Effects of Astragaloside IV on Hearing, Inflammatory Factors, and Intestinal Flora in Mice Exposed to Noise
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Groups
2.2. Noise Exposure and Procedures
2.3. Auditory Brainstem Response (ABR) Audiometry
2.4. Observation of Cochlear Hair Cells
2.5. Analysis of Inflammatory Indicators
2.6. Analysis of the Microbiota
2.7. Statistical Analysis
3. Results
3.1. AS-IV Improves Hearing in Noise-Exposed Mice
3.2. AS-IV Reduces the Loss of OHCs in Noise-Exposed Mice
3.3. AS-IV Reduces the Levels of Inflammatory Factors in Noise-Exposed Mice
3.4. AS-IV Alleviates the Disorder of Intestinal Flora in Noise-Exposed Mice
3.5. Correlation Analysis between Gut Microbiota and Inflammatory Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Natarajan, N.; Batts, S.; Stankovic, K.M. Noise-Induced Hearing Loss. J. Clin. Med. 2023, 12, 2347. [Google Scholar] [CrossRef]
- Daniel, E. Noise and hearing loss: A review. J. Sch. Health 2007, 77, 225–231. [Google Scholar] [CrossRef]
- Lie, A.; Skogstad, M.; Johannessen, H.A.; Tynes, T.; Mehlum, I.S.; Nordby, K.C.; Engdahl, B.; Tambs, K. Occupational noise exposure and hearing: A systematic review. Int. Arch. Occup. Environ. Health 2016, 89, 351–372. [Google Scholar] [CrossRef]
- Liu, S.; Zou, H.; Lei, S.; Xin, J.; Qian, P.; Liu, Y.; Chen, Y.; Yu, K.; Zhang, M. The role of kurtosis and kurtosis-adjusted energy metric in occupational noise-induced hearing loss among metal manufacturing workers. Front. Public Health 2023, 11, 1159348. [Google Scholar] [CrossRef]
- Mutlu, A.; Ocal, F.C.A.; Erbek, S.; Ozluoglu, L. The protective effect of adrenocorticotropic hormone treatment against noise-induced hearing loss. Auris Nasus Larynx 2018, 45, 929–935. [Google Scholar] [CrossRef]
- Müller, M.; Tisch, M.; Maier, H.; Löwenheim, H. Reduction of permanent hearing loss by local glucocorticoid application : Guinea pigs with acute acoustic trauma. Hno 2017, 65 (Suppl. S1), 59–67. [Google Scholar] [CrossRef]
- Soyaliç, H.; Gevrek, F.; Karaman, S. Curcumin protects against acoustic trauma in the rat cochlea. Int. J. Pediatr. Otorhinolaryngol. 2017, 99, 100–106. [Google Scholar] [CrossRef]
- Seidman, M.D.; Tang, W.; Bai, V.U.; Ahmad, N.; Jiang, H.; Media, J.; Patel, N.; Rubin, C.J.; Standring, R.T. Resveratrol decreases noise-induced cyclooxygenase-2 expression in the rat cochlea. Otolaryngol. Head Neck Surg. 2013, 148, 827–833. [Google Scholar] [CrossRef]
- Yamashita, D.; Jiang, H.-Y.; Le Prell, C.; Schacht, J.; Miller, J. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience 2005, 134, 633–642. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, J.E.; Chung, B.Y.; Lee, H.M.; Kang, H.H.; Chung, J.W.; Pak, J.H. Involvement of retinoic acid-induced peroxiredoxin 6 expression in recovery of noise-induced temporary hearing threshold shifts. Environ. Toxicol. Pharmacol. 2013, 36, 463–471. [Google Scholar] [CrossRef]
- Staecker, H.; Jokovic, G.; Karpishchenko, S.; Kienle-Gogolok, A.; Krzyzaniak, A.; Lin, C.D.; Navratil, P.; Tzvetkov, V.; Wright, N.; Meyer, T. Efficacy and Safety of AM-111 in the Treatment of Acute Unilateral Sudden Deafness-A Double-blind, Randomized, Placebo-controlled Phase 3 Study. Otol. Neurotol. 2019, 40, 584–594. [Google Scholar] [CrossRef]
- Koch, M.; Eßinger, T.M.; Stoppe, T.; Lasurashvili, N.; Bornitz, M.; Zahnert, T. Fully implantable hearing aid in the incudostapedial joint gap. Hear. Res. 2016, 340, 169–178. [Google Scholar] [CrossRef]
- Frosolini, A.; Badin, G.; Sorrentino, F.; Brotto, D.; Pessot, N.; Fantin, F.; Ceschin, F.; Lovato, A.; Coppola, N.; Mancuso, A.; et al. Application of Patient Reported Outcome Measures in Cochlear Implant Patients: Implications for the Design of Specific Rehabilitation Programs. Sensors 2022, 22, 8770. [Google Scholar] [CrossRef]
- Groves, A.K. The challenge of hair cell regeneration. Exp. Biol. Med. 2010, 235, 434–446. [Google Scholar] [CrossRef]
- Gittleman, S.N.; Le Prell, C.G.; Hammill, T.L. Octave band noise exposure: Laboratory models and otoprotection efforts. J. Acoust. Soc. Am. 2019, 146, 3800. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear. Res. 2015, 330 Pt B, 191–199. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Mizushima, Y.; Fujimoto, C.; Kashio, A.; Kondo, K.; Yamasoba, T. Macrophage recruitment, but not interleukin 1 beta activation, enhances noise-induced hearing damage. Biochem. Biophys. Res. Commun. 2017, 493, 894–900. [Google Scholar] [CrossRef]
- Möhrle, D.; Reimann, K.; Wolter, S.; Wolters, M.; Varakina, K.; Mergia, E.; Eichert, N.; Geisler, H.S.; Sandner, P.; Ruth, P.; et al. NO-Sensitive Guanylate Cyclase Isoforms NO-GC1 and NO-GC2 Contribute to Noise-Induced Inner Hair Cell Synaptopathy. Mol. Pharmacol. 2017, 92, 375–388. [Google Scholar] [CrossRef]
- Fan, B.; Lu, F.; Du, W.J.; Chen, J.; An, X.G.; Wang, R.F.; Li, W.; Song, Y.L.; Zha, D.J.; Chen, F.Q. PTEN inhibitor bisperoxovanadium protects against noise-induced hearing loss. Neural Regen. Res. 2023, 18, 1601–1606. [Google Scholar]
- Wang, X.; Zhu, Y.; Long, H.; Pan, S.; Xiong, H.; Fang, Q.; Hill, K.; Lai, R.; Yuan, H.; Sha, S.H. Mitochondrial Calcium Transporters Mediate Sensitivity to Noise-Induced Losses of Hair Cells and Cochlear Synapses. Front. Mol. Neurosci. 2018, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xiong, H.; Sha, S. Noise-induced loss of sensory hair cells is mediated by ROS/AMPKα pathway. Redox Biol. 2020, 29, 101406. [Google Scholar] [CrossRef]
- Liberman, M.C.; Dodds, L.W. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984, 16, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Beil, D.G. Hair cell condition and auditory nerve response in normal and noise-damaged cochleas. Acta Oto-Laryngol. 1979, 88, 161–176. [Google Scholar] [CrossRef]
- Nordmann, A.S.; Bohne, B.A.; Harding, G.W. Histopathological differences between temporary and permanent threshold shift. Hear. Res. 2000, 139, 13–30. [Google Scholar] [CrossRef]
- Liberman, M.C.; Dodds, L.W. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates. Hear. Res. 1984, 16, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear. Res. 2019, 377, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; He, Q.; Lai, H.; Wang, J. Astragaloside IV inhibits apoptotic cell death in the guinea pig cochlea exposed to impulse noise. Acta Oto-Laryngol. 2012, 132, 467–474. [Google Scholar] [CrossRef]
- Yang, K.; Xie, Q.; Tang, T.; Zhao, N.; Liang, J.; Shen, Y.; Li, Z.; Liu, B.; Chen, J.; Cheng, W.; et al. Astragaloside IV as a novel CXCR4 antagonist alleviates osteoarthritis in the knee of monosodium iodoacetate-induced rats. Phytomedicine 2023, 108, 154506. [Google Scholar] [CrossRef]
- Huang, D.; Shi, S.; Wang, Y.; Wang, X.; Shen, Z.; Wang, M.; Pei, C.; Wu, Y.; He, Y.; Wang, Z. Astragaloside IV alleviates PM2.5-caused lung toxicity by inhibiting inflammasome-mediated pyroptosis via NLRP3/caspase-1 axis inhibition in mice. Biomed. Pharmacother. 2022, 150, 112978. [Google Scholar] [CrossRef]
- Weng, S.; Huang, L.; Cai, B.; He, L.; Wen, S.; Li, J.; Zhong, Z.; Zhang, H.; Huang, C.; Yang, Y.; et al. Astragaloside IV ameliorates experimental autoimmune myasthenia gravis by regulating CD4+ T cells and altering gut microbiota. Chin. Med. 2023, 18, 97. [Google Scholar] [CrossRef]
- Huang, P.; Lu, X.; Yuan, B.; Liu, T.; Dai, L.; Liu, Y.; Yin, H. Astragaloside IV alleviates E. coli-caused peritonitis via upregulation of neutrophil influx to the site of infection. Int. Immunopharmacol. 2016, 39, 377–382. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, C.; Liu, R.; Li, H.; Zhang, J.; Mao, C.; Chen, C. Quantitative determination of Astragaloside IV, a natural product with cardioprotective activity, in plasma, urine and other biological samples by HPLC coupled with tandem mass spectrometry. J. Chromatogr. B 2005, 822, 170–177. [Google Scholar] [CrossRef]
- Du, X.Q.; Shi, L.P.; Chen, Z.W.; Hu, J.Y.; Zuo, B.; Xiong, Y.; Cao, W.F. Astragaloside IV Ameliorates Isoprenaline-Induced Cardiac Fibrosis in Mice via Modulating Gut Microbiota and Fecal Metabolites. Front. Cell. Infect. Microbiol. 2022, 12, 836150. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Cui, Y.; Wu, H.; Yu, Y.; Yu, S. Dysregulations of metabolites and gut microbes and their associations in rats with noise induced hearing loss. Front. Microbiol. 2023, 14, 1229407. [Google Scholar] [CrossRef]
- Cui, B.; Su, D.; Li, W.; She, X.; Zhang, M.; Wang, R.; Zhai, Q. Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: Implications for Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 190. [Google Scholar] [CrossRef]
- Chi, H.; Cao, W.; Zhang, M.; Su, D.; Yang, H.; Li, Z.; Li, C.; She, X.; Wang, K.; Gao, X.; et al. Environmental noise stress disturbs commensal microbiota homeostasis and induces oxi-inflammmation and AD-like neuropathology through epithelial barrier disruption in the EOAD mouse model. J. Neuroinflamm. 2021, 18, 9. [Google Scholar] [CrossRef]
- Xiong, M.; Lai, H.; He, Q.; Wang, J. Astragaloside IV attenuates impulse noise-induced trauma in guinea pig. Acta Oto-Laryngol. 2011, 131, 809–816. [Google Scholar] [CrossRef]
- Li, Z.; Hu, E.; Zheng, F.; Wang, S.; Zhang, W.; Luo, J.; Tang, T.; Huang, Q.; Wang, Y. The effects of astragaloside IV on gut microbiota and serum metabolism in a mice model of intracerebral hemorrhage. Phytomedicine 2023, 121, 155086. [Google Scholar] [CrossRef]
- Wu, S.; Wen, F.; Zhong, X.; Du, W.; Chen, M.; Wang, J. Astragaloside IV ameliorate acute alcohol-induced liver injury in mice via modulating gut microbiota and regulating NLRP3/caspase-1 signaling pathway. Ann. Med. 2023, 55, 2216942. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Liang, D.; Quan, X.; Gu, R.; Meng, Z.; Gan, H.; Wu, Z.; Sun, Y.; Liu, S.; et al. Pharmacological Effects of Astragaloside IV: A Review. Molecules 2023, 28, 6118. [Google Scholar] [CrossRef]
- Lai, R.; Fang, Q.; Wu, F.; Pan, S.; Haque, K.; Sha, S.H. Prevention of noise-induced hearing loss by calpain inhibitor MDL-28170 is associated with upregulation of PI3K/Akt survival signaling pathway. Front. Cell. Neurosci. 2023, 17, 1199656. [Google Scholar] [CrossRef]
- Fulian, W.; Xuying, J.; Shuang, F.; Changzhi, S. The Changes of the Electrophysiological Characteristics in the Pathway from Auditory Nerve to Cochlear Nucleus after Noise-exposure in Mouse. J. Audiol. Speech Pathol. 2023, 31, 349–354. [Google Scholar]
- Duque, D.; Pais, R.; Malmierca, M.S. Stimulus-specific adaptation in the anesthetized mouse revealed by brainstem auditory evoked potentials. Hear. Res. 2018, 370, 294–301. [Google Scholar] [CrossRef]
- Nieto-Diego, J.; Malmierca, M.S. Topographic Distribution of Stimulus-Specific Adaptation across Auditory Cortical Fields in the Anesthetized Rat. PLoS Biol. 2016, 14, e1002397. [Google Scholar] [CrossRef]
- Whittaker, A.L.; Barker, T.H. The Impact of Common Recovery Blood Sampling Methods, in Mice (Mus Musculus), on Well-Being and Sample Quality: A Systematic Review. Animals 2020, 10, 989. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, C.; Zhang, X.; Li, J.; Li, Z.; Zhu, J.; Zhou, Q.; Yang, J.; Chen, Q.; Meng, X. Combined effects of lead and manganese on locomotor activity and microbiota in zebrafish. Ecotoxicol. Environ. Saf. 2023, 263, 115260. [Google Scholar] [CrossRef]
- Kennedy, C.L.; Shuster, B.; Amanipour, R.; Milon, B.; Patel, P.; Elkon, R.; Hertzano, R. Metformin Protects Against Noise-Induced Hearing Loss in Male Mice. Otol. Neurotol. 2023, 44, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Hequembourg, S.J.; Atencio, C.A.; Rosowski, J.J.; Liberman, M.C. Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss. Hear. Res. 2000, 141, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Hemmi, T.; Maekawa, M.; Watanabe, M.; Inada, H.; Ikushima, H.; Oishi, T.; Ikeda, R.; Honkura, Y.; Kagawa, Y.; et al. Fatty acid binding protein type 7 deficiency preserves auditory function in noise-exposed mice. Sci. Rep. 2023, 13, 21494. [Google Scholar] [CrossRef] [PubMed]
- Paciello, F.; Pisani, A.; Rolesi, R.; Escarrat, V.; Galli, J.; Paludetti, G.; Grassi, C.; Troiani, D.; Fetoni, A.R. Noise-Induced Cochlear Damage Involves PPAR Down-Regulation through the Interplay between Oxidative Stress and Inflammation. Antioxidants 2021, 10, 1188. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, H.; Xing, Z.; Liu, Y.; Han, L.; Wang, Z.; Yu, L. The circadian timing of noise exposure influences noise-induced inflammatory responses in the mouse cochlea. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S3), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Paciello, F.; Di Pino, A.; Rolesi, R.; Troiani, D.; Paludetti, G.; Grassi, C.; Fetoni, A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: In vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020, 143, 111555. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.A.; Lyu, A.R.; Jeong, S.H.; Kim, T.H.; Park, M.J.; Park, Y.H. Acoustic Trauma Modulates Cochlear Blood Flow and Vasoactive Factors in a Rodent Model of Noise-Induced Hearing Loss. Int. J. Mol. Sci. 2019, 20, 5316. [Google Scholar] [CrossRef] [PubMed]
- Dhukhwa, A.; Bhatta, P.; Sheth, S.; Korrapati, K.; Tieu, C.; Mamillapalli, C.; Ramkumar, V.; Mukherjea, D. Targeting Inflammatory Processes Mediated by TRPVI and TNF-α for Treating Noise-Induced Hearing Loss. Front. Cell. Neurosci. 2019, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Yoo, J.E.; Hong, J.W.; Park, H.R.; Noh, B.; Kim, H.; Kang, M.; Hyun, Y.M.; Gee, H.Y.; Choi, J.Y.; et al. LCCL peptide cleavage after noise exposure exacerbates hearing loss and is associated with the monocyte infiltration in the cochlea. Hear. Res. 2021, 412, 108378. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.K.; Woo, J.I.; Lim, D.J. Involvement of TNF-α and IFN-γ in Inflammation-Mediated Cochlear Injury. Ann. Otol. Rhinol. Laryngol. 2019, 128 (Suppl. S6), 8s–15s. [Google Scholar] [CrossRef]
- Marshall, K.D.; Baines, C.P. Necroptosis: Is there a role for mitochondria? Front. Physiol. 2014, 5, 323. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Lin, X.; Zhu, X.; Huang, J.; Xu, W.; Tan, M.; Su, J. Melatonin Suppresses Cyclic GMP-AMP Synthase-Stimulator of Interferon Genes Signaling and Delays the Development of Hearing Loss in the C57BL/6J Presbycusis Mouse Model. Neuroscience 2023, 517, 84–95. [Google Scholar] [CrossRef]
- Ochoa, C.E.; Mirabolfathinejad, S.G.; Ruiz, V.A.; Evans, S.E.; Gagea, M.; Evans, C.M.; Dickey, B.F.; Moghaddam, S.J. Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev. Res. 2011, 4, 51–64. [Google Scholar] [CrossRef]
- Hashizume, M.; Hayakawa, N.; Suzuki, M.; Mihara, M. IL-6/sIL-6R trans-signalling, but not TNF-alpha induced angiogenesis in a HUVEC and synovial cell co-culture system. Rheumatol. Int. 2009, 29, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Q.; Pan, X.; Li, W.; Liu, W.; Jiang, W.; Huang, L.; Peng, A.; Zhang, Z. Up-Regulated Expression of Interferon-Gamma, Interleukin-6 and Tumor Necrosis Factor-Alpha in the Endolymphatic Sac of Meniere’s Disease Suggesting the Local Inflammatory Response Underlies the Mechanism of This Disease. Front. Neurol. 2022, 13, 781031. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, L.; Chen, M.; Liu, W.; Li, Y.; Wang, X.; Li, S.; Zhang, J.; Ni, X. Evaluation of Caspase-1, Interleukin-1β, and Interleukin-18, in the Middle Ear Effusion in Children with Otitis Media with Effusion. Front. Pediatr. 2021, 9, 732973. [Google Scholar] [CrossRef]
- Wang, P.; Qian, H.; Xiao, M.; Lv, J. Role of signal transduction pathways in IL-1β-induced apoptosis: Pathological and therapeutic aspects. Immun. Inflamm. Dis. 2023, 11, e762. [Google Scholar] [CrossRef] [PubMed]
- Sai, N.; Yang, Y.Y.; Ma, L.; Liu, D.; Jiang, Q.Q.; Guo, W.W.; Han, W.J. Involvement of NLRP3-inflammasome pathway in noise-induced hearing loss. Neural Regen. Res. 2022, 17, 2750–2754. [Google Scholar] [PubMed]
- Zhang, D.G.; Yu, W.Q.; Liu, J.H.; Kong, L.G.; Song, Y.D.; Li, X.F.; Fan, Z.M.; Lyu, Y.F.; Li, N.; Wang, H.-B. Serum/glucocorticoid-inducible kinase 1 deficiency induces NLRP3 inflammasome activation and autoinflammation of macrophages in a murine endolymphatic hydrops model. Nat. Commun. 2023, 14, 1249. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, T.; Chen, D.; Xu, S.; Ye, R.; Zhang, Y. Astragaloside IV Regulates Insulin Resistance and Inflammatory Response of Adipocytes via Modulating MIR-21/PTEN/PI3K/AKT Signaling. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 1538–1547. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Tang, X.H.; Jiang, S.L.; Pang, J.Q.; Xu, Y.B.; Yuan, D.D.; Zhang, L.L.; Liu, H.M.; Fan, Q. Astragaloside IV improves the pharmacokinetics of febuxostat in rats with hyperuricemic nephropathy by regulating urea metabolism in gut microbiota. Front. Pharmacol. 2022, 13, 1031509. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Long, X.; Mu, S.; Zhang, J.; Xiang, H.; Wei, W.; Sun, J.; Kuang, Z.; Yang, Y.; Chen, Y.; Zhao, H.; et al. Global signatures of the microbiome and metabolome during hospitalization of septic patients. Shock 2023, 59, 716–724. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, X.; Guo, F.; Wu, Y.; Deng, D.; Lv, L.; Dong, D.; Shang, D.; Xiang, H. Tryptophan metabolite norharman secreted by cultivated Lactobacillus attenuates acute pancreatitis as an antagonist of histone deacetylases. BMC Med. 2023, 21, 329. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Han, C.; Huang, L.; Yang, H.; Hu, J.; Chen, H.; Dou, R.; Ren, D.; Lin, H. Astragaloside IV alleviates mouse slow transit constipation by modulating gut microbiota profile and promoting butyric acid generation. J. Cell. Mol. Med. 2020, 24, 9349–9361. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Cheng, L.; Wu, Y.; Wu, T.; Wen, J.; Huang, D.; Liao, Z.; Tan, C.; Luo, Y.; et al. Gut microbiota dysbiosis characterized by abnormal elevation of Lactobacillus in patients with immune-mediated necrotizing myopathy. Front. Cell. Infect. Microbiol. 2023, 13, 1243512. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680.e7. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Patnode, M.L.; Beller, Z.W.; Han, N.D.; Cheng, J.; Peters, S.L.; Terrapon, N.; Henrissat, B.; Le Gall, S.; Saulnier, L.; Hayashi, D.K.; et al. Interspecies Competition Impacts Targeted Manipulation of Human Gut Bacteria by Fiber-Derived Glycans. Cell 2019, 179, 59–73.e13. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Rao, C.; Coyte, K.Z.; Kuziel, G.A.; Zhang, Y.; Huang, W.; Franzosa, E.A.; Weng, J.-K.; Huttenhower, C.; Rakoff-Nahoum, S. Strain-level fitness in the gut microbiome is an emergent property of glycans and a single metabolite. Cell 2022, 185, 513–529.e21. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef]
- Sarin, S.K.; Pande, A.; Schnabl, B. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol. 2019, 70, 260–272. [Google Scholar] [CrossRef]
- Wu, B.; Luo, Y.; Wu, D.; Wang, Y.; Shen, M. Phenotypic and genotypic characterization of Chinese adult patients with NLRP3-associated autoinflammatory disease with hearing loss. Rheumatology 2023, 1–9. [Google Scholar] [CrossRef]
- Koçdor, P.; Özkan, E.; Akpunar, F.; Hızal, E.; Özdemir, Y.G. Protective Effects of Infliximab Against Kanamycin-Induced Ototoxicity in Rats. Otol. Neurotol. 2023, 44, e463–e470. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Sun, R.; Wang, Q.; Liu, F.; Tang, D.; Chang, X. Standardized Astragalus Mongholicus Bunge-Curcuma Aromatica Salisb. Extract Efficiently Suppresses Colon Cancer Progression Through Gut Microbiota Modification in CT26-Bearing Mice. Front. Pharmacol. 2021, 12, 714322. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Schulz, H.M.; Regner, E.H.; Severs, E.L.; Hendrickson, J.D.; Mehta, G.; Whitney, A.K.; Ir, D.; Ohri, N.; Robertson, C.E.; et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018, 11, 357–368. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Xu, H.M.; Liang, Y.J.; Xu, J.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Yao, J.; Wang, L.S.; Nie, Y.Q.; et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4(+)CD8(+)T cells expansion. J. Nanobiotechnol. 2023, 21, 309. [Google Scholar] [CrossRef]
- Hidalgo-García, L.; Ruiz-Malagon, A.J.; Huertas, F.; Rodríguez-Sojo, M.J.; Molina-Tijeras, J.A.; Diez-Echave, P.; Becerra, P.; Mirón, B.; Morón, R.; Rodríguez-Nogales, A.; et al. Administration of intestinal mesenchymal stromal cells reduces colitis-associated cancer in C57BL/6J mice modulating the immune response and gut dysbiosis. Pharmacol. Res. 2023, 195, 106891. [Google Scholar] [CrossRef] [PubMed]

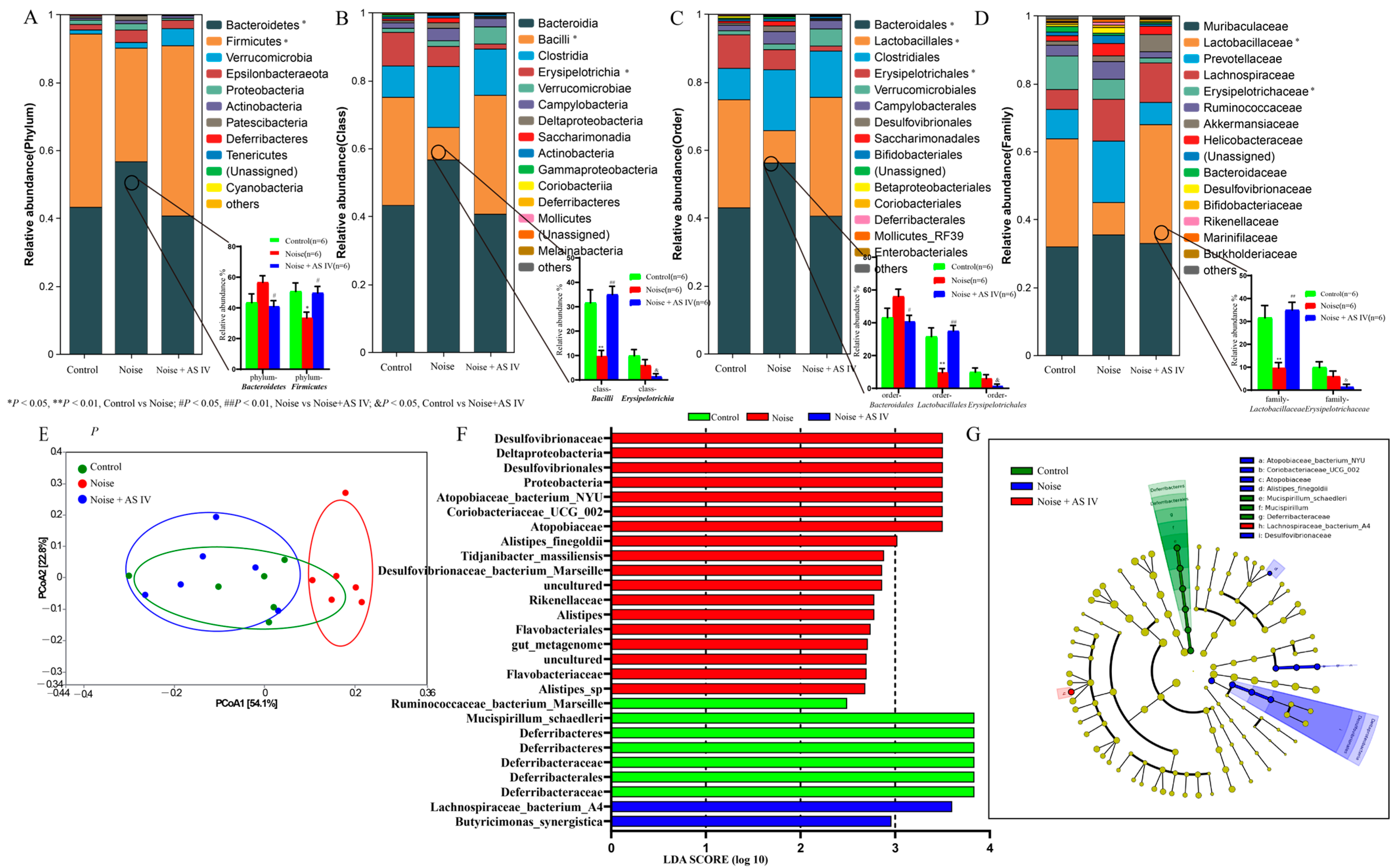
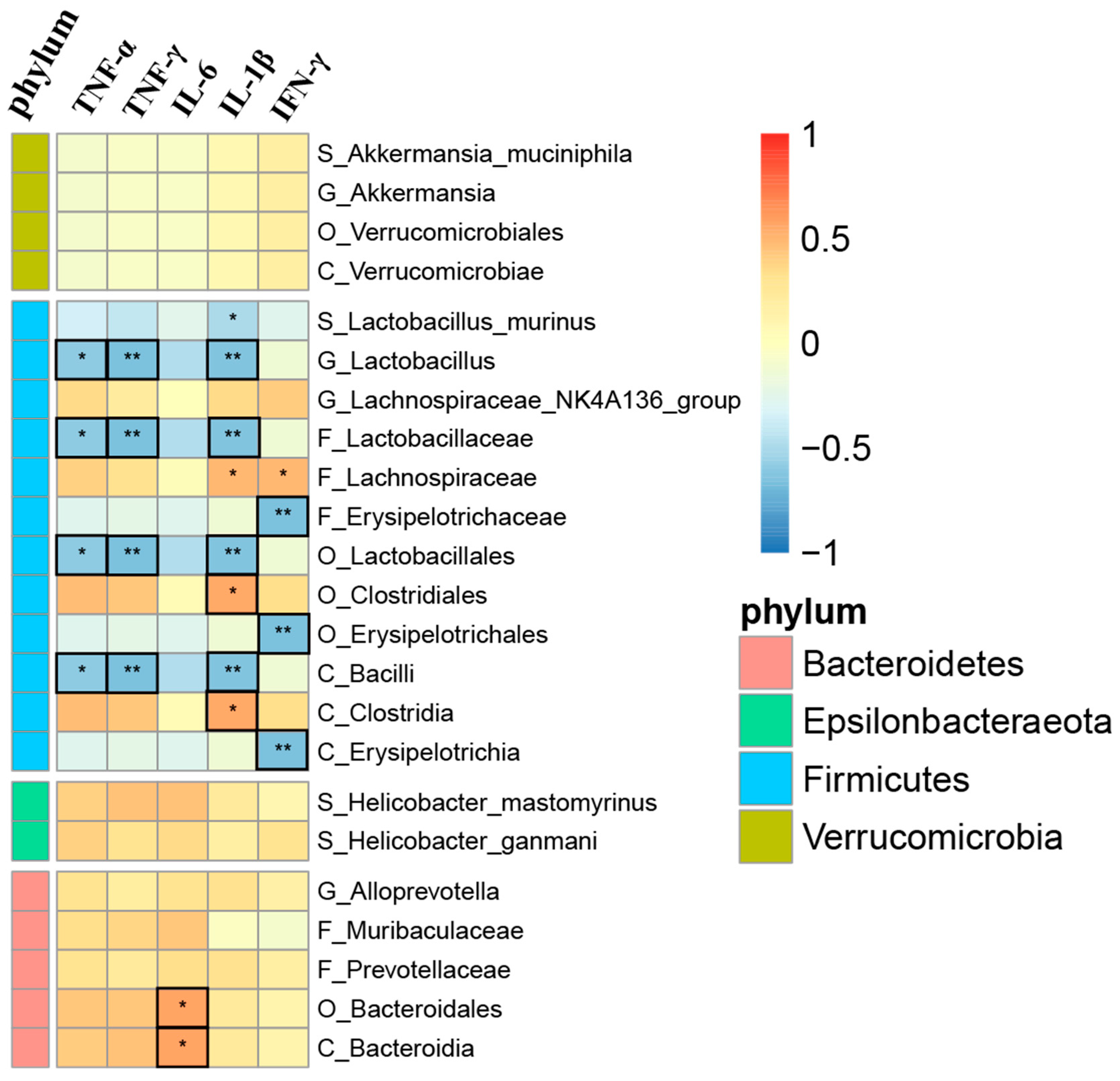
| Group | No. of Ears | Pre-Exposure | Post-Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Click | 4 kHz | 8 kHz | 16 kHz | Click | 4 kHz | 8 kHz | 16 kHz | ||
| Control | 12 | 25.3 ± 5.5 | 31.1 ± 2.9 | 20.8 ± 12.5 | 22.1 ± 8.5 | 21.3 ± 5.7 | 26.9 ± 6.8 | 23.8 ± 7.9 | 27.1 ± 8.7 |
| Noise | 12 | 19.7 ± 6.0 | 25.6 ± 6.8 | 16.2 ± 3.6 | 16.7 ± 5.0 | 42.5 ± 6.1 a | 54.4 ± 11.6 a | 37.7 ± 7.1 a | 43.3 ± 9.1 a |
| Noise + AS-IV | 12 | 23.3 ± 5.5 | 27.4 ± 6.7 | 14.9 ± 6.8 | 18.5 ± 5.2 | 29.4 ± 10.1 bc | 38.1 ± 10.7 bc | 26.0 ± 8.3 b | 32.5 ± 10.6 b |
| F | 3.03 | 2.82 | 1.63 | 2.15 | 24.18 | 23.26 | 11.07 | 9.11 | |
| p value | 0.062 | 0.074 | 0.212 | 0.133 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Group | No. of Mice | TNF-α | TNF-γ | IL-6 | IL-1β | IFN-γ |
|---|---|---|---|---|---|---|
| Control | 6 | 152.2 ± 15.7 | 33.1 ± 10.8 | 77.3 ± 15.7 | 71.9 ± 6.8 | 585.3 ± 50.0 |
| Noise | 6 | 393.8 ± 51.3 a | 59.1 ± 8.3 a | 112.7 ± 13.6 a | 102.2 ± 13.0 a | 812.4 ± 93.3 a |
| Noise + AS-IV | 6 | 277.8 ± 86.7 bc | 46.6 ± 6.9 bc | 96.4 ± 17.4 | 83.5 ± 6.2 bc | 820.4 ± 22.8 c |
| F | 25.31 | 13.02 | 7.71 | 16.59 | 27.38 | |
| p value | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, J.; Xia, Y.; Wang, J.; Xia, Y. Effects of Astragaloside IV on Hearing, Inflammatory Factors, and Intestinal Flora in Mice Exposed to Noise. Metabolites 2024, 14, 122. https://doi.org/10.3390/metabo14020122
Li J, Yang J, Xia Y, Wang J, Xia Y. Effects of Astragaloside IV on Hearing, Inflammatory Factors, and Intestinal Flora in Mice Exposed to Noise. Metabolites. 2024; 14(2):122. https://doi.org/10.3390/metabo14020122
Chicago/Turabian StyleLi, Junyi, Jian Yang, Yun Xia, Junyi Wang, and Yuan Xia. 2024. "Effects of Astragaloside IV on Hearing, Inflammatory Factors, and Intestinal Flora in Mice Exposed to Noise" Metabolites 14, no. 2: 122. https://doi.org/10.3390/metabo14020122
APA StyleLi, J., Yang, J., Xia, Y., Wang, J., & Xia, Y. (2024). Effects of Astragaloside IV on Hearing, Inflammatory Factors, and Intestinal Flora in Mice Exposed to Noise. Metabolites, 14(2), 122. https://doi.org/10.3390/metabo14020122





