Creatine Acts as a Mediator of the Causal Effect of Obesity on Puberty Onset in Girls: Evidence from Mediation Mendelian Randomization Study
Abstract
1. Introduction
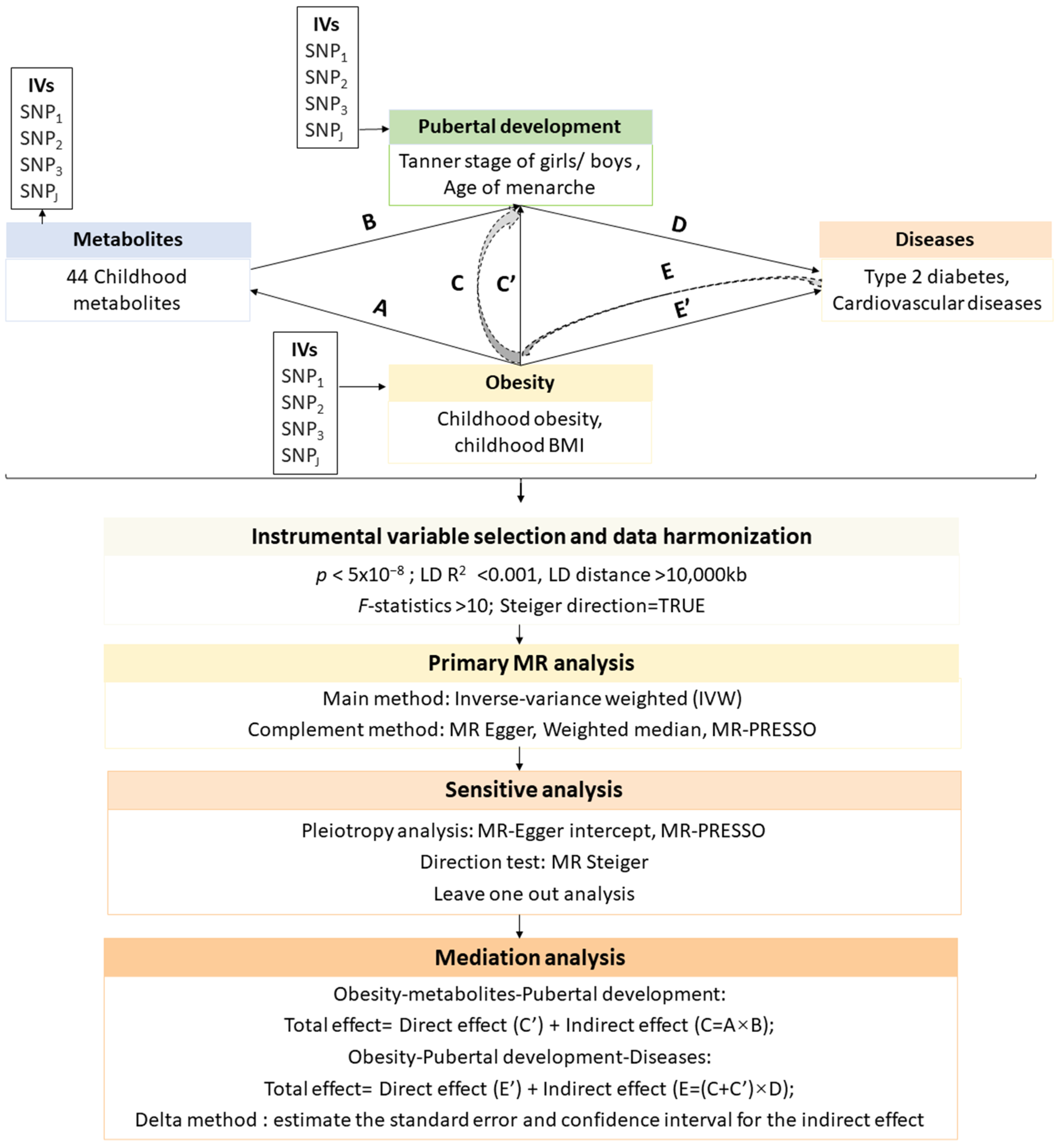
2. Materials and Methods
2.1. Data Sources
2.2. Instrumental Variable Selection and Data Harmonization
2.3. Primary MR Analysis
2.4. Mediation Analysis
2.5. Sensitive Analysis
2.6. Statistical Analysis
3. Results
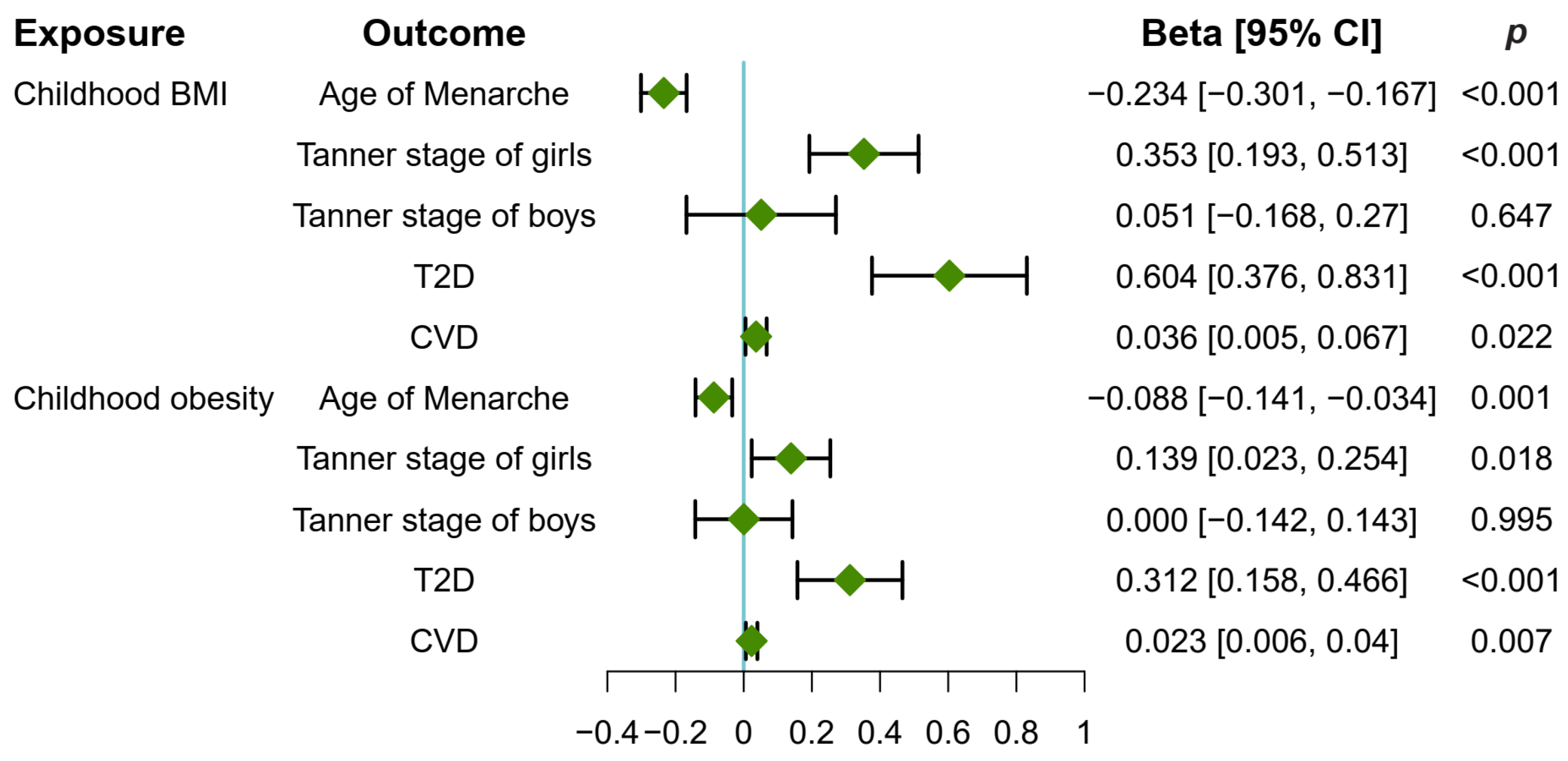
3.1. Childhood Obesity Causally Promoted Pubertal Development in Girls
3.2. Childhood Obesity Causally Increased T2D and CVD Risk
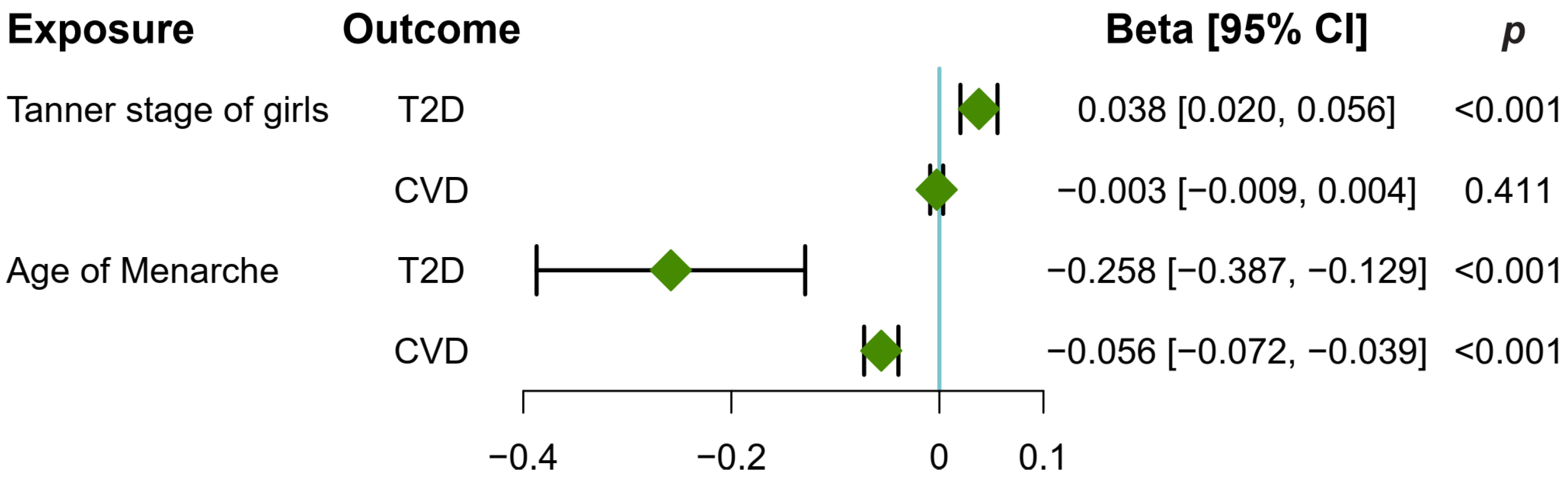
3.3. Early Puberty in Girls Causally Increased T2D and CVD Risk
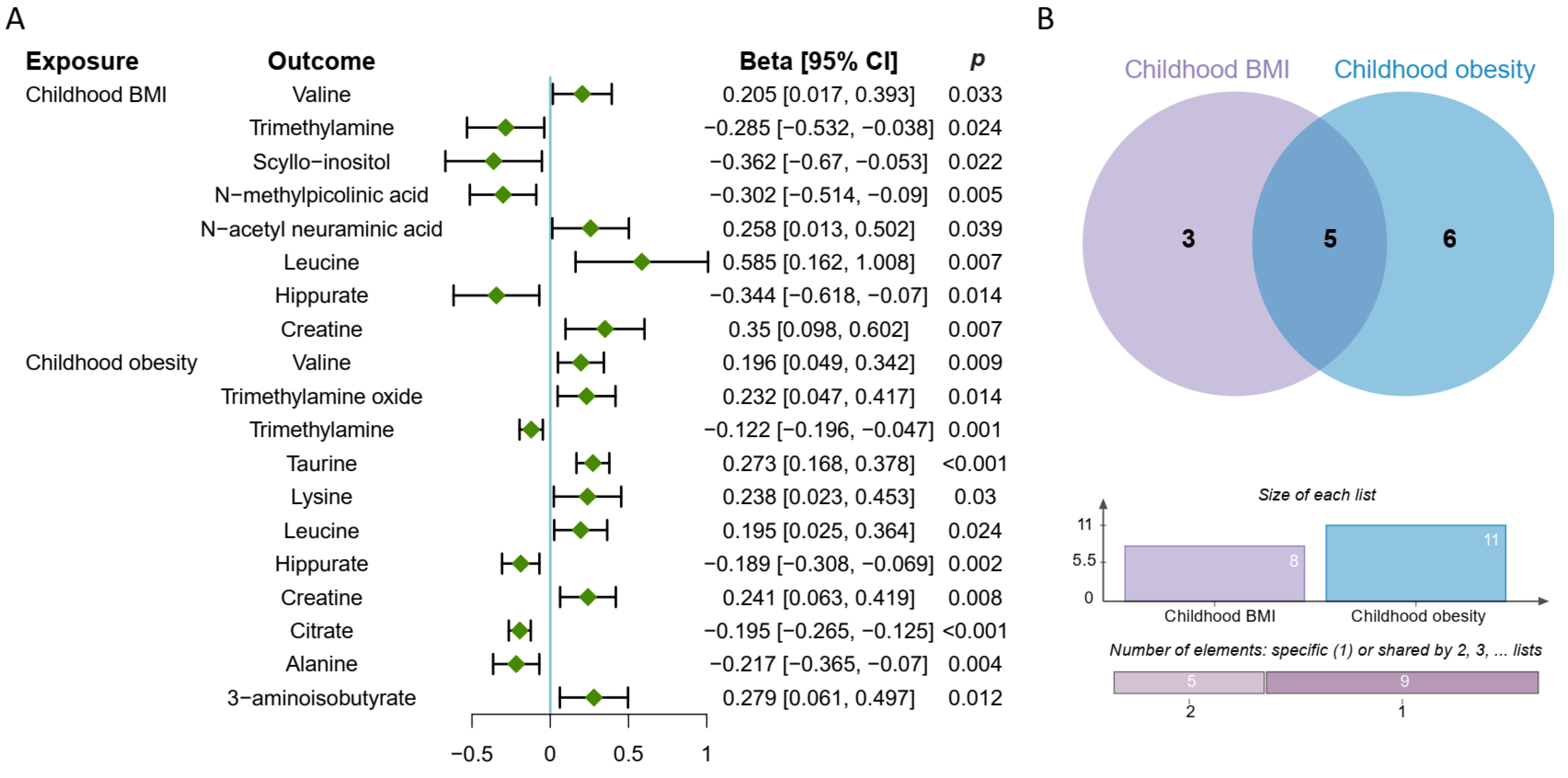
3.4. Causal Effect of Childhood Obesity on Metabolites
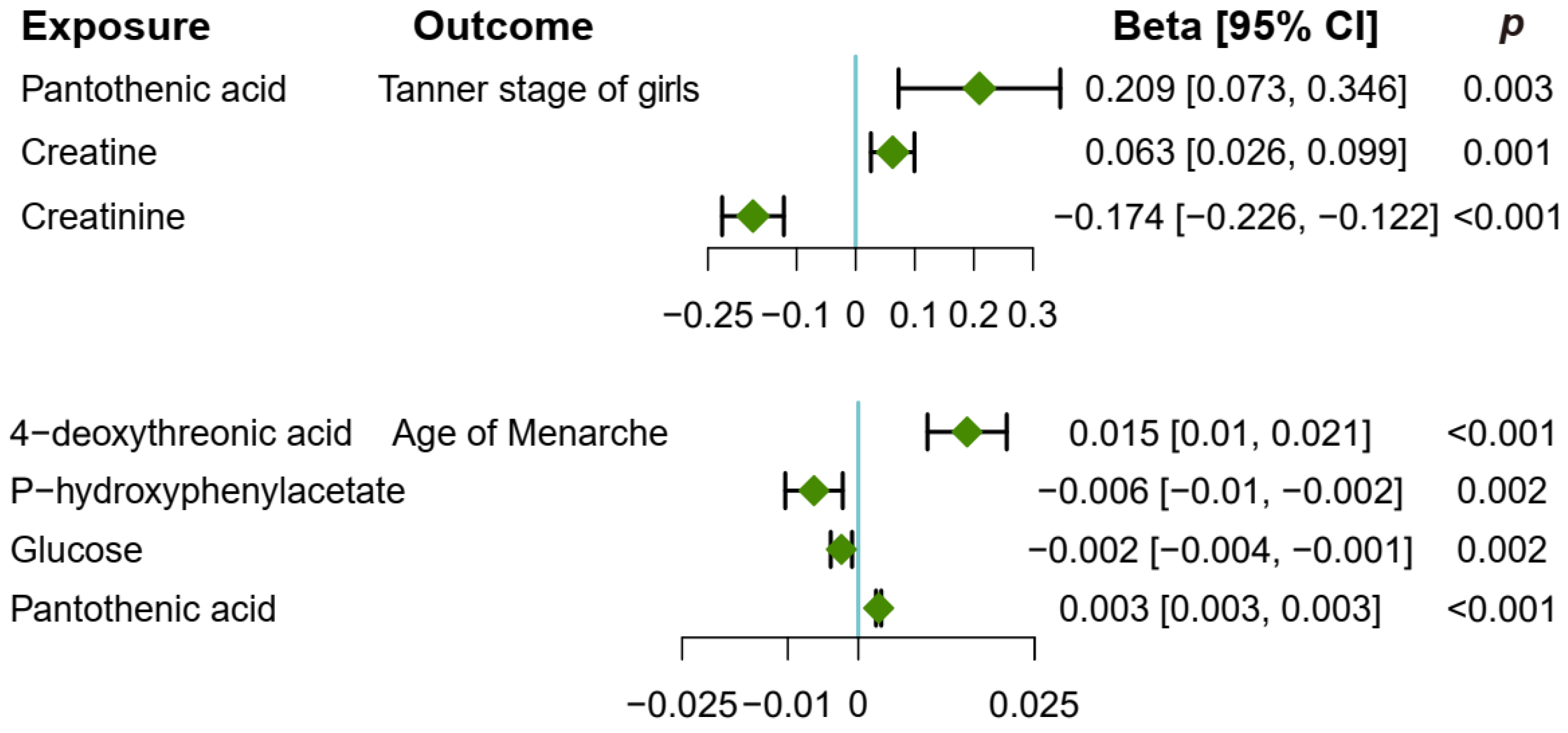
3.5. Causal Effect of Metabolites on Pubertal Development
3.6. Puberty in Girls Mediates the Causal Pathway from Childhood Obesity to T2D and CVD Risk
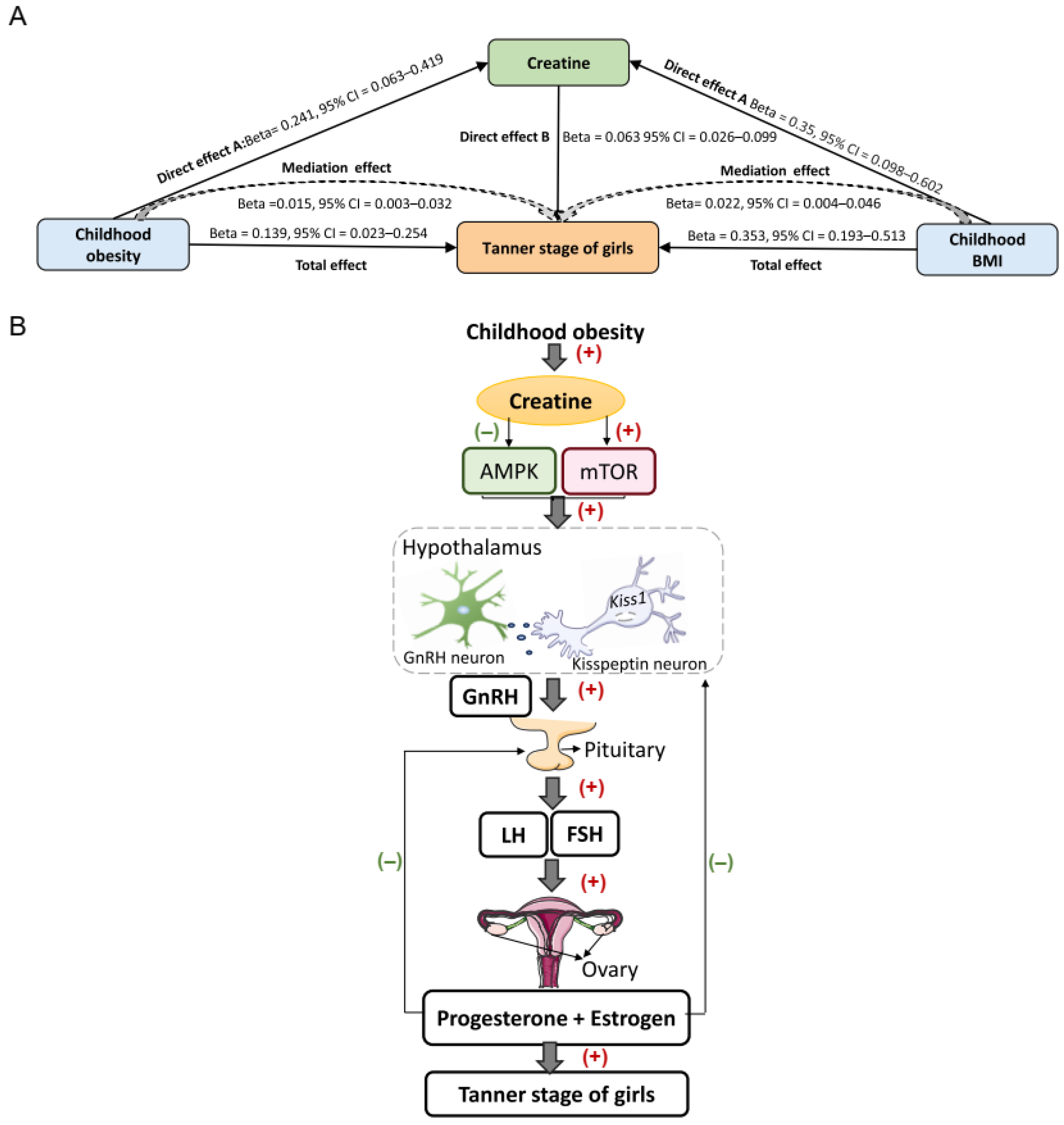
3.7. Creatine Mediates the Causal Pathway from Childhood Obesity to Pubertal Development in Girls
3.8. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burt Solorzano, C.M.; McCartney, C.R. Obesity and the pubertal transition in girls and boys. Reproduction 2010, 140, 399–410. [Google Scholar] [CrossRef]
- Mastromauro, C.; Giannini, C.; Chiarelli, F. Short stature related to Growth Hormone Insensitivity (GHI) in childhood. Front. Endocrinol. 2023, 14, 1141039. [Google Scholar] [CrossRef] [PubMed]
- Temelturk, R.D.; Ilcioglu Ekici, G.; Beberoglu, M.; Siklar, Z.; Kilic, B.G. Managing precocious puberty: A necessity for psychiatric evaluation. Asian J. Psychiatry 2021, 58, 102617. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, M.; Bokor, B.R. Tanner Stages. In StatPearls; StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, L.; Xu, F.; Zhang, Q.; Chen, J.; Zhou, Q.; Sun, C. Causal relationships between birth weight, childhood obesity and age at menarche: A two-sample Mendelian randomization analysis. Clin. Endocrinol. 2023, 98, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Wong, A.H.C.; Hon, K.L. Childhood Obesity: An Updated Review. Curr. Pediatr. Rev. 2024, 20, 2–26. [Google Scholar] [CrossRef]
- Heras, V.; Castellano, J.M.; Fernandois, D.; Velasco, I.; Rodríguez-Vazquez, E.; Roa, J.; Vazquez, M.J.; Ruiz-Pino, F.; Rubio, M.; Pineda, R.; et al. Central Ceramide Signaling Mediates Obesity-Induced Precocious Puberty. Cell Metab. 2020, 32, 951–966. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, X.; Yang, X.; Lin, X.; Cai, C.; Chen, S.; Ai, Z.; ShangGuan, H.; Wu, W.; Chen, R. Associations of Obesity With Growth and Puberty in Children: A Cross-S ectional Study in Fuzhou, China. Int. J. Public Health 2023, 68, 1605433. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, J.; Zhang, X.; Lu, Y.; Miao, J.; Xue, H. Obesity is a risk factor for central precocious puberty: A case-contro l study. BMC Pediatr. 2021, 21, 509. [Google Scholar] [CrossRef]
- Ortega, M.T.; McGrath, J.A.; Carlson, L.; Flores Poccia, V.; Larson, G.; Douglas, C.; Sun, B.Z.; Zhao, S.; Beery, B.; Vesper, H.W.; et al. Longitudinal Investigation of Pubertal Milestones and Hormones as a Function of Body Fat in Girls. J. Clin. Endocrinol. Metab. 2021, 106, 1668–1683. [Google Scholar] [CrossRef]
- Jasik, C.B.; Lustig, R.H. Adolescent obesity and puberty: The “perfect storm”. Ann. N. Y. Acad. Sci. 2008, 1135, 265–279. [Google Scholar] [CrossRef]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.T.; Arah, O.A.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansena, C.H. Childhood overweight and obesity and timing of puberty in boys and gir ls: Cohort and sibling-matched analyses. Int. J. Epidemiol. 2020, 49, 834–844. [Google Scholar] [CrossRef]
- Song, Y.; Kong, Y.; Xie, X.; Wang, Y.; Wang, N. Association between precocious puberty and obesity risk in children: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1226933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hu, Y.; Yang, Z.; Gong, Z.; Zhang, S.; Liu, X.; Chen, Y.; Ye, C.; Chen, L.; Wang, T. Overweight/Obesity in Childhood and the Risk of Early Puberty: A Syste matic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 795596. [Google Scholar] [CrossRef] [PubMed]
- Mohsenipour, R.; Abbasi, F.; Setoodeh, A.; Sayarifard, F.; Rostami, P.; Moinfar, Z.; Amoli, M.M.; Tajdini, P.; Rabbani, A. Early and delayed puberty among Iranian children with obesity. Minerva Endocrinol. 2022, 47, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Wasserman, R.; Kaciroti, N.; Gebremariam, A.; Steffes, J.; Dowshen, S.; Harris, D.; Serwint, J.; Abney, D.; Smitherman, L.; et al. Timing of Puberty in Overweight Versus Obese Boys. Pediatrics 2016, 137, e20150164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.S.; Day, F.R.; Lakshman, R.; Ong, K.K. Association of puberty timing with type 2 diabetes: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003017. [Google Scholar] [CrossRef] [PubMed]
- Day, F.R.; Elks, C.E.; Murray, A.; Ong, K.K.; Perry, J.R. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: The UK Biobank study. Sci. Rep. 2015, 5, 11208. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.T.; Krenek, A.; Magge, S.N. Childhood Obesity and Cardiovascular Disease Risk. Curr. Atheroscler. Rep. 2023, 25, 405–415. [Google Scholar] [CrossRef]
- Salama, M.; Balagopal, B.; Fennoy, I.; Kumar, S. Childhood Obesity, Diabetes. and Cardiovascular Disease Risk. J. Clin. Endocrinol. Metab. 2023, 108, 3051–3066. [Google Scholar] [CrossRef]
- Calcaterra, V.; Magenes, V.C.; Hruby, C.; Siccardo, F.; Mari, A.; Cordaro, E.; Fabiano, V.; Zuccotti, G. Links between Childhood Obesity, High-Fat Diet, and Central Precocious Puberty. Children 2023, 10, 241. [Google Scholar] [CrossRef]
- Townsend, L.K.; Steinberg, G.R. AMPK and the Endocrine Control of Metabolism. Endocr. Rev. 2023, 44, 910–933. [Google Scholar] [CrossRef]
- Shi, L.; Jiang, Z.; Zhang, L. Childhood obesity and central precocious puberty. Front. Endocrinol. 2022, 13, 1056871. [Google Scholar] [CrossRef]
- Mathew, H.; Castracane, V.D.; Mantzoros, C. Adipose tissue and reproductive health. Metab. Clin. Exp. 2018, 86, 18–32. [Google Scholar] [CrossRef]
- Hur, J.H.; Park, S.; Jung, M.K.; Kang, S.J.; Kwon, A.; Chae, H.W.; Kim, H.S.; Kim, D.H. Insulin resistance and bone age advancement in girls with central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 176–182. [Google Scholar] [CrossRef]
- Moret, M.; Stettler, R.; Rodieux, F.; Gaillard, R.C.; Waeber, G.; Wirthner, D.; Giusti, V.; Tappy, L.; Pralong, F.P. Insulin modulation of luteinizing hormone secretion in normal female volunteers and lean polycystic ovary syndrome patients. Neuroendocrinology 2009, 89, 131–139. [Google Scholar] [CrossRef]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef]
- Thankamony, A.; Ong, K.K.; Ahmed, M.L.; Ness, A.R.; Holly, J.M.; Dunger, D.B. Higher levels of IGF-I and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J. Clin. Endocrinol. Metab. 2012, 97, E786–E790. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F. Hyper-androgenemia and obesity in early-pubertal girls. J. Endocrinol. Investig. 2022, 45, 1577–1585. [Google Scholar] [CrossRef]
- Stathori, G.; Tzounakou, A.M.; Mastorakos, G.; Vlahos, N.F.; Charmandari, E.; Valsamakis, G. Alterations in Appetite-Regulating Hormones in Girls with Central Early or Precocious Puberty. Nutrients 2023, 15, 4306. [Google Scholar] [CrossRef]
- Maly, M.A.; Edwards, K.L.; Koester, D.C.; Farin, C.E.; Crosier, A.E. Assessing puberty in female cheetahs (Acinonyx jubatus) via faecal hormone metabolites and body weight. Reprod. Fertil. Dev. 2021, 33, 841–854. [Google Scholar] [CrossRef]
- Puljiz, Z.; Kumric, M.; Vrdoljak, J.; Martinovic, D.; Ticinovic Kurir, T.; Krnic, M.O.; Urlic, H.; Puljiz, Z.; Zucko, J.; Dumanic, P.; et al. Obesity, Gut Microbiota, and Metabolome: From Pathophysiology to Nutritional Interventions. Nutrients 2023, 15, 2236. [Google Scholar] [CrossRef]
- Calvani, R.; Miccheli, A.; Capuani, G.; Tomassini Miccheli, A.; Puccetti, C.; Delfini, M.; Iaconelli, A.; Nanni, G.; Mingrone, G. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. Int. J. Obes. 2010, 34, 1095–1098. [Google Scholar] [CrossRef]
- Roa, J.; Barroso, A.; Ruiz-Pino, F.; Vázquez, M.J.; Seoane-Collazo, P.; Martínez-Sanchez, N.; García-Galiano, D.; Ilhan, T.; Pineda, R.; León, S.; et al. Metabolic regulation of female puberty via hypothalamic AMPK-kisspeptin signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E10758–E10767. [Google Scholar] [CrossRef]
- Roa, J.; Garcia-Galiano, D.; Varela, L.; Sánchez-Garrido, M.A.; Pineda, R.; Castellano, J.M.; Ruiz-Pino, F.; Romero, M.; Aguilar, E.; López, M.; et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 2009, 150, 5016–5026. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Ghanbari, F.; Otomo, N.; Gamache, I.; Iwami, T.; Koike, Y.; Khanshour, A.M.; Ikegawa, S.; Wise, C.A.; Terao, C.; Manousaki, D. Interrogating Causal Effects of Body Composition and Puberty-Related Risk Factors on Adolescent Idiopathic Scoliosis: A Two-Sample Mendelian Randomization Study. JBMR Plus 2023, 7, e10830. [Google Scholar] [CrossRef]
- Vogelezang, S.; Bradfield, J.P.; Ahluwalia, T.S.; Curtin, J.A.; Lakka, T.A.; Grarup, N.; Scholz, M.; van der Most, P.J.; Monnereau, C.; Stergiakouli, E.; et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 2020, 16, e1008718. [Google Scholar] [CrossRef]
- Cousminer, D.L.; Stergiakouli, E.; Berry, D.J.; Ang, W.; Groen-Blokhuis, M.M.; Körner, A.; Siitonen, N.; Ntalla, I.; Marinelli, M.; Perry, J.R.; et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum. Mol. Genet. 2014, 23, 4452–4464. [Google Scholar] [CrossRef]
- Calvo-Serra, B.; Maitre, L.; Lau, C.E.; Siskos, A.P.; Gützkow, K.B.; Andrušaitytė, S.; Casas, M.; Cadiou, S.; Chatzi, L.; González, J.R.; et al. Urinary metabolite quantitative trait loci in children and their interaction with dietary factors. Hum. Mol. Genet. 2021, 29, 3830–3844. [Google Scholar] [CrossRef]
- Xu, L.; Hao, Y.T. Effect of handgrip on coronary artery disease and myocardial infarction: A Mendelian randomization study. Sci. Rep. 2017, 7, 954. [Google Scholar] [CrossRef]
- Yu, T.; Yu, Y.; Li, X.; Xue, P.; Yu, X.; Chen, Y.; Kong, H.; Lin, C.; Wang, X.; Mei, H.; et al. Effects of childhood obesity and related genetic factors on precocious puberty: Protocol for a multi-center prospective cohort study. BMC Pediatr. 2022, 22, 310. [Google Scholar] [CrossRef]
- Pan, D.; Fu, S.; Li, X.; Yu, T.; Huang, S.; Zhang, B.; Lai, X.; Liu, Y.; Yu, X.; Lin, C.; et al. The concordance between ultrasonographic stage of breast and Tanner stage of breast for overweight and obese girls: A school population-based study. J. Pediatr. Endocrinol. Metab. JPEM 2021, 34, 1549–1558. [Google Scholar] [CrossRef]
- Perry, J.R.; Day, F.; Elks, C.E.; Sulem, P.; Thompson, D.J.; Ferreira, T.; He, C.; Chasman, D.I.; Esko, T.; Thorleifsson, G.; et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 2014, 514, 92–97. [Google Scholar] [CrossRef]
- Giglione, E.; Lapolla, R.; Cianfarani, S.; Faienza, M.F.; Fintini, D.; Weber, G.; Delvecchio, M.; Valerio, G. Linear growth and puberty in childhood obesity: What is new? Minerva Pediatr. 2021, 73, 563–571. [Google Scholar] [CrossRef]
- Yao, H.; Li, K.; Wei, J.; Lin, Y.; Liu, Y. The contradictory role of branched-chain amino acids in lifespan and insulin resistance. Front. Nutr. 2023, 10, 1189982. [Google Scholar] [CrossRef]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D.; et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genom. 2007, 29, 99–108. [Google Scholar] [CrossRef]
- Heianza, Y.; Sun, D.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Qi, L. Changes in Gut Microbiota-Related Metabolites and Long-term Successful Weight Loss in Response to Weight-Loss Diets: The POUNDS Lost Trial. Diabetes Care 2018, 41, 413–419. [Google Scholar] [CrossRef]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e12993. [Google Scholar] [CrossRef]
- Gao, X.; Sun, G.; Randell, E.; Tian, Y.; Zhou, H. Systematic investigation of the relationships of trimethylamine N-oxide and L-carnitine with obesity in both humans and rodents. Food Funct. 2020, 11, 7707–7716. [Google Scholar] [CrossRef]
- Smith-Ryan, A.E.; Cabre, H.E.; Eckerson, J.M.; Candow, D.G. Creatine Supplementation in Women’s Health: A Lifespan Perspective. Nutrients 2021, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Cohen, P. Creatine metabolism: Energy homeostasis, immunity and cancer biology. Nat. Rev.. Endocrinol. 2020, 16, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Rajcsanyi, L.S.; Hoffmann, A.; Ghosh, A.; Matrisch-Dinkler, B.; Zheng, Y.; Peters, T.; Sun, W.; Dong, H.; Noé, F.; Wolfrum, C.; et al. Genetic variants in genes involved in creatine biosynthesis in patients with severe obesity or anorexia nervosa. Front. Genet. 2023, 14, 1128133. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.U.; Nabuurs, C.; Stockebrand, M.C.; Neu, A.; Nunes, P.; Morellini, F.; Sauter, K.; Schillemeit, S.; Hermans-Borgmeyer, I.; Marescau, B.; et al. L-arginine:glycine amidinotransferase deficiency protects from metabolic syndrome. Hum. Mol. Genet. 2013, 22, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.J.; Velasco, I.; Tena-Sempere, M. Novel mechanisms for the metabolic control of puberty: Implications for pubertal alterations in early-onset obesity and malnutrition. J. Endocrinol. 2019, 242, R51–R65. [Google Scholar] [CrossRef]
- Coyral-Castel, S.; Tosca, L.; Ferreira, G.; Jeanpierre, E.; Rame, C.; Lomet, D.; Caraty, A.; Monget, P.; Chabrolle, C.; Dupont, J. The effect of AMP-activated kinase activation on gonadotrophin-releasing hormone secretion in GT1-7 cells and its potential role in hypothalamic regulation of the oestrous cyclicity in rats. J. Neuroendocrinol. 2008, 20, 335–346. [Google Scholar] [CrossRef]
- Wen, J.P.; Liu, C.; Bi, W.K.; Hu, Y.T.; Chen, Q.; Huang, H.; Liang, J.X.; Li, L.T.; Lin, L.X.; Chen, G. Adiponectin inhibits KISS1 gene transcription through AMPK and specificity protein-1 in the hypothalamic GT1-7 neurons. J. Endocrinol. 2012, 214, 177–189. [Google Scholar] [CrossRef]
- Roa, J.; Tena-Sempere, M. Energy balance and puberty onset: Emerging role of central mTOR signaling. Trends Endocrinol. Metab. TEM 2010, 21, 519–528. [Google Scholar] [CrossRef]
- Duan, B.B.; Xu, J.W.; Xing, T.; Li, J.L.; Zhang, L.; Gao, F. Creatine nitrate supplementation strengthens energy status and delays glycolysis of broiler muscle via inhibition of LKB1/AMPK pathway. Poult. Sci. 2022, 101, 101653. [Google Scholar] [CrossRef]
- Cunha, M.P.; Budni, J.; Ludka, F.K.; Pazini, F.L.; Rosa, J.M.; Oliveira, Á.; Lopes, M.W.; Tasca, C.I.; Leal, R.B.; Rodrigues, A.L.S. Involvement of PI3K/Akt Signaling Pathway and Its Downstream Intracellular Targets in the Antidepressant-Like Effect of Creatine. Mol. Neurobiol. 2016, 53, 2954–2968. [Google Scholar] [CrossRef]
- Mao, X.; Kelty, T.J.; Kerr, N.R.; Childs, T.E.; Roberts, M.D.; Booth, F.W. Creatine Supplementation Upregulates mTORC1 Signaling and Markers of Synaptic Plasticity in the Dentate Gyrus While Ameliorating LPS-Induced Cognitive Impairment in Female Rats. Nutrients 2021, 13, 2758. [Google Scholar] [CrossRef] [PubMed]
- Pazini, F.L.; Cunha, M.P.; Rosa, J.M.; Colla, A.R.; Lieberknecht, V.; Oliveira, Á.; Rodrigues, A.L. Creatine, Similar to Ketamine, Counteracts Depressive-Like Behavior Induced by Corticosterone via PI3K/Akt/mTOR Pathway. Mol. Neurobiol. 2016, 53, 6818–6834. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, R.; Moura, E.G.; Dos Santos, V.C.; Caldeira, E.J.; Conte, M.; Matsumura, C.Y.; Pertille, A.; Mosqueira, M. High-fat diet suppresses the positive effect of creatine supplementation on skeletal muscle function by reducing protein expression of IGF-PI3K-AKT-mTOR pathway. PLoS ONE 2018, 13, e0199728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-S.; Li, M.; Wang, Y.; Li, X.; Zong, Y.; Long, S.; Zhang, M.; Feng, J.-W.; Wei, X.; Liu, Y.-H.; et al. The aldolase inhibitor aldometanib mimics glucose starvation to activa te lysosomal AMPK. Nat. Metab. 2022, 4, 1369–1401. [Google Scholar] [CrossRef]
- Walker, J.B. Creatine: Biosynthesis, regulation, and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1979, 50, 177–242. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
| Phenotype | nSNPs | Sample Size (Case) | Consortium | PMID |
|---|---|---|---|---|
| Obesity | ||||
| Childhood obesity | 2,442,739 | 5530 (8318) | EGG | 22484627 |
| Childhood BMI | 8,173,382 | 39,620 | EGG | 33045005 |
| Tanner stage | ||||
| Tanner stage of boys | 2,183,191 | 3769 | EGG | 24770850 |
| Tanner stage of girls | 2,183,191 | 6147 | EGG | 24770850 |
| Age of menarche | 9,851,867 | 243,944 | MRC-IEU | NA |
| Childhood urinary metabolite | 6,143,757 | 996 | HELIX | 33283231 |
| T2D | 24,167,560 | 490,089 (38,841) | GWAS catalog | 34594039 |
| CVD | 11,973,400 | 459,324 (146,524) | UK Biobank | 29892013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Zhao, G. Creatine Acts as a Mediator of the Causal Effect of Obesity on Puberty Onset in Girls: Evidence from Mediation Mendelian Randomization Study. Metabolites 2024, 14, 137. https://doi.org/10.3390/metabo14030137
Jin C, Zhao G. Creatine Acts as a Mediator of the Causal Effect of Obesity on Puberty Onset in Girls: Evidence from Mediation Mendelian Randomization Study. Metabolites. 2024; 14(3):137. https://doi.org/10.3390/metabo14030137
Chicago/Turabian StyleJin, Chuandi, and Guoping Zhao. 2024. "Creatine Acts as a Mediator of the Causal Effect of Obesity on Puberty Onset in Girls: Evidence from Mediation Mendelian Randomization Study" Metabolites 14, no. 3: 137. https://doi.org/10.3390/metabo14030137
APA StyleJin, C., & Zhao, G. (2024). Creatine Acts as a Mediator of the Causal Effect of Obesity on Puberty Onset in Girls: Evidence from Mediation Mendelian Randomization Study. Metabolites, 14(3), 137. https://doi.org/10.3390/metabo14030137





