, P.; Weber, M.; Ouellet, J.A.; Haglund, L. Curcumin and o-Vanillin Exhibit Evidence of Senolytic Activity in Human IVD Cells In Vitro. J. Clin. Med. 2019, 8, 433. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Zhou, Y.; Liang, Z.; Chen, C.; Han, C.; Cao, X.; He, W.; Zhang, K.; Qin, A.; et al. Dehydrocostus Lactone Attenuates the Senescence of Nucleus Pulposus Cells and Ameliorates Intervertebral Disc Degeneration via Inhibition of STING-TBK1/NF-κB and MAPK Signaling. Front. Pharmacol. 2021, 12, 641098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, C.; Zhao, X.; Tan, H.; Li, C.; Deng, Y.; Chen, X.; Wu, Y.; Tian, N.; Zhang, X.; et al. 20-Deoxyingenol alleviates intervertebral disc degeneration by activating TFEB in nucleus pulposus cells. Biochem. Pharmacol. 2023, 218, 115865. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, X.; Rong, K.; Liang, J.; Wang, Z.; Zhao, J.; Zhang, P.; Li, Y.; Wang, L.; Ma, H.; et al. Eupatilin attenuates the senescence of nucleus pulposus cells and mitigates intervertebral disc degeneration via inhibition of the MAPK/NF-κB signaling pathway. Front. Pharmacol. 2022, 13, 940475. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Gu, X.; Pan, R.; Huang, W.; Dong, S. Evodiamine ameliorates intervertebral disc degeneration through the Nrf2 and MAPK pathways. Cytotechnology 2023, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Nisar, M.; Huang, C.; Pan, X.; Lin, D.; Zheng, G.; Jin, H.; Chen, D.; Tian, N.; Huang, Q.; et al. Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Liu, F.; Wang, J.; Liu, F.; Zhang, Q.; Li, J. Pharmacological network analysis of the functions and mechanism of kaempferol from Du Zhong in intervertebral disc degeneration (IDD). J. Orthop. Transl. 2023, 39, 135–146. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Y.; Li, M. Systematic Elaboration of the Pharmacological Targets and Potential Mechanisms of ZhiKe GanCao Decoction for Preventing and Delaying Intervertebral Disc Degeneration. Evid.-Based Complement. Altern. Med. 2022, 2022, 8786052. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, R.; Wang, Z.; Luo, L.; Wu, J.; Zhang, C.; Liu, M.; Shi, C.; Zhou, Y. Kinsenoside ameliorates intervertebral disc degeneration through the activation of AKT-ERK1/2-Nrf2 signaling pathway. Aging 2019, 11, 7961–7977. [Google Scholar] [CrossRef]
- Xie, T.; Yuan, J.; Mei, L.; Li, P.; Pan, R. Luteolin suppresses TNF-α-induced inflammatory injury and senescence of nucleus pulposus cells via the Sirt6/NF-κB pathway. Exp. Ther. Med. 2022, 24, 469. [Google Scholar] [CrossRef]
- Zhou, C.; Yao, S.; Fu, F.; Bian, Y.; Zhang, Z.; Zhang, H.; Luo, H.; Ge, Y.; Chen, Y.; Ji, W.; et al. Morroniside attenuates nucleus pulposus cell senescence to alleviate intervertebral disc degeneration via inhibiting ROS-Hippo-p53 pathway. Front. Pharmacol. 2022, 13, 942435. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, X.; Zhu, X.; Wang, C.; Xu, W. Myricetin alleviated hydrogen peroxide-induced cellular senescence of nucleus pulposus cell through regulating SERPINE1. J. Orthop. Surg. Res. 2023, 18, 143. [Google Scholar] [CrossRef]
- Xie, T.; Pan, R.; Huang, W.; Dong, S.; Wu, S.; Ye, Y. Myricetin alleviates H2O2-induced senescence and apoptosis in rat nucleus pulposus-derived mesenchymal stem cells. Folia Histochem. Cytobiol. 2023, 61, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, C.; Lin, Z.; Pan, X.; Chen, J.; Zheng, G.; Tian, N.; Yan, Y.; Zhang, Z.; Hu, J.; et al. Polydatin suppresses nucleus pulposus cell senescence, promotes matrix homeostasis and attenuates intervertebral disc degeneration in rats. J. Cell. Mol. Med. 2018, 22, 5720–5731. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Liu, M.Q.; Zhang, G.Z.; Zhang, C.Y.; Wang, Z.H.; Lin, A.X.; Kang, J.H.; Liu, W.Z.; Guo, X.D.; Wang, Y.D.; et al. Proanthocyanidins inhibit the apoptosis and aging of nucleus pulposus cells through the PI3K/Akt pathway delaying intervertebral disc degeneration. Connect. Tissue Res. 2022, 63, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Wang, B.; Shi, Y.; Xie, C.; Huang, C.; Chen, B.; Zhang, H.; Zeng, G.; Liang, H.; Wu, Y.; et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthr. Cartil. 2021, 29, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Liu, X.; Hu, M.; Zhang, Y.; Shi, P.Z.; Wang, J.W.; Lu, X.H.; Cheng, X.F.; Tao, Y.P.; Feng, X.M.; et al. Quercetin ameliorates oxidative stress-induced senescence in rat nucleus pulposus-derived mesenchymal stem cells via the miR-34a-5p/SIRT1 axis. World J. Stem Cells 2023, 15, 842–865. [Google Scholar] [CrossRef]
- Guo, J.; Shao, M.; Lu, F.; Jiang, J.; Xia, X. Role of Sirt1 Plays in Nucleus Pulposus Cells and Intervertebral Disc Degeneration. Spine 2017, 42, E757–E766. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, F.; Wu, Y.; Liu, N.; Wang, J.; Chen, R.; Lu, Z. Resveratrol attenuates inflammation environment-induced nucleus pulposus cell senescence in vitro. Biosci. Rep. 2019, 39, BSR20190126. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.; Xu, J.; Wu, X.; Guo, Z.; Fan, L.; Song, R.; Wang, J.; Wei, L.; Teng, H. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Biosci. Rep. 2018, 38, BSR20171454. [Google Scholar] [CrossRef]
- Jiang, Y.; Dong, G.; Song, Y. Nucleus pulposus cell senescence is alleviated by resveratrol through regulating the ROS/NF-κB pathway under high-magnitude compression. Biosci. Rep. 2018, 38, BSR20180670. [Google Scholar] [CrossRef]
- Cherif, H.; Bisson, D.G.; Mannarino, M.; Rabau, O.; Ouellet, J.A.; Haglund, L. Senotherapeutic drugs for human intervertebral disc degeneration and low back pain. eLife 2020, 9, e54693. [Google Scholar] [CrossRef]
- Mannarino, M.; Cherif, H.; Li, L.; Sheng, K.; Rabau, O.; Jarzem, P.; Weber, M.H.; Ouellet, J.A.; Haglund, L. Toll-like receptor 2 induced senescence in intervertebral disc cells of patients with back pain can be attenuated by o-vanillin. Arthritis Res. Ther. 2021, 23, 117. [Google Scholar] [CrossRef]
- Li, L.; Sheng, K.; Mannarino, M.; Jarzem, P.; Cherif, H.; Haglund, L. o-Vanillin Modulates Cell Phenotype and Extracellular Vesicles of Human Mesenchymal Stem Cells and Intervertebral Disc Cells. Cells 2022, 11, 3589. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant flavone apigenin: An emerging anticancer agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef]
- Sharma, A.; Ghani, A.; Sak, K.; Tuli, H.S.; Sharma, A.K.; Setzer, W.N.; Sharma, S.; Das, A.K. Probing into Therapeutic Anti-cancer Potential of Apigenin: Recent Trends and Future Directions. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Park, H.; Kim, H.P. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Perrott, K.M.; Wiley, C.D.; Desprez, P.Y.; Campisi, J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. GeroScience 2017, 39, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Shoara, R.; Hashempur, M.H.; Ashraf, A.; Salehi, A.; Dehshahri, S.; Habibagahi, Z. Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2015, 21, 181–187. [Google Scholar] [CrossRef]
- Chen, H.W.; Zhou, J.W.; Zhang, G.Z.; Luo, Z.B.; Li, L.; Kang, X.W. Emerging role and therapeutic implication of mTOR signalling in intervertebral disc degeneration. Cell Prolif. 2023, 56, e13338. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; Wang, T.; Zhang, K.; Zhang, Y.; Kang, X. Oxidative stress in intervertebral disc degeneration: Molecular mechanisms, pathogenesis and treatment. Cell Prolif. 2023, 56, e13448. [Google Scholar] [CrossRef]
- Zheng, G.; Pan, Z.; Zhan, Y.; Tang, Q.; Zheng, F.; Zhou, Y.; Wu, Y.; Zhou, Y.; Chen, D.; Chen, J.; et al. TFEB protects nucleus pulposus cells against apoptosis and senescence via restoring autophagic flux. Osteoarthr. Cartil. 2019, 27, 347–357. [Google Scholar] [CrossRef]
- Hu, S.; Chen, L.; Al Mamun, A.; Ni, L.; Gao, W.; Lin, Y.; Jin, H.; Zhang, X.; Wang, X. The therapeutic effect of TBK1 in intervertebral disc degeneration via coordinating selective autophagy and autophagic functions. J. Adv. Res. 2021, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Li, X. Apigenin Mitigates Intervertebral Disc Degeneration through the Amelioration of Tumor Necrosis Factor α (TNF-α) Signaling Pathway. Med. Sci. Monit. 2020, 26, e924587. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Butein: From ancient traditional remedy to modern nutraceutical. Phytochem. Lett. 2015, 11, 188–201. [Google Scholar] [CrossRef]
- Song, Z.; Shanmugam, M.K.; Yu, H.; Sethi, G. Butein and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015, 22, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, H.; Jin, Y.; Wang, Q.; Chen, L.; Feng, Z.; Chen, H.; Wu, Y. Butein inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes and slows the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, X.; Zheng, X.; Ru, A.; Ni, X.; Wu, Y.; Tian, N.; Huang, Y.; Xue, E.; Wang, X.; et al. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J. Orthop. Res. 2013, 31, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.G.; Park, J.B.; Lee, D.; Park, E.Y. Effect of high glucose on stress-induced senescence of nucleus pulposus cells of adult rats. Asian Spine J. 2015, 9, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ni, B.; Zhang, F.; Hu, Y.; Zhao, J. High Glucose-Induced Oxidative Stress Mediates Apoptosis and Extracellular Matrix Metabolic Imbalances Possibly via p38 MAPK Activation in Rat Nucleus Pulposus Cells. J. Diabetes Res. 2016, 2016, 3765173. [Google Scholar] [CrossRef] [PubMed]
- Illien-Jünger, S.; Lu, Y.; Qureshi, S.A.; Hecht, A.C.; Cai, W.; Vlassara, H.; Striker, G.E.; Iatridis, J.C. Chronic ingestion of advanced glycation end products induces degenerative spinal changes and hypertrophy in aging pre-diabetic mice. PLoS ONE 2015, 10, e0116625. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- Xia, X.; Guo, J.; Lu, F.; Jiang, J. SIRT1 Plays a Protective Role in Intervertebral Disc Degeneration in a Puncture-induced Rodent Model. Spine 2015, 40, E515–E524. [Google Scholar] [CrossRef]
- Zaman, A.; Hasnat, H.; Noman, Z.; Islam, M.; Nakib, A.; Mukherjee, S.; Saha, K.; Ahmed, N.; Ashrafi, S.; Saha, T.; et al. Exploring Pharmacological Potentials of p-Coumaric Acid: A Prospective Phytochemical for Drug Discovery. Bangladesh Pharm. J. 2023, 26, 2023. [Google Scholar] [CrossRef]
- Abazari, M.F.; Nasiri, N.; Karizi, S.Z.; Nejati, F.; Haghi-Aminjan, H.; Norouzi, S.; Piri, P.; Estakhr, L.; Faradonbeh, D.R.; Kohandani, M.; et al. An Updated Review of Various Medicinal Applications of p-Co umaric Acid: From Antioxidative and Anti-Inflammatory Properties to Effects on Cell Cycle and Proliferation. Mini Rev. Med. Chem. 2021, 21, 2187–2201. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Victorelli, F.D.; Fonseca-Santos, B.; Chorilli, M. A Review of Analytical Methods for p-Coumaric Acid in Plant-Based Products, Beverages, and Biological Matrices. Crit. Rev. Anal. Chem. 2019, 49, 21–31. [Google Scholar] [CrossRef]
- An, S.M.; Koh, J.S.; Boo, Y.C. p-coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother. Res. PTR 2010, 24, 1175–1180. [Google Scholar] [CrossRef]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef]
- Navaneethan, D.; Rasool, M. p-Coumaric acid, a common dietary polyphenol, protects cadmium chloride-induced nephrotoxicity in rats. Ren. Fail. 2014, 36, 244–251. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Huang, X.; You, Y.; Xi, Y.; Ni, B.; Chu, X.; Zhang, R.; You, H. p-Coumaric Acid Attenuates IL-1β-Induced Inflammatory Responses and Cellular Senescence in Rat Chondrocytes. Inflammation 2020, 43, 619–628. [Google Scholar] [CrossRef]
- Neog, M.K.; Joshua Pragasam, S.; Krishnan, M.; Rasool, M. p-Coumaric acid, a dietary polyphenol ameliorates inflammation and curtails cartilage and bone erosion in the rheumatoid arthritis rat model. BioFactors 2017, 43, 698–717. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Murunikkara, V.; Sabina, E.P.; Rasool, M. Ameliorative effect of p-coumaric acid, a common dietary phenol, on adjuvant-induced arthritis in rats. Rheumatol. Int. 2013, 33, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liang, Q.H.; Xiong, X.G.; Wang, Y.; Zhang, Z.H.; Sun, M.J.; Lu, X.; Wu, D. Anti-Inflammatory Effects of p-Coumaric Acid, a Natural Compound of Oldenlandia diffusa, on Arthritis Model Rats. Evid.-Based Complement. Altern. Med. 2018, 2018, 5198594. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, E.; Penolazzi, L.; Notarangelo, M.P.; Fiorito, S.; Epifano, F.; Pandolfi, A.; Piva, R. Pro-differentiating compounds for human intervertebral disc cells are present in Violina pumpkin leaf extracts. Int. J. Mol. Med. 2023, 51, 39. [Google Scholar] [CrossRef] [PubMed]
- Sundar Dhilip Kumar, S.; Houreld, N.N.; Abrahamse, H. Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules 2018, 23, 835. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, Ł.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.G.; Xu, N.; Wang, W.B.; Pan, S.H.; Li, K.S.; Liu, J.K. Interleukin-1 inhibits Sox9 and collagen type II expression via nuclear factor-kappaB in the cultured human intervertebral disc cells. Chin. Med. J. 2009, 122, 2483–2488. [Google Scholar]
- Klawitter, M.; Quero, L.; Klasen, J.; Gloess, A.N.; Klopprogge, B.; Hausmann, O.; Boos, N.; Wuertz, K. Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J. Inflamm. 2012, 9, 29. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Chen, W.F.; Zhou, B.; Yang, L.; Liu, Z.L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim. Biophys. Acta 2006, 1760, 70–77. [Google Scholar] [CrossRef]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin Inhibits Apoptosis of Chondrocytes through Activation ERK1/2 Signaling Pathways Induced Autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Gao, Y.; Zou, X.; Wei, F. Oxidative Stress and Intervertebral Disc Degeneration: Pathophysiology, Signaling Pathway, and Therapy. Oxidative Med. Cell. Longev. 2022, 2022, 1984742. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Shang, L.; Mao, Y. GelMA hydrogel scaffold containing curcumin-loaded solid lipid nanoparticles promotes the regeneration of degenerative discs. SN Appl. Sci. 2023, 5, 243. [Google Scholar] [CrossRef]
- Xiao, L.; Ding, B.; Gao, J.; Yang, B.; Wang, J.; Xu, H. Curcumin prevents tension-induced endplate cartilage degeneration by enhancing autophagy. Life Sci. 2020, 258, 118213. [Google Scholar] [CrossRef]
- Ma, T.; Guo, C.J.; Zhao, X.; Wu, L.; Sun, S.X.; Jin, Q.H. The effect of curcumin on NF-κB expression in rat with lumbar intervertebral disc degeneration. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1305–1314. [Google Scholar]
- Hu, Y.; Tang, J.S.; Hou, S.X.; Shi, X.X.; Qin, J.; Zhang, T.S.; Wang, X.J. Neuroprotective effects of curcumin alleviate lumbar intervertebral disc degeneration through regulating the expression of iNOS, COX-2, TGF-β1/2, MMP-9 and BDNF in a rat model. Mol. Med. Rep. 2017, 16, 6864–6869. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Su, C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int. J. Mol. Sci. 2015, 16, 10888–10906. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Z.; Wang, C.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Ma, J.; Zhang, B.; et al. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-κB/COX-2 signaling pathway by targeting IKKβ. Am. J. Cancer Res. 2017, 7, 1270–1284. [Google Scholar] [PubMed]
- Li, W.; Ma, Y.B.; Mao, Y.Q.; Lin, T. Dehydrocostus lactone suppresses cell growth and induces apoptosis in recombinant human papilloma virus-18 HaCaT cells via the PI3K/Akt signaling pathway. Mol. Med. Rep. 2018, 17, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Singireesu, S.; Mondal, S.K.; Misra, S.; Yerramsetty, S. Dehydrocostus lactone induces prominent apoptosis in kidney distal tubular epithelial cells and interstitial fibroblasts along with cell cycle arrest in ovarian epithelial cells. Biomed. Pharmacother. 2018, 99, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wu, F.; Shi, Z.; He, B.; Zhao, X.; Wu, H.; Yan, S. Dehydrocostus lactone attenuates osteoclastogenesis and osteoclast-induced bone loss by modulating NF-κB signalling pathway. J. Cell. Mol. Med. 2019, 23, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wang, Z.; Chai, G.; Xiong, Y.; Li, B.; Zhang, H.; Xin, R.; Qian, X.; Tang, Z.; Wu, J.; et al. Dehydrocostus Lactone Suppresses LPS-induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by p38 MAPK and Akt. Molecules 2019, 24, 1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gu, Z.; Fu, Z.; Jiang, D. High-quality genome assembly of an important biodiesel plant, Euphorbia lathyris L. DNA Res. 2021, 28, dsab022. [Google Scholar] [CrossRef]
- Gu, M.; Jin, J.; Ren, C.; Chen, X.; Pan, Z.; Wu, Y.; Tian, N.; Sun, L.; Wu, A.; Gao, W.; et al. 20-Deoxyingenol alleviates osteoarthritis by activating TFEB in chondrocytes. Pharmacol. Res. 2021, 165, 105361. [Google Scholar] [CrossRef] [PubMed]
- Nageen, B.; Sarfraz, I.; Rasul, A.; Hussain, G.; Rukhsar, F.; Irshad, S.; Riaz, A.; Selamoglu, Z.; Ali, M. Eupatilin: A natural pharmacologically active flavone compound with its wide range applications. J. Asian Nat. Prod. Res. 2020, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Song, E.H.; Chung, K.S.; Kang, Y.M.; Lee, J.H.; Lee, M.; An, H.J. Eupatilin suppresses the allergic inflammatory response in vitro and in vivo. Phytomedicine 2018, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Sun, T.; Lu, F.; Shen, Y.; Zhang, Y.; Zhang, B.; Yu, G.; Li, H.; Hao, J. Eupatilin Suppresses OVA-Induced Asthma by Inhibiting NF-κB and MAPK and Activating Nrf2 Signaling Pathways in Mice. Int. J. Mol. Sci. 2022, 23, 1582. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.C.; Choi, Y.; Lee, S.; Kang, K.S.; Kim, Y.K.; Kim, S.N. Eupatilin with PPARα agonistic effects inhibits TNFα-induced MMP signaling in HaCaT cells. Biochem. Biophys. Res. Commun. 2017, 493, 220–226. [Google Scholar] [CrossRef]
- Lu, Y.; Li, D.; Huang, Y.; Sun, Y.; Zhou, H.; Ye, F.; Yang, H.; Xu, T.; Quan, S.; Pan, J. Pretreatment with Eupatilin Attenuates Inflammation and Coagulation in Sepsis by Suppressing JAK2/STAT3 Signaling Pathway. J. Inflamm. Res. 2023, 16, 1027–1042. [Google Scholar] [CrossRef]
- Jeong, J.H.; Moon, S.J.; Jhun, J.Y.; Yang, E.J.; Cho, M.L.; Min, J.K. Eupatilin Exerts Antinociceptive and Chondroprotective Properties in a Rat Model of Osteoarthritis by Downregulating Oxidative Damage and Catabolic Activity in Chondrocytes. PLoS ONE 2015, 10, e0130882. [Google Scholar] [CrossRef]
- Li, X.; Ge, J.; Zheng, Q.; Zhang, J.; Sun, R.; Liu, R. Evodiamine and rutaecarpine from Tetradium ruticarpum in the treatment of liver diseases. Phytomedicine 2020, 68, 153180. [Google Scholar] [CrossRef]
- Xu, D.; Qiu, C.; Wang, Y.; Qiao, T.; Cui, Y.L. Intranasal co-delivery of berberine and evodiamine by self-assembled thermosensitive in-situ hydrogels for improving depressive disorder. Int. J. Pharm. 2021, 603, 120667. [Google Scholar] [CrossRef]
- Wang, M.X.; Lin, L.; Chen, Y.D.; Zhong, Y.P.; Lin, Y.X.; Li, P.; Tian, X.; Han, B.; Xie, Z.Y.; Liao, Q.F. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol. Res. 2020, 159, 104978. [Google Scholar] [CrossRef]
- Li, C.G.; Zeng, Q.Z.; Chen, M.Y.; Xu, L.H.; Zhang, C.C.; Mai, F.Y.; Zeng, C.Y.; He, X.H.; Ouyang, D.Y. Evodiamine Augments NLRP3 Inflammasome Activation and Anti-bacterial Responses Through Inducing α-Tubulin Acetylation. Front. Pharmacol. 2019, 10, 290. [Google Scholar] [CrossRef]
- Zhang, W.D.; Chen, X.Y.; Wu, C.; Lian, Y.N.; Wang, Y.J.; Wang, J.H.; Yang, F.; Liu, C.H.; Li, X.Y. Evodiamine reduced peripheral hypersensitivity on the mouse with nerve injury or inflammation. Mol. Pain 2020, 16, 1744806920902563. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hua, Q.; Li, N.; Zhao, M.; Cui, Y. Protective Effects of Evodiamine against LPS-Induced Acute Kidney Injury through Regulation of ROS-NF-κB-Mediated Inflammation. Evid.-Based Complement. Altern. Med. 2019, 2019, 2190847. [Google Scholar] [CrossRef] [PubMed]
- Kuai, J.; Zhang, N. Upregulation of SIRT1 by Evodiamine activates PI3K/AKT pathway and blocks intervertebral disc degeneration. Mol. Med. Rep. 2022, 26, 265. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef]
- Syed, D.N.; Adhami, V.M.; Khan, N.; Khan, M.I.; Mukhtar, H. Exploring the molecular targets of dietary flavonoid fisetin in cancer. Semin. Cancer Biol. 2016, 40–41, 130–140. [Google Scholar] [CrossRef]
- Maher, P. How fisetin reduces the impact of age and disease on CNS function. Front. Biosci. (Sch. Ed.) 2015, 7, 58–82. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, J.; Liu, X.; Zhang, X.; Zhang, S.; Zhang, X.; Zhou, D.; Zheng, G. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging 2016, 8, 2915–2926. [Google Scholar] [CrossRef] [PubMed]
- Myrianthopoulos, V. The emerging field of senotherapeutic drugs. Future Med. Chem. 2018, 10, 2369–2372. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, K.; Nelson, A.L.; Nishimura, H.; Rutledge, J.C.; Ravuri, S.K.; Bahney, C.; Philippon, M.J.; Huard, J. The effects of fisetin on bone and cartilage: A systematic review. Pharmacol. Res. 2022, 185, 106504. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Hambright, W.S.; Mu, X.; Gao, X.; Guo, P.; Kawakami, Y.; Mitchell, J.; Mullen, M.; Nelson, A.L.; Bahney, C.; Nishimura, H.; et al. The Senolytic Drug Fisetin Attenuates Bone Degeneration in the Zmpste24−/− Progeria Mouse Model. J. Osteoporos. 2023, 2023, 5572754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhu, C.; Xuan, A.; Zhang, J.; Zhu, Z.; Tang, L.; Ruan, D. Fisetin regulates the biological effects of rat nucleus pulposus mesenchymal stem cells under oxidative stress by sirtuin-1 pathway. Immun. Inflamm. Dis. 2023, 11, e865. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Olatunde, A.; Imran, M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Khan, S.A.; Uddin, M.S.; Mitra, S.; Emran, T.B.; Khayrullin, M.; et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine 2021, 90, 153647. [Google Scholar] [CrossRef] [PubMed]
- Chuang, D.Y.; Chan, M.H.; Zong, Y.; Sheng, W.; He, Y.; Jiang, J.H.; Simonyi, A.; Gu, Z.; Fritsche, K.L.; Cui, J.; et al. Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. J. Neuroinflamm. 2013, 10, 15. [Google Scholar] [CrossRef]
- Chen, P.J.; Wang, Y.L.; Kuo, L.M.; Lin, C.F.; Chen, C.Y.; Tsai, Y.F.; Shen, J.J.; Hwang, T.L. Honokiol suppresses TNF-α-induced neutrophil adhesion on cerebral endothelial cells by disrupting polyubiquitination and degradation of IκBα. Sci. Rep. 2016, 6, 26554. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, H.; Jia, C.; Zhang, R.; Shen, C. Targeting Oxidative Stress and Inflammation in Intervertebral Disc Degeneration: Therapeutic Perspectives of Phytochemicals. Front. Pharmacol. 2022, 13, 956355. [Google Scholar] [CrossRef]
- Huang, P.P.; Fu, J.; Liu, L.H.; Wu, K.F.; Liu, H.X.; Qi, B.M.; Liu, Y.; Qi, B.L. Honokiol antagonizes doxorubicin-induced cardiomyocyte senescence by inhibiting TXNIP-mediated NLRP3 inflammasome activation. Int. J. Mol. Med. 2020, 45, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Facchini, G.; Pinheiro, A.; da Silva, M.S.; Bonner, M.Y.; Arbiser, J.; Eberlin, S. Honokiol protects skin cells against inflammation, collagenolysis, apoptosis, and senescence caused by cigarette smoke damage. Int. J. Dermatol. 2017, 56, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Tsai, K.S.; Chan, D.C.; Lan, K.C.; Chen, C.F.; Yang, R.S.; Liu, S.H. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J. Orthop. Res. 2014, 32, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Gu, J.M.; Xie, Z.A.; Gu, Y.; Jie, Z.W.; Huang, K.M.; Wang, J.Y.; Fan, S.W.; Jiang, X.S.; Hu, Z.J. Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radic. Biol. Med. 2018, 120, 368–379. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Lyu, F.J.; Cui, H.; Pan, H.; Mc Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7. [Google Scholar] [CrossRef]
- Nepal, M.; Li, L.; Cho, H.K.; Park, J.K.; Soh, Y. Kaempferol induces chondrogenesis in ATDC5 cells through activation of ERK/BMP-2 signaling pathway. Food Chem. Toxicol. 2013, 62, 238–245. [Google Scholar] [CrossRef]
- Yuan, X.; Ni, H.; Hou, Y.; Lai, M.-T.; Hu, S.-Q. Efficient short extraction and purification procedures of kinsenoside from Anoectochilus roxburghii with deep eutectic solvent by column chromatographic extraction. Ind. Crops Prod. 2022, 182, 114866. [Google Scholar] [CrossRef]
- Hsiao, H.B.; Wu, J.B.; Lin, H.; Lin, W.C. Kinsenoside isolated from Anoectochilus formosanus suppresses LPS-stimulated inflammatory reactions in macrophages and endotoxin shock in mice. Shock 2011, 35, 184–190. [Google Scholar] [CrossRef]
- Hsiao, H.B.; Hsieh, C.C.; Wu, J.B.; Lin, H.; Lin, W.C. Kinsenoside inhibits the inflammatory mediator release in a type-II collagen induced arthritis mouse model by regulating the T cells responses. BMC Complement. Altern. Med. 2016, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Gu, S.; Zhang, Y.; Zhang, J. Kinsenoside Ameliorates Oxidative Stress-Induced RPE Cell Apoptosis and Inhibits Angiogenesis via Erk/p38/NF-κB/VEGF Signaling. Front. Pharmacol. 2018, 9, 240. [Google Scholar] [CrossRef]
- Xiang, M.; Liu, T.; Tan, W.; Ren, H.; Li, H.; Liu, J.; Cao, H.; Cheng, Q.; Liu, X.; Zhu, H.; et al. Effects of kinsenoside, a potential immunosuppressive drug for autoimmune hepatitis, on dendritic cells/CD8(+) T cells communication in mice. Hepatology 2016, 64, 2135–2150. [Google Scholar] [CrossRef]
- Hsiao, H.B.; Lin, H.; Wu, J.B.; Lin, W.C. Kinsenoside prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis by regulating classical NF-κB pathways. Osteoporos. Int. 2013, 24, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Ruan, H.; Pi, H.; Wu, J. Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J. Ethnopharmacol. 2007, 114, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Liu, Q.; Xiao, B.; Zhou, J.; Zhang, J.G.; Li, Y. The vascular protective properties of kinsenoside isolated from Anoectochilus roxburghii under high glucose condition. Fitoterapia 2013, 86, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Bing, W.; Di, Y.; Hua, L.; Shi-He, L.; Yu-Hua, Z.; Xiu-Guo, H.; Yu-Gang, W.; Qi-Ming, F.; Shih-Mo, Y.; et al. Kinsenoside screening with a microfluidic chip attenuates gouty arthritis through inactivating NF-κB signaling in macrophages and protecting endothelial cells. Cell Death Dis. 2016, 7, e2350. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Muruganathan, N.; Dhanapal, A.R.; Baskar, V.; Muthuramalingam, P.; Selvaraj, D.; Aara, H.; Shiek Abdullah, M.Z.; Sivanesan, I. Recent Updates on Source, Biosynthesis, and Therapeutic Potential of Natural Flavonoid Luteolin: A Review. Metabolites 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Kang, L.; Hu, J.; Weng, Y.; Jia, J.; Zhang, Y. Sirtuin 6 prevents matrix degradation through inhibition of the NF-κB pathway in intervertebral disc degeneration. Exp. Cell Res. 2017, 352, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Dong, L.; Gao, Q.; Yin, L.; Quan, H.; Chen, R.; Fu, X.; Lin, D. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J. Ethnopharmacol. 2018, 213, 280–301. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cheng, W.; Zhang, G.; Ma, Q.; Li, X.; Zhang, B.; Hu, T.; Song, G. Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-ĸB pathway. Int. Immunopharmacol. 2020, 81, 106240. [Google Scholar] [CrossRef] [PubMed]
- Pi, W.X.; Feng, X.P.; Ye, L.H.; Cai, B.C. Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis. Molecules 2017, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, X.; Xu, J.; Shi, Y.; Hu, R.; Ren, Z.; Qi, Q.; Lü, H.; Cheng, X.; Hu, J. Morroniside protects OLN-93 cells against H(2)O(2)-induced injury through the PI3K/Akt pathway-mediated antioxidative stress and antiapoptotic activities. Cell Cycle 2021, 20, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Han, Q.; Yu, Z.; Liu, M.; Sun, J.; Wu, M.; Yin, H.; Fu, J.; Guo, Y.; Wang, L.; et al. Morroniside Inhibits Inflammatory Bone Loss through the TRAF6-Mediated NF-κB/MAPK Signalling Pathway. Pharmaceuticals 2023, 16, 1438. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, W. Cardioprotective Effects of Morroniside in Rats Following Acute Myocardial Infarction. Inflammation 2018, 41, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Tao, J.; Qian, Y.; Feng, F.; Hu, X.; Xu, T.; Jin, H.; Ruan, H.; Zheng, H.F.; Tong, P. Morroniside ameliorates inflammatory skeletal muscle atrophy via inhibiting canonical and non-canonical NF-κB and regulating protein synthesis/degradation. Front. Pharmacol. 2022, 13, 1056460. [Google Scholar] [CrossRef]
- Li, B.; Lei, S.; Xiong, S.; Chen, S.; Zhang, Z. Pharmacokinetics and Pharmacodynamics of Morroniside: A Review. Nat. Prod. Commun. 2019, 14, 1934578X19856526. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, S.; Zhang, Z.; Zhou, C.; Fu, F.; Bian, Y.; Luo, H.; Li, Y.; Yan, S.; Ge, Y.; et al. Network Pharmacology and Experimental Validation to Reveal the Pharmacological Mechanisms of Liuwei Dihuang Decoction Against Intervertebral Disc Degeneration. Drug Des. Dev. Ther. 2021, 15, 4911–4924. [Google Scholar] [CrossRef] [PubMed]
- Zheng-Wei, S.; Yuan, T.; Chao-Shuai, F.; Lei, Z.; Zong-Rang, S.; Tuan-Jiang, L.; Ding-Jun, H. Roles of Hippo-YAP/TAZ signalling in intervertebral disc degeneration. Biomed. Pharmacother. 2023, 159, 114099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, F.; Xie, Z.; Chen, L.; Sinkemani, A.; Yu, H.; Wang, K.; Mao, L.; Wu, X. Dysregulation of YAP by the Hippo pathway is involved in intervertebral disc degeneration, cell contact inhibition, and cell senescence. Oncotarget 2018, 9, 2175–2192. [Google Scholar] [CrossRef]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, M.A.; Ahmad, M.A.; Arshad, M.; Khushtar, M. Current Pharmacological Trends on Myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef] [PubMed]
- Kenouche, S.; Sandoval-Yañez, C.; Martínez-Araya, J.I. The antioxidant capacity of myricetin. A molecular electrostatic potential analysis based on DFT calculations. Chem. Phys. Lett. 2022, 801, 139708. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.S.; Kang, N.J.; Bode, A.M.; Dong, Z.; et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef]
- Pan, X.; Chen, T.; Zhang, Z.; Chen, X.; Chen, C.; Chen, L.; Wang, X.; Ying, X. Activation of Nrf2/HO-1 signal with Myricetin for attenuating ECM degradation in human chondrocytes and ameliorating the murine osteoarthritis. Int. Immunopharmacol. 2019, 75, 105742. [Google Scholar] [CrossRef]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yang, Z.; Wang, Y.; Zhao, T.; Luo, W.; Liang, T.; Yang, Z. Traditional herbs: Mechanisms to combat cellular senescence. Aging 2023, 15, 14473–14505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Xu, Z. SIRT1 provides new pharmacological targets for polydatin through its role as a metabolic sensor. Biomed. Pharmacother. 2021, 139, 111549. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Gao, J.P.; Han, Y.L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine 2015, 22, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Posa, F.; De Maria, S.; Ravagnan, G.; Ballini, A.; Porro, C.; Trotta, T.; Grano, M.; Muzio, L.L.; Mori, G. Polydatin, Natural Precursor of Resveratrol, Promotes Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 944–952. [Google Scholar] [CrossRef]
- Huang, K.; Chen, C.; Hao, J.; Huang, J.; Wang, S.; Liu, P.; Huang, H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol. Cell. Endocrinol. 2015, 399, 178–189. [Google Scholar] [CrossRef]
- Tang, S.; Tang, Q.; Jin, J.; Zheng, G.; Xu, J.; Huang, W.; Li, X.; Shang, P.; Liu, H. Polydatin inhibits the IL-1β-induced inflammatory response in human osteoarthritic chondrocytes by activating the Nrf2 signaling pathway and ameliorates murine osteoarthritis. Food Funct. 2018, 9, 1701–1712. [Google Scholar] [CrossRef]
- Hu, L.; Luo, D.; Zhang, H.; He, L. Polydatin inhibits IL-1β-mediated chondrocyte inflammation and ameliorates cartilage degradation: Involvement of the NF-κB and Wnt/β-catenin pathways. Tissue Cell 2022, 78, 101865. [Google Scholar] [CrossRef]
- Kang, L.; Liu, S.; Li, J.; Tian, Y.; Xue, Y.; Liu, X. Parkin and Nrf2 prevent oxidative stress-induced apoptosis in intervertebral endplate chondrocytes via inducing mitophagy and anti-oxidant defenses. Life Sci. 2020, 243, 117244. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. BioFactors 2010, 36, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Tie, F.; Wang, J.; Liang, Y.; Zhu, S.; Wang, Z.; Li, G.; Wang, H. Proanthocyanidins Ameliorated Deficits of Lipid Metabolism in Type 2 Diabetes Mellitus Via Inhibiting Adipogenesis and Improving Mitochondrial Function. Int. J. Mol. Sci. 2020, 21, 2029. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Abdel-Mageed, W.M.; Shahat, A.A.; Parvez, M.K.; Al-Dosari, M.S.; Malik, A.; Abdel-Kader, M.S.; Alsaid, M.S. Proanthocyanidins from the stem bark of Rhus tripartita ameliorate methylgloxal-induced endothelial cell apoptosis. J. Food Drug Anal. 2019, 27, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Chacón, L.D.M.; Yanes-Díaz, J.; de Lucas, B.; Riestra-Ayora, J.I.; Madrid-García, R.; Sanz-Fernández, R.; Sánchez-Rodríguez, C. Cocoa Polyphenol Extract Inhibits Cellular Senescence via Modulation of SIRT1 and SIRT3 in Auditory Cells. Nutrients 2023, 15, 544. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhu, W.; Wu, Y.; Long, Y.; Liu, H.; Wan, W.; Wan, G.; Yu, J. Grape Seed Proanthocyanidin Extract Moderated Retinal Pigment Epithelium Cellular Senescence Through NAMPT/SIRT1/NLRP3 Pathway. J. Inflamm. Res. 2021, 14, 3129–3143. [Google Scholar] [CrossRef]
- Mével, E.; Merceron, C.; Vinatier, C.; Krisa, S.; Richard, T.; Masson, M.; Lesoeur, J.; Hivernaud, V.; Gauthier, O.; Abadie, J.; et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1β activities before and after oral consumption. Sci. Rep. 2016, 6, 33527. [Google Scholar] [CrossRef]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; Cho, M.L.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp. Mol. Med. 2011, 43, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.A.; Zeitlin, S.I.; Shahed, A.; Rajfer, J. Quercetin in men with category III chronic prostatitis: A preliminary prospective, double-blind, placebo-controlled trial. Urology 1999, 54, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Wang, D.; He, X.; Wang, D.; Peng, P.; Xu, X.; Gao, B.; Zheng, C.; Wang, H.; Jia, H.; Shang, Q.; et al. Quercetin Suppresses Apoptosis and Attenuates Intervertebral Disc Degeneration via the SIRT1-Autophagy Pathway. Front. Cell Dev. Biol. 2020, 8, 613006. [Google Scholar] [CrossRef]
- Pallauf, K.; Rimbach, G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013, 12, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.J.; Frei, B. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 2016, 9, 104–113. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jiang, K.; Ogrodnik, M.; Chen, X.; Zhu, X.Y.; Lohmeier, H.; Ahmed, L.; Tang, H.; Tchkonia, T.; Hickson, L.J.; et al. Increased renal cellular senescence in murine high-fat diet: Effect of the senolytic drug quercetin. Transl. Res. J. Lab. Clin. Med. 2019, 213, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, W.; Abulizi, Y.; Xu, T.; Cao, R.; Xun, C.; Zhang, J.; Sheng, W. Quercetin Alleviates Intervertebral Disc Degeneration by Modulating p38 MAPK-Mediated Autophagy. BioMed Res. Int. 2021, 2021, 6631562. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Li, N.; Zhang, F.; Yu, P.; Meng, Q. Resveratrol Suppresses Annulus Fibrosus Cell Apoptosis through Regulating Oxidative Stress Reaction in an Inflammatory Environment. BioMed Res. Int. 2021, 2021, 9100444. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Yu, X.; Yang, W.; Wang, Q. Natural phytoalexin stilbene compound resveratrol and its derivatives as anti-tobacco mosaic virus and anti-phytopathogenic fungus agents. Sci. Rep. 2021, 11, 16509. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.L.; Kosmeder, J.W.; Pezzuto, J.M. Biological Effects of Resveratrol. Antioxid. Redox Signal. 2001, 3, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Zhang, L.; Zang, W.D.; Zhang, K.G.; Li, H.J.; Gao, Y.Z. Pharmacological Effects of Resveratrol in Intervertebral Disc Degeneration: A Literature Review. Orthop. Surg. 2022, 14, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and related stilbenes: Their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kwak, D.W.; Lin, S.K.; Kim, H.M.; Kim, Y.M.; Park, O.J. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann. N. Y. Acad. Sci. 2007, 1095, 441–448. [Google Scholar] [CrossRef]
- Kamali, A.; Ziadlou, R.; Lang, G.; Pfannkuche, J.; Cui, S.; Li, Z.; Richards, R.G.; Alini, M.; Grad, S. Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions. Theranostics 2021, 11, 27–47. [Google Scholar] [CrossRef]
- Wu, C.; Ge, J.; Yang, M.; Yan, Q.; Wang, Y.; Yu, H.; Yang, H.; Zou, J. Resveratrol protects human nucleus pulposus cells from degeneration by blocking IL-6/JAK/STAT3 pathway. Eur. J. Med. Res. 2021, 26, 81. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, K.; Quero, L.; Sekiguchi, M.; Klawitter, M.; Nerlich, A.; Konno, S.; Kikuchi, S.; Boos, N. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus-mediated pain in vitro and in vivo. Spine 2011, 36, E1373–E1384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xie, Z.; Yu, J.; Fu, L. Resveratrol inhibits IL-1β-mediated nucleus pulposus cell apoptosis through regulating the PI3K/Akt pathway. Biosci. Rep. 2019, 39, BSR20190043. [Google Scholar] [CrossRef]
- Wang, D.; Hu, Z.; Hao, J.; He, B.; Gan, Q.; Zhong, X.; Zhang, X.; Shen, J.; Fang, J.; Jiang, W. SIRT1 inhibits apoptosis of degenerative human disc nucleus pulposus cells through activation of Akt pathway. Age 2013, 35, 1741–1753. [Google Scholar] [CrossRef]
- Bai, X.; Guo, X.; Zhang, F.; Zheng, L.; Ding, W.; Yang, S. Resveratrol Combined with 17β-Estradiol Prevents IL-1β Induced Apoptosis in Human Nucleus Pulposus Via The PI3K/AKT/Mtor and PI3K/AKT/GSK-3β Pathway. J. Investig. Surg. 2021, 34, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhu, L.; Hong, X.; Wang, Y.T.; Wang, F.; Bao, J.P.; Xie, X.H.; Liu, L.; Wu, X.T. Resveratrol attenuated TNF-α-induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Exp. Biol. Med. 2016, 241, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, L.; Zhuo, N.; Shen, J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem. Biophys. Res. Commun. 2017, 493, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Phillips, F.M.; An, H.S.; Ellman, M.; Thonar, E.J.; Wu, W.; Park, D.; Im, H.J. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine 2008, 33, 2586–2595. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Leng, X.; Zhao, M.; Wu, M.; Chen, A.; Hong, G.; Sun, P. Resveratrol increases nucleus pulposus matrix synthesis through activating the PI3K/Akt signaling pathway under mechanical compression in a disc organ culture. Biosci. Rep. 2017, 37, BSR20171319. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J. Resveratrol has anabolic effects on disc degeneration in a rabbit model. J. Korean Med. Sci. 2013, 28, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yu, H.; He, Y.; Xu, Y.; Zhang, W.; Lu, C.; Ao, Q. Protective effects of resveratrol on autologous nucleus pulposus model of radiculopathy. Exp. Ther. Med. 2016, 12, 3917–3922. [Google Scholar] [CrossRef] [PubMed]
- Triller, P.; Bachorz, J.; Synowitz, M.; Kettenmann, H.; Markovic, D. O-Vanillin Attenuates the TLR2 Mediated Tumor-Promoting Phenotype of Microglia. Int. J. Mol. Sci. 2020, 21, 2959. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, L.; Wang, Y.; Yao, J.; Jin, F.; Tao, T.; Yuan, H.; Shi, L.; Lu, S. Treatment with toll-like receptor 2 inhibitor ortho-vanillin alleviates lipopolysaccharide-induced acute kidney injury in mice. Exp. Ther. Med. 2019, 18, 4829–4837. [Google Scholar] [CrossRef]
- Krock, E.; Rosenzweig, D.H.; Currie, J.B.; Bisson, D.G.; Ouellet, J.A.; Haglund, L. Toll-like Receptor Activation Induces Degeneration of Human Intervertebral Discs. Sci. Rep. 2017, 7, 17184. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Q.; Wu, J.; Han, X.; Qian, T.; Zhang, Z.; Wang, J.; Pan, X.; Wu, A.; Wang, X. Baicalein Inhibits the IL-1β-Induced Inflammatory Response in Nucleus Pulposus Cells and Attenuates Disc Degeneration in vivo. Inflammation 2019, 42, 1032–1044. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.K.; Wang, Z.W.; Zhao, C.; Miao, J. Baicalein alleviates TNF-α-induced apoptosis of human nucleus pulposus cells through PI3K/AKT signaling pathway. J. Orthop. Surg. Res. 2023, 18, 292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, Z.; Wang, J.; Tang, C.; Khor, S.; Chen, J.; Chen, X.; Zhang, Z.; Tang, Q.; Wang, C.; et al. Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model. Int. J. Biol. Sci. 2018, 14, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, K.; Song, H.; Zhu, R.; Ding, S.; Yang, H.; Sun, J.; Wen, X.; Sun, L. Celastrol ameliorates osteoarthritis via regulating TLR2/NF-κB signaling pathway. Front. Pharmacol. 2022, 13, 963506. [Google Scholar] [CrossRef] [PubMed]
- Cascão, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.H.; Cheng, Y.; Chen, W.P.; Zhong, H.M.; Wang, X.H. Celastrol, an inhibitor of heat shock protein 90β potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur. J. Pharmacol. 2013, 708, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Wu, D.; Liu, X.M.; Xu, J.T.; Ma, B.J.; Ji, Y.; Jin, Y.Y.; Wu, S.Y.; Wu, T.; Ma, K. Intra-articular delivery of celastrol by hollow mesoporous silica nanoparticles for pH-sensitive anti-inflammatory therapy against knee osteoarthritis. J. Nanobiotechnol. 2020, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xuan, J.; Gu, Y.T.; Shi, K.S.; Xie, J.J.; Chen, J.X.; Zheng, Z.M.; Chen, Y.; Chen, X.B.; Wu, Y.S.; et al. Celastrol reduces IL-1β induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo. Biomed. Pharmacother. 2017, 91, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, H.; Wang, M.; Zhang, Y.; Zhang, Z.; Lin, Z. Comparative transcriptomics and network pharmacology analysis to identify the potential mechanism of celastrol against osteoarthritis. Clin. Rheumatol. 2021, 40, 4259–4268. [Google Scholar] [CrossRef]
- Ge, Q.; Ying, J.; Shi, Z.; Mao, Q.; Jin, H.; Wang, P.E.; Chen, J.; Yuan, W.; Tong, P.; Li, J. Chlorogenic Acid retards cartilaginous endplate degeneration and ameliorates intervertebral disc degeneration via suppressing NF-κB signaling. Life Sci. 2021, 274, 119324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Cheng, X.; Wei, H.; Li, P.; Fan, L.; Liu, K.; Zhang, S.; Wang, H. The antioxidant Glycitin protects against intervertebral disc degeneration through antagonizing inflammation and oxidative stress in nucleus pulposus cells. Aging 2023, 15, 13693–13709. [Google Scholar] [CrossRef]
- Wang, W.; Yang, R.; Zhang, M.; Li, J.; Peng, J.; Xu, M.; Zhao, Y.; Li, H.; Pan, X. Glycitin Suppresses Cartilage Destruction of Osteoarthritis in Mice. Inflammation 2020, 43, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, S.; Cao, Z.; Yang, L.; Lu, F.; Li, Y.; Hu, L.; Bai, X. Higenamine mitigates interleukin-1β-induced human nucleus pulposus cell apoptosis by ROS-mediated PI3K/Akt signaling. Mol. Cell. Biochem. 2021, 476, 3889–3897. [Google Scholar] [CrossRef]
- Bai, X.; Ding, W.; Yang, S.; Guo, X. Higenamine inhibits IL-1β-induced inflammation in human nucleus pulposus cells. Biosci. Rep. 2019, 39, BSR20190857. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Wang, Z.; Wu, X.; Guo, H.; Jiao, X.; Zhang, H.; Qi, C.; Li, X. Mangiferin exert protective effects on joints of adjuvant-induced arthritis rats by regulating the MAPKs/NF-κB pathway of fibroblast-like synoviocytes. Int. Immunopharmacol. 2021, 101, 108352. [Google Scholar] [CrossRef]
- Yu, H.; Hou, G.; Cao, J.; Yin, Y.; Zhao, Y.; Cheng, L. Mangiferin Alleviates Mitochondrial ROS in Nucleus Pulposus Cells and Protects against Intervertebral Disc Degeneration via Suppression of NF-κB Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 6632786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Shangguan, W.J.; Zhao, Z.J.; Zhou, F.C.; Liu, H.T.; Liang, Z.H.; Song, J.; Shao, J. Naringin Inhibits Apoptosis Induced by Cyclic Stretch in Rat Annular Cells and Partially Attenuates Disc Degeneration by Inhibiting the ROS/NF-κB Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 6179444. [Google Scholar] [CrossRef]
- Chen, R.; Gao, S.; Guan, H.; Zhang, X.; Gao, Y.; Su, Y.; Song, Y.; Jiang, Y.; Li, N. Naringin protects human nucleus pulposus cells against TNF-α-induced inflammation, oxidative stress, and loss of cellular homeostasis by enhancing autophagic flux via AMPK/SIRT1 activation. Oxidative Med. Cell. Longev. 2022, 2022, 7655142. [Google Scholar] [CrossRef]
- Gao, G.; Chang, F.; Zhang, T.; Huang, X.; Yu, C.; Hu, Z.; Ji, M.; Duan, Y. Naringin Protects Against Interleukin 1β (IL-1β)-Induced Human Nucleus Pulposus Cells Degeneration via Downregulation Nuclear Factor kappa B (NF-κB) Pathway and p53 Expression. Med. Sci. Monit. 2019, 25, 9963–9972. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, Z. The Effect of Naringin on the Apoptosis of Degenerative Nucleus Pulposus Cells: A Study on the Function and Mechanism. Drug Des. Dev. Ther. 2022, 16, 499–508. [Google Scholar] [CrossRef]
- Meiners, F.; Secci, R.; Sueto, S.; Fuellen, G.; Barrantes, I. Computational identification of natural senotherapeutic compounds that mimic dasatinib based on gene expression data. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kapoor, N.; Bhattacharjee, A.; Chakraborty, S.; Katti, D.S. Piperlongumine mediates amelioration of osteoarthritis via inhibition of chondrocyte senescence and inflammation in a goat ex vivo model. Eur. J. Pharmacol. 2023, 961, 176136. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xue, J.; He, Z.; Jia, H.; Yang, X. The mechanism by which Naru 3 pill protects against intervertebral disc cartilage endplate degeneration based on network pharmacology and experimental verification. J. Orthop. Surg. Res. 2023, 18, 552. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Y.; Xu, B.; Mao, L.; Zhao, J. Sesamin inhibits lipopolysaccharide-induced inflammation and extracellular matrix catabolism in rat intervertebral disc. Connect. Tissue Res. 2016, 57, 347–359. [Google Scholar] [CrossRef]
- Li, K.; Lv, C. Intradiscal injection of sesamin protects from lesion-induced degeneration. Connect. Tissue Res. 2020, 61, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhou, X.; Wang, J.; Xu, L.; Zhou, L.; Yu, W.; Tao, Y.; Zhu, J.; Hu, B.; Liang, C.; et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int. Immunopharmacol. 2018, 65, 539–549. [Google Scholar] [CrossRef]
- Gao, W.; Bao, J.; Zhang, Y.; He, D.; Zhang, L.; Zhang, J.; Pan, H.; Wang, D. Injectable kaempferol-loaded fibrin glue regulates the metabolic balance and inhibits inflammation in intervertebral disc degeneration. Sci. Rep. 2023, 13, 20001. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, R.; Hu, H.; Zhan, M.; Wang, T.; Huang, F.; Wei, F.; Chai, Y.; Ling, Z.; Zou, X. Elimination of Senescent Cells by Senolytics Facilitates Bony Endplate Microvessel Formation and Mitigates Disc Degeneration in Aged Mice. Front. Cell Dev. Biol. 2022, 10, 853688. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Q.; Li, H.; Han, F.; Guo, Q.; Sun, H.; Zhao, H.; Tu, Z.; Liu, Z.; Zhu, C.; et al. Vanillin-based functionalization strategy to construct multifunctional microspheres for treating inflammation and regenerating intervertebral disc. Bioact. Mater. 2023, 28, 167–182. [Google Scholar] [CrossRef]
- Kohli, J.; Wang, B.; Brandenburg, S.M.; Basisty, N.; Evangelou, K.; Varela-Eirin, M.; Campisi, J.; Schilling, B.; Gorgoulis, V.; Demaria, M. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat. Protoc. 2021, 16, 2471–2498. [Google Scholar] [CrossRef]
- Wang, W.; Jing, X.; Du, T.; Ren, J.; Liu, X.; Chen, F.; Shao, Y.; Sun, S.; Yang, G.; Cui, X. Iron overload promotes intervertebral disc degeneration via inducing oxidative stress and ferroptosis in endplate chondrocytes. Free Radic. Biol. Med. 2022, 190, 234–246. [Google Scholar] [CrossRef]
- Sloan, S.R., Jr.; Wipplinger, C.; Kirnaz, S.; Navarro-Ramirez, R.; Schmidt, F.; McCloskey, D.; Pannellini, T.; Schiavinato, A.; Härtl, R.; Bonassar, L.J. Combined nucleus pulposus augmentation and annulus fibrosus repair prevents acute intervertebral disc degeneration after discectomy. Sci. Transl. Med. 2020, 12, eaay2380. [Google Scholar] [CrossRef]
- Benneker, L.M.; Heini, P.F.; Alini, M.; Anderson, S.E.; Ito, K. 2004 Young Investigator Award Winner: Vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine 2005, 30, 167–173. [Google Scholar] [CrossRef]
- Ashinsky, B.G.; Bonnevie, E.D.; Mandalapu, S.A.; Pickup, S.; Wang, C.; Han, L.; Mauck, R.L.; Smith, H.E.; Gullbrand, S.E. Intervertebral Disc Degeneration Is Associated With Aberrant Endplate Remodeling and Reduced Small Molecule Transport. J. Bone Miner. Res. 2020, 35, 1572–1581. [Google Scholar] [CrossRef]
- Hwang, M.H.; Son, H.G.; Kim, J.; Choi, H. In vitro model of distinct catabolic and inflammatory response patterns of endothelial cells to intervertebral disc cell degeneration. Sci. Rep. 2020, 10, 20596. [Google Scholar] [CrossRef]
- Ling, Z.; Li, L.; Chen, Y.; Hu, H.; Zhao, X.; Wilson, J.; Qi, Q.; Liu, D.; Wei, F.; Chen, X.; et al. Changes of the end plate cartilage are associated with intervertebral disc degeneration: A quantitative magnetic resonance imaging study in rhesus monkeys and humans. J. Orthop. Transl. 2020, 24, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Cohen, S.B.; Berenbaum, F.; Lufkin, J.; Johnson, J.R.; Bodick, N. Brief Report: A Phase IIb Trial of a Novel Extended-Release Microsphere Formulation of Triamcinolone Acetonide for Intraarticular Injection in Knee Osteoarthritis. Arthritis Rheumatol. 2018, 70, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Christophoridis, C.; Kouroumalis, A.; Kletsas, D. Accumulation of zoledronic acid in rabbit intervertebral discs. J. Chromatogr. B 2022, 1197, 123229. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Lin, G.; Xie, Y.; Duan, J.; Ma, P.; Li, G.; Ji, G. Quantitative determination of myricetin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and its absolute bioavailability. Drug Res. 2014, 64, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chiang, C.J.; Wu, L.C.; Yang, C.H.; Kuo, Y.J.; Tsai, T.H. In vitro Penetration and in vivo Distribution of Honokiol into the Intervertebral Disc in Rat. Anal. Sci. 2015, 31, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Meng, H.; Li, J.; Zhao, J.; Xu, Y.; Wang, A.; Xu, W.; Peng, J.; Lu, S. Potential and recent advances of microcarriers in repairing cartilage defects. J. Orthop. Transl. 2021, 27, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Seaquist, E.R.; Blonde, L.; McGill, J.B.; Heller, S.R.; Kendall, D.M.; Bumpass, J.B.; Pompilio, F.M.; Grant, M.L. Hypoglycaemia is reduced with use of inhaled Technosphere(®) Insulin relative to insulin aspart in type 1 diabetes mellitus. Diabet. Med. 2020, 37, 752–759. [Google Scholar] [CrossRef] [PubMed]
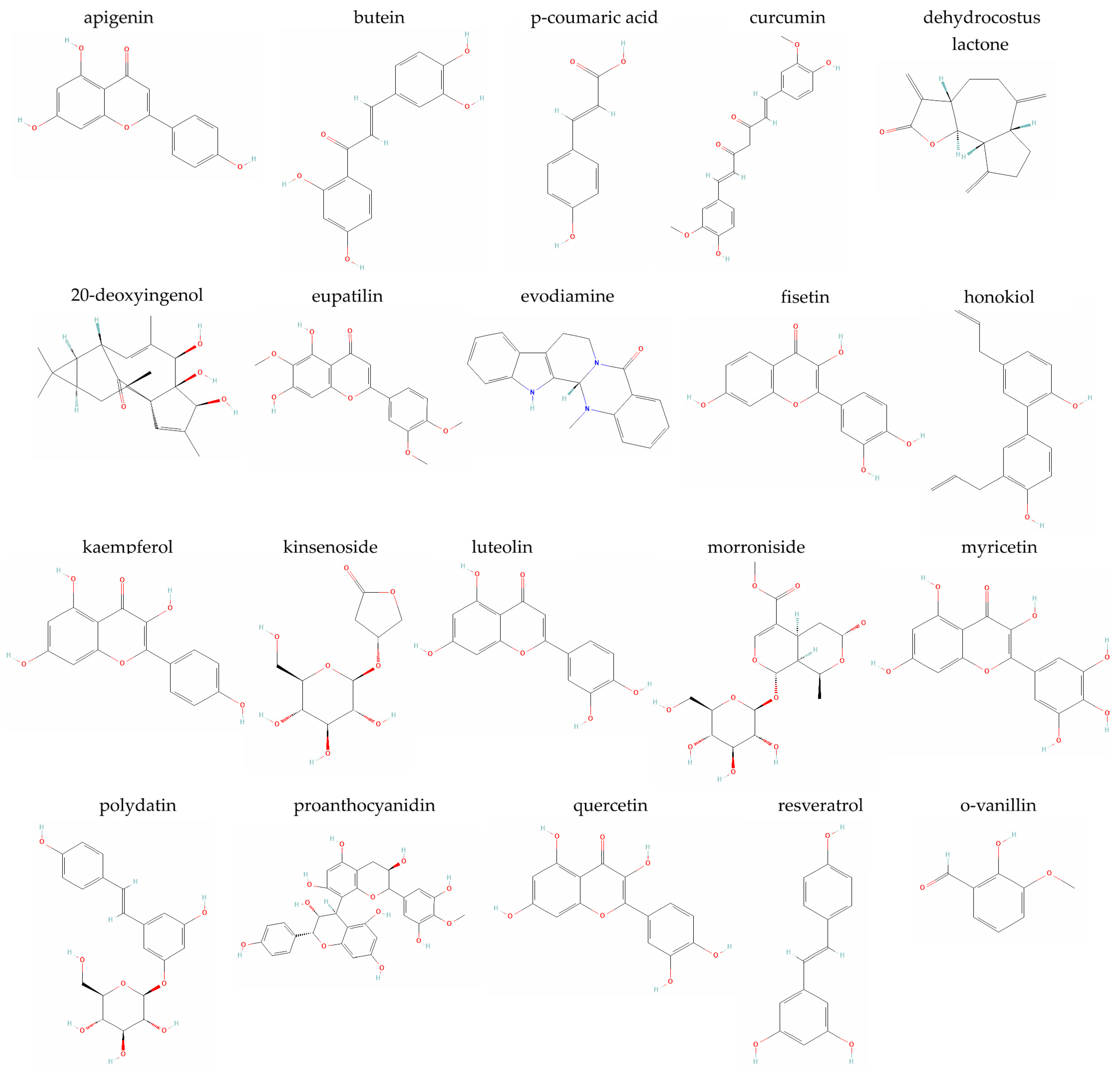

| Plant-Derived Compound | Senotherapeutic Activity | Implicated Signaling Pathway(s) | Reference(s) |
|---|---|---|---|
| Apigenin | Senomorphic | AMPK-mTOR-TFEB | [45] |
| Butein | Senomorphic | Sirt1-p53 | [46] |
| p-Coumaric acid | Senomorphic | ND * | [47] |
| Curcumin | Senomorphic | AMPK-mTOR-ULK1 | [48] |
| p70/S6K, Akt-LC3-II-SQSTM1/p62 | [49] | ||
| Senolytic | JNK | [50] | |
| Dehydrocostus lactone | Senomorphic | STING-TBK1-NF-κB, MAPK | [51] |
| 20-Deoxyingenol | Senomorphic | TFEB-autophagy/lysosome pathway | [52] |
| Eupatilin | Senomorphic | MAPK-NF-κB | [53] |
| Evodiamine | Putative senomorphic | Nrf2-HO-1, MAPK | [54] |
| Fisetin | Senomorphic | ND | [43] |
| Honokiol | Senomorphic | AMPK-PGC-1α-SIRT3 | [55] |
| Kaempferol | Senomorphic (network pharmacology analysis/in vitro) | MAPK | [56] |
| ND | [57] | ||
| Kinsenoside | Senomorphic | Akt-ERK1/2-Nrf2 | [58] |
| Luteolin | Senomorphic | SIRT6-NF-κB | [59] |
| Morroniside | Senomorphic | ROS-Hippo-Mst1/2 and Lats1/2-YAP/TAZ-p53 | [60] |
| Myricetin | Senomorphic | SERPINE1 | [61] |
| SIRT1-PGC-1α | [62] | ||
| Polydatin | Senomorphic | Nrf2-HO-1 | [63] |
| Proanthocyanidins | Senomorphic | PI3K-Akt | [64] |
| Quercetin | Senolytic (quercetin/dasatinib combination) | ND | [65] |
| Senomorphic | Nrf2-NF-κB | [66] | |
| miR-34a-5p-SIRT1 | [67] | ||
| Resveratrol | Senomorphic | SIRT1 | [68] |
| ND | [69] | ||
| ROS-PI3K-Akt | [70] | ||
| ROS-NF-κB | [71] | ||
| o-Vanillin | Senolytic (o-vanillin/RG-7112 combination) | ND | [4] |
| Senolytic/senomorphic | JNK, Nrf2, NF-κB | [50] | |
| ND | [72] | ||
| TLR-2 | [73] | ||
| ND | [74] |
| Plant-Derived Compound | Setting | Cell/Animal Model | Reference(s) |
|---|---|---|---|
| Apigenin | In vitro | Rat NP IVD cells | [45,87] |
| In vivo | Puncture-induced rat IDD model | ||
| Baicalein | In vitro | Rat and human NP IVD cells | [262,263] |
| In vivo | Puncture-induced rat IDD model | [262] | |
| Berberine | In vitro | Rat NP IVD cells | [264] |
| In vivo | Puncture-induced rat IDD model | ||
| Butein | In vitro | Rat NP IVD cells | [46] |
| In vivo | Streptozotocin-/puncture-induced rat diabetes and IDD model | ||
| Celastrol | In vitro | Human NP IVD cells | [269] |
| In vivo | Puncture-induced rat IDD model | ||
| Chlorogenic acid | In vivo | Lumbar spine instability-induced IDD mouse model | [271] |
| p-Coumaric acid | In vitro | Human NP IVD cells | [47] |
| Human degenerated IVD cells | [110] | ||
| Curcumin | In vitro | Human IVD cells | [50,115] |
| Rat NP IVD cells (curcumin/SLNs mixed with GelMA hydrogel) | [120] | ||
| Human NP IVD cells | [48,49] | ||
| Human cartilaginous endplate cells | [121] | ||
| In vivo | Puncture-induced rat IDD model | [48,123] | |
| Surgically-induced lumbar rat IDD model | [122] | ||
| Dehydrocostus lactone | In vitro | Rat NP IVD cells | [51] |
| In vivo | Spinal instability-induced mouse model | ||
| 20-Deoxyingenol | In vitro | Rat NP IVD cells | [52] |
| In vivo | Puncture-induced rat IDD model | ||
| Eupatilin | In vitro | Rat NP IVD cells | [53] |
| In vivo | Puncture-induced caudal rat IDD model | ||
| Evodiamine | In vitro | Immortalized human NP IVD cells | [144] |
| Rat NP IVD cells | [54] | ||
| In vivo | Puncture-induced rat IDD model | ||
| Fisetin | In vitro | Rat NP IVD cells | [43] |
| Primary rat NP mesenchymal stem cells | [156] | ||
| In vivo | Puncture-induced rat IDD model | [43] | |
| Glycitin | In vitro | Human NP IVD cells | [272] |
| In vivo | Puncture-induced rat IDD model | ||
| Higenamine | In vitro | Human NP IVD cells | [274,275] |
| Honokiol | In vitro | Rat NP IVD cells | [55,164] |
| In vivo | Puncture-induced rat IDD model | ||
| Kaempferol | Network pharmacology analysis/In vitro | Human NP IVD cells | [56] |
| Network pharmacology analysis | - | [57] | |
| In vitro | Rat NP IVD cells (kaempferol-loaded fibrin glue) | [288] | |
| In vivo | Puncture-induced rat IDD model (kaempferol-loaded fibrin glue) | ||
| Kinsenoside | In vitro | Rat NP IVD cells | [58] |
| In vivo | Puncture-induced caudal rat IDD model | ||
| Luteolin | In vitro | Immortalized human NP IVD cells | [59] |
| Mangiferin | In vitro | Human NP IVD cells | [277] |
| Ex vivo | Cultured mouse IVD tissues | ||
| In vivo | Puncture-induced rat IDD model | ||
| Morroniside | Network pharmacology analysis/In vivo | Lumbar spine instability-induced IDD rat model | [191] |
| In vitro | Rat NP IVD cells | [60] | |
| In vivo | Lumbar spine instability-induced IDD mouse model | ||
| Myricetin | In vitro | Human NP IVD cells | [61] |
| Rat NP mesenchymal stem cells | [62] | ||
| Naringin | In vitro | Rat AF IVD cells | [278] |
| Human NP IVD cells | [279,280] | ||
| Human degenerated NP IVD cells | [281] | ||
| In vivo | Static and dynamic imbalance-induced IDD rat model | [278] | |
| Polydatin | In vitro | Rat NP IVD cells | [63] |
| Human endplate chondrocytes | [212] | ||
| In vivo | Puncture-induced rat IDD model | [63,212] | |
| Proanthocyanidins | In vitro | Rat NP IVD cells | [64] |
| Quercetin | In vitro | Rat and human NP IVD cells | [66,227,239] |
| Rat NP-derived mesenchymal stem cells | [67] | ||
| Human umbilical vein endothelial cells (quercetin/dasatinib combination) | [289] | ||
| In vivo | Naturally aged mice (quercetin/dasatinib combination) | [65,289] | |
| Ercc1−/Δ mice (quercetin/dasatinib combination) | [224] | ||
| Puncture-induced rat IDD model | [66,67,227,239] | ||
| Resveratrol | In vitro | Rat and human NP IVD cells | [68,69,70,71,248,249,250,251,252,253,254] |
| Rat AF IVD cells | [240] | ||
| Bovine NP IVD cells | [255] | ||
| Ex vivo | Porcine disc organ culture | [256] | |
| In vivo | Puncture-induced mouse IDD model | [98] | |
| Puncture-induced rabbit IDD model | [254,257] | ||
| Rat model of radiculopathy | [249,258] | ||
| Sesamin | Network pharmacology analysis/In vitro | ATDC5 cell line | [284] |
| In vitro | Rat NP IVD cells | [285] | |
| Ex vivo | Rat lumbar IVD organ cultures | ||
| In vivo | Lesion-induced rat IDD model | [286] | |
| o-Vanillin | In vitro | Human degenerated IVD cells and pellet cultures (o-vanillin/RG-7112 combination) | [4] |
| Human IVD cells and pellet cell cultures | [50,72] | ||
| Non-degenerate and degenerated human IVD cells | [73] | ||
| Human mesenchymal stem cells | [74] | ||
| NP IVD cells (GelMA microspheres with vanillin/TGFβ3) | [290] | ||
| Ex vivo | Human IVD organ cultures | [72] | |
| In vivo | Puncture-induced rat IDD model (GelMA microspheres with vanillin/TGFβ3) | [290] | |
| Wogonin | In vitro | Rat NP IVD cells | [287] |
| In vivo | Rat caudal vertebrae needle-stab model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrogonatou, E.; Kletsas, D. Plant-Derived Senotherapeutics for the Prevention and Treatment of Intervertebral Disc Degeneration and Aging. Metabolites 2024, 14, 146. https://doi.org/10.3390/metabo14030146
Mavrogonatou E, Kletsas D. Plant-Derived Senotherapeutics for the Prevention and Treatment of Intervertebral Disc Degeneration and Aging. Metabolites. 2024; 14(3):146. https://doi.org/10.3390/metabo14030146
Chicago/Turabian StyleMavrogonatou, Eleni, and Dimitris Kletsas. 2024. "Plant-Derived Senotherapeutics for the Prevention and Treatment of Intervertebral Disc Degeneration and Aging" Metabolites 14, no. 3: 146. https://doi.org/10.3390/metabo14030146
APA StyleMavrogonatou, E., & Kletsas, D. (2024). Plant-Derived Senotherapeutics for the Prevention and Treatment of Intervertebral Disc Degeneration and Aging. Metabolites, 14(3), 146. https://doi.org/10.3390/metabo14030146










